Abstract
Background: Increased breast density is a strong risk factor for breast cancer and also decreases the sensitivity of mammographic screening. The purpose of our study was to compare breast density for black and white women using quantitative measures.
Methods: Breast density was assessed among 5282 black and 4216 white women screened using digital mammography. Breast Imaging-Reporting and Data System (BI-RADS) density was obtained from radiologists’ reports. Quantitative measures for dense area, area percent density (PD), dense volume, and volume percent density were estimated using validated, automated software. Breast density was categorized as dense or nondense based on BI-RADS categories or based on values above and below the median for quantitative measures. Logistic regression was used to estimate the odds of having dense breasts by race, adjusted for age, body mass index (BMI), age at menarche, menopause status, family history of breast or ovarian cancer, parity and age at first birth, and current hormone replacement therapy (HRT) use. All statistical tests were two-sided.
Results: There was a statistically significant interaction of race and BMI on breast density. After accounting for age, BMI, and breast cancer risk factors, black women had statistically significantly greater odds of high breast density across all quantitative measures (eg, PD nonobese odds ratio [OR] = 1.18, 95% confidence interval [CI] = 1.02 to 1.37, P = .03, PD obese OR = 1.26, 95% CI = 1.04 to 1.53, P = .02). There was no statistically significant difference in BI-RADS density by race.
Conclusions: After accounting for age, BMI, and other risk factors, black women had higher breast density than white women across all quantitative measures previously associated with breast cancer risk. These results may have implications for risk assessment and screening.
Breast density is a strong risk factor for breast cancer; women with high breast density have four to six times increased risk of breast cancer compared with women with low density ( 1–12 ). This higher risk is partly explained by the fact that dense tissue can mask tumors, making mammography less sensitive among women with dense breasts ( 13 ) while tumor size at diagnosis also increases and prognosis worsens with increasing breast density ( 14 ). In addition, hormonal risk factors and genetics may contribute to breast density ( 15–19 ). How these and other factors influence breast density and form the pathways by which density increases breast cancer risk are areas of active research.
Breast density has taken on added importance recently. As of March 2016, 25 states have passed legislation mandating notification of breast density in order to identify women that may benefit from supplemental screening, encompassing nearly two-thirds of screening-eligible US women. It is unclear what effect such policy will have on breast cancer disparities. Racial differences in breast density would have implications for breast cancer risk assessment and could lead to different screening practices for women in many states.
Compared with white women, black women have higher breast cancer mortality, are diagnosed with later stage disease, and have higher incidence of poor prognosis and triple-negative breast cancers ( 20 , 21 ). Studies investigating racial differences in breast density have had mixed results ( 22–31 ). Comparisons of breast density by race may be confounded by factors such as age ( 32 ), body mass index (BMI) ( 33 ), use of hormone replacement therapy (HRT) ( 34 ), and reproductive factors ( 32 , 35 ), all of which are known to impact breast density and to differ substantially by race, particularly BMI ( 36 ). Not all studies have fully controlled for these factors, making it difficult to identify whether there are racial differences in breast density at the population level and whether these are fully accounted for by BMI or other hormonal factors.
An additional complication is that there are several ways to measure breast density. Breast density is typically estimated clinically by radiologists’ qualitative visual assessment using the American College of Radiology Breast Imaging-Reporting and Data System (BI-RADS) categories ( 37 ), but this measure has limited reproducibility ( 38–42 ). Quantitative breast density measures, such as percent density, can be measured using semi-automated software such as Cumulus ( 43 , 44 ), which provides a continuous score and has been used in many studies. Fully automated quantitative tools have also been developed to improve reproducibility of density measurement ( 45 , 46 ). These various methods quantify the amount of dense breast tissue in different ways. The two-dimensional (2D) assessments of breast density from a conventional mammogram can provide an estimate of the dense tissue (dense area) or the percent of breast tissue that is dense (area percent density). However, while these 2D area measures may capture measures of density that are predictive of cancer risk and the “masking effect” because of increased breast density, such measures may not fully capture the actual volume of dense tissue in the breast. Toward this end, three-dimensional (volumetric) estimations of both dense area and percent density have also been developed to more accurately quantify fibroglandular tissue ( 8 , 47 ). All of these measures have been associated with breast cancer risk, though the magnitude of the associations differs across measures and studies ( 46 ). Lastly, most prior studies have used film rather than digital mammograms, which are now the standard of care ( 48 ).
The purpose of our study was to investigate the association of race with breast density by comparing novel quantitative breast density measures for black and white women while also taking into account differences in age, BMI, hormone use, and reproductive factors.
Methods
Study Population
From September 1, 2012, through August 31, 2013, a total of 11 141 women underwent routine screening mammography at the Hospital of the University of Pennsylvania, of whom 11 117 had raw digital mammogram images available for analysis. We selected 10 216 women identified in electronic medical records as white or black/African American. We excluded women with a prior history of breast cancer (n = 96, 0.9%), women missing weight or height (n = 485, 4.8%), women with breast implants (n = 109, 1.1%), women missing a BI-RADS breast density category in their screening reports (n = 17, 0.2%), and those for whom quantitative density measurements could not be obtained (n = 10, 0.1%). This resulted in a final study population of 9498 individual women. Self-report of demographic and reproductive breast cancer risk factors including age, menopause status, prior biopsy, atypical hyperplasia, age at first birth, age at menarche, family history of breast or ovarian cancer, and use of hormone replacement therapy (HRT) were available from a mammography screening questionnaire administered as part of routine practice. Weight and height were extracted from electronic medical records recorded on the screening date if available and if not from within one year prior to screening date. The study was HIPAA-compliant and approved by the Institutional Review Board of the University of Pennsylvania, and a waiver of informed consent was granted for this review of existing clinical data.
Breast Density Measurements
Visual BI-RADS breast density estimates given by the interpreting radiologist were obtained from mammography screening reports. The estimates were based on the ACR BI-RADS Atlas 4th Edition ( 37 ) definitions and given as an overall assessment of a woman’s breast density corresponding to standard categories of 1) fatty, 2) scattered densities, 3) heterogeneously dense, and 4) extremely dense.
For each woman, raw (ie, “For Processing”) bilateral, two-view screening digital mammograms were retrospectively analyzed. From these images, two types of quantitative mammographic density measures were obtained: area-based and volume-based measures.
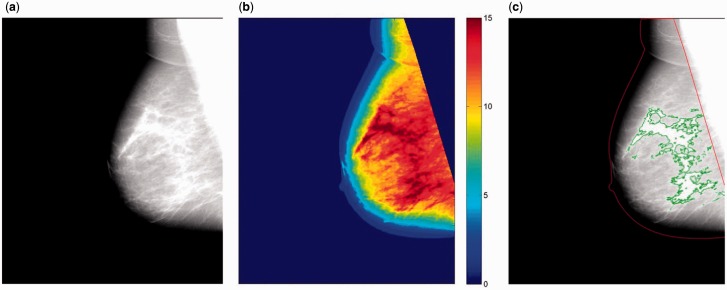
Dense area was measured on a per-image basis using fully automated, publicly available software (Laboratory for Individualized Breast Radiodensity Assessment [LIBRA], v.1.1.0) ( Figure 1 ) ( 49 ), which has been previously validated against Cumulus ( 45 ) as well as breast cancer ( 50 ). The details of the LIBRA algorithm have been previously described ( 45 ). Briefly, LIBRA first identifies the air-breast boundary and the edge of the pectoral muscle (when present). Within the breast region, the algorithm identifies the dense areas of the mammogram using fuzzy c-means clustering and support vector machine classification. Finally, absolute breast area (cm 2 ) and absolute dense area (cm 2 ) are derived, and area percent density (%) is obtained from the ratio of absolute dense area to the total breast area. A per-woman score of each measure was generated by averaging estimates from each image.
Figure 1.
A representative example of the Laboratory for Individualized Breast Radiodensity Assessment breast density estimation algorithm applied on a digital mediolateral-oblique view mammogram from a black woman age 50 years with American College of Radiology Breast Imaging Reporting and Data System density category 2 breasts (ie, scattered densities). A) Original digital mammogram. B) Cluster-based identification or regions of similar density. C) Final dense tissue segmentation, corresponding to a dense area of 26.6 cm 2 and an area percent density of 13.0%.
Mammographic dense volume was obtained using a commercially available software package (Quantra, v.2.0). Based on an adaptation of the validated Highnam and Brady method ( 51 , 52 ) for digital mammography, the software estimates the fraction of each pixel that contains fibroglandular tissue using known tissue x-ray attenuation properties (ie, adipose vs fibroglandular). An estimate of the absolute dense volume is then obtained by summing the fibroglandular tissue thickness of all pixels within the breast, and an estimate of breast volume is obtained by summing the overall thickness of each pixel within the breast, accounting for the breast edge. An estimate of volume percent density is derived from the ratio of these two measures. Finally, as with area density above, a per-woman composite score of each volume density measure was generated by averaging the density estimates from each image. A description of the breast density measures used in our study is provided in Table 1 .
Table 1.
Breast density measures evaluated in the study
| Breast density measure | Type of measurement | Source of measurement |
|---|---|---|
| BI-RADS density | Subjective | Radiologist interpretation on mammogram report |
| Dense area, cm 2 | Quantitative, area based | LIBRA software* |
| Area percent density, % | Quantitative, area based | LIBRA software* |
| Dense volume, cm 3 | Quantitative, volume based | Quantra software† |
| Volume percent density, % | Quantitative, volume based | Quantra software† |
*University of Pennsylvania Center for Biomedical Image Computing and Analytics, https://www.cbica.upenn.edu/sbia/software/LIBRA/index.html . BI-RADS = American College of Radiology Breast Imaging Reporting and Data System; LIBRA = Laboratory for Individualized Breast Radiodensity Assessment.
†Quantra breast density assessment software, Hologic, Inc.
Statistical Analysis
BMI was calculated from weight and height ( 53 ). Women missing menopause status (n = 790, 7%) were assumed to be premenopausal if younger than age 50 years and postmenopausal if age 50 years or older. Parity and age at first birth were categorized as nulliparous, older than 20, 20 to 24, 25 to 29, and 30 years or older. BI-RADS breast density was categorized as dense (heterogeneously dense or extremely dense) or nondense (almost entirely fatty or scattered fibroglandular densities). Characteristics of the study sample were compared by race using the t test and Chi-square tests, and density measures were compared by race using the Wilcoxon rank-sum test. Scatter plots of log-transformed BMI and log-transformed quantitative breast density measures were compared by race. Linear regression lines were fit, and the interaction of race and BMI on density was tested using linear regression with the inclusion of cross-product terms for the race-BMI interaction. For multivariable analysis, quantitative breast density measures above the median were categorized as dense and below the median as nondense based on the median value of each measure in the full sample. Logistic regression was performed separately for each density measure to estimate the odds of having dense breasts by race, adjusted for the following factors: age, BMI (continuous), age at menarche, menopause status, family history (first-degree relative with breast or ovarian cancer), parity and age at first birth, and current HRT use. The interaction of race and BMI on the density measures was also tested in the logistic regression models by including cross-product terms and analyses stratified by BMI of less than 30 kg/m 2 (nonobese) and BMI of 30 kg/m 2 or greater (obese) were performed. All statistical tests were two-sided, with statistical significance level of an α value of .05. Analyses were performed using STATA 12 (StataCorp LP, College Station, TX).
Results
The study sample included 55.6% black (n = 5282) and 44.4% white (n = 4216) women ( Table 2 ). The mean age was 57 years for both groups (white = 57.3 years, SD = 10.9; black = 57.0 years, SD = 11.0, P = .20). Black women had higher mean BMIs than whites (32.4 kg/m 2 , SD = 7.6 vs 26.4 kg/m 2 , SD = 7.6, P < .001). Women excluded because of missing BMI were similar to women with complete BMI data in terms of their age, race, BI-RADS breast density, and quantitative breast density measures. With the exception of menopause status, the distribution of breast cancer risk factors differed statistically significantly for black and white women.
Table 2.
Descriptive characteristics of the study population (n = 9498)
| Characteristic | White | Black | P * |
|---|---|---|---|
| (n = 4216) | (n = 5282) | ||
| Age, mean ± SD, y | 57.3 ± 10.9 | 57.0 ± 11.0 | .20 |
| Age categories, No. (%) | |||
| <40 y | 130 (3.1) | 89 (1.7) | <.001 |
| 40–49 y | 1034 (24.5) | 1517 (28.7) | |
| 50–59 y | 1351 (32.0) | 1683 (31.9) | |
| 60–69 y | 1147 (27.2) | 1258 (23.8) | |
| ≥70 y | 554 (13.1) | 735 (13.9) | |
| BMI, mean ± SD | 26.4 ± 6.0 | 32.4 ± 7.6 | <.001 |
| BMI categories, No. (%) | |||
| <18.5 kg/m 2 | 96 (2.3) | 37 (0.7) | <.001 |
| 18.5–24.9 kg/m 2 | 1975 (46.9) | 728 (13.8) | |
| 25.0–29.9 kg/m 2 | 1206 (28.6) | 1425 (27.0) | |
| ≥30 kg/m 2 | 939 (22.3) | 3092 (58.5) | |
| Menopause status, No. (%) | |||
| Premenopause | 1374 (32.6) | 1662 (31.5) | .24 |
| Postmenopause | 2842 (67.4) | 3620 (68.5) | |
| Prior biopsy, No. (%) | 1282 (33.4) | 1133 (23.5) | <.001 |
| Atypical hyperplasia, No. (%) | 57 (1.4) | 11 (0.2) | <.001 |
| Age at first birth, No. (%) | |||
| Nulliparous | 1206 (28.6) | 821 (15.5) | <.001 |
| <20 y | 172 (4.1) | 1616 (30.6) | |
| 20-24 y | 573 (13.6) | 1215 (23.0) | |
| 25-29 y | 815 (19.3) | 543 (10.3) | |
| ≥30 y | 1091 (25.9) | 403 (7.6) | |
| Missing/unknown | 359 (8.5) | 684 (13.0) | |
| Age at menarche, No. (%) | |||
| 7–11 y | 576 (13.7) | 927 (17.6) | <.001 |
| 12–13 y | 1957 (46.4) | 1969 (37.3) | |
| ≥14 y | 825 (19.6) | 993 (18.8) | |
| Missing/unknown | 858 (20.4) | 1393 (26.4) | |
| Family history†, No. (%) | 1046 (24.8) | 952 (18.0) | <.001 |
| Ever used HRT, No. (%) | 1108 (26.3) | 577 (10.9) | <.001 |
| Current HRT use, No. (%) | 263 (6.2) | 76 (1.4) | <.001 |
* P values from two-sided t tests for continuous variables and Chi square test for categorical variables. BMI = body mass index; HRT = hormone replacement therapy.
†First-degree relative(s) diagnosed with breast or ovarian cancer.
When examining the unadjusted distributions of the different breast density measures for black and white women, differences were observed across most measures ( Tables 3 and 4 ). Based on BI-RADS density, 22.0% of black women had density in the highest two categories (21.0% heterogeneously dense, 1.0% extremely dense) compared with 40.9% of white women (37.8% heterogeneously dense, 3.1% extremely dense, P < .001). When comparing quantitative measures, black and white women had similar levels of dense area (22.2 cm 2 vs 22.3 cm 2 , P = .24), but black women had lower area percent density than white women (12.3% vs 17.1%, P < .001). Black women, however, had a greater volume of dense tissue (266.9 cm 3 vs 196.1 cm 3 , P < .001) but lower volume percent density compared with white women (9.8% vs 11.6%, P < .001).
Table 3.
Distribution of BI-RADS breast density by race
| BI-RADS density | White (n = 4216) | Black (n = 5282) | P * |
|---|---|---|---|
| No. (%) | No. (%) | ||
| 1 Almost entirely fatty | 315 (7.5) | 980 (18.6) | <.001 |
| 2 Scattered fibroglandular Densities | 2177 (51.6) | 3140 (59.5) | |
| 3 Heterogeneously dense | 1594 (37.8) | 1107 (21.0) | |
| 4 Extremely dense | 130 (3.1) | 55 (1.0) |
* P values from two-sided Chi square test. BI-RADS = Breast Imaging-Reporting and Data System.
Table 4.
Distributions of quantitative breast density measures by race
| Quantitative breast density | Mean (SD) | Mean (SD) | P * |
|---|---|---|---|
| Dense area, cm 2 | 22.3 (12.3) | 22.2 (11.3) | .03 |
| Area percent density, % | 17.1 (10.8) | 12.3 (7.8) | <.001 |
| Dense volume, cm 3 | 196.1 (136.5) | 266.9 (185.7) | <.001 |
| Volume percent density, % | 11.6 (7.7) | 9.8 (5.5) | <.001 |
* P values from two-sided Wilcoxon rank-sum tests.
When scatter plots of quantitative density measures and BMI were examined ( Supplementary Figure 1 , available online), we observed differences in the association of BMI with quantitative density measures for white and black women, and the interaction of race and BMI was statistically significant for all quantitative density measures ( P < .001). Furthermore, in the logistic regression models estimating the odds of high density, the interaction of race and BMI was also statistically significant for all quantitative density measures ( P ≤ .01). Therefore, logistic regression models were stratified by BMI ( Table 5 ). Models adjusting for age only and age and BMI are displayed in Supplementary Table 1 (available online). After accounting for age, BMI, and breast cancer risk factors, there was no statistically significant racial difference in the odds of high density using BI-RADS density for nonobese (OR = 1.01, 95% CI = 0.88 to 1.17, P = .86) or obese women (OR = 1.00, 95% CI = 0.78 to 1.26, P = .97). However, for all quantitative breast density measures, black women had statistically significantly greater odds of high breast density than white women. Specifically, for dense area, black women had 40% to 51% higher odds of high density than white women (nonobese: OR = 1.40, 95% CI = 1.23 to 1.60, P < .001; obese: OR = 1.51, 95% CI = 1.28 to 1.77, P < .001). For area percent density, black women had 18% to 26% higher odds of high breast density than white women (nonobese: OR = 1.18, 95% CI = 1.02 to 1.37, P = .03; obese: OR = 1.26, 95% CI = 1.04 to 1.53, P = .02). For dense volume, black women had 27% to 55% higher odds of high breast density than white women (nonobese: OR = 1.27, 95% CI = 1.10 to 1.45, P = .001; obese: OR = 1.55, 95% CI = 1.30 to 1.85, P < .001). Finally, for volume percent density, black women had 32% to 65% higher odds of high breast density than white women (nonobese: OR = 1.32, 95% CI = 1.15 to 1.52, P < .001; obese: OR = 1.73, 95% CI = 1.45 to 2.05, P < .001).
Table 5.
Logistic regression of having dense breasts by race after adjusting for age, BMI, and breast cancer risk factors *
| Outcome and characteristics | OR (95% CI) | P † |
|---|---|---|
| BI-RADS density (category 3-4 vs 1-2) | ||
| BMI <30 kg/m 2 | ||
| Black vs white | 1.01 (0.88 to 1.17) | .86 |
| Age | 0.95 (0.94 to 0.96) | <.001 |
| BMI | 0.81 (0.79 to 0.82) | <.001 |
| BMI ≥30 kg/m 2 | ||
| Black vs white | 1.00 (0.78 to 1.26) | .97 |
| Age | 0.96 (0.95 to 0.97) | <.001 |
| BMI | 0.87 (0.85 to 0.89) | <.001 |
| Dense area (Q3-4 vs Q1-2) | ||
| BMI <30 kg/m 2 | ||
| Black vs white | 1.40 (1.23 to 1.60) | <.001 |
| Age | 0.98 (0.97 to 0.98) | <.001 |
| BMI | 0.88 (0.86 to 0.90) | <.001 |
| BMI ≥30 kg/m 2 | ||
| Black vs white | 1.51 (1.28 to 1.77) | <.001 |
| Age | 0.98 (0.98 to 0.99) | <.001 |
| BMI | 1.03 (1.02 to 1.05) | <.001 |
| Area percent density (Q3-4 vs Q1-2) | ||
| BMI <30 kg/m 2 | ||
| Black vs white | 1.18 (1.02 to 1.37) | .03 |
| Age | 0.97 (0.96 to 0.98) | <.001 |
| BMI | 0.71 (0.70 to 0.73) | <.001 |
| BMI ≥30 kg/m 2 | ||
| Black vs white | 1.26 (1.04 to 1.53) | .02 |
| Age | 0.98 (0.97 to 0.99) | <.001 |
| BMI | 0.90 (0.88 to 0.91) | <.001 |
| Dense volume (Q3-4 vs Q1-2) | ||
| BMI <30 kg/m 2 | ||
| Black vs white | 1.27 (1.10 to 1.45) | .001 |
| Age | 0.97 (0.96 to 0.97) | <.001 |
| BMI | 1.10 (1.08 to 1.13) | <.001 |
| BMI ≥30 kg/m 2 | ||
| Black vs white | 1.55 (1.30 to 1.85) | <.001 |
| Age | 0.97 (0.96 to 0.98) | <.001 |
| BMI | 1.13 (1.11 to 1.15) | <.001 |
| Volume percent density (Q3-4 vs Q1-2) | ||
| BMI <30 kg/m 2 | ||
| Black vs white | 1.32 (1.15 to 1.52) | <.001 |
| Age | 0.95 (0.95 to 0.96) | <.001 |
| BMI | 0.81 (0.79 to 0.83) | <.001 |
| BMI ≥30 kg/m 2 | ||
| Black vs white | 1.73 (1.45 to 2.05) | <.001 |
| Age | 0.97 (0.97 to 0.98) | <.001 |
| BMI | 1.02 (1.01 to 1.04) | <.001 |
*Additionally adjusted for current hormone replacement therapy use, age menarche, menopause status, first-degree relative breast or ovarian, age first birth. BI-RADS = Breast Imaging-Reporting and Data System; BMI = body mass index; CI = confidence interval; OR = odds ratio; Q = Quartile.
† P values from Wald tests. All statistical tests were two-sided.
Discussion
We observed statistically significant differences in breast density for black and white women in a large screening mammography cohort. When quantitative breast density measures were examined, black women had higher breast density than whites after accounting for age, BMI, HRT use, age at menarche, menopause status, family history of breast or ovarian cancer, parity, and age at first birth while there was no statistically significant difference in BI-RADS density.
The BI-RADS categories and area percent density are the most widely used metrics to assess breast density. A recent meta-analysis found that both dense area and area percent density are strong risk factors for breast cancer though the association was stronger for percent density ( 54 ). However, it is unclear whether these two-dimensional measures fully capture the variation in breast tissue volume and composition across individuals, and therefore efforts have been most recently geared towards developing fully automated, quantitative volumetric methods, which could provide a more accurate representation of the dense breast tissue ( 50 , 55–59 ). Our study therefore examines both established area-based and novel volumetric quantitative breast density measures, which are shown to be correlated with breast cancer risk ( 46 , 60 ). To the best of our knowledge, this is the largest study to specifically examine breast density among black women while also quantitatively measuring both area and volume density.
The vast majority of studies investigating breast density have been performed in predominantly white or European populations, and it may be possible that volumetric breast density measures may be more sensitive for risk stratification in black women, given the large differences in breast size and BMI between black and white populations ( 36 ). Breast density, specifically BI-RADS density, has also been associated with breast cancer risk in black women. However, the magnitude of this association was smaller for black compared with white women in two large studies ( 30 , 61 ). Interestingly, we observed higher density for black women across all quantitative measures even after adjusting for factors known to be associated with density. These racial differences could be because of differences in hormonal exposures across life-course for black and white women ( 62–65 ), genetic differences ( 16–19 ), or other unmeasured factors.
Our results highlight the importance of carefully controlling for confounding factors, particularly BMI, when comparing breast density by race. We found a statistically significant interaction between race and BMI on density and therefore subsequently performed analyses stratified by BMI. Our findings are consistent with three prior studies that found higher breast density among black compared with white women ( 22 , 23 , 26 ), two of which adjusted for age, BMI, and reproductive factors ( 22 , 26 ). All three of these studies included fewer than 1000 black women; two examined area percent density ( 23 , 26 ) and the third only BI-RADS density ( 22 ). Several additional studies found black women to have similar or lower breast density than whites using BI-RADS density or area percent density ( 24 , 25 , 27–31 ), but only two studies ( 27 , 29 ) adjusted for age, BMI, and reproductive factors. In general, BMI has been shown to be inversely associated with percent density measures, partly because women with higher BMIs tend to have larger breasts with more nondense tissue. However, while higher BMI has also been associated with lower dense area in several studies ( 16 ), our results suggest that there is an interplay between race, BMI, and both area-based and volumetric breast density measures, indicating the need to better understand how to best measure and interpret density quantitatively in diverse populations.
A small proportion of women in our study were classified as having extremely dense breasts by BI-RADS (3.1% of white and 1.0% of black women). This is lower than some of the estimates reported by other studies, including the Breast Cancer Surveillance Consortium (BCSC), where 7.3% to 8.5% of women undergoing screening mammography from 2000 through 2009 in the United States were classified as having extremely dense breasts ( 66 ). However, our results are consistent with previous studies both from our site ( 67 ) and from another large study performed in Vermont ( 42 ). This variation could be because of differences in populations across sites and/or differences in radiologists’ practices. The small proportion of women in the extremely dense category could be partly because of the high prevalence of obesity at our institution, particularly among black women, or the differences in the age distributions of women screened across sites. In addition, BI-RADS density is known to have modest inter-radiologist reliability ( 68 , 69 ), and our lower prevalence of women with extremely dense breasts may also reflect differences in radiologist interpretation across sites and populations.
The question of the most useful metrics for breast density assessment has become increasingly important, as it has direct implications for personalized screening. If BI-RADS categories alone are used to identify which women have dense breasts, black women would be less likely than whites to be considered as having dense breasts and therefore not triaged to supplemental screening. Black women have higher breast cancer mortality than white women, are diagnosed with later stage disease, and have higher incidence of poor prognosis and triple-negative breast cancers ( 20 , 21 ), making early detection critical. If black women have a greater quantity of dense breast tissue than whites once BMI is accounted for, this may have implications for their breast cancer risk. To date, density has been associated with breast cancer risk regardless of tumor subtype ( 70 ) though there is some evidence that the association is stronger for estrogen receptor–negative cancers and large tumors ( 71 ). Existing studies of screening performance have primarily assessed the association of BI-RADS density with screening sensitivity and interval cancers ( 72–76 ) while there is little data on quantitative breast density measures and sensitivity of screening mammography ( 13 , 71 , 77 ). Quantitative measures could alleviate issues of reproducibility of subjective BI-RADS measurements; however, it is unknown which quantitative measure is best at predicting screening outcomes or cancer diagnosis. These data emphasize the need to further investigate breast density measurements and their relationship to both screening and cancer outcomes if breast density will be used in large-scale policy interventions for breast cancer prevention and early detection.
A few limitations should be considered when interpreting our findings. First, BMI was obtained from medical records at the time of mammography screening or shortly before and may have been a combination of both self-report and measured weight and height. We excluded women with missing BMI data, though this was less than 5.0% of the baseline study population, and no statistically significant differences in race distribution or breast density levels for women missing BMI were observed. There was also a large number of statistical comparisons performed in our study. However, using a Bonferroni-corrected statistical significance level of .005 (ie, for 10 comparisons), the racial difference would not be statistically significant for area percent density, but the racial difference in all other quantitative measures would remain statistically significant. Though we found statistically significant racial differences across all quantitative breast density measures examined, additional work is needed to determine which measure is most strongly associated with breast cancer risk and most useful for making personalized screening recommendations.
Our study also has several strengths. To the best of our knowledge, this is the largest study to quantitatively assess breast density in black women using digital mammography. Over half of our study population was black, which provided statistical power to compare density levels by race while accounting for important confounders such as age, BMI, and reproductive risk factors. We had digital mammograms for the entire cohort of women undergoing screening mammography for one contiguous year at our institution along with detailed data on breast cancer risk factors such as BMI, reproductive history, family history, and HRT use.
In summary, we found that there was no statistically significant difference in BI-RADS density between black and white women after accounting for age, BMI, and breast cancer risk factors. However, when quantitative measures were used to assess breast density, black women had statistically significantly higher breast density than white women across all quantitative area-based and volumetric density measures examined. Future work will assess how quantitative breast density measures relate to breast cancer outcomes and whether more comprehensive measures of breast density patterns, such as parenchymal texture and complexity, could further explain racial differences in breast cancer incidence, tumor subtype, and stage at diagnosis.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health, Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) program (U54 CA163313) and 5R01CA161749-04.
Notes
The funder had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors thank Younji Kim for her assistance preparing the manuscript.
The publicly available LIBRA breast density estimation software tool used in this study can be downloaded at https://www.cbica.upenn.edu/sbia/software/LIBRA/index.html .
Supplementary Material
References
- 1. Boyd NF, Guo H, Martin LJ , et al. . Mammographic density and the risk and detection of breast cancer . N Engl J Med. 2007. ; 356 ( 3 ): 227 – 236 . [DOI] [PubMed] [Google Scholar]
- 2. Vacek PM, Geller BM. A prospective study of breast cancer risk using routine mammographic breast density measurements . Cancer Epidemiol Biomarkers Prev . 2004. ; 13 ( 5 ): 715 – 722 . [PubMed] [Google Scholar]
- 3. Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk . Radiology. 2004. ; 230 ( 1 ): 29 – 41 . [DOI] [PubMed] [Google Scholar]
- 4. Vachon CM, Brandt KR, Ghosh K , et al. . Mammographic breast density as a general marker of breast cancer risk . Cancer Epidemiol Biomarkers Prev. 2007. ; 16 ( 1 ): 43 – 49 . [DOI] [PubMed] [Google Scholar]
- 5. McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis . Cancer Epidemiol Biomarkers Prev. 2006. ; 15 ( 6 ): 1159 – 1169 . [DOI] [PubMed] [Google Scholar]
- 6. Keller B, Conant E, Oh H , et al. . Breast cancer risk prediction via area and volumetric estimates of breast density . In: Maidment ADA, Bakic PR, Gavenonis S , eds. 11th International Workshop on Breast Imaging (IWDM), Lecture Notes in Computer Science (LNCS) : Springer-Verlag; Berlin Heidelberg: ; 2012. , 236 – 243 . [Google Scholar]
- 7. Ding J, Warren R, Warsi I , et al. . Evaluating the effectiveness of using standard mammogram form to predict breast cancer risk: case-control study . Cancer Epidemiol Biomarkers Prev. 2008. ; 17 ( 5 ): 1074 – 1081 . [DOI] [PubMed] [Google Scholar]
- 8. Boyd N, Martin L, Gunasekara A , et al. . Mammographic density and breast cancer risk: evaluation of a novel method of measuring breast tissue volumes . Cancer Epidemiol Biomarkers Prev. 2009. ; 18 ( 6 ): 1754 – 1762 . [DOI] [PubMed] [Google Scholar]
- 9. Aitken Z, McCormack VA, Highnam RP , et al. . Screen-film mammographic density and breast cancer risk: a comparison of the volumetric standard mammogram form and the interactive threshold measurement methods . Cancer Epidemiol Biomarkers Prev. 2010. ; 19 ( 2 ): 418 – 428 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shepherd JA, Kerlikowske K, Ma L , et al. . Volume of mammographic density and risk of breast cancer . Cancer Epidemiol Biomarkers Prev. 2011. ; 20 ( 7 ): 1473 – 1482 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolfe JN. Breast patterns as an index of risk for developing breast cancer . AJR Am J Roentgenol. 1976. ; 126 ( 6 ): 1130 – 1137 . [DOI] [PubMed] [Google Scholar]
- 12. Byrne C, Schairer C, Wolfe J , et al. . Mammographic features and breast cancer risk: effects with time, age, and menopause status . J Natl Cancer Inst. 1995. ; 87 ( 21 ): 1622 – 1629 . [DOI] [PubMed] [Google Scholar]
- 13. Yaghjyan L, Colditz GA, Collins LC , et al. . Mammographic breast density and subsequent risk of breast cancer in postmenopausal women according to tumor characteristics . J Natl Cancer Inst. 2011. ; 103 ( 15 ): 1179 – 1189 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bertrand KA, Tamimi RM, Scott CG , et al. . Mammographic density and risk of breast cancer by age and tumor characteristics . Breast Cancer Res. 2013. ; 15 ( 6 ): R104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyd NF, Martin LJ, Bronskill M , et al. . Breast tissue composition and susceptibility to breast cancer . J Natl Cancer Inst. 2010. ; 102 ( 16 ): 1224 – 1237 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huo CW, Chew GL, Britt KL , et al. . Mammographic density-a review on the current understanding of its association with breast cancer . Breast Cancer Res Treat. 2014. ; 144 ( 3 ): 479 – 502 . [DOI] [PubMed] [Google Scholar]
- 17. Vachon CM, Scott CG, Fasching PA , et al. . Common breast cancer susceptibility variants in LSP1 and RAD51L1 are associated with mammographic density measures that predict breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2012. ; 21 ( 7 ): 1156 – 1166 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Varghese JS, Thompson DJ, Michailidou K , et al. . Mammographic breast density and breast cancer: evidence of a shared genetic basis . Cancer Res. 2012. ; 72 ( 6 ): 1478 – 1484 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keller BM, McCarthy AM, Chen J , et al. . Associations between breast density and a panel of single nucleotide polymorphisms linked to breast cancer risk: a cohort study with digital mammography . BMC Cancer. 2015. ; 15 : 143 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Howlader N, Altekruse SF, Li CI , et al. . US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status . J Natl Cancer Inst. 2014. ; 106 ( 5 ):dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kohler BA, Sherman RL, Howlader N , et al. . Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state . J Natl Cancer Inst. 2015. ; 107 ( 6 ):djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. El-Bastawissi AY, White E, Mandelson MT , et al. . Variation in mammographic breast density by race . Ann Epidemiol. 2001. ; 11 ( 4 ): 257 – 263 . [DOI] [PubMed] [Google Scholar]
- 23. Chen Z, Wu AH, Gauderman WJ , et al. . Does mammographic density reflect ethnic differences in breast cancer incidence rates? Am J Epidemiol. 2004. ; 159 ( 2 ): 140 – 147 . [DOI] [PubMed] [Google Scholar]
- 24. del Carmen MG, Halpern EF, Kopans DB , et al. . Mammographic breast density and race . AJR Am J Roentgenol. 2007. ; 188 ( 4 ): 1147 – 1150 . [DOI] [PubMed] [Google Scholar]
- 25. del Carmen MG, Hughes KS, Halpern E , et al. . Racial differences in mammographic breast density . Cancer. 2003. ; 98 ( 3 ): 590 – 596 . [DOI] [PubMed] [Google Scholar]
- 26. Habel LA, Capra AM, Oestreicher N , et al. . Mammographic density in a multiethnic cohort . Menopause. 2007. ; 14 ( 5 ): 891 – 899 . [DOI] [PubMed] [Google Scholar]
- 27. McCormack VA, Dowsett M, Folkerd E , et al. . Sex steroids, growth factors and mammographic density: a cross-sectional study of UK postmenopausal Caucasian and Afro-Caribbean women . Breast Cancer Res. 2009. ; 11 ( 3 ): R38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stuedal A, Ma H, Bernstein L , et al. . Does breast size modify the association between mammographic density and breast cancer risk? Cancer Epidemiol Biomarkers Prev. 2008. ; 17 ( 3 ): 621 – 627 . [DOI] [PubMed] [Google Scholar]
- 29. Tehranifar P, Reynolds D, Flom J , et al. . Reproductive and menstrual factors and mammographic density in African American, Caribbean, and white women . Cancer Causes Control. 2011. ; 22 ( 4 ): 599 – 610 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ursin G, Ma H, Wu AH , et al. . Mammographic density and breast cancer in three ethnic groups . Cancer Epidemiol Biomarkers Prev. 2003. ; 12 ( 4 ): 332 – 338 . [PubMed] [Google Scholar]
- 31. Zhang S, Ivy JS, Diehl KM , et al. . The association of breast density with breast cancer mortality in African American and white women screened in community practice . Breast Cancer Res Treat. 2013. ; 137 ( 1 ): 273 – 283 . [DOI] [PubMed] [Google Scholar]
- 32. Kelemen LE, Pankratz VS, Sellers TA , et al. . Age-specific trends in mammographic density: the Minnesota Breast Cancer Family Study . Am J Epidemiol. 2008. ; 167 ( 9 ): 1027 – 1036 . [DOI] [PubMed] [Google Scholar]
- 33. Boyd NF, Martin LJ, Sun L , et al. . Body size, mammographic density, and breast cancer risk . Cancer Epidemiol Biomarkers Prev. 2006. ; 15 ( 11 ): 2086 – 2092 . [DOI] [PubMed] [Google Scholar]
- 34. Rutter CM, Mandelson MT, Laya MB , et al. . Changes in breast density associated with initiation, discontinuation, and continuing use of hormone replacement therapy . JAMA. 2001. ; 285 ( 2 ): 171 – 176 . [DOI] [PubMed] [Google Scholar]
- 35. El-Bastawissi AY, White E, Mandelson MT , et al. . Reproductive and hormonal factors associated with mammographic breast density by age (United States) . Cancer Causes Control. 2000. ; 11 ( 10 ): 955 – 963 . [DOI] [PubMed] [Google Scholar]
- 36. Flegal KM, Carroll MD, Kit BK , et al. . Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010 . JAMA. 2012. ; 307 ( 5 ): 491 – 497 . [DOI] [PubMed] [Google Scholar]
- 37. D'Orsi CJ, Bassett LW, Berg WA , et al. . BI-RADS: Mammography, 4th edition . In: D'Orsi CJ, Mendelson EB, Ikeda DM , et al. : Breast Imaging Reporting and Data System: ACR BI-RADS – Breast Imaging Atlas . In: Reston, VA: : American College of Radiology; ; 2003. . [Google Scholar]
- 38. Kerlikowske K, Grady D, Barclay J , et al. . Variability and accuracy in mammographic interpretation using the American College of Radiology Breast Imaging Reporting and Data System . J Natl Cancer Inst. 1998. ; 90 ( 23 ): 1801 – 1809 . [DOI] [PubMed] [Google Scholar]
- 39. Ciatto S, Houssami N, Apruzzese A , et al. . Categorizing breast mammographic density: intra- and interobserver reproducibility of BI-RADS density categories . Breast. 2005. ; 14 ( 4 ): 269 – 275 . [DOI] [PubMed] [Google Scholar]
- 40. Gard CC, Aiello Bowles EJ, Miglioretti DL , et al. . Misclassification of Breast Imaging Reporting and Data System (BI-RADS) Mammographic Density and Implications for Breast Density Reporting Legislation . Breast J. 2015. ; 21 ( 5 ): 481 – 489 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ooms EA, Zonderland HM, Eijkemans MJ , et al. . Mammography: interobserver variability in breast density assessment . Breast. 2007. ; 16 ( 6 ): 568 – 576 . [DOI] [PubMed] [Google Scholar]
- 42. Spayne MC, Gard CC, Skelly J , et al. . Reproducibility of BI-RADS breast density measures among community radiologists: a prospective cohort study . Breast J. 2012. ; 18 ( 4 ): 326 – 333 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boyd NF, Byng JW, Jong RA , et al. . Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study . J Natl Cancer Inst. 1995. ; 87 ( 9 ): 670 – 675 . [DOI] [PubMed] [Google Scholar]
- 44. Byng JW, Boyd NF, Fishell E , et al. . The quantitative analysis of mammographic densities . Phys Med Biol. 1994. ; 39 ( 10 ): 1629 – 1638 . [DOI] [PubMed] [Google Scholar]
- 45. Keller BM, Nathan DL, Wang Y , et al. . Estimation of breast percent density in raw and processed full field digital mammography images via adaptive fuzzy c-means clustering and support vector machine segmentation . Med Phys. 2012. ; 39 ( 8 ): 4903 – 4917 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eng A, Gallant Z, Shepherd J , et al. . Digital mammographic density and breast cancer risk: a case-control study of six alternative density assessment methods . Breast Cancer Res. 2014. ; 16 ( 5 ): 439 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yaffe MJ, Boone JM, Packard N , et al. . The myth of the 50-50 breast . Med Phys. 2009. ; 36 ( 12 ): 5437 – 5443 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Food and Drug Administration . Mammography Quality and Standards Act and Program Facility Scorecard. http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/FacilityScorecard/ucm113858.htm . Accessed January 10, 2015.
- 49. Center for Biomedical Image Computing and Analytics . Laboratory for Individualized Breast Radiodensity Assessment (LIBRA) homepage. https://www.cbica.upenn.edu/sbia/software/LIBRA/index.html . Accessed January 10, 2015.
- 50. Keller BM, Chen J, Daye D , et al. . Preliminary evaluation of the publicly available Laboratory for Breast Radiodensity Assessment (LIBRA) software tool: comparison of fully automated area and volumetric density measures in a case-control study with digital mammography . Breast Cancer Res. 2015. ; 17 ( 1 ): 117 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Highnam R, Jeffreys M, McCormack V , et al. . Comparing measurements of breast density . Phys Med Biol. 2007. ; 52 ( 19 ): 5881 – 5895 . [DOI] [PubMed] [Google Scholar]
- 52. Hartman K, Highnam R, Warren R, Jackson V. Volumetric Assessment of Breast Tissue Composition from FFDM Images . In: 9th International Workshop on Digital Mammography (IWDM), Lecture Notes in Computer Science (LNCS) . Krupinski EA , ed. Vol. 5116 : Springer-Verlag Berlin; Heidelberg: ; 2008. : 33 – 39 . [Google Scholar]
- 53. FerroLuzzi A, Garza C, Haas J , et al. . Physical status: The use and interpretation of anthropometry - Introduction . Physical Status: The Use and Interpretation of Anthropometry; 1995. ; 854 : 1 – 3 . [PubMed] [Google Scholar]
- 54. Pettersson A, Graff RE, Ursin G , et al. . Mammographic density phenotypes and risk of breast cancer: a meta-analysis . J Natl Cancer Inst. 2014. ; 106 ( 5 ):dju078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shepherd JA, Kerlikowske K, Ma L , et al. . Volume of Mammographic Density and Risk of Breast Cancer . Cancer Epidemiol Biomarkers Prev. 2011. ; 20 ( 7 ): 1473 – 1482 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van Engeland S, Snoeren PR, Huisman H , et al. . Volumetric breast density estimation from full-field digital mammograms . IEEE Trans Med Imaging. 2006. ; 25 ( 3 ): 273 – 282 . [DOI] [PubMed] [Google Scholar]
- 57. Lokate M, Kallenberg MG, Karssemeijer N , et al. . Volumetric breast density from full-field digital mammograms and its association with breast cancer risk factors: a comparison with a threshold method . Cancer Epidemiol Biomarkers Prev. 2010. ; 19 ( 12 ): 3096 – 3105 . [DOI] [PubMed] [Google Scholar]
- 58. Gubern-Merida A, Kallenberg M, Platel B , et al. . Volumetric breast density estimation from full-field digital mammograms: a validation study . PLoS One. 2014. ; 9 ( 1 ): e85952 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brand JS, Czene K, Shepherd JA , et al. . Automated measurement of volumetric mammographic density: a tool for widespread breast cancer risk assessment. Cancer Epidemiol Biomarkers Prev. 2014. ; 23 ( 9 ): 1764 – 1772 . [DOI] [PubMed] [Google Scholar]
- 60. Brandt KR, Scott CG, Ma L , et al. . Comparison of Clinical and Automated Breast Density Measurements: Implications for Risk Prediction and Supplemental Screening . Radiology . 2015. ; in press. [DOI] [PMC free article] [PubMed]
- 61. Razzaghi H, Troester MA, Gierach GL , et al. . Mammographic density and breast cancer risk in White and African American Women . Breast Cancer Res Treat. 2012. ; 135 ( 2 ): 571 – 580 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Butler LM, Gold EB, Greendale GA , et al. . Menstrual and reproductive factors in relation to mammographic density: the Study of Women's Health Across the Nation (SWAN) . Breast Cancer Res Treat. 2008. ; 112 ( 1 ): 165 – 174 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bernstein L, Teal CR, Joslyn S , et al. . Ethnicity-related variation in breast cancer risk factors . Cancer. 2003. ; 97 ( 1 Suppl ): 222 – 229 . [DOI] [PubMed] [Google Scholar]
- 64. Titus-Ernstoff L, Tosteson AN, Kasales C , et al. . Breast cancer risk factors in relation to breast density (United States) . Cancer Causes Control. 2006. ; 17 ( 10 ): 1281 – 1290 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Woolcott CG, Koga K, Conroy SM , et al. . Mammographic density, parity and age at first birth, and risk of breast cancer: an analysis of four case-control studies . Breast Cancer Res Treat. 2012. ; 132 ( 3 ): 1163 – 1171 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Distribution of Key Variables, Breast Cancer Surveillance Consortium . http://breastscreening.cancer.gov/data/variables/ . Accessed December 29, 2015.
- 67. McCarthy AM, Kontos D, Synnestvedt M , et al. . Screening outcomes following implementation of digital breast tomosynthesis in a general-population screening program . J Natl Cancer Inst. 2014. ; 106 ( 11 ):dju316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Keller BM, Nathan DL, Gavenonis SC , et al. . Reader variability in breast density estimation from full-field digital mammograms: the effect of image postprocessing on relative and absolute measures . Acad Radiol. 2013. ; 20 ( 5 ): 560 – 568 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Redondo A, Comas M, Macia F , et al. . Inter- and intraradiologist variability in the BI-RADS assessment and breast density categories for screening mammograms . Br J Radiol. 2012. ; 85 ( 1019 ): 1465 – 1470 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Antoni S, Sasco AJ, dos Santos Silva I , et al. . Is mammographic density differentially associated with breast cancer according to receptor status? A meta-analysis . Breast Cancer Res Treat. 2013. ; 137 ( 2 ): 337 – 347 . [DOI] [PubMed] [Google Scholar]
- 71. Bertrand KA, Scott CG, Tamimi RM , et al. . Dense and nondense mammographic area and risk of breast cancer by age and tumor characteristics . Cancer Epidemiol Biomarkers Prev. 2015. ; 24 ( 5 ): 798 – 809 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pisano ED, Gatsonis C, Hendrick E , et al. . Diagnostic performance of digital versus film mammography for breast-cancer screening . N Engl J Med. 2005. ; 353 ( 17 ): 1773 – 1783 . [DOI] [PubMed] [Google Scholar]
- 73. Kerlikowske K, Zhu W, Tosteson AN , et al. . Identifying women with dense breasts at high risk for interval cancer: a cohort study . Ann Intern Med. 2015. ; 162 ( 10 ): 673 – 681 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kerlikowske K, Zhu W, Hubbard RA , et al. . Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy . JAMA Intern Med. 2013. ; 173 ( 9 ): 807 – 816 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pisano ED, Hendrick RE, Yaffe MJ , et al. . Diagnostic accuracy of digital versus film mammography: exploratory analysis of selected population subgroups in DMIST . Radiology. 2008. ; 246 ( 2 ): 376 – 383 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nelson HD, O'Meara ES, Kerlikowske K , et al. . Factors Associated With Rates of False-Positive and False-Negative Results From Digital Mammography Screening: An Analysis of Registry Data . Ann Intern Med. 2016. ; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Holm J, Humphreys K, Li J , et al. . Risk factors and tumor characteristics of interval cancers by mammographic density . J Clin Oncol. 2015. ; 33 ( 9 ): 1030 – 1037 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.