Abstract
Background/Objectives
Vitamin B12 (VB12) deficiency is a common complication after total gastrectomy which may be associated with megaloblastic anemia and potentially irreversible neurologic symptoms. Intramuscular supplementation of VB12 has been considered the standard treatment, although it is associated with high costs and patient discomfort.
Patients/Methods
We performed a prospective uncontrolled study (ACTRN12614000107628) in order to evaluate the clinical and laboratory efficacy of long-term oral VB12 supplementation in patients submitted to total gastrectomy. All patients received daily oral VB12 (1 mg/day) and were evaluated every 3 months (clinical and laboratory evaluation: hemoglobin, VB12, total iron, ferritin, and folate).
Results
A total of 26 patients were included with a mean age of 64 years (29–79). Patients were included with a mean period of 65 months (3–309) after total gastrectomy. At inclusion time, 17/26 patients were under intramuscular VB12, and 9 had not started supplementation yet. There were normal serum VB12 levels in 25/26 patients (mean VB12 serum levels: 657 pg/mL). The mean follow-up period was 20 (8.5–28) months. During follow-up, all patients had normal VB12 levels and there was no need for intramuscular supplementation. The patient with low VB12 levels had an increase to adequate levels, which remained stable. There were no differences with statistical significance among VB12 levels at 6 (867 pg/mL), 12 (1,008 pg/mL), 18 (1,018 pg/mL), and 24 (1,061 pg/mL) months. Iron and folate supplementation was necessary in 21 and 7 patients, respectively.
Conclusions
Oral VB12 supplementation is effective and safe in patients who underwent total gastrectomy and should be considered the preferential form of supplementation.
Keywords: Vitamin B12 deficiency, Oral supplementation, Total gastrectomy, Prospective study
Resumo
Introdução
O défice de vitamina B12 (vitB12) ocorre de forma quase universal e precocemente após gastrectomia total (GT), podendo associar-se a anemia megaloblástica e alterações neurológicas potencialmente irreversíveis. A administração intramuscular de vitB12 é considerada a forma de suplementação adequada, sendo, contudo, desconfortável, dispendiosa e, atualmente, de acesso difícil.
Métodos/Objetivo
Estudo prospetivo, não controlado (ACTRN12614000107628), cujo objetivo principal foi avaliar a eficácia clínica e laboratorial a longo prazo da suplementação oral com vitB12 em doentes com GT. O objetivo secundário foi avaliar outros défices nutricionais (ferro e folatos). Os doentes foram medicados com vitB12 oral (1mg/dia) e sujeitos a avaliação clínica e laboratorial trimestral (hemoglobina, vitB12, ferro, ferritina e ácido fólico). SPSS 23 (Wilcoxon test).
Resultados
Incluídos 26 doentes (M-18; F-8), idade média 64 anos (29–79), com diagnósticos de adenocarcinoma (n = 25) e linfoma MALT (n = 1). Os doentes foram incluídos em média 65 meses (3–309) após GT. À data de inclusão, 17/25 doentes encontravam-se medicados com vitB12 intramuscular e 9 ainda não tinham iniciado suplementação, verificando-se níveis séricos adequados de vitB12 em 25/26 doentes (1/26 com níveis de vitB12 baixos por incumprimento da terap'utica intramuscular), sendo o valor médio de 657 pg/mL (136–1,642). Os doentes foram avaliados durante uma mediana de 23 meses (IQR 8,8). No follow-up todos os doentes apresentaram níveis normais de vitB12, não sendo necessária terap'utica intramuscular. O doente com vitB12 baixa registou um incremento para níveis adequados, que se mantiveram estáveis. Não houve diferenças com significado estatístico nos níveis de vitB12 aos 6 (867 pg/mL), 12 (1,008 pg/mL) e 24 (1,061 pg/mL) meses, embora com aumento progressivo dos mesmos. A suplementação com ferro e folatos foi necessária em 21 e 7 doentes, respetivamente.
Conclusão
A suplementação oral de vitB12 é eficaz e segura em doentes com GT, pelo que esta deve ser considerada a forma preferencial de administração neste grupo de doentes.
Palavras Chave: Défice de vitamina B12, Suplementação oral, Gastrectomia total, Estudo prospetivo
Introduction
Anemia is a frequent complication after total gastrectomy, and deficiencies of iron, vitamin B12 (VB12) or folate, either alone or in combination, are common after the surgical procedure [1]. Notably, VB12 deficiency, which causes megaloblastic anemia and a spectrum of neuropsychiatric disorders, is one of the common long-term nutritional sequelae after total gastrectomy [2].
VB12 deficiency develops as a consequence of the elimination of the source of intrinsic factor which is essential for VB12 absorption in the distal small bowel and also because of a defect in the separation of VB12 from its transporter protein [3]. Due to the elimination of intrinsic factor source after total gastrectomy, it has been generally accepted that the only appropriate and effective therapy is the intramuscular (IM) administration of VB12 [4]. However, this is associated with high costs and patient discomfort and, therefore, alternative routes of administration tend to be equated.
Previous studies showed that oral VB12 successfully treats patients with pernicious anemia who lack the intrinsic factor-dependent pathway as a result of the presence of autoantibodies specific for intrinsic factor [5, 6]. However, the evidence about the long-term efficacy of oral VB12 supplementation in patients after total gastrectomy is still sparse [7, 8].
Additionally, in several countries, including Portugal, a suspension in the supply of injectable VB12 was verified [9]. This disruption forced physicians to find alternative sources or make therapeutic approaches with clinical outcomes which are not fully clarified.
That shortage, however, presented an opportunity to find an evidence-based therapeutic alternative for VB12 deficiency. Thus, the primary objective of this study was to evaluate the clinical and laboratory efficacy of long-term oral VB12 supplementation in patients who underwent total gastrectomy. The secondary objective was to assess other nutritional deficiencies, namely iron and folate deficiency.
Materials and Methods
To address the outcomes, we performed an observational, prospective, and uncontrolled study at the Portuguese Institute of Oncology of Lisbon. It started in January 2014 and had the following inclusion criteria: (1) adult patients (≥18 years) who underwent total gastrectomy; (2) oral feeding capacity; (3) no evidence of active malignant disease; (4) able to provide informed consent. Exclusion criteria were as follows: (1) unable to provide informed consent; (2) metastatic disease.
From the time of study enrolment, all patients received daily oral VB12 (dosage: 1 mg of cobalamin); in the group of patients who had already started IM supplementation, that route was stopped, and patients were started on oral supplementation (group A), and in the group of patients who had recently undergone total gastrectomy, oral VB12 was promptly started (group B). All patients were prospectively followed every 3 months until April 2016. The follow-up study consisted of interim history taking, physical examination, and blood tests. Our main outcome measure was serum VB12 levels. Additional outcome measures were hemoglobin, serum iron, serum ferritin, total iron-binding capacity, and folate levels. During the regular medical visits, patients were asked about symptoms associated with VB12 deficiency including asthenia, dyspnea or dizziness, stomatitis, loss of appetite, palpitation, tingling sensations and/or paresthesia of the hands or feet, tremor, memory impairment, and irritability [8].
Anemia was defined by World Health Organization criteria [10] (hemoglobin <12 g/dL in women and <13 g/dL in men). Iron, VB12, and folate deficiency were defined as serum ferritin <20 μg/dL, VB12 <200 pg/mL, and folate levels <3.0 ng/mL, respectively. Iron deficiency anemia was defined as anemia with concomitant iron deficiency, and anemia from VB12 deficiency was defined as megaloblastic anemia (MCV >100 fL) with VB12 deficiency. All the patients whose serum VB12 levels were <200 pg/mL were candidates for IM VB12 replacement. Iron (oral or parenteral) or folate supplementation was started at the attending physician discretion in evidence of iron or folate deficiency, respectively.
The study was approved by our hospital's ethical committee, and written informed consent was obtained from every patient. The study was registered on the Australian New Zealand Clinical Trials Registry (ACTRN12614000107628).
Data handling and statistical analysis were performed with IBM SPSS software package version 22.0 (SPSS Inc., Chicago, IL, USA). Continuous data are presented as means or median and range, and categorical data are presented as proportions. Data were analyzed using the Wilcoxon test to compare the difference between serum VB12 levels during follow-up. A p value <0.05 was considered statistically significant.
Results
A total of 26 patients with a mean age of 64 years (29–79) were included in this study with a mean period of 65 months (3–309) after total gastrectomy. Patients' characteristics are shown in Table 1.
Table 1.
Patient characteristics (n = 26)
| Gender | |
| Male | 18 (69%) |
| Female | 8 (31%) |
| Age, years | 64 (29–79) |
| Total gastrectomy | |
| Gastric adenocarcinoma | 25 (96%) |
| Stage I/II/III | 8/5/12 |
| Gastric MALT lymphoma | 1 (4%) |
| Stage EII1 | 1 |
| Time after total gastrectomy - T0, months | 65 (3–309) |
At the beginning of the study (T0), 17/26 patients were under IM VB12 supplementation (group A), and 9/26 patients had not started VB12 supplementation (group B) (Table 2). The frequency of IM therapy in group A was as follows: 1,000 μg every 3 months - 4 patients; 1,000 μg every 2 months - 6 patients; 1,000 μg monthly - 7 patients. The mean serum VB12 levels among patients in group A and B were 821 pg/mL (186–2,642) and 395 pg/mL (236–657), respectively. Despite having no symptoms, the mean VB12 concentration was below the lower limit of normal (200 pg/mL) in one patient from group A due to noncompliance with IM supplementation.
Table 2.
Serum VB12 levels (T0)
| Mean serum VB12 levels, pg/mL | 657 |
| Group A (with IM supplementation) | |
| Patients, n | 17 |
| Time after total gastrectomy, months | 102 (17–309) |
| Serum VB12 levels, pg/mL | 821 (186–2,642) |
| Group B (without IM supplementation) | |
| Patients, n | 9 |
| Time after total gastrectomy, months | 6 (3–7) |
| Serum VB12 levels, pg/mL | 395 (236–657) |
The mean and median follow-up period was 20 (8.5–28) and 23 months (IQR = 8.8), respectively. Three patients died during follow-up: 2 due to malignancy recurrence and 1 due to unrelated cause.
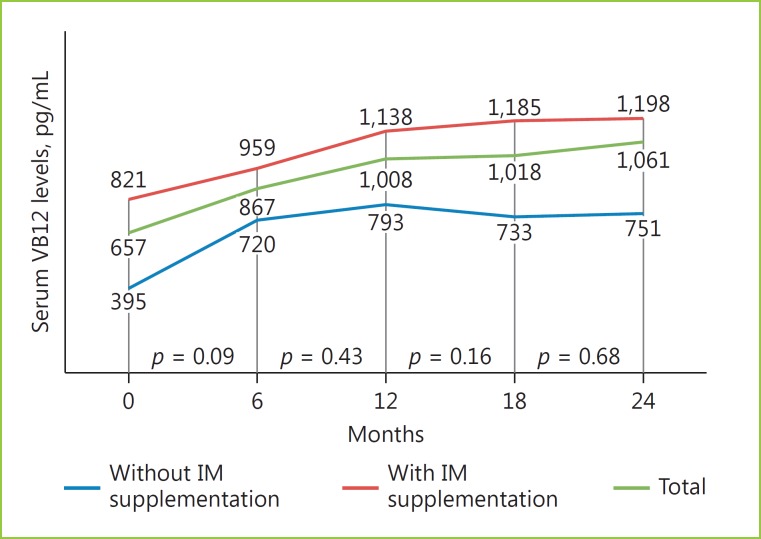
In both groups, there was a progressive increase in serum VB12 levels within the first 12 months, which remained stable thereafter (Fig. 1). None of the patients presented symptoms associated to VB12 deficiency. Remarkably, all patients had normal serum VB12 levels and none was submitted to IM supplementation. Furthermore, there were no side effects or adverse events. The patient with low serum VB12 levels had an increase to adequate levels, which kept stable. There were no differences with statistical significance among VB12 levels at 6 (867 pg/mL), 12 (1,008 pg/mL), 18 (1,018 pg/mL), and 24 (1,061 pg/mL) months, although there was a trend for progressive increase (Fig. 1).
Fig. 1.
Serum VB12 levels during follow-up.
The incidence of anemia was 19% (n = 5) in the third month of follow-up but decreased to 7% in the in the twelfth month (n = 2) (Table 3). There were no patients with megaloblastic anemia. Oral iron and folate supplementation was necessary in 21 and 7 patients, respectively (Table 3).
Table 3.
Laboratory results during the follow-up
| 0 months | 6 months | 12 months | 18 months | 24 months | |
| Patients, n | 26 | 26 | 24 | 19 | 15 |
| VB12, pg/mL | 657 (136–2,642) | 867 (453–3,500) | 1,008 (428–3,765) | 1,018 (301–3,828) | 1,078 (395–3,920) |
| IM VB12, n | 0 | 0 | 0 | 0 | 0 |
| Folate, ng/mL | 8.9 (3–12) | 8.1 (2.4–13) | 7.8 (2.9–14) | 8.7 (2.8–22) | 6.7 (2.3–14) |
| Oral folate, n | 0 | 1 | 2 | 2 | 2 |
| Iron, mg/L | 80 (24–146) | 99 (54–159) | 92 (39–184) | 103 (52–208) | 102 (52–148) |
| Ferritin, ng/mL | 105 (105–391) | 130 (5–641) | 128 (5–357) | 98 (5–352) | 86 (5–392) |
| Oral/i.v. iron, n | 5/0 | 4/0 | 4/0 | 5/0 | 3/0 |
| Hg, g/dL | 13.0 (11.9–16.2) | 13.7 (11.9–16.4) | 13.9 (11.5–15.6) | 13.9 (11.6–15.4) | 13.8 (11.5–15.3) |
Discussion
The proper management of functional and nutritional impairments after gastric surgery has become a primary treatment concern in patients submitted to total gastrectomy [11]. Anemia is a frequent complication after total gastrectomy, and deficiencies of iron, VB12, or folate, either alone or in combination, are also common during follow-up [1, 12].
Impaired iron absorption is explained by the following mechanisms: bypass of the duodenum, increased intestinal transit time and reduction in gastric acid which is necessary for the absorption of food iron [3]. The VB12 deficiency develops as a consequence of the decreased production of intrinsic factor, which is essential for VB12 absorption in the distal small bowel, and also because of a defect in the separation of VB12 from its transporter protein [3].
VB12 plays an important role in DNA synthesis and neurologic function [2]. VB12 deficiency is associated with hematologic, neurologic, and psychiatric manifestations [2]. In addition, VB12 deficiency may exert indirect cardiovascular effects since it is associated with hyperhomocysteinemia, which is an independent risk factor for atherosclerotic disease [13].
VB12 deficiency can develop as early as 1 year after total gastrectomy even before the onset of anemia [1, 14], and it should be routinely prescribed in this group of patients [8]. The standard treatment of VB12 deficiency has been parenteral administration, usually by IM injection, under the form of cyanocobalamin, hydroxocobalamin, or methylcobalamin [15, 16]. However, IM injections burden patients' and caregivers' time, cause unnecessary discomfort and contribute to considerable costs to the health system [9]. Thus, alternative routes of VB12 administration tend to be proposed.
An evidence-based analysis supports the efficacy of oral VB12 supplementation in several scenarios [5, 7, 8, 15, 17, 18, 19, 20]. Two randomized controlled trials, although with a follow up shorter than 4 months, showed that oral VB12 was as effective as IM supplementation in improving VB12 levels and associated biochemical markers, anemia and neurologic symptoms in patients with megaloblastic anemia due to VB12 deficiency [5, 18]. Two other studies showed that even in patients submitted to gastrectomy, VB12 deficiency could be easily reversed with oral supplementation [7, 8]. However, the role of oral VB12 supplementation therapy in patients who underwent gastrectomy has not yet been completely validated in clinical practice, most notably the long-term efficacy. In fact, in the available studies, the length of the follow-up (no longer than 4 months) is considered insufficient because of the biological half-time of body stores of VB12, which is estimated to be more than 480 days [21].
With this study, we were able to show the efficacy of long-term oral supplementation with VB12, particularly in asymptomatic patients with normal VB12 levels. At the beginning of our study, almost all patients had adequate hepatic stores of VB12 (only 1 patient had low VB12 levels, and 17 patients were under IM VB12 supplementation). During a mean follow-up of 20 months, the serum levels progressively increased, which is in accordance with the preliminary study of Kim et al. [7], and remained stable thereafter, supporting the long-term efficacy of this administration route. The actual transport mechanism used in this pathway remains unclear. A possible explanation is given by the alternative pathway for VB12 absorption, the passive diffusion, which is independent of intrinsic factor [22]. The absorption rate of this pathway is only about 1%, whereas that of the intrinsic factor-dependent pathway is 60% [17]. The intrinsic factor-independent pathway is insufficient to meet the daily requirement of 1–2 μg of VB12 with usual food intake [2]. Exceptionally, large amounts of oral VB12, can, however, meet the daily requirement of VB12 by the intrinsic factor-independent pathway despite the low absorption rate [7]. Another possible explanation is the ectopic production of intrinsic factor in the duodenum and jejunum [8].
A long-term follow-up study showed that anemia was common after gastrectomy and increased from 25% at 3 months to 37% at 48 months after surgery, iron deficiency being the major cause [1]. In our study, the incidence of anemia was lower and decreased during follow-up, showing that a close monitoring and appropriate nutritional supplementation can easily prevent the incidence of anemia after gastrectomy.
Our study has some limitations which have to be pointed out. This study was a single-center study and we did not measure serum methylmalonic acid and homocysteine levels, which are more sensitive in the early diagnosis of VB12 deficiency [23]. However, most of the patients had levels >400 pg/ml, which definitively excludes VB12 deficiency [3]. The major strength of our study is the tightly controlled long-term follow-up, with systematically and prospectively scheduled serial follow-up visits.
In conclusion, our study showed that oral VB12 should be offered to asymptomatic patients who underwent total gastrectomy and had normal VB12 levels. This therapy is inexpensive, convenient, and effective, preventing the symptoms and anemia associated with VB12 deficiency.
Statement of Ethics
This study did not require informed consent nor review/approval by the appropriate ethics committee.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Lim C-H, Kim SW, Kim WC, Kim JS, Cho YK, Park JM, et al. Anemia after gastrectomy for early gastric cancer: long-term follow-up observational study. World J Gastroenterol. 2012;14:6114–6119. doi: 10.3748/wjg.v18.i42.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh R, Brown DL. Vitamin B12 deficiency. Am Fam Physician. 2003;67:979–986. [PubMed] [Google Scholar]
- 3.Fernández-Bañares F, Monzón H, Forné M. A short review of malabsorption and anemia. World J Gastroenterol. 2009;15:4644–4652. doi: 10.3748/wjg.15.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernánde Sabiston DC, Townsend CM. Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. ed 18. Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 5.Kuzminski AM, Del Giacco EJ, Allen RH, Stabler SP, Lindenbaum J. Effective treatment of cobalamin deficiency with oral cobalamin. Blood. 1998;92:1191–1198. [PubMed] [Google Scholar]
- 6.Berlin R, Berlin H, Brante G, Pilbrant A. Vitamin B12 body stores during oral and parenteral treatment of pernicious anaemia. Acta Med Scand. 1978;204:81–84. doi: 10.1111/j.0954-6820.1978.tb08402.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim H, Hyung WJ, Song KJ, Choi SH, Kim C-B, Noh SH. Oral vitamin B12 replacement: an effective treatment for vitamin B12 deficiency after total gastrectomy in gastric cancer patients. Ann Surg Oncol. 2011;18:3711–3717. doi: 10.1245/s10434-011-1764-6. [DOI] [PubMed] [Google Scholar]
- 8.Adachi S, Kawamoto T, Otsuka M, Todoroki T, Fukao K. Enteral vitamin B12 supplements reverse postgastrectomy B12 deficiency. Ann Surg. 2000;232:199–201. doi: 10.1097/00000658-200008000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolber M, Houle S. Oral vitamin B12: a cost-effective alternative. Can Fam Physician. 2014;60:111–112. [PMC free article] [PubMed] [Google Scholar]
- 10.Cable CT, Colbert CY, Showalter T, Ahluwalia R, Song J, Whitfield P, Rodriguez J. Prevalence of anemia after Roux-en-Y gastric bypass surgery: what is the right number? Surg Obes Relat Dis. 2011;7:134–139. doi: 10.1016/j.soard.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Schölmerich J. Postgastrectomy syndromes - diagnosis and treatment. Best Pract Res Clin Gastroenterol. 2004;18:917–933. doi: 10.1016/j.bpg.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Beyan C, Beyan E, Kaptan K, Ifran A, Uzar AI. Postgastrectomy anemia: evaluation of 72 cases with postgastrectomy anemia. Hematology. 2007;12:81–84. doi: 10.1080/10245330600938554. [DOI] [PubMed] [Google Scholar]
- 13.Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337:230–236. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 14.Bae JM, Park JW, Yang HK, et al. Nutritional status of gastric cancer patients after total gastrectomy. World J Surg. 1998;22:254–261. doi: 10.1007/s002689900379. [DOI] [PubMed] [Google Scholar]
- 15.Andrès E, Loukili NH, Noel E, Kaltenbach G, Abdelgheni MB, Perrin AE, et al. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ. 2004;171:251–259. doi: 10.1503/cmaj.1031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dali-Youcef N, Andrès E. An update on cobalamin deficiency in adults. QJM. 2009;102:17–28. doi: 10.1093/qjmed/hcn138. [DOI] [PubMed] [Google Scholar]
- 17.Elia M. Oral or parenteral therapy for B12 deficiency. Lancet. 1998;352:7211–7212. doi: 10.1016/S0140-6736(05)79821-4. [DOI] [PubMed] [Google Scholar]
- 18.Bolaman Z, Kadikoylu G, Yukselen V, Yavasoglu I, Barutca S, Senturk T. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center, prospective, randomized, open-label study. Clin Ther. 2003;25:3124–3134. doi: 10.1016/s0149-2918(03)90096-8. [DOI] [PubMed] [Google Scholar]
- 19.Lederle FA. Oral cobalamin for pernicious anemia. Medicine's best kept secret? JAMA. 1991;265:94–95. [PubMed] [Google Scholar]
- 20.Butler C, Vidal-Alaball J, Cannings-John R, McCaddon A, Hood K, Papaioannou A, McdowelI I, Goringe A. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials. Fam Pract. 2006;23:279–285. doi: 10.1093/fampra/cml008. [DOI] [PubMed] [Google Scholar]
- 21.Basu TK, Dickerson JWT. Vitamins in Human Health and Disease. Wallingford: CAB International; 1996. [Google Scholar]
- 22.Doscherholmen A, Hagen PS. A dual mechanism of vitamin B12 plasma absorption. J Clin Invest. 1957;36:1551–1155. doi: 10.1172/JCI103552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savage DG, Lindenbaum J, Stabler SP, Allen RH. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med. 1994;96:239–246. doi: 10.1016/0002-9343(94)90149-x. [DOI] [PubMed] [Google Scholar]