Abstract
Aims
Coronary microvascular ischaemia, cardiomyocyte injury and stiffness may play an important role in the pathophysiology of heart failure with preserved ejection fraction (HFpEF). To date, the relationship between coronary flow reserve (CFR), myocardial injury, diastolic dysfunction, and future HFpEF risk is unknown.
Methods and results
Consecutive patients (n = 201) undergoing evaluation for suspected coronary artery disease (CAD) with stress myocardial perfusion positron emission tomography, serum troponin, and transthoracic echocardiography who did not have flow-limiting CAD or reduced left ventricular ejection fraction were identified. Patients were followed up (median 4.1 years) for cardiovascular death and hospitalization for non-fatal myocardial infarction or heart failure. Coronary flow reserve was quantified as stress/rest myocardial blood flow. Early diastolic flow (E) and relaxation (e′) velocities were obtained via transmitral and tissue Doppler, respectively. Patients with impaired CFR (<2, n = 108) demonstrated linearly decreasing e′ and increasing E/e′ consistent with worsening diastolic function (P for trend <0.0001). A detectable troponin was associated with diastolic dysfunction only in the presence of impaired CFR (interaction P = 0.002). In adjusted analyses, impaired CFR was independently associated with diastolic dysfunction (E/e′septal > 15, adjusted OR 2.58, 95%CI 1.22–5.48) and composite cardiovascular outcomes or HFpEF hospitalization alone (adjusted HR 2.47, 95%CI 1.09–5.62). Patients with both impaired CFR and diastolic dysfunction demonstrated >five-fold increased risk of HFpEF hospitalization (P < 0.001).
Conclusion
In symptomatic patients without overt CAD, impaired CFR was independently associated with diastolic dysfunction and adverse events, especially HFpEF hospitalization. The presence of both coronary microvascular and diastolic dysfunctions was associated with a markedly increased risk of HFpEF events.
Keywords: Coronary microvascular ischaemia, Coronary flow reserve, Diastolic dysfunction, Cardiac troponin, Heart failure with preserved ejection fraction
Introduction
Despite its escalating prevalence worldwide, heart failure with preserved ejection fraction (HFpEF) remains a poorly understood clinical syndrome without effective targeted therapies.1,2 Although the presence of elevated left ventricular (LV) filling pressure, as a function of increased myocardial stiffness, is thought to be a hallmark of HFpEF, diastolic dysfunction as assessed by echocardiography has not been a unifying feature of patients with HFpEF3 and may be ubiquitous in older individuals.4 The association of detectable levels of cardiac troponins, signifying subclinical cardiomyocyte injury, with incident heart failure events in patients without overt acute coronary syndromes or cardiac structural abnormalities5 underscores that a complex pathophysiology may underlie HFpEF. Recent discussions2,6–8 have implicated coronary microvascular dysfunction (CMD) as a possible driver in HFpEF, but clinical data supporting this hypothesis are limited by few cross-sectional observations.9,10
Coronary flow reserve (CFR), quantified as the ratio of hyperemic to rest myocardial blood flow, is a functional measure of large- and small-vessel ischaemia, and in the absence of overt coronary artery disease (CAD), is a marker of CMD.11,12 CFR measurements by non-invasive cardiac positron emission tomography (PET) imaging distinguish patients at low or high risk for major adverse cardiovascular events (MACE), including cardiac death,13,14 beyond comprehensive clinical assessment, left ventricular ejection fraction (LVEF), traditional measures of stress-induced ischaemia, and angiographic CAD severity.15
In patients with stable CAD and preserved LVEF, chronic circulating levels of high-sensitivity troponins have been associated with increased incidence of cardiovascular death and heart failure,5 but the mechanism for this increased risk is unclear. In otherwise low-risk patients with anginal symptoms and minimally elevated troponin, only those with CMD demonstrated significant risk of adverse events.16 Coronary microvascular ischaemia leading to cardiomyocyte injury and myocardial stiffness may play an important role in the pathophysiology of HFpEF.
To date, the relationship between CMD, myocardial injury, diastolic dysfunction, and future risk of HFpEF in symptomatic patients without overt CAD is not known. We sought to test the hypothesis that HFpEF is a disorder of cardiac functional reserve in which (i) CMD, as measured by impaired CFR, is associated with diastolic dysfunction, especially in patients with detectable myocardial injury and (ii) both coronary microvascular and diastolic dysfunctions are independently associated with adverse cardiovascular events, especially HFpEF.
Methods
Study population
Study participants were consecutive patients without prior history of CAD who were undergoing evaluation for suspected CAD with stress cardiac PET, serum cardiac troponin testing, and transthoracic echocardiography at Brigham and Women’s Hospital between 1 January 2006 and 31 July 2011. The most common indication for testing was the evaluation of chest pain, dyspnoea, or their combination. Patient history, medication use, and select laboratory values were ascertained at the time of PET imaging. From 522 patients, a final cohort of 201 was established after excluding those with known CAD, including prior revascularization and/or myocardial infarction, prior history of heart failure or severe valvular disease, and PET evidence of flow-limiting CAD (semiquantitative perfusion summed stress score > 2) or LVEF < 40%. No patients with positive troponin demonstrated a rise and fall of troponin values in concert with ECG changes or symptoms to prompt an early invasive clinical strategy of angiography and revascularization for an acute coronary syndrome. The study population therefore included patients in whom significant CAD was ruled out by conventional clinical diagnostics. The estimated glomerular filtration rate (eGFR) was determined with the abbreviated Modification of Diet in Renal Disease formula. The study was approved by the Partners Healthcare Institutional Review Board and conducted in accordance with institutional guidelines.
Positron emission tomography imaging
Patients were imaged with a whole-body PET–computed tomography scanner (Discovery RX or STE LightSpeed 64, GE Healthcare, Milwaukee, WI, USA) with 82Rb (1480–2200 MBq) as the flow tracer at rest and pharmacological stress, as previously described.17 Computed tomography was used for the purpose of attenuation correction only. For semiquantitative assessment of myocardial scarring and ischaemia, 17-segment visual interpretation of gated myocardial perfusion images was performed by experienced operators using a standard five-point scoring system. Summed rest, stress, and difference scores, with higher scores reflecting larger areas of myocardial scar, scar plus ischaemia, or ischaemia, respectively, were computed; summed stress scores ≤2 were considered normal.18 Rest LVEFs were calculated from gated myocardial perfusion images with commercially available software (Corridor4DM; Ann Arbor, MI, USA).
Absolute global myocardial blood flow (MBF, in mL/min/g) was quantified at rest and at peak hyperaemia using automated factor analysis and a validated two-compartment kinetic model, as previously described.17 Coronary hyperaemia was achieved predominantly through regadenoson or dipyridamole-induced vasodilation. Per-patient global CFR was calculated as the ratio of stress to rest absolute MBF for the whole left ventricle. Rest MBF and CFR were corrected for rest rate–pressure product (heart rate × systolic blood pressure), an index of baseline cardiac work. Radiation exposure per study was ≤4.6 mSV. Quantitative measures of CFR were recorded by a single operator blinded to patient data and obtained in patients undergoing PET myocardial perfusion at no additional clinical cost, imaging time, or radiation exposure.
Echocardiography
All patients underwent clinically indicated transthoracic echocardiography within median 1.5 days (Q1–Q3 0.6–7.3) of PET imaging. The most common indication for echocardiography was the evaluation of dyspnoea. There were no intervening cardiovascular events between echocardiography and PET imaging. Resting two-dimensional transthoracic echocardiograms were performed and analysed using standard clinical techniques. Early (E) diastolic transmitral filling velocities were measured at the mitral leaflet tips by pulsed-wave Doppler, and spectral tissue Doppler imaging was used to obtain early (e′) diastolic relaxation velocities at the septal and lateral mitral annulus from the apical four-chamber view.19 These measures were used to calculate E/e′. All measurements were averaged over consecutive cardiac cycles at end-expiration (3 and 5 beats for patients in sinus rhythm and atrial fibrillation, respectively) and recorded by a single experienced operator blinded to patient data.
Serum cardiac troponins
All patients underwent serial assessment of serum cardiac troponin using the clinically available local assay within 14 days prior to PET imaging. Serial assessment involved three consecutive blood draws approximately every 6–8 h over a 24-h period. From 2006 to 2011, three different troponin assays were clinically utilized sequentially: cTnI (Siemens Healthcare Diagnostics, initially introduced by Bayer HealthCare LLC, Diagnostics Division) with reference range <0.10 µg/L reflecting a 99th percentile cut-off point of 0.16 µg/L; TnI-Ultra (Siemens Healthcare Diagnostics) with reference range <0.04 µg/L reflecting a 99th percentile cut-off point of 0.04 µg/L; and cTnT fourth-generation Elecsys (Roche Diagnostics) with reference range <0.01 µg/L reflecting a 99th percentile cut-off point of less than 0.01 µg/L. Values above the reference range indicated detectable troponin. The peak value from serial assessment for each patient was used.
Outcomes
Subjects were followed up for a median of 4.1 years (Q1–Q3 1.4–6.6) for the occurrence of MACE, including cardiovascular death and hospitalization for non-fatal myocardial infarction or heart failure. The date of the last consultation was used to determine follow-up. Time to first event was analysed. Ascertainment of clinical endpoints was determined by blinded expert committee adjudication of the integrated electronic longitudinal medical record, Partners Healthcare Research Patient Data Registry, the National Death Index, mail surveys, and telephone calls. For an event to be classified as admission for non-fatal myocardial infarction or heart failure, discharge with a primary hospitalization diagnosis of myocardial infarction or heart failure, respectively, was required. In addition, only events meeting the 2012 Third Universal Definition of Myocardial Infarction20 or defined clinical criteria for the presence of symptoms, signs, and escalation of therapy for heart failure2 were classified as such. All hospitalization events occurred more than 30 days following imaging.
Statistical analysis
Baseline characteristics are reported as rates with percentages (%) for categorical variables and medians with interquartile ranges for continuous variables. We used the Fisher’s exact test and the Wilcoxon rank-sum test to assess the differences in categorical and continuous baseline characteristics. Spearman’s correlation was used to describe the association between the continuous variables of CFR and e′ or E/e′, in the overall cohort and also stratified by troponin detectability. Linear regression models of e′ and E/e′ were used to evaluate for significant interactions between CFR and troponin detectability. Restricted cubic splines were used to model a non-linear relationship between CFR and diastolic dysfunction. Impaired CFR was defined as CFR < 2, which is associated with worse cardiovascular outcomes in patients undergoing evaluation for suspected CAD14 and approximately served as a median cut point in this clinical cohort. A positive peak troponin value (e.g. above the reference range for the specific assay) was used as a dichotomous variable to accommodate the three different assays with varying detection thresholds in clinical use throughout the study period. Logistic regression was used to assess for the independent relationship between impaired CFR and E/e′septal > 15, an established non-invasive marker of elevated LV filling pressures (and stand-alone evidence of diastolic LV function21) in patients with suspected cardiac disease.19 Candidate variables tested included demographic characteristics, medical history and medication use, and non-invasive imaging parameters, with the most clinically important covariates or significant univariable associations included in the multivariable model. To avoid overfitting, demographic and medical history variables (age, gender, chest pain, type, hypertension, diabetes, hyperlipidaemia, smoking history, family history of premature CAD, body mass index, and oestrogen status) were incorporated into the final model using a validated pretest clinical risk score for diagnosing significant CAD (with values 0–8, 9–15, and 16–24 indicating low, intermediate, and high pretest risk, respectively), as previously described.22 Results are shown for e′septal, but similar findings were obtained for e′lateral.
Cumulative event-free survival curves for the MACE endpoint of cardiovascular death or hospitalization for non-fatal myocardial infarction or heart failure, as well as HFpEF hospitalization alone, were compared across dichotomous categories of impaired CFR using the log-rank test. Cox proportional hazards models were used to examine the association between impaired CFR and outcome events after controlling for effects of clinically important covariates. Univariate associations were tested and Cox models sequentially added pretest clinical score and laboratory and imaging variables, with the collinearity index used to check for linear combinations among covariates, and the Akaike information criterion assessed to avoid overfitting. The proportional hazards assumption was confirmed with the use of martingale residuals. The final model with impaired CFR and elevated E/e′ was adjusted for pretest clinical score, history of atrial fibrillation, reduced eGFR, detectable troponin, and LVEF. Interaction terms for CFR and diastolic dysfunction were tested for significance in the adjusted model.
To further investigate the presence of effect modification between coronary microvascular ischaemia and diastolic dysfunction on HFpEF outcomes, we performed an exploratory analysis where we stratified patients by impaired CFR and diastolic dysfunction. Event-free survival curves were compared across dichotomous categories with the log-rank test and plotted after adjustment for pretest clinical score and troponin detectability. Binary categories of e′ (high vs. low) were defined according to patient age (<50 years, e′septal > 7 vs. ≤7 cm; 50–64 years, >6 vs. ≤6 cm; ≥65 yo, >5 vs. ≤5 cm).4 Finally, Poisson regression was performed to compute annualized rates of HFpEF across categories of CMD and diastolic dysfunction. Model fit was assessed with the goodness-of-fit χ2 test, with a non-significant result indicating adequate fit. A P-value of <0.05 was considered to indicate statistical significance, and all tests were two-sided. The SAS analysis system version 9.4 was used for all analyses (SAS Institute).
Results
Baseline characteristics
Distribution of baseline characteristics is shown by categories of preserved vs. impaired CFR (Table 1). The median (Q1–Q3) age of patients in the overall cohort was 66 (57–79) years, 65% were women, 50% were White, and median pretest clinical score was 12 (9–15), consistent with intermediate risk. Three-quarters of patients had history of hypertension, and nearly two-thirds and one-third had dyslipidaemia and diabetes mellitus, respectively. The median (Q1–Q3) LVEF was 60% (55–63%) by echocardiogram and 61% (54–67%) by PET, and 25% of patients had a troponin value minimally elevated above the reference range of the clinical assay. Compared to patients with preserved CFR, those with impaired CFR (<2, n = 108) had higher rates of detectable troponin (18.3 vs. 31.5%, P = 0.03) and worse diastolic function, including both lower e′ and higher E/e′ (P < 0.01 for both, septal and lateral).
Table 1.
Baseline characteristics of patients by coronary flow reserve
| Characteristics | Overall (N = 201) | Coronary flow reserve |
P-valuea | |
|---|---|---|---|---|
| ≥2 (n = 93) | <2 (n = 108) | |||
| Demographic characteristics | ||||
| Age,b years (Q1–Q3) | 66 (57–79) | 64 (57–75) | 67 (57–81) | 0.30 |
| Female gender (%) | 130 (64.7) | 63 (67.7) | 67 (62.0) | 0.46 |
| White race (%) | 100 (49.8) | 50 (53.8) | 50 (46.3) | 0.32 |
| Body mass indexb (kg/m2) | 28.7 (24.9–34.3) | 29.2 (25.2–32.9) | 27.7 (24.7–35.9) | 0.96 |
| Pretest clinical scoreb,c | 12 (9–15) | 12 (10–15) | 13 (9–15) | 0.67 |
| Medical history | ||||
| Hypertension (%) | 152 (75.6) | 71 (76.3) | 81 (75.0) | 0.87 |
| Dyslipidaemia (%) | 129 (64.2) | 55 (59.1) | 74 (68.5) | 0.19 |
| Diabetes mellitus (%) | 66 (32.8) | 33 (35.5) | 33 (30.6) | 0.55 |
| Current smoker (%) | 16 (8.0) | 8 (8.6) | 8 (7.4) | 0.80 |
| Family history of CAD (%) | 38 (18.9) | 17 (18.3) | 21 (19.4) | 0.86 |
| Atrial fibrillation (%) | 18 (9.0) | 6 (6.5) | 12 (11.1) | 0.32 |
| Renal hemodialysis (%) | 5 (2.5) | 1 (1.1) | 4 (3.7) | 0.38 |
| Medications | ||||
| Aspirin (%) | 129 (64.2) | 52 (55.9) | 77 (71.3) | 0.03 |
| Statin (%) | 119 (59.2) | 49 (52.7) | 70 (64.8) | 0.09 |
| Beta-blocker (%) | 123 (61.2) | 54 (58.1) | 69 (63.9) | 0.47 |
| Nitrate (%) | 18 (9.0) | 11 (11.8) | 7 (6.5) | 0.22 |
| Angiotensin inhibitor (%) | 66 (32.8) | 36 (38.7) | 30 (27.8) | 0.13 |
| Insulin (%) | 37 (18.4) | 20 (21.5) | 17 (15.7) | 0.36 |
| Laboratory values | ||||
| eGFR <60 mL•min−1•1.73m−2 (%) | 68 (33.8) | 26 (28.0) | 42 (38.9) | 0.13 |
| Troponin detectabled (%) | 51 (25.4) | 17 (18.3) | 34 (31.5) | 0.03 |
| Non-invasive imaging parameters | ||||
| Left ventricular ejection fraction (%) | ||||
| By echocardiogram | 60 (55–63) | 60 (58–63) | 60 (55–63) | 0.76 |
| By positron emission tomography | 61 (54–67) | 61 (56–68) | 60 (52–67) | 0.27 |
| Early diastolic mitral flow (E) velocityb (cm/s) | 73 (63–90) | 73 (65–90) | 74 (60–94) | 0.72 |
| Tissue Doppler imaging (e′) velocityb (cm/s) | ||||
| Septal mitral annulus (e′septal) | 6.5 (5.0–8.0) | 7.0 (6.0–9.0) | 6.0 (4.8–8.0) | <0.01 |
| Lateral mitral annulus (e′lateral) | 9.0 (7.0–11.0) | 9.0 (8.0–11.0) | 7.8 (6.0–11.0) | <0.01 |
| E/e′septal | 11.6 (8.9–15.6) | 10.8 (8.7–12.8) | 13.0 (9.3–16.1) | <0.01 |
| E/e′lateral | 8.7 (6.5–11.7) | 7.8 (6.3–10.3) | 9.5 (7.0–12.3) | <0.01 |
| Rest heart rateb (bpm) | 69 (62–80) | 74 (65–85) | 66 (59–73) | <0.01 |
| Rest systolic blood pressureb (mmHg) | 148 (130–168) | 158 (140–176) | 145 (124–161) | <0.01 |
| Rest myocardial blood flowb,e, mL/min/g | 1.1 (0.9–1.4) | 1.0 (0.8–1.3) | 1.2 (1.0–1.6) | <0.01 |
| Stress myocardial blood flowb (ml/min/g) | 2.1 (1.5–2.8) | 2.7 (2.0–3.2) | 1.8 (1.3–2.4) | <0.01 |
| Coronary flow reserveb,e (%) | 1.9 (1.5–2.5) | 2.6 (2.3–3.0) | 1.5 (1.2–1.7) | <0.01 |
The P-value is for the comparison between groups and is based on the Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
Continuous variables are presented as medians (quartile 1–3).
Pretest clinical score integrates age, gender, presence of hypertension, dyslipidaemia, diabetes, BMI > 27, oestrogen status, smoking history, family history, and angina history into a pretest risk score for coronary artery disease: Risk: low (0–8), intermediate (9–15), and high (>15).22
Cardiac troponin T or I, as determined by clinically available local assay.
Rest myocardial blood flow and coronary flow reserve are corrected for rest rate pressure product (heart rate ⋅ systolic pressure).
Association between coronary flow reserve and markers of diastolic dysfunction
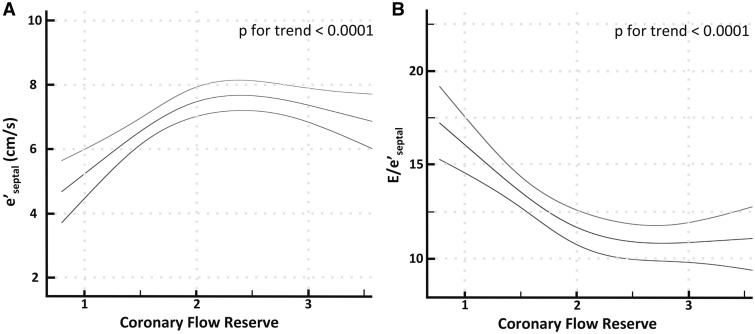
There was a direct relationship between CFR and e′ and an inverse relationship between CFR and E/e′ (P for trend <0.0001 for both), such that diastolic function declined in patients with CFR < 2 (Figure 1). Stratifying patients by troponin detectability revealed a significant interaction between troponin and CFR on diastolic function (P for interaction P = 0.026 and 0.002 for e′ and E/e′, respectively), with stronger correlations observed between CFR and e′ (rs = 0.49, P < 0.001) and E/e′ (rs = −0.50, P < 0.001), respectively, in patients with a detectable troponin (see Supplementary material online, Figure S1).
Figure 1.
Relationship between coronary flow reserve (CFR) and markers of diastolic dysfunction. There is a direct relationship between CFR and e′ (A) and an inverse relationship between CFR and E/e′ (B) such that diastolic function sharply declined in patients with CFR < 2. Non-linear relationship is modelled using restricted cubic splines with 95% confidence intervals. Results are shown for e′septal, but similar findings were obtained for e′lateral.
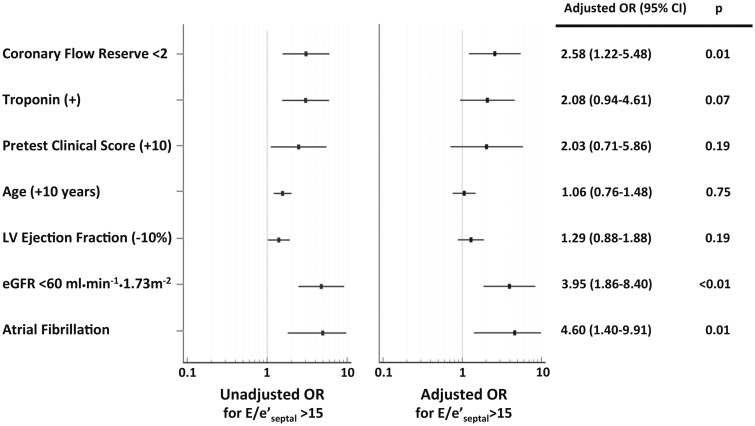
In univariable analysis, there was a significant association between impaired CFR and elevated E/e′septal > 15 (odds ratio for CFR < 2, 3.06, 95% CI 1.56–6.02, P = 0.001). In multivariable logistic regression modelling incorporating pretest clinical score, age, history of atrial fibrillation, reduced eGFR, LVEF, detectable troponin and impaired CFR, this association remained significant for impaired CFR (odds ratio 2.58, 95% CI 1.22–5.48, P = 0.01) (Figure 2). Impaired CFR was independently associated with E/e′septal > 15, a specific non-invasive marker of elevated cardiac filling pressures, in patients without flow-limiting CAD.
Figure 2.
Univariable and multivariable-adjusted associations for elevated E/e′ (E/e′septal > 15 as compared to ≤ 15). Odds ratios (OR) with 95% confidence intervals (95% CI) are presented for binary covariates, as well as a 10-unit increase in pretest clinical score and age, and a 10-unit decrease in left ventricular ejection fraction. Pretest clinical score incorporates age, gender, presence of hypertension, dyslipidaemia, diabetes, tobacco use, family history of premature CAD, body mass index ≥27 kg/m2, oestrogen status, and anginal history into a risk score for diagnosing significant CAD in a population of symptomatic patients presenting for stress testing: low (0–8), intermediate (9–15), and high (>15).
Coronary flow reserve, diastolic dysfunction, and cardiovascular events
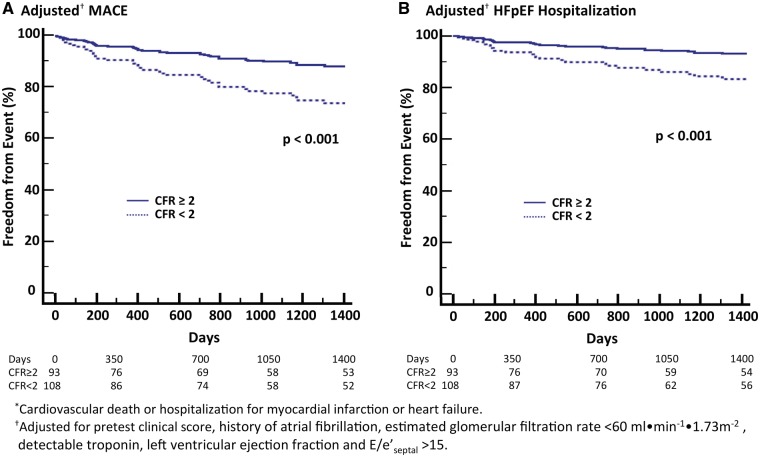
During follow-up over a median of 4.1 years (Q1–Q3, 1.4–6.6 years), 51 patients met the primary composite endpoint of cardiovascular death or hospitalization for non-fatal myocardial infarction or heart failure (Table 2). All hospitalization events occurred >30 days following imaging. These included 36 patients admitted for heart failure, all in the setting of preserved ejection fraction. In univariable modelling, the cumulative rate of MACE or HFpEF hospitalization was significantly associated with impaired CFR (hazard ratio for CFR < 2, 2.86; 95% CI 1.52–5.41; P = 0.001 for MACE and 3.01; 95% CI 1.41–6.44; P = 0.005 for HFpEF hospitalization). These associations remained significant after the addition of clinically and statistically important covariates into a multivariable model, including pretest clinical score, age, history of atrial fibrillation, reduced eGFR, detectable troponin, LVEF, and E/e′septal>15 (adjusted hazard ratio for CFR < 2, 2.38; 95% CI 1.21–4.67; P = 0.01 for MACE and 2.47; 95% CI 1.09–5.62; P = 0.03 for HFpEF hospitalization) (Table 3). Accordingly, patients with impaired CFR experienced worse event-free survival in comparison to those with preserved CFR, in composite MACE (Figure 3A) or HFpEF hospitalization (Figure 3B). Impaired CFR, here reflecting CMD, was as strongly associated with MACE and HFpEF events as non-invasive measures of elevated cardiac filling pressures (adjusted hazard ratio for E/e′septal>15, 2.24; 95% CI 1.18–4.27; P = 0.01 for MACE and 2.32; 95% CI 1.09–4.91; P = 0.03 for HFpEF hospitalization) (Table 3).
Table 2.
Patients meeting cardiovascular endpointa
| Outcomes | No. of patients (cumulative event, %)b (N = 201) |
|---|---|
| Cardiovascular death, myocardial infarction or heart failurec | 51 (23.7) |
| Cardiovascular death | 18 (7.0) |
| Myocardial infarction | 24 (11.3) |
| Heart failured | 36 (16.9) |
Median (Q1–Q3) follow-up time was 4.1 years (1.4–6.6 years).
Denotes cumulative event rate (%) from Kaplan–Meier estimates.
Time to first event was analysed. Myocardial infarction or heart failure denotes hospitalization for myocardial infarction or heart failure, respectively. All hospitalization events occurred >30 days following imaging.
All heart failure hospitalizations occurred in the setting of preserved ejection fraction.
Table 3.
Association between impaired coronary flow reserve or elevated E/e′ and clinical outcomes
| Outcomes | Univariable model hazard ratio (95% CI) |
Multivariable modela hazard ratio (95% CI) |
||
|---|---|---|---|---|
| CFR < 2b | E/e′ > 15c | CFR < 2b | E/e′ > 15c | |
| Cardiovascular death, myocardial infarction, or heart failured | 2.86 (1.52–5.41) | 3.28 (1.89–5.71) | 2.38 (1.21–4.67) | 2.24 (1.18–4.27) |
| Heart failure with preserved ejection fractiond | 3.01 (1.41–6.44) | 4.00 (2.07–7.76) | 2.47 (1.09–5.62) | 2.32 (1.09–4.91) |
Adjusted for pretest clinical score, history of atrial fibrillation, estimated glomerular filtration rate <60 ml•min−1•1.73 m−2, detectable troponin, left ventricular ejection fraction, coronary flow reserve <2 and E/e′septal > 15.
CFR denotes coronary flow reserve <2 relative to ≥ 2.
E/e′ denotes E/e′septal > 15 relative to ≤ 15.
Myocardial infarction or heart failure denotes hospitalization for myocardial infarction or heart failure, respectively. All heart failure hospitalizations occurred in the setting of preserved ejection fraction.
Figure 3.
Adjusted freedom from major adverse cardiovascular events (MACE)*, (A) and hospitalization for heart failure with preserved ejection fraction (HFpEF, (B) by coronary flow reserve (CFR). Event-free survival, adjusted for pretest clinical score, history of atrial fibrillation, estimated glomerular filtration rate <60 mL•min−1•1.73 m−2, detectable troponin, left ventricular ejection fraction and E/e′septal > 15. Freedom from events differed significantly among subgroups stratified by CFR such that patients with low CFR experienced higher rates of composite or HFpEF events (overall P < 0.001 in unadjusted and adjusted analyses).
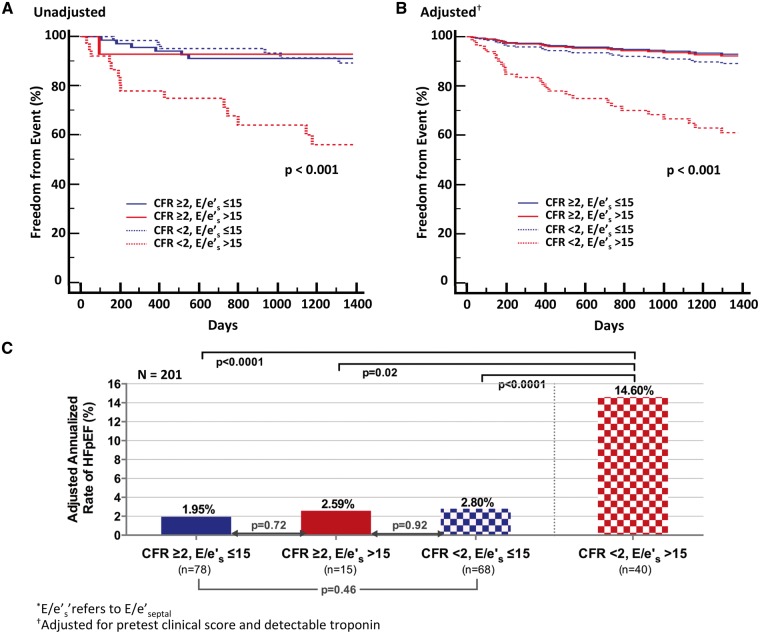
In an exploratory analysis of the relationship between CFR, diastolic dysfunction, and HFpEF hospitalization, we stratified probability of HFpEF hospitalization by CFR and diastolic dysfunction. In stratified analysis, those patients with elevated E/e′ and impaired CFR experienced the highest cumulative rate of HFpEF hospitalization (P < 0.001 unadjusted and adjusted for pretest clinical score and detectable troponin) (Figure 4A and B). The adjusted annualized rate of HFpEF hospitalization in this subgroup was 14.6% when compared with 2–3% for all other subgroups (P < 0.001) (Figure 4C). Findings were similar for reduced e′ (adjusted for age) and impaired CFR, where CFR further modified the effect of e′ on future risk of HFpEF hospitalization (P for interaction = 0.03, see Supplementary material online, Figure S2). Thus, for the same apparent level of diastolic dysfunction, patients with coronary microvascular ischaemia demonstrated a greater than five-fold increased risk of HFpEF hospitalization.
Figure 4.
Freedom from hospitalization for heart failure with preserved ejection fraction (HFpEF) by coronary flow reserve (CFR) and E/e′septal(*). (A) The Kaplan–Meier (unadjusted) analysis of time to first event. (B) Event-free survival, adjusted for pretest clinical score and detectable troponin. (C) Adjusted annualized rates of events. Freedom from HFpEF hospitalization differed significantly among subgroups stratified by CFR and E/e′, such that only those patients with elevated E/e′ and impaired CFR demonstrated the highest risk of hospitalization for HFpEF (P < 0.001).
Discussion
We demonstrate that in symptomatic patients without flow-limiting epicardial CAD, impaired CFR is independently associated with diastolic dysfunction and adverse cardiovascular outcomes, including HFpEF hospitalization. The latter was observed even after adjustment for the presence of detectable myocardial injury and diastolic dysfunction. CFR < 2, here reflecting CMD, was as strongly associated with MACE and HFpEF events as was E/e′septal > 15, a non-invasive echocardiographic marker with high specificity for increased LV filling pressures. After adjusting for clinical covariates and a detectable troponin, we show that the risk of HFpEF was significantly increased only in those patients with both diastolic dysfunction and impaired CFR. Finally, we provide evidence via significant interactions for effect modification of the association between: (i) myocardial injury and CMD on diastolic dysfunction, including noninvasive measures of impaired cardiac relaxation and elevated filling pressures, and (ii) diastolic function and CMD on future risk of HFpEF hospitalization.
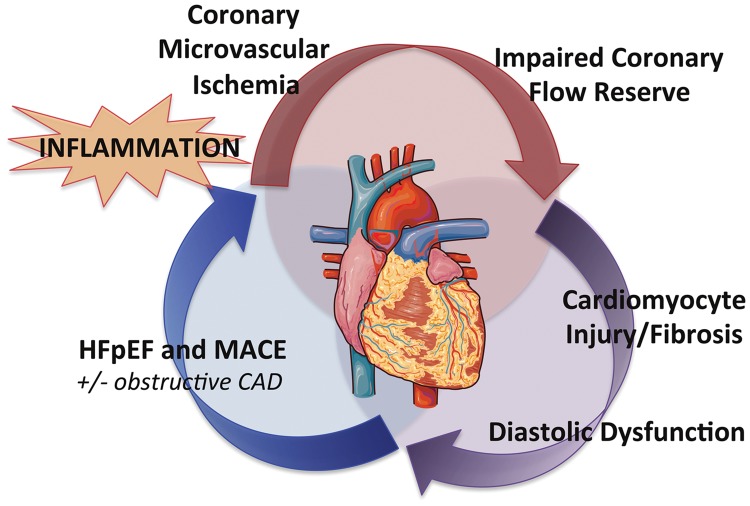
Angina without obstructive CAD has been associated with increased risk of MACE.23 In the setting of increased oxygen demand, impaired CFR, even absent obstructive CAD, reflects downstream myocardial ischaemia from an upset supply–demand relationship, which may predispose the myocardium to injury and worsened global ventricular mechanics and dysfunction. Our data demonstrate that coronary microvascular ischaemia was independently associated with diastolic dysfunction and that a detectable troponin was significantly associated with diastolic dysfunction only in the presence of coronary microvascular ischaemia. This suggests that factors tipping the balance towards cardiomyocyte injury in patients with existing CMD may worsen myocardial mechanics and increase risk of HFpEF outcomes, even absent overt structural abnormalities or obstructive CAD (Take home figure). In particular, microvascular endothelial dysfunction, decreased nitric oxide bioavailability, and increased profibrotic cytokine signaling may contribute to reduced coronary microvascular density or rarefaction, and increased myocardial fibrosis observed in HFpEF.6,24 Such an interplay of insults—precisely in the comorbid population of older, hypertensive, diabetic, and frequently female patients at risk for chronic kidney disease and atrial fibrillation as reflected in this study cohort—may synergize to propagate the vascular–ventricular stiffening thought to be central to the emerging epidemic of HFpEF.8,25,26
Take home figure.
Conceptual model of the pathophysiology linking coronary microvascular ischaemia, low-level cardiomyocyte injury, and myocardial stiffness to major adverse cardiovascular outcomes (MACE), especially heart failure with preserved ejection fraction (HFpEF). This process may occur even in the absence of obstructive coronary artery or overt structural heart disease. Heart image is adapted from Servier Medical Art. CAD, coronary artery disease.
We found that in the presence of coronary microvascular ischaemia, a detectable troponin was associated with exacerbated diastolic dysfunction, and patients with CFR < 2 and E/e′septal > 15 demonstrated a greater than five-fold increased adjusted risk of HFpEF hospitalization. These findings support a likely interplay of chronic CMD and subclinical myocardial injury in the pathway to diastolic dysfunction and HFpEF outcomes. They also advance prior observations that chronic circulating levels of high-sensitivity troponins are associated with increased incidence of cardiovascular death or heart failure (but not acute coronary syndromes) in patients with stable CAD and preserved LVEF,5 and that impaired CFR is independently associated with detectable troponin levels and modifies their effect on adverse outcomes in otherwise low-risk patients without obstructive CAD.16 The pathway to heart failure in patients without overt CAD and preserved ejection fraction may involve systemic inflammation,27 endothelial dysfunction, and increased cardiomyocyte oxygen demand with ensuing microvascular ischaemia, myocardial injury,16 interstitial fibrosis,28,29 and impaired cardiac mechanics6 (Take home figure). Clearer understanding of the relationship between coronary vasomotor dysfunction and CAD co-morbid conditions, including ischaemia with no obstructive CAD (INOCA),25,30 and heart failure1 may guide development of novel systemic therapies to restore coronary vascular function, especially for HFpEF outcomes.
Study limitations
This study must be interpreted in the context of its single-centre observational design, in which patients were clinically referred for PET myocardial perfusion imaging with echocardiography and serial cardiac troponin assessment. Despite best attempts at multivariable adjustment of associations, unmeasured confounding is possible. Our modest sample size limits extensive subgroup analysis, particularly for specific outcomes, and high-sensitivity troponin assays were not available. The study cohort was specifically defined so as to exclude flow-limiting CAD, by excluding patients with prior myocardial infarction, coronary revascularization, and abnormal semiquantitative perfusion using a stringent cut-off of SSS > 2 on index PET stress imaging, one of the most sensitive and diagnostically accurate tests available for non-invasive evaluation of ischaemia.31 Coronary microvascular dysfunction was therefore defined as the presence of impaired CFR in the absence of flow-limiting CAD. CFR results, which were not available to referring clinicians at the time of testing, did not affect downstream management decisions. To minimize overt structural abnormalities, we also excluded patients with cardiomoypathy or severe valvular disease. Recognizing important limitations, this hypothesis-generating work links the associations of functional biomarkers of coronary microvascular ischaemia, cardiomyocyte injury, and diastolic dysfunction with cardiovascular outcomes, particularly HFpEF, in symptomatic patients without flow-limiting CAD.
Conclusions
In symptomatic patients without overt CAD and with preserved LVEF, impaired CFR was independently associated with diastolic dysfunction and future MACE, especially HFpEF events. Impaired CFR also modified the effect of a detectable troponin on diastolic dysfunction severity. Independently of troponin, patients with both diastolic dysfunction and impaired CFR demonstrated a greater than five-fold increased risk of HFpEF hospitalization. Coronary microvascular ischaemia, alongside myocardial stiffness, may play an important role in the pathophysiology of HFpEF. Prospective studies are needed to investigate the role of impaired CFR and CMD for patient selection or as a target for intervention in clinical trials for HFpEF outcomes.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
National Institutes of Health [K23HL135438] to V.R.T.; National Institutes of Health [K08HL116792] and Americal Heart Association [14CRP20380422] to A.M.S.; National Institutes of Health [T32HL076136] to M.T.O.; and National Institutes of Health [5R01HL132021] to M.F.D.C.
Conflict of interest: A.M.S. received research support from Novartis, and consulting fees from Myocardia and GlaskoSmithKline. A.S.D. received research support from Novartis and consulting fees from AstraZeneca, Janssen, Novartis, Relypsa, Sanofi, and St. Jude Medical. J.D.G. received research support from Amgen and consulting fees from Takeda. R.B. received research support from Amgen and Gilead. S.D. received research grant support from Astellas Global Pharma Development. The other authors declared that they have no relevant relationships to disclose.
Supplementary Material
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med 2016;375:1868–1877. [DOI] [PubMed] [Google Scholar]
- 3. Kelly JP, Mentz RJ, Mebazaa A, Voors AA, Butler J, Roessig L, Fiuzat M, Zannad F, Pitt B, O’Connor CM, Lam CSP.. Patient selection in heart failure with preserved ejection fraction clinical trials. J Am Coll Cardiol 2015;65:1668–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shah AM, Claggett B, Kitzman D, Biering-Sorensen T, Jensen JS, Cheng S, Matsushita K, Konety S, Folsom AR, Mosley TH, Wright JD, Heiss G, Solomon SD.. Contemporary assessment of left ventricular diastolic function in older adults: the atherosclerosis risk in communities study. Circulation 2017;135:426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Røsjø H, Šaltytė Benth J, Maggioni A, Domanski MJ, Rouleau JL, Sabatine MS, Braunwald E; PEACE Investigators. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol 2013;61:1240–1249. [DOI] [PubMed] [Google Scholar]
- 6. Paulus WJ, Tschope C.. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 7. Taqueti VR, Ridker PM.. Inflammation, coronary flow reserve, and microvascular dysfunction: moving beyond cardiac syndrome X. JACC Cardiovasc Imaging 2013;6:668–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crea F, Bairey Merz CN, Beltrame JF, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Camici PG; Coronary Vasomotion Disorders International Study Group. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J 2017;38:473–477. [DOI] [PubMed] [Google Scholar]
- 9. Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Fukui K, Futaki M, Iwasawa T, Kimura K, Umemura S.. Impairment of coronary flow reserve evaluated by phase contrast cine-magnetic resonance imaging in patients with heart failure with preserved ejection fraction. J Am Heart Assoc 2016;5:e002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Srivaratharajah K, Coutinho T, deKemp R, Liu P, Haddad H, Stadnick E, Davies RA, Chih S, Dwivedi G, Guo A, Wells GA, Bernick J, Beanlands R, Mielniczuk LM.. Reduced myocardial flow in heart failure patients with preserved ejection fraction. Circ Heart Fail 2016;9:e002562. [DOI] [PubMed] [Google Scholar]
- 11. Taqueti VR, Di Carli MF.. Clinical significance of noninvasive coronary flow reserve assessment in patients with ischemic heart disease. Curr Opin Cardiol 2016;31:662–669. [DOI] [PubMed] [Google Scholar]
- 12. Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, Camici PG, Cerqueira MD, Chow BJ, Di Carli MF, Dorbala S, Gewirtz H, Gropler RJ, Kaufmann PA, Knaapen P, Knuuti J, Merhige ME, Rentrop KP, Ruddy TD, Schelbert HR, Schindler TH, Schwaiger M, Sdringola S, Vitarello J, Williams KA Sr, Gordon D, Dilsizian V, Narula J.. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol 2013;62:1639–1653. [DOI] [PubMed] [Google Scholar]
- 13. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF.. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE, Beanlands RS.. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740–748. [DOI] [PubMed] [Google Scholar]
- 15. Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF.. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taqueti VR, Everett BM, Murthy VL, Gaber M, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF.. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation 2015;131:528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, Fischman A, Coughlan M, Yasuda T, Di Carli MF.. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J Nucl Med 2009;50:1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, Gropler RJ, Knuuti J, Schelbert HR, Travin MI.. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol 2016;23:1187–1226. [DOI] [PubMed] [Google Scholar]
- 19. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston T, Oslo N, Phoenix A, Nashville T, Hamilton OC, Uppsala S, Liege B, Cleveland O, Novara I, Rochester M, Bucharest R; St. Louis, Missouri. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 20. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD Writing Group on the Joint ESC/ACCF/AAH/AWHF/Task Force for the Universal Documentation of Myocardial Infarction Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S; Guidelines ESC Committee for Practice Guidelines. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551–2567.22922414 [Google Scholar]
- 21. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL.. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007;28:2539–2550. [DOI] [PubMed] [Google Scholar]
- 22. Morise AP, Haddad WJ, Beckner D.. Development and validation of a clinical score to estimate the probability of coronary artery disease in men and women presenting with suspected coronary disease. Am J Med 1997;102:350–356. [DOI] [PubMed] [Google Scholar]
- 23. Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E.. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 24. Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM.. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015;131:550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF.. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation 2017;135:566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. EUGenMed Cardiovascular Clinical Study Group; Regitz-Zagrosek V, Oertelt-Prigione S, Prescott E, Franconi F, Gerdts E, Foryst-Ludwig A, Maas AH, Kautzky-Willer A, Knappe-Wegner D, Kintscher U, Ladwig KH, Schenck-Gustafsson K, Stangl V.. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J 2016;37:24–34. [DOI] [PubMed] [Google Scholar]
- 27. AlBadri A, Lai K, Wei J, Landes S, Mehta PK, Li Q, Johnson D, Reis SE, Kelsey SF, Bittner V, Sopko G, Shaw LJ, Pepine CJ, Bairey Merz CN, Bugiardini R.. Inflammatory biomarkers as predictors of heart failure in women without obstructive coronary artery disease: a report from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE). PLoS One 2017;12:e0177684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mascherbauer J, Marzluf BA, Tufaro C, Pfaffenberger S, Graf A, Wexberg P, Panzenbock A, Jakowitsch J, Bangert C, Laimer D, Schreiber C, Karakus G, Hulsmann M, Pacher R, Lang IM, Maurer G, Bonderman D.. Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging 2013;6:1056–1065. [DOI] [PubMed] [Google Scholar]
- 29. Rommel KP, von Roeder M, Latuscynski K, Oberueck C, Blazek S, Fengler K, Besler C, Sandri M, Lucke C, Gutberlet M, Linke A, Schuler G, Lurz P.. Extracellular volume fraction for characterization of patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2016;67:1815–1825. [DOI] [PubMed] [Google Scholar]
- 30. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL.. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Danad I, Raijmakers PG, Driessen RS, Leipsic J, Raju R, Naoum C, Knuuti J, Maki M, Underwood RS, Min JK, Elmore K, Stuijfzand WJ, van Royen N, Tulevski II, Somsen AG, Huisman MC, van Lingen AA, Heymans MW, van de Ven PM, van Kuijk C, Lammertsma AA, van Rossum AC, Knaapen P.. Comparison of coronary CT angiography, SPECT, PET, and hybrid imaging for diagnosis of ischemic heart disease determined by fractional flow reserve. JAMA Cardiol 2017;2:1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.