Abstract
Heterozygous loss-of-function mutations in GRN, the progranulin gene, which result in progranulin (PGRN) protein haploinsufficiency, are a major cause of frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP). PGRN is composed of seven and a half repeats of a highly conserved granulin motif that is cleaved to produce the granulin peptides A–G and paragranulin. To better understand the role of PGRN and granulin (Grn) peptides in the pathogenesis of neurodegeneration, we evaluated PGRN/Grn in brains of patients with Alzheimer disease, FTLD-TDP type A with or without GRN mutations, and normal individuals, using a panel of monoclonal antibodies against Grn peptides A–G. In the neocortex, Grn peptide-specific immunostains were observed, for example, membranous Grn E immunopositivity in pyramidal neurons, and Grn C immunopositivity in ramified microglia. In the hippocampus, Grn immunopositivity in the CA1 and CA2 regions showed disease-specific changes in both neurons and microglia. Most interestingly, in FTLD-TDP type A with GRN mutations, there is a 60% decrease in the density of Grn-positive microglia in the hippocampal CA1, suggesting that haploinsufficiency of the GRN mutations also extends to PGRN expression in microglia. This study provides important insights into future studies of the pathogenesis and treatment of FTLD-TDP.
Keywords: Alzheimer disease, Frontotemporal lobar degeneration, Granulin, Hippocampal sclerosis, Microglia, Neuroinflammation, Progranulin
INTRODUCTION
Frontotemporal dementia is the second most common dementia in patients younger than 65 years of age (1, 2). Pathologically, most cases have frontotemporal lobar degeneration (FTLD) with neuronal and glial inclusions containing either tau or transactive response DNA-binding protein of 43 kDa (TDP-43, FTLD-TDP) (3–10). Heterozygous loss-of-function mutations in GRN, the progranulin gene, are associated with FTLD-TDP type A (11–5).
GRN encodes progranulin (PGRN), a 593 amino acid (aa) cysteine-rich, secreted protein with a predicted molecular mass of 63.5 kDa (16–8). PGRN is expressed in multiple peripheral tissues, and has roles in cancer, inflammation, and metabolic disease (19, 20). In the CNS it functions as a growth factor (21, 22) or anti-inflammatory agent (23, 24). The PGRN holoprotein can be cleaved by proteolytic enzymes into seven and a half individual granulin (Grn) peptides (19), whose functions in the peripheral tissues seem to oppose those of full-length PGRN in processes such as inflammation, cell growth, and survival (19, 22, 25, 26). The existence and functions of Grn peptides in the brain are unknown. However, studies suggest that Grn peptides may play important roles in the pathogenesis of neurodegenerative diseases (27). Using a Caenorhabditis elegans FTLD-TDP model, Salazar et al showed that Gran 3 (equivalent to human Grn E) had toxic effects (27). The same group also showed that a granulin cleavage product was overrepresented in diseased brain regions of Alzheimer disease (AD) and FTLD patients (27). In addition, heterozygous loss of the GRN gene, which is believed to act through a haploinsufficiency mechanism (28), leads to FTLD-TDP, most often type A (3–8, 29). However, homozygous mutations in GRN are associated with a distinct disease, neuronal ceroid lipofuscinosis, which is characterized by the storage of abnormal lipopigment in lysosomes (30). Grn peptides are present in GRN haploinsufficiency but not null states, which suggests that the presence of Grn peptides might modulate disease phenotype.
Neuropathologically, GRN associated FTLD-TDP type A cases show cerebral atrophy that is most prominent in the frontal lobes. Most cases have significant loss of pyramidal neurons in the hippocampal CA1 region and subiculum (hippocampal sclerosis [HS]). In addition, caudate atrophy and loss of pigmented neurons in the substantia nigra are consistent findings as well (31, 32). Immunohistochemically, the neocortex contains TDP-43-immunopositive inclusions, which are mostly localized in the upper layers, and composed of short dystrophic neurites, neuronal cytoplasmic inclusions, and lentiform to round neuronal intranuclear inclusions. Similar TDP-43 pathology can also be found in striatum. In hippocampus, while neuronal cytoplasmic in the dentate granule cells vary in numbers, it is common to find frequent TDP-43-positive dystrophic neurites in the CA1 region (31–3). In addition to FTLD-TDP, TDP-43 pathology has now been detected in 25%–50% of cases of AD (34–6), especially those with greater AD pathology, as well as AD with HS. HS is manifested by selective neuronal loss affecting CA1 sector of the hippocampus, and 85% of HS cases, with or without AD, have TDP-43 inclusions (35, 37–9).
The link between Grn peptides and FTLD-TDP type A pathology is still unknown. We hypothesized that if Grn peptides are present in the brain, and contribute to the pathogenesis of FTLD-TDP type A, they would likely exhibit different immunostaining patterns in vulnerable regions of brain tissue from patients with FTLD-TDP with and without GRN mutations and from patients with AD, with and without HS. To better understand the roles of PGRN/Grn peptides in the pathogenesis of neurodegeneration, the distributions of PGRN and Grn peptides were evaluated in frontal cortex and hippocampus from patients with AD, AD with HS (ADHS), FTLD-TDP type A with or without GRN mutations, and normal controls. In our previous studies, we produced monoclonal antibodies (mAbs) against each Grn peptide: A, B, C, D, E, F, and G (40, 41). These antibodies all showed sensitivity and specificity for immunohistochemistry, Western blot, and ELISA (40, 41). With the aid of the full panel of anti-Grn antibodies, we showed disease-, region-, and Grn peptide-specific Grn immunopositivity in AD and FTLD-TDP brains.
MATERIALS AND METHODS
Human Samples
Paraformaldehyde-fixed, paraffin-embedded human brain samples from 31 cases were acquired from the Neuropathology Core of the Northwestern University Cognitive Neurology and Alzheimer Disease Center. Demographic and neuropathologic data for these cases is presented in Table 1. The cases were subdivided into five groups: Normal control (CON), AD, ADHS, FTLD-TDP type A with or without GRN mutation. Pathologic characterization was made by board-certified neuropathologists following consensus criteria (6, 9, 10, 42). GRN gene mutations are described in Table 2, which also includes the ABC scores of AD neuropathologic change (43, 44) of each case. Informed consent was obtained for all studies.
TABLE 1.
Sample Demographics
| Neuropathologic diagnosis | Number of cases | Gender M/F | Age at death (mean ± SD) |
|---|---|---|---|
| CON | 6 | 3/3 | 77.8 ± 7.8 |
| AD | 5 | 2/3 | 77.3 ± 8.4 |
| ADHS | 6 | 2/4 | 78.3 ± 5.2 |
| FTLD-TDP type A | 6 | 4/2 | 71.5 ± 6.9 |
| GRN | 8 | 5/3 | 59.7 ± 3.8 |
CON, normal control; AD, Alzheimer disease; ADHS, AD with hippocampal sclerosis; FTLD-TDP type A, FTLD-TDP type A without GRN mutation; GRN, FTLD-TDP with GRN mutation.
TABLE 2.
Study Subjects With Identified GRN Mutations
| Case | Sex | Clinical diagnosis | ADNC score (A, B, C) | Death (years) | Duration (years) | GRN mutation |
|---|---|---|---|---|---|---|
| 1 | M | FTD | 0, 1, 0 | 53 | 3.0 | IVS6 + 2 del TGAG |
| 2 | M | FTD | 0, 1, 0 | 61 | 2.0 | c.1477C>T |
| 3 | M | FTD | 0, 1, 0 | 64 | 6.5 | IVS6 + 2 del TGAG |
| 4 | F | PPA | 1, 1, 0 | 61 | 4.0 | c.675_676delCA |
| 5 | F | PPA | 1, 1, 2 | 67 | 6.0 | c.1477 C > T |
| 6 | F | PPA | 0, 0, 0 | 56 | 6.0 | c.910_911insTG |
| 7 | M | PPA | 0, 0, 0 | 61 | 8.0 | c.5913 A > G |
| 8 | M | PPA | 0, 0, 0 | 64 | 4.0 | c.3240C>T |
FTD, frontotemporal dementia; PPA, primary progressive aphasia; ADNC, Alzheimer disease neuropathologic change.
Tissue Staining
Paraffin-embedded tissue sections from middle frontal gyrus and hippocampus were cut to a thickness of 5 μm for immunohistochemical analysis. First, antigen retrieval was performed in the Decloaking Chamber (Biocare medical, Pacheco, CA) for 15 minutes using a citrate buffer, pH 6.0. Sections were then placed in 3% H2O2 in methanol for 30 minutes. Following washes in distilled water, sections were blocked in 5% goat serum at room temperature for 1 hour. Sections were then incubated in primary antibodies including IBA-1 (goat polyclonal, 1:1000, Abcam, Boston, MA), Grn A, B, C, D, E, F, G (mAbs, 1:100, homemade [41, 42]), tau (AT8, mAb, 1:500, Thermo, Waltham, MA), phosphorylated TDP-43 (pS409/410-2, rabbit polyclonal, 1:2500, CosmoBio, Carlsbad, CA), and beta amyloid (4G8, mAb, 1:1000, Biolegend, San Diego, CA) overnight at 4 °C. Biotinylated secondary antibodies (DAKO. Carpinteria, CA) were amplified using avidin-biotin substrate (ABC solution, DAKO), followed by color development in DAB chromogen (K4007, DAKO).
MAbs against Grn A, B, C, D, E, F, G were home-made, and will be available upon request. The production of these antibodies was demonstrated in the previously published papers (40, 41). MAbs against Grn A, B, and C were shown to be specific for its own Grn domain (40). In this study, we confirmed that mAbs against Grn D, E, F, and G also only recognized its own Grn domain (Supplementary Data Fig. S1). In addition, absorption control was used to further confirm the specificity of these antibodies. Specifically, each of the 7 mAbs was preabsorbed with the corresponding antigenic peptide (1 µg/mL) (Sigma-Aldrich, St. Louis, MO) prior to the application of the antibody to the brain tissue. The immunostaining results of these antibodies only showed weak background signals.
Quantitation
Cases were examined by neuropathologists blinded to clinical and pathologic diagnoses as well as GRN status. Regions of the hippocampus individually analyzed were the 4 subdivisions of Ammon’s horn, C1, CA2, CA3, and CA4. The delineation of the 4 subdivisions was performed according to the previously described method (45). Microglia were counted using a 40× objective with a grid (250 × 250 µm2) in a minimum of 5 microscopic grid fields per subdivision (46). Field selection was performed by choosing 5 evenly spaced fields along the hippocampal pyramidal layer of each subdivision. Results are given as mean objects per unit area (mm2). Microglial cells that had stained cytoplasmic processes and contained a nucleus in the plane of section were counted.
Group effects within and between subdivisions of the hippocampus were evaluated using the Student t test, one-way analysis of variance, or both. Differences between means were considered significant at p < 0.05.
RESULTS
Immunostaining Pattern of Anti-Grn mAbs in the Normal Cerebral Cortex
The immunostaining pattern for each anti-Grn mAb was evaluated in the neocortex of normal controls (Figs. 1, 2). All of the antibodies demonstrated diffuse cytoplasmic reactivity in cortical neurons, with stronger intensity in the pyramidal neurons. In addition, the anti-Grn E mAb also showed immunopositivity of the cytoplasmic membrane in scattered pyramidal neurons. Microglia and macrophages had granular cytoplasmic positivity with all of the mAbs. In addition, the anti-Grn C mAb also revealed a population of ramified microglial cells with abundant processes that were not labeled by the other anti-Grn antibodies. Overall, the immunostains of the anti-Grn A and B mAbs were stronger in neurons; those of anti-Grn D, F, and G mAbs were stronger in microglial cells; the anti-Grn C mAb had strong labeling in both neuronal and microglial cells; and the anti-Grn E mAb strongly labeled cytoplasmic membrane of occasional pyramidal neurons.
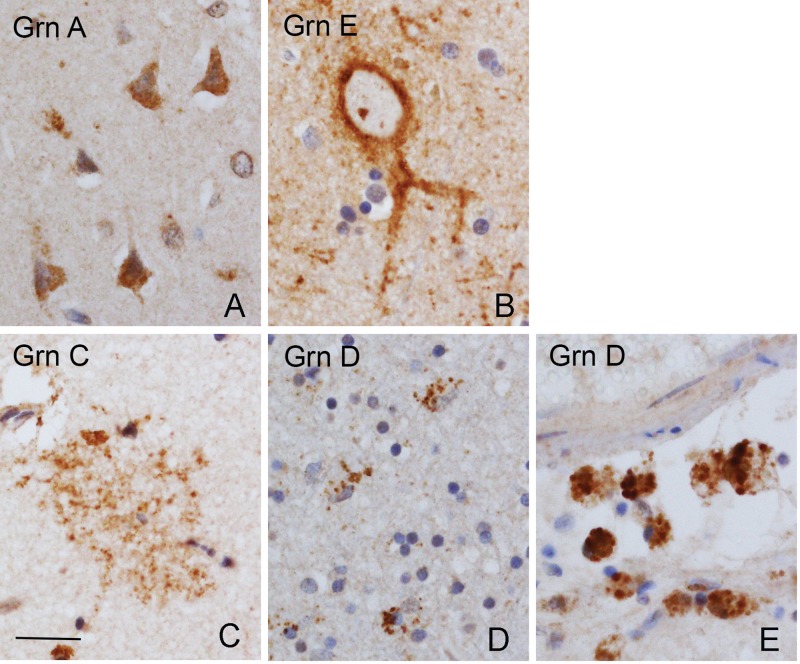
FIGURE 1.
Cellular immunostaining patterns of anti-Grn mAbs. All of the anti-Grn mAbs revealed diffuse cytoplasmic positivity in neocortical neurons, which were stronger in the pyramidal neurons (A, illustrated by anti-Grn A antibody). The anti-Grn E mAb also showed a membranous staining in scattered neurons (B). Except for a ramified microglial population revealed by the anti-Grn C mAb (C), all of the anti-Grn mAbs showed granular cytoplasmic positivity of microglia and macrophages (D, E, illustrated by anti-Grn D antibody). Scale bar: 100 µm.
FIGURE 2.
Immunostains with anti-Grn mAbs of normal neocortex. The labeling of anti-Grn A and anti-Grn B mAbs was stronger in neurons; that of anti-Grn D, F, and G mAbs were stronger in microglial cells; and that of anti-Grn C was strong in both neuronal and microglial cells. Arrow shows a Grn C-immunopositive, ramified microglial cell with abundant processes. Anti-Grn E shows membranous immunopositivity in the pyramidal neurons. Scale bar: 100 µm.
Immunostaining Pattern of Anti-Grn mAbs in the AD and FTLD-TDP Type a Neocortex
Tissues from the frontal cortices of patients with AD and FTLD-TDP type A with or without GRN mutation were evaluated immunohistochemically with anti-Grn mAbs (Fig. 3). Compared with normal controls, overall, Grn immunopositivities in the neurons were decreased in AD and FTLD-TDP type A with/without the GRN mutation samples; and, as expected, the changes were in line with the severity of neuronal loss and gliosis. The densities of Grn-immunopositive microglial cells were greater in AD and FTLD-TDP type A with or without GRN mutation compared with normal controls. Furthermore, these changes were proportional to the increase in densities of the total microglial population, as revealed by IBA-1 immunostains (47–9) (semiquantification data not shown). In addition, we observed that Grn C-positive ramified microglia showed different distribution patterns in these diseases (Fig. 4). In the frontal cortices of normal controls and AD patients there were patchy transcortical Grn C-positive ramified microglia, seemingly corresponding to amyloid/tau deposits. In some GRN mutation-associated FTLD-TDP cases, especially those without amyloid deposits in the neocortex (Table 2), Grn C-positive ramified microglial cells were mainly located in cortical layer 3, while TDP-43-positive inclusions were mostly located in layer 2. Grn C-positive ramified microglial cells were overall rare in the other FTLD-TDP type A cases with or without GRN mutation. There were only scattered pyramidal neurons with membranous Grn E-immunopositivity in normal controls, AD, and FTLD-TDP type A without GRN mutation groups, but in FTLD-TDP type A with GRN mutation, both the densities and staining intensities of neurons with such Grn E-immunopositivity were markedly increased (Fig. 3).
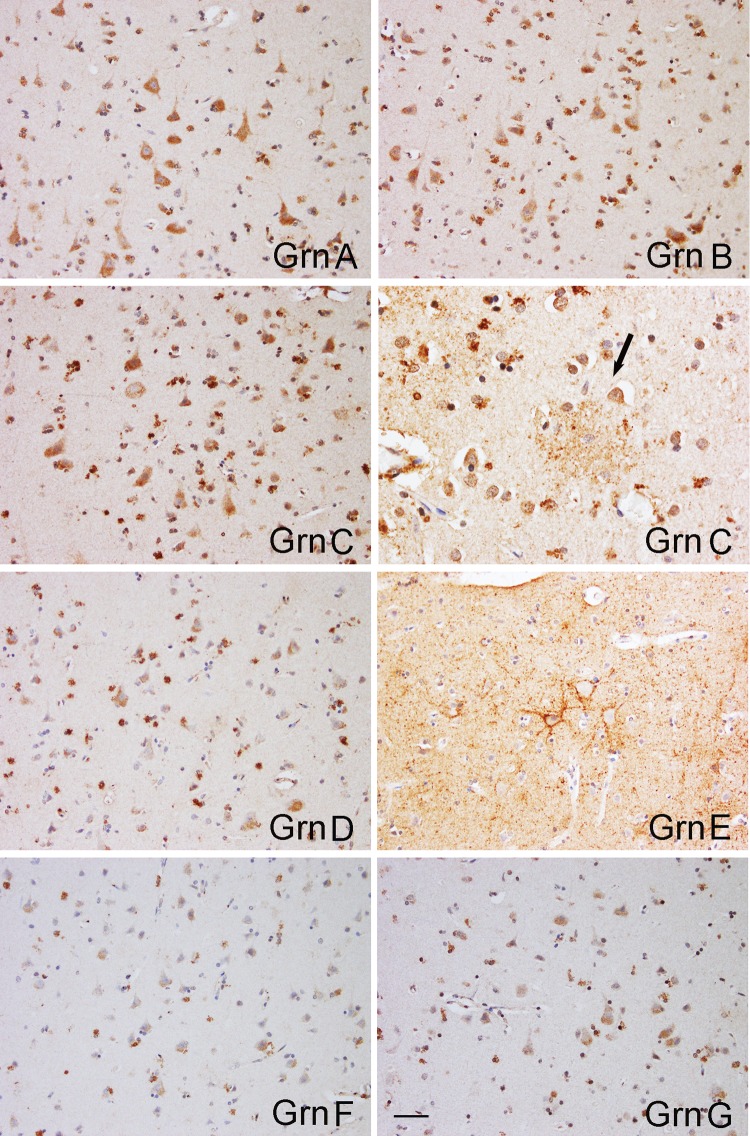
FIGURE 3.
Immunohistochemical evaluation with anti-Grn mAbs of middle frontal cortices from patients with different diseases. Representative images (the upper 4 panels) of immunostains by the anti-Grn C mAb showed decreased immunopositivity in neurons, and increased numbers of microglia cells in the middle frontal cortices of patients with AD, and FTLD-TDP type A with or without GRN mutation, when compared with the controls. Similar results were obtained from all other mAbs. In the cortex from AD, and FTLD-TDP type A with GRN mutation, anti-Grn C mAb also labeled a group of ramified microglia cells with abundant processes (arrows). The bottom 2 panels showed that Grn E-positive neurons were markedly increased in number in FTLD-TDP type A with GRN mutation, when compared with controls and the other diseases. Scale bar: 100 µm. CON, control; AD, Alzheimer disease; FTLD-TDP type A, FTLD-TDP type A without GRN mutation; GRN, FTLD-TDP type A with GRN mutation; Grn C, anti-Grn C mAb; Grn E, anti-Grn E mAb.
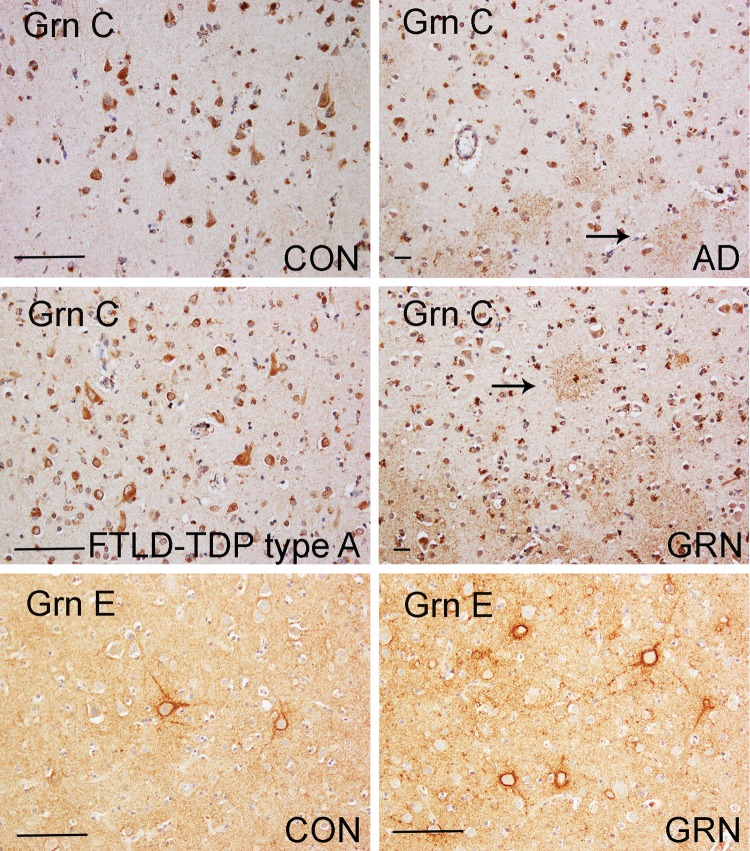
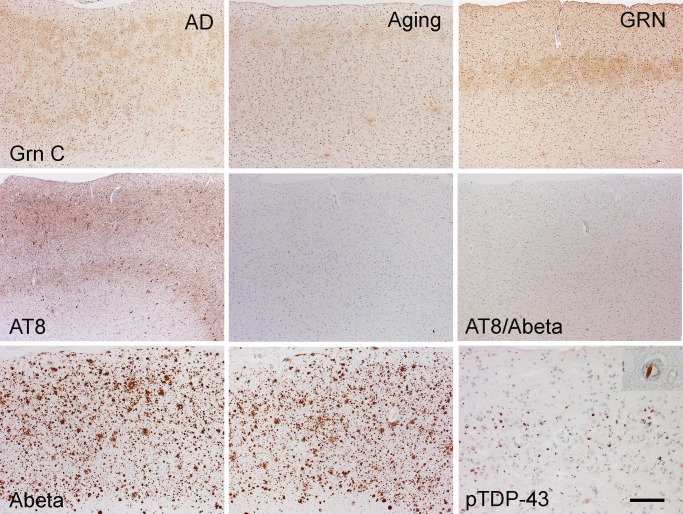
FIGURE 4.
Grn C-immunopositive ramified microglia had different distribution patterns in the frontal cortices of patients with different diseases. In the frontal cortices of AD patients and normal aging controls, Grn C-positive ramified microglia had a transcortical distribution that may correlate to amyloid/tau deposits, as revealed by Abeta and AT8 immunostaining. In some FTLD-TDP type A with GRN mutation cases, Grn C-immunopositive ramified microglia cells were localized in layer 3 of the frontal cortex, while TDP-43-positive inclusions were mainly in layer 2. Inset in the right lower panel, TDP-43 neuronal intranuclear inclusion. Bar, 100 µm. GRN, FTLD-TDP type A with the GRN mutation; AD, Alzheimer disease.
Immunostaining Pattern of Anti-Grn mAbs in AD and FTLD-TDP Hippocampal Neurons
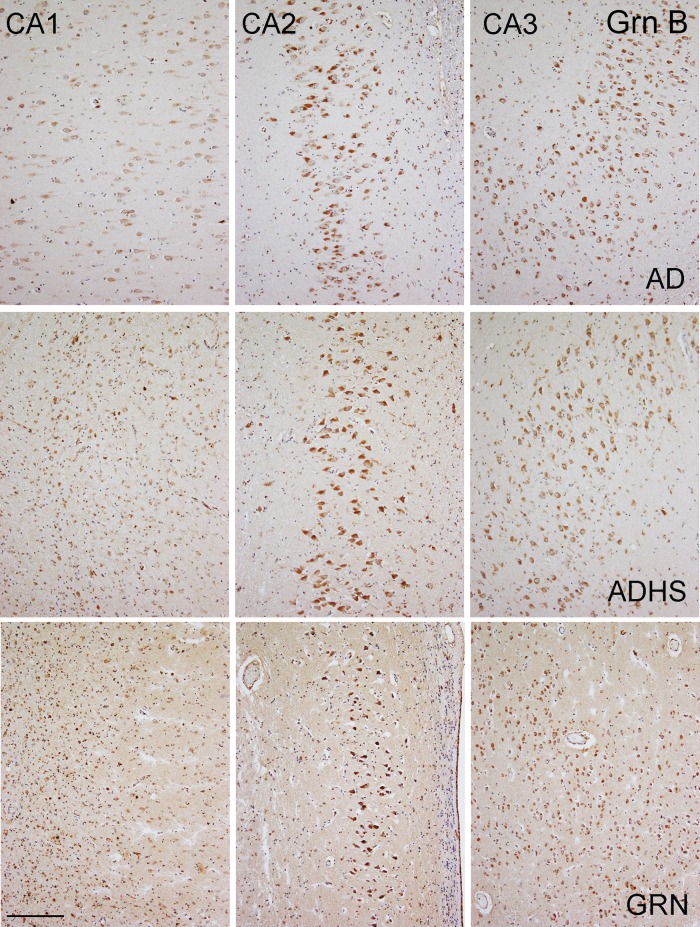
Immunohistochemistry using anti-Grn mAbs revealed labeling of hippocampal pyramidal neurons. All anti-Grn mAbs revealed diffuse cytoplasmic reactivity of hippocampal pyramidal neurons, with the anti-Grn B mAb having the strongest staining intensity (Fig. 5). In control individuals, anti-Grn B mAb revealed moderate immunopositivity in hippocampal regions CA1, 2, 3, and 4, with a staining intensity slightly lower in CA1 region. The staining intensity of the neurons in CA1 was further decreased in those cases with HS; however, interestingly, the staining in CA2 of these cases tended to increase (Fig. 5). The staining intensity in CA3 and CA4 showed no significant differences between diseases. The anti-Grn A mAb, another antibody showing good sensitivity for neurons, revealed changes in the hippocampus similar to those of the anti-Grn B mAb.
FIGURE 5.
Neuronal PGRN expression in the hippocampi of the different diseases, evaluated by the anti-Grn B mAb. Anti-Grn B mAb reveals weak immunopositivity in hippocampal CA1, and moderate immunopositivity in hippocampal CA2, 3, and 4 of the control (not shown) or AD. Compared with the control (not shown) or to AD, the intensity of the immunostains was lower in the CA1 regions of ADHS, FTLD-TDP type A without GRN mutation (not shown), and FTLD-TDP type A with GRN mutation samples due to neuronal loss and gliosis in this region. Compared with the control (not shown) and to AD, the immunostaining intensity was higher in the CA2 regions in FTLD-TDP type A without GRN mutation (not shown) and FTLD-TDP type A with GRN mutation samples, while no significant difference was found between ADHS and AD or the controls. CA3 and CA4 showed no significant differences in immunostaining intensity between the different diseases. Bar, 100 µm. AD, Alzheimer disease; ADHS, Alzheimer disease with hippocampal sclerosis; GRN, FTLD-TDP type A with GRN mutation.
Immunostaining Pattern of Anti-Grn mAbs in AD and FTLD-TDP Hippocampal Microglia
The anti-Grn D mAb was used to evaluate the microglial population because this antibody was found to be the most sensitive microglia cell marker among the anti-Grn mAbs, although the anti-Grn G mAb was also a good microglial marker. Furthermore, anti-IBA-1 antibody (47–9) was used to reveal the total microglial population.
IBA-1-positive microglia showed morphologic heterogeneity in the hippocampus, consistent with findings previously described by Bachstetter et al (50). Specifically, ramified microglial cells were mainly seen in normal control and AD hippocampi; hypertrophic microglial cells were more commonly seen in ADHS hippocampi, and scattered, rod-shaped microglia were observed in all disease groups. The densities of IBA-1 positive microglia (number/mm2) showed no significant differences between the control and AD samples, nor between ADHS and FTLD-TDP type A with or without GRN mutation, in all 4 hippocampal regions studied. There were more IBA-1-positive microglial cells in the CA1, CA2, CA3, and CA4 regions of ADHS and FTLD-TDP type A with or without GRN mutation, compared with control and AD hippocampi (p < 0.05).
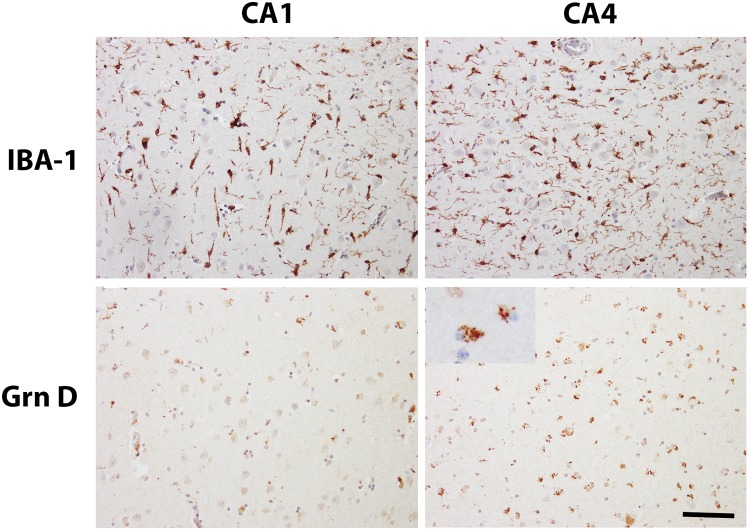
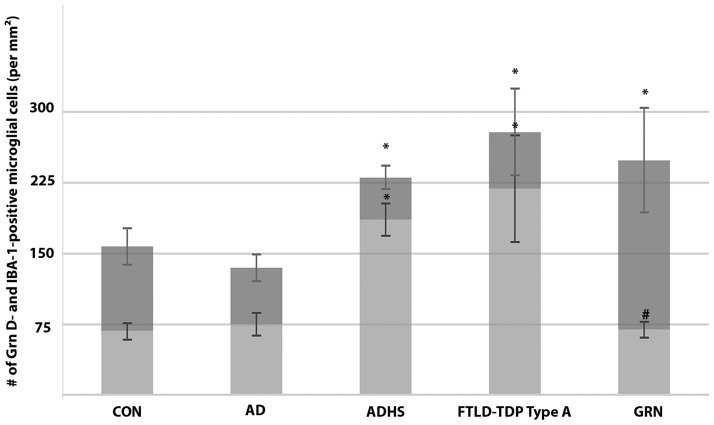
Grn D-positive microglial cells had fine granular cytoplasmic immunostaining (Fig. 6). Quantitative analysis showed that the densities (number/mm2) of Grn D- and IBA-1-positive microglial cells were not significantly different in any of the 4 regions, except CA1. In the CA1 regions of the control and AD hippocampi, the ratios of the densities of Grn D- to IBA-1-positive microglial cells were about 0.5 (Table 3). This ratio was slightly increased up to 0.8 in ADHS and FTLD-TDP type A without GRN mutation, at the same time that the total number of microglia was increased, indicating an overall microglial activation in CA1. In FTLD-TDP with GRN mutation, the CA1 region also showed increased total microglial number, but the Grn D-positive microglia represented only 30% of the total population (Figs. 6, 7; Table 3). In addition, the anti-Grn G mAb, another antibody showing good sensitivity for microglia, revealed similar changes in hippocampi. TDP43 immunostaining revealed frequent CA1 fine dystrophic neurites in 5 out of 6 cases of ADHS, 3 out of 6 cases of FTLD-TDP type A without GRN mutation, and 6 out of 8 cases of FTLD-TDP type A with GRN mutation. The remaining FTLD-TDP type A with or without GRN mutation cases had no or sparse TDP-43 positive CA1 dystrophic neurites, and all had severe HS. There was no linear correlation between the density of the Grn-positive microglia in CA1 region and the severity of HS, and CA1 TDP-43 pathology.
FIGURE 6.
IBA-1- and Grn D-positive microglia in the hippocampi of FTLD-TDP with the GRN mutation. Representative photos from IBA-1- and Grn D-positive microglia in CA1 and CA4. Inset in the lower right panel shows higher power image of Grn D-positive microglia. Scale bar: 100 µm.
TABLE 3.
The Ratio of the Number of grn D- to IBA-1-Positive Microglial Cells in the Hippocampal CA1-4 Regions of Different Diseases
| CA1 | CA2 | CA3 | CA4 | |
|---|---|---|---|---|
| CON | 0.4±0.02 | 0.9±0.10 | 1.0±0.14 | 0.9±0.14 |
| AD | 0.5±0.05 | 0.9±0.08 | 0.9±0.03 | 0.9±0.11 |
| ADHS | 0.8±0.05* | 0.9±0.11 | 0.9±0.17 | 0.8±0.08 |
| FTLD-TDP type A | 0.8±0.11* | 0.8±0.19 | 0.9±0.20 | 0.8±0.12 |
| GRN | 0.3±0.06*,# | 0.9±0.13 | 0.9±0.11 | 0.8±0.13 |
Values are mean ± SD.
p < 0.05 compared with the control group.
p < 0.05, GRN versus FTLD-TDP type A without GRN mutation. CON, normal control; AD, Alzheimer disease; ADHS, Alzheimer disease with hippocampal sclerosis; FTLD-TDP type A, FTLD-TDP type A without GRN mutation; GRN, FTLD-TDP type A with GRN mutation.
FIGURE 7.
Density of IBA-1- and Grn D-positive microglia in hippocampal CA1 regions of patients with the different diseases. The full height of each bar (both dark and light gray) represents the average number of IBA-1-positive microglia per mm2 in CA1, while the light gray bar represents that of Grn D-positive microglia. Values are mean ± SD. The densities of IBA-1-positive microglia were compared with the control value by ANOVA with Dunnett's post hoc test (*p < 0.05). The density of Grn D-positive microglia was compared between FTLD-TDP type A with and without GRN mutation, and statistical significance was determined by Student’s t-test (#p < 0.05). CON, normal control; AD, Alzheimer disease; ADHS, Alzheimer disease with hippocampal sclerosis; FTLD-TDP type A, FTLD-TDP type A without GRN mutation; GRN, FTLD-TDP type A with GRN mutation; Grn D, anti-Grn D mAb.
DISCUSSION
In this study, neocortical and hippocampal tissues of patients with AD, ADHS, and FTLD-TDP type A with or without GRN mutation were evaluated immunohistochemically with mAbs created against different Grn peptides. Results showed Grn peptide-, disease-, and region-specific immunostaining patterns in the neocortex and hippocampus of patients with these diseases.
Consistent with previous immunohistochemical studies with different anti-PGRN antibodies (14, 51, 52), all of our mAbs against Grn peptides revealed diffuse cytoplasmic positivity in neurons, and strong staining of microglia. These staining patterns most likely represent the expression and distribution of full-length PGRN in the neural tissue, which we expected all of these anti-Grn mAbs to recognize. We also noticed variation in the staining intensities of different anti-Grn antibodies in the neurons and microglia of normal control neocortices. Whether this indicates Grn peptide-specific staining is not clear.
In peripheral tissues, full-length PGRN is involved in multiple biological functions, such as wound healing, tumorigenesis, and inflammation (19, 20). The holoprotein can be cleaved into individual Grn peptides, whose functions seem to oppose those of PGRN (19, 22, 25, 26). In the central nervous system, full-length PGRN has been reported to be related to neurite outgrowth and neuroinflammation (21, 22, 53–6), though the existence and function of Grn peptides are still unknown. In a C. elegans FTLD-TDP model, expression of Gran 3 (the equivalent of human Grn E) has been shown to impair the reproduction, development, and growth of the animals (27). Using an anti-Grn E antibody in Western blot studies, the same group also revealed a 33 kDa PGRN cleavage product in diseased brain regions from AD and FTLD patients, suggesting that a PGRN cleavage product exists (27). In this study, Grn peptide-specific immunostains demonstrated, for example, membranous Grn E positivity in pyramidal neurons in the neocortex, the pattern of which was only seen with the anti-Grn E (and not with the other) mAb. Our study also showed that Grn E positivity was higher in the frontal cortex of FTLD-TDP type A with GRN mutation only, suggesting that the Grn E peptide or PGRN cleavage product containing Grn E not only exists in the human brain, but also plays a role in the pathophysiology of neurodegeneration. It is known that neurite outgrowth is mediated by extracellular PGRN (22, 54, 55–9), which can be endocytosed through binding to the sortilin receptor via its C terminus, and delivered to lysosomes (58, 59). Interestingly, the C terminal Grn peptide, Grn E, has been shown to be fully sufficient for binding to sortilin (59). Hence, it will be interesting to see if sortilin colocalizes with the membranous Grn E immunopositivity in future invitro and invivo studies.
In microglia, PGRN is constitutively expressed and secreted (24, 60). It has an anti-inflammatory role: The absence of PGRN in microglia causes increased production and release of multiple cytokines, such as interleukin-6, in response to an inflammatory stimulus (24, 60). So far it is unclear whether single Grn peptides mediate neuroinflammation in human brains. In the current study, the anti-Grn C mAb revealed a unique, ramified microglial population, which had a distinct band-like distribution pattern in the frontal cortex of FTLD-TDP type A with GRN mutation. These data suggest the existence of individual Grn peptides in microglial cells. The presence of a single peptide out of the set of peptides as well as the absence of the precursor, raises the possibility that these cells have taken this particular granulin up from the surrounding environment, rather than generating it themselves. This might be biologically meaningful but could also represent nonspecific phagocytosis.
The hippocampus is an ideal structure for performing a regional analysis. If the PGRN/Grn peptides contribute significantly to neuropathologic changes, then vulnerable regions of brain tissue would likely exhibit different PGRN/Grn peptide distributions. In this study, both the anti-Grn A and anti-Grn B mAbs revealed similar changes in hippocampal neurons, hence, these changes might represent that of the expression of the full-length PGRN. We found that the intensity of the anti-Grn B immunopositivity in the pyramidal neurons was lower in the CA1 regions of patients with ADHS and FTLD-TDP type A with or without GRN mutation. These changes correlate with neuronal loss and gliosis in these regions. Interestingly, the Grn B immunostaining intensity was markedly increased in the CA2 regions of these cases. Whether it is a compensatory response inside the hippocampal circuitry of CA2 to CA1 injury is unknown.
Microglial regionalization in the hippocampus has been previously reported. Vinet et al reported that in response to N-methyl-d-aspartate (NMDA) challenge, microglia within CA1 adopt an activated morphology, while microglia within protected regions retain a ramified appearance (61, 62). Sanchez-Mejias et al recently demonstrated the existence of a significant microglial degenerative process in the human AD hippocampus, which was more prominent in the hilar region of the dentate gyrus and the CA3 region of the hippocampus than in other regions (63). Region- and disease-specific activity of Grn D-immunopositive microglial cells was also noted in this study. These changes might represent that of full-length PGRN, because similar findings were observed by using the anti-Grn G antibody. We found that in 2 settings with abnormal hippocampal TDP-43—AD with HS and FTLD-TDP without GRN mutation—both Grn-immunopositive and total microglia were activated in CA1, suggesting that TDP-43 deposition in the hippocampus is associated with microglial proliferation. In FTLD-TDP with GRN mutation, the CA1 region also showed increased total microglial number, but the number of Grn-positive microglia was only 30% of the total population. In other words, there is a 60% decrease in the density of Grn-positive microglia in the hippocampal CA1, when compared with FTLD-TDP without GRN mutation. These data suggest that the haploinsufficiency of GRN mutations also extends to PGRN expression in microglia. Interestingly, Cases 1, 3, 4, and 6 (Table 2), which have mutations that are predicted to reduce the levels of progranulin message upstream of the Grn D region, showed overall lower levels of Grn D immunopositivity in microglia in the hippocampi than the other 4 cases, but there was no statistically significant difference because of the small sample size (data not shown). In addition, the granular cytoplasmic staining in microglia, clearly demonstrated by anti-Grn D antibody, strongly suggested a lysosomal distribution of full-length PGRN or Grn peptides in microglia, for which we will confirm by double immunofluorescence staining in our future study.
A pathologic consequence of decreased PGRN-positive microglia in the CA1 region of patients with the GRN mutation is the possible loss of immunological protection. Studies have shown that microglial cells are implicated in the maintenance of synaptic integrity (64), and play a role in removing damaged neurons and neuronal components. Deficiencies in key genes for microglial survival and/or proliferation lead to compromised functions. For example, deficiencies in the key genes CSF1R or TREM2 are associated with the rare hereditary neurodegenerative diseases, adult-onset leukoencephalopathy with axonal spheroids, and Nasu–Hakola disease, respectively (65–7). The microglial dysfunction in GRN mutants has been primarily associated with overactivation and cytotoxicity of these cells. Chen-Plotkin et al observed that GRN mutation carriers have increased levels of mRNA transcription in brain regions severely affected by disease, for example, frontal and temporal cortices. Moderate microglial infiltration was observed in these regions, and is the most likely cellular source of this message (51). Yin et al demonstrated that brains of PGRN-deficient mice displayed greater activation of microglia and astrocytes than aged wild type mice. PGRN-deficient macrophages and microglia were cytotoxic to hippocampal cells invitro (23). Furthermore, Martens et al showed that when exposed to the neurotoxin MPTP, both global GRN knockout and microglia-specific GRN knockout mutant mice showed a much more robust increase in microglial activation and neuronal loss (24).
We do not know what determines the regionalization of PGRN-positive microglial cells and neurons. However, the decreased neuronal and microglial Grn immunopositivity specifically in the CA1 region of GRN mutation carriers might indicate reduced neurotrophic support together with deficient immunoprotection in this region. These changes might be related to the development of HS and/or TDP-43 pathology in CA1 of these patients, which would indeed contribute to the progression of FTLD pathology and cognitive impairment.
In summary, disease-, region-, and Grn peptide-specific immunopositivity in different neurodegenerative diseases was demonstrated in this study. These data will provide important insights into the pathogenesis of FTLD-TDP, and the future development of PGRN-based treatments for dementia.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the patients and their families who donated brains to help the understanding of neurodegeneration. We gratefully acknowledge the assistance of the Northwestern Alzheimer Disease Center and its participants.
REFERENCES
- 1. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ratnavalli E, Brayne C, Dawson K, et al. The prevalence of frontotemporal dementia. Neurology 2002;58:1615–21 [DOI] [PubMed] [Google Scholar]
- 3. Arai T, Hasegawa M, Akiyama H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 2006;351:602–11 [DOI] [PubMed] [Google Scholar]
- 4. Cairns NJ, Neumann M, Bigio EH, et al. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 2007;171:227–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol 2006;59:952–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol 2009;117:15–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006;314:130–3 [DOI] [PubMed] [Google Scholar]
- 8. Rosso SM, Donker Kaat L, Baks T, et al. Frontotemporal dementia in The Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain 2003;126:2016–22 [DOI] [PubMed] [Google Scholar]
- 9. Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 2007;114:5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 2010;119:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gass J, Cannon A, Mackenzie IR, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 2006;15:2988–3001 [DOI] [PubMed] [Google Scholar]
- 12. Huey ED, Grafman J, Wassermann EM, et al. Characteristics of frontotemporal dementia patients with a progranulin mutation. Ann Neurol 2006;60:374–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Le Ber I, van der Zee J, Hannequin D, et al. Progranulin null mutations in both sporadic and familial frontotemporal dementia. Hum Mutat 2007;28:846–55 [DOI] [PubMed] [Google Scholar]
- 14. Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006;442:916–9 [DOI] [PubMed] [Google Scholar]
- 15. Cruts M, Gijselinck I, van der Zee J, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 2006;442:920–4 [DOI] [PubMed] [Google Scholar]
- 16. Bateman A, Belcourt D, Bennett H, et al. Granulins, a novel class of peptide from leukocytes. Biochem Biophys Res Commun 1990;173:1161–8 [DOI] [PubMed] [Google Scholar]
- 17. Bhandari V, Palfree RG, Bateman A.. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci USA 1992;89:1715–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shoyab M, McDonald VL, Byles C, et al. Epithelins 1 and 2: isolation and characterization of two cysteine-rich growth-modulating proteins. Proc Natl Acad Sci USA 1990;87:7912–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He Z, Bateman A.. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med 2003;81:600–12 [DOI] [PubMed] [Google Scholar]
- 20. Petkau TL, Leavitt BR.. Progranulin in neurodegenerative disease. Trends Neurosci 2014;37:388–98 [DOI] [PubMed] [Google Scholar]
- 21. Tapia L, Milnerwood A, Guo A, et al. Progranulin deficiency decreases gross neural connectivity but enhances transmission at individual synapses. J Neurosci 2011;31:11126–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Damme P, Van Hoecke A, Lambrechts D, et al. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol 2008;181:37–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yin F, Banerjee R, Thomas B, et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J Exp Med 2010;207:117–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martens LH, Zhang J, Barmada SJ, et al. Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J Clin Invest 2012;122:3955–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Plowman GD, Green JM, Neubauer MG, et al. The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. J Biol Chem 1992;267:13073–8 [PubMed] [Google Scholar]
- 26. Zhu J, Nathan C, Jin W, et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell 2002;111:867–78 [DOI] [PubMed] [Google Scholar]
- 27. Salazar DA, Butler VJ, Argouarch AR, et al. The progranulin cleavage products, granulins, exacerbate TDP-43 toxicity and increase TDP-43 levels. J Neurosci 2015;35:9315–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cruts M, Van Broeckhoven C.. Loss of progranulin function in frontotemporal lobar degeneration. Trends Genet 2008;24:186–94 [DOI] [PubMed] [Google Scholar]
- 29. Mackenzie IR, Neumann M, Baborie A, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 2011;122:111–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith KR, Damiano J, Franceschetti S, et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet 2012;90:1102–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mackenzie IR, Baker M, Pickering-Brown S, et al. The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 2006;129:3081–90 [DOI] [PubMed] [Google Scholar]
- 32. Josephs KA, Ahmed Z, Katsuse O, et al. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol 2007;66:142–51 [DOI] [PubMed] [Google Scholar]
- 33. Hatanpaa KJ, Bigio EH, Cairns NJ, et al. TAR DNA-binding protein 43 immunohistochemistry reveals extensive neuritic pathology in FTLD-U: a midwest-southwest consortium for FTLD study. J Neuropathol Exp Neurol 2008;67:271–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uryu K, Nakashima-Yasuda H, Forman MS, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 2008;67:555–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol 2007;61:435–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kadokura A, Yamazaki T, Lemere CA, et al. Regional distribution of TDP-43 inclusions in Alzheimer disease (AD) brains: their relation to AD common pathology. Neuropathology 2009;29:566–73 [DOI] [PubMed] [Google Scholar]
- 37. Dickson DW, Baker M, Rademakers R.. Common variant in GRN is a genetic risk factor for hippocampal sclerosis in the elderly. Neurodegener Dis 2010;7:170–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zarow C, Sitzer TE, Chui HC.. Understanding hippocampal sclerosis in the elderly: epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep 2008;8:363–70 [DOI] [PubMed] [Google Scholar]
- 39. Yokota O, Davidson Y, Bigio EH, et al. Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol 2010;120:55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chai L, Mao Q, Liu S, et al. Domain-specific monoclonal antibodies produced against human PGRN. Hybridoma (Larchmt) 2011;30:271–8 [DOI] [PubMed] [Google Scholar]
- 41. Li Y, Li Y, Ye M, et al. Biological function analysis of monoclonal antibodies against human granulins invitro using U251 cells as a model. Protein Expr Purif 2017;130:55–62 [DOI] [PubMed] [Google Scholar]
- 42. McKhann GM, Albert MS, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol 2001;58:1803–9 [DOI] [PubMed] [Google Scholar]
- 43. Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 2012;8:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012;123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hatanpaa KJ, Raisanen JM, Herndon E, et al. Hippocampal sclerosis in dementia, epilepsy, and ischemic injury: differential vulnerability of hippocampal subfields. J Neuropathol Exp Neurol 2014;73:136–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. DiPatre PL, Gelman BB.. Microglial cell activation in aging and Alzheimer disease: partial linkage with neurofibrillary tangle burden in the hippocampus. J Neuropathol Exp Neurol 1997;56:143–9 [DOI] [PubMed] [Google Scholar]
- 47. Ito D, Imai Y, Ohsawa K, et al. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res 1998;57:1–9 [DOI] [PubMed] [Google Scholar]
- 48. Okere CO, Kaba H.. Heterogenous immunohistochemical expression of microglia-specific ionized calcium binding adaptor protein (Iba1) in the mouse olfactory bulb. Brain Res 2000;877:85–90 [DOI] [PubMed] [Google Scholar]
- 49. Hirayama A, Okoshi Y, Hachiya Y, et al. Early immunohistochemical detection of axonal damage and glial activation in extremely immature brains with periventricular leukomalacia. Clin Neuropathol 2001;20:87–91 [PubMed] [Google Scholar]
- 50. Bachstetter AD, Van Eldik LJ, Schmitt FA, et al. Disease-related microglia heterogeneity in the hippocampus of Alzheimer's disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol Commun 2015;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen-Plotkin AS, Xiao J, Geser F, et al. Brain progranulin expression in GRN-associated frontotemporal lobar degeneration. Acta Neuropathol 2010;119:111–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Daniel R, He Z, Carmichael KP, et al. Cellular localization of gene expression for progranulin. J Histochem Cytochem 2000;48:999–1009 [DOI] [PubMed] [Google Scholar]
- 53. Ryan CL, Baranowski DC, Chitramuthu BP, et al. Progranulin is expressed within motor neurons and promotes neuronal cell survival. BMC Neuroscience 2009;10:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gao X, Joselin AP, Wang L, et al. Progranulin promotes neurite outgrowth and neuronal differentiation by regulating GSK-3beta. Protein & Cell 2010;1:552–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gass J, Lee WC, Cook C, et al. Progranulin regulates neuronal outgrowth independent of sortilin. Mol Neurodegeneration 2012;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Petkau TL, Neal SJ, Milnerwood A, et al. Synaptic dysfunction in progranulin-deficient mice. Neurobiol Dis 2012;45:711–22 [DOI] [PubMed] [Google Scholar]
- 57. De Muynck L, Herdewyn S, Beel S, et al. The neurotrophic properties of progranulin depend on the granulin E domain but do not require sortilin binding. Neurobiol Aging 2013;34:2541–7 [DOI] [PubMed] [Google Scholar]
- 58. Hu F, Padukkavidana T, Vaegter CB, et al. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 2010;68:654–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zheng Y, Brady OA, Meng PS, et al. C-terminus of progranulin interacts with the beta-propeller region of sortilin to regulate progranulin trafficking. PLoS One 2011;6:e21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suh HS, Choi N, Tarassishin L, et al. Regulation of progranulin expression in human microglia and proteolysis of progranulin by matrix metalloproteinase-12 (MMP-12). PLoS One 2012;7:e35115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Howe ML, Barres BA.. A novel role for microglia in minimizing excitotoxicity. BMC Biology 2012;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vinet J, Weering HR, Heinrich A, et al. Neuroprotective function for ramified microglia in hippocampal excitotoxicity. J Neuroinflammation 2012;9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sanchez-Mejias E, Navarro V, Jimenez S, et al. Soluble phospho-tau from Alzheimer's disease hippocampus drives microglial degeneration. Acta Neuropathol 2016;132:897–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang X, Zhao L, Zhang J, et al. Requirement for microglia for the maintenance of synaptic function and integrity in the mature retina. J Neurosci 2016;36:2827–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chitu V, Gokhan S, Nandi S, et al. Emerging Roles for CSF-1 Receptor and its Ligands in the Nervous System. Trends Neurosci 2016;39:378–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Paloneva J, Kestila M, Wu J, et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet 2000;25:357–61 [DOI] [PubMed] [Google Scholar]
- 67. Paloneva J, Manninen T, Christman G, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet 2002;71:656–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.