A longitudinal cohort study of multispecies Plasmodium infections in mothers and children in Uganda has revealed there to be both persistent and increasing parasitemia of certain species, which has clinical significance, despite regular access to frontline antimalarial treatments.
Keywords: malaria, Plasmodium malariae, Plasmodium ovale spp, Uganda, artemisinin combination therapy
Abstract
As part of a longitudinal cohort investigation of intestinal schistosomiasis and malaria in Ugandan children and their mothers on the shorelines of Lakes Victoria and Albert, we documented risk factors and morbidity associated with nonfalciparum Plasmodium infections and the longitudinal dynamics of Plasmodium species in children. Host age, household location, and Plasmodium falciparum infection were strongly associated with nonfalciparum Plasmodium infections, and Plasmodium malariae infection was associated with splenomegaly. Despite regular artemisinin combination therapy treatment, there was a 3-fold rise in P. malariae prevalence, which was not accountable for by increasing age of the child. Worryingly, our findings reveal the consistent emergence of nonfalciparum infections in children, highlighting the complex dynamics underlying multispecies infections here. Given the growing body of evidence that nonfalciparum malaria infections cause significant morbidity, we encourage better surveillance for nonfalciparum Plasmodium infections, particularly in children, with more sensitive DNA detection methods and improved field-based diagnostics.
Despite progress, control of malaria is a substantial challenge in parts of sub-Saharan Africa [1]. Although Plasmodium falciparum is the leading cause of malaria, other species—namely, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale curtisi and Plasmodium ovale wallikeri—circulate concurrently, although Duffy-negative individuals curtail P. vivax distributions [2–5]. Diagnosis of P. malariae and P. ovale spp. by light microscopy can be problematic because parasitemias often occur below detection thresholds for expert microscopy or are masked by more visible, concurrent P. falciparum infections [6]. Introduction of molecular/serological techniques has revealed that P. malariae and P. ovale spp. are more common than previously thought [7–11]. In southwestern Uganda, nearly half of asymptomatic children with malaria harbored nonfalciparum species [12].
Despite often being considered benign, a growing body of evidence reports overt disease and morbidity associated with P. malariae and P. ovale spp. infections [13–17]. In southern Papua, Indonesia, P. malariae infection is associated with a high burden of anemia [18, 19], and in Papua New Guinea, where at least 4 Plasmodium species cocirculate in humans, detrimental epidemiological interactions occur [20]. A number of other studies have suggested that mixed P. falciparum/P. malariae infections were associated with increased P. falciparum gametocytemia [21–23]. However, evidence of the clinical importance of nonfalciparum Plasmodium infections can be conflicting; Black et al demonstrated an inverse relationship between mixed-species infections and fever in Ivory Coast [24], whereas in Nigeria, anemia was shown to be more severe in mixed-species Plasmodium infections [25]. In Malawi, Bruce et al concluded that interactions among Plasmodium coinfecting species could protect against certain clinical outcomes but was contingent on the local seasonality and intensity of malaria transmission [8].
Commencing field surveillance in 2009 in Uganda, the longitudinal cohort study Schistosomiasis in Mothers and Infants project (SIMI) investigated the dynamics of intestinal schistosomiasis and malaria in young children and their mothers during an 18-month period with regular treatment follow-ups [26]. At baseline, although the general prevalence of noncomplicated P. falciparum in children across the 6 SIMI villages was high (>75%), P. malariae and P. ovale spp. could be found in young children at prevalences of up to 15% and 9%, respectively [27].
In the present study, based on a detailed molecular analysis of the SIMI dried blood spot archive, we identify risk factors for Plasmodium species infection, comparing multispecies and single-species infections, and assess interactions among species in terms of clinical outcomes. In Bukoba village, where the prevalence of nonfalciparum Plasmodium infections was highest, we conducted a longitudinal and geospatial analysis of all malaria infections that tested for clustering of infection in time and/or space.
METHODS
Ethical Statement and Recruitment
The London School of Hygiene and Tropical Medicine, United Kingdom (application no LSHTM 5538.09) and the Ugandan National Council of Science and Technology approved this study. Before enrollment, informed consent was obtained from mothers on their own behalf or on behalf of their children and was documented in writing or by thumbprint (in cases of illiteracy).
Study Sites, Participants, and Sampling
The longitudinal, closed-cohort SIMI study was conducted in communities of 6 villages on the shores of Lakes Albert and Victoria in Uganda [26]. In total, 662 mothers were enrolled together with 1211 young children (1 or 2 children per mother) aged 5 months to 6 years (49.1% were female). Mothers (or guardians) were aged 15–60 years (see Supplementary Table 1). The SIMI study aimed to investigate the infection dynamics of intestinal schistosomiasis, malaria, and soil-transmitted helminthiases over a period of 18 months, with follow-ups at 6 months, 12 months, and 18 months (Lake Victoria communities only). At each time point, a dried blood spot archive was collected onto Whatman 3M filter paper. A qualified nurse examined each participant on site, carrying out an abdominal examination to assess hepatosplenomegaly and measuring weight, height, and temperature. Each mother was interviewed in the local language to determine their own and their childrens’ exposure to risk factors for infection. The GPS coordinates of study participants’ households were collected as described [28].
On-Site Diagnosis and Treatment
During each survey, malaria diagnosis was carried out using rapid diagnostic tests (Paracheck-Pf, Orchid Biomedical Systems, Goa, India; or First Response, Premier Medical Corporation, Watchung, NJ) and microscopy on Giemsa-stained blood films [29]. Hemoglobin levels were recorded using a HemoCue spectrometer (HemoCue AB, Angelholm, Sweden). Egg-patent Schistosoma mansoni and soil-transmitted helminth infections were also diagnosed on site by microscopic detection of eggs in stool using the Kato-Katz method [26], with diagnosis of intestinal schistosomiasis bolstered by assessing serum antibodies to soluble egg antigen by enzyme-linked immunosorbent assay and circulating cathodic antigen in urine using rapid tests [30].
On the basis of a positive malaria rapid diagnostic test or blood film, children were treated with Lonart (20 mg/120 mg artemether/lumefrantrine; Cipla, Mumbai, India). Praziquantel (40 mg/kg) for treatment of intestinal schistosomiasis was offered to all study participants at baseline and the final survey. For interim surveys, praziquantel was administered on the basis of a positive circulating cathodic antigen urine test. In addition, participants were treated with albendazole (400 mg) at each survey time point. The project nurse supervised all treatment, and participants were monitored for side effects [31].
Molecular Analysis of Dried Blood Spots
Blood samples on filter paper were stored at 4°C with desiccant prior to genomic DNA extraction using the chelex method [32]. Real-time polymerase chain reaction (PCR) to detect Plasmodium species infections was carried out on all baseline samples. Plasmodium falciparum infections were detected using a SYBR® green-based real-time PCR assay followed by melt-curve analysis [33]. A probe-based real-time PCR assay [34] was used to detect P. malariae and P. ovale spp. infections on a Rotorgene RG3000 thermocycler (Corbett, Sydney, Australia). No attempt was made to detect P. vivax because analysis of preliminary data demonstrated its absence. For longitudinal analysis of dried blood spots from children in Bukoba village, the probe-based real-time PCR [34] was used to detect P. falciparum, P. malariae, and P. ovale spp. infections at baseline, 6 months, 12 months, and 18 months using the Mx3000P qPCR System (Agilent, Santa Clara, CA).
Epidemiological and Statistical Analyses
Epidemiological data were analyzed using Stata v9.2 (StatCorp, College Station, TX) and R v2.10.1 (The R Foundation for Statistical Computing, Vienna, Austria). Anemia in children was categorized based on hemoglobin levels as follows: mild, 10–11 g/dL; moderate, 7–10 g/dL; and severe, <7 g/dL. Multispecies malaria infections were categorized as >2 Plasmodium species. Intensity of P. falciparum infection was either categorized based on Giemsa-stained blood films as high (>5000 parasites/µL) or low (≤5000 parasites/µL) or based on cycle threshold (Ct) values as negative, >40; low, >30–40; medium, >22–30; or high, ≤22 cycles. Univariable regression analysis was performed to identify risk factors associated with P. falciparum, P. malariae, or P. ovale spp. infection as detected by real-time PCR. Factors identified as being statistically associated with a malaria infection (P > .05) were incorporated stepwise into a multivariable logistic regression model, and likelihood ratio tests were used to compare models. Random effects were included to control for clustering at household (for P. falciparum and P. malariae) or village level (P. ovale spp.), and interactions among variables were investigated. A similar analysis was carried out to investigate risk factors associated with multispecies malaria versus single-species infections and to investigate associations between morbidity markers and infections with different Plasmodium species.
A multiple-kind lottery model lottery-kind model [35] was used to determine whether there was evidence of a departure from random distribution of single and multispecies malaria infections. A generalized linear mixed model with random intercept to account for within-subject correlation was fitted to the time series data from children in Bukoba village to determine the effects of age, time period, and previous infection on current Plasmodium infection status.
Geospatial Analysis
Geospatial analysis of Plasmodium species infections among children in Bukoba was carried out based on household GPS locations. Global tests for clustering were undertaken, using the log ratio of spatial densities method proposed by Kelsall and Diggle (1995) to determine whether cases of each species were more clustered than noncases across the village [36]; this testing was conducted using the R package smacpod. This method was also used to identify and map putative local clusters using a Monte-Carlo simulation envelope approach. Coinfection of malaria species at baseline was also examined (ie, P. falciparum plus P. malariae and P. falciparum plus P. ovale spp.). There were too few coinfections with all 3 species to explore this spatial structure.
RESULTS
Multispecies Malaria Infections
Overall malaria infection prevalence as assessed by microscopy or real-time PCR was substantially raised in children compared with mothers (72.2% [95% confidence interval {CI}, 69.4%–74.8%] vs 24.2% [95% CI, 21.0%–27.7%] by microscopy; 74.9% [95% CI, 72.4%–77.4%] vs 38.6% [95% CI, 34.8%–42.4%] by real-time PCR), and in children infection prevalence was higher in villages along Lake Victoria than along Lake Albert (82.7% [95% CI, 79.5%–85.5%) vs 60.5% [95% CI, 56.4%–64.6%] by microscopy; 82.9% [95% CI, 79.8%–85.8 vs 66.0% [95% CI, 61.9%–69.9% by real-time PCR) (see Supplementary Table 1). Plasmodium falciparum was the most common species, with an overall prevalence of 74.6% (95% CI, 72.1%–77.0%) in children and 37.7% (95% CI, 34.0%–41.5%) in mothers. Eighty-nine (7.4%) children were infected with P. malariae, and 34 children (2.8%) were infected with P. ovale spp. with a higher prevalence along Lake Victoria than along Lake Albert. The majority of children infected with P. malariae and/or P. ovale spp. were also infected with P. falciparum. Only 9 mothers were infected with P. malariae, and only 2 were infected with P. ovale spp. No individual harbored P. malariae/P. ovale spp. coinfection in the absence of P. falciparum.
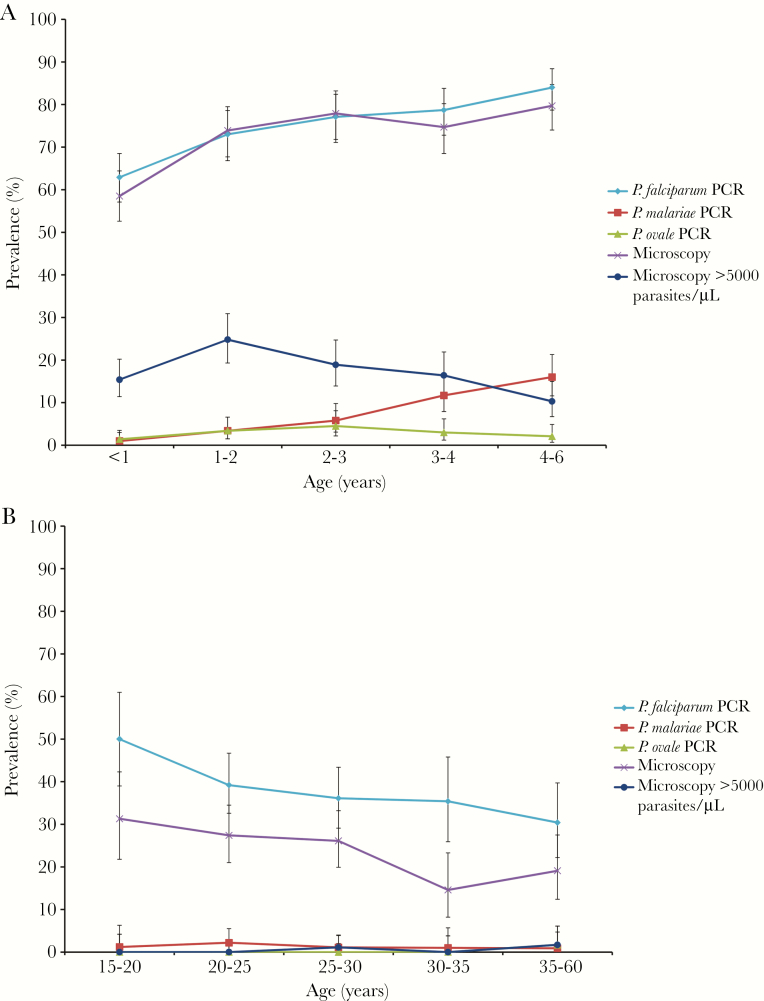
The relationship between age and malaria infection prevalence was investigated in children and mothers (Figure 1). The prevalence of slide-positive malaria, P. falciparum, and P. malariae infections increased with increasing age in children; however the fold difference between the youngest and oldest age category was substantially larger for P. malariae infections than P. falciparum infections (15.3-fold vs 1.3-fold). In contrast, there was no significant difference in P. ovale spp. infection prevalence among the different age groups. The prevalence of highly parasitemic infections (≥5000 parasites/µL) peaked in children aged 1–2 years, then declined in older age groups. In mothers, the prevalence of P. malariae, P. ovale spp., and highly parasitemic infections was very low and did not vary among age groups. For slide-positive malaria and P. falciparum infections, there was a general downward trend in prevalence with increasing age group in mothers. The prevalence of P. falciparum infections as detected by real-time PCR was higher than the prevalence of slide-positive malaria, demonstrative of submicroscopic carriage of Plasmodium parasites in the mothers.
Figure 1.
Plasmodium infection prevalence in Ugandan lakeshore communities varies with host age. A, Plasmodium infection prevalence at baseline in children enrolled in the Schistosomiasis in Mothers and Infants (SIMI) study. B, Plasmodium infection prevalence at baseline in mothers enrolled in the SIMI study. “PCR” refers to infection status determined by real-time polymerse chain reaction performed on DNA extracted from dried blood spots. “Microscopy” refers to presence of parasites in peripheral blood as determined by microscopy on Giemsa-stained blood smears. Error bars represent 95% confidence intervals. Abbreviations: P. falciparum, Plasmodium falciparum; P. malariae, Plasmodium malariae; P. ovale, Plasmodium ovale; PCR, polymerse chain reaction.
Risk Factors Associated With Plasmodium Infections
Risk factors associated with P. falciparum, P. malariae, and P. ovale spp. infections in children were investigated using logistic regression analysis. This analysis was not carried out in mothers due to the low prevalence of nonfalciparum infections. In univariable analysis, infection with 1 malaria species was associated with infection with each of the other 2 species and also with hookworm infection (Supplementary Table 2). For both P. falciparum and P. malariae, there was a strong association with age group, lake system, village, and being inside the house at night. For both infections, owning >1 insecticide-treated bednets (ITNs) was associated with reduced odds of infection, as was sleeping under a bednet in the case of P. falciparum. There was also a positive association between P. falciparum infection and living in a household with goats or sheep (Supplementary Table 2). Plasmodium ovale spp. infection was associated with lake system and living in a household owning goats, sheep, or cows. The final multivariable model for P. falciparum included age group, village, P. malariae infection, and P. ovale spp. infection (Table 1). The model for P. malariae was similar but also included hookworm infection and owning an ITN. In contrast, the model for P. ovale spp. contained only lake system, P. falciparum infection, and P. malariae infection (Table 1).
Table 1.
Multivariable Analysis of Risk Factors for Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, or Multispecies Malaria Infection in Children at Baseline
| Species | Variable | Category | Odds ratio | 95% CI | P value |
|---|---|---|---|---|---|
| P. falciparum | Age, y | <2 | 1.00 | … | … |
| 2–4 | 1.81 | 1.27–2.57 | .001 | ||
| 4–6 | 2.71 | 1.70–4.32 | <.0001 | ||
| Village | Bugoigo | 1.00 | … | … | |
| Walukuba | 0.89 | .53–1.50 | .67 | ||
| Piida | 1.09 | .63–1.90 | .76 | ||
| Bugoto | 2.12 | 1.25–3.57 | .005 | ||
| Bukoba | 4.01 | 2.21–7.29 | <.0001 | ||
| Lwanika | 2.11 | 1.12–3.97 | .02 | ||
| P. malariae | Negative | 1.00 | … | … | |
| Positive | 7.32 | 2.10–25.52 | .002 | ||
| P. ovale spp. | Negative | 1.00 | … | … | |
| Positive | 8.24 | .96–70.62 | .054 | ||
| P. malariae | Age, y | <2 | 1.00 | … | … |
| 2–4 | 6.13 | 2.49–15.09 | <.0001 | ||
| 4–6 | 14.82 | 5.20–42.25 | <.0001 | ||
| Village | Bugoigo | 1.00 | … | … | |
| Walukuba | 6.08 | 1.27–26.10 | .02 | ||
| Piida | 5.03 | 1.02–24.97 | .048 | ||
| Bugoto | 7.86 | 1.73–35.71 | .008 | ||
| Bukoba | 12.07 | 2.56–56.90 | .002 | ||
| Lwanika | 1.74 | .30–10.18 | .54 | ||
| P. falciparum | Negative | 1.00 | … | … | |
| Positive | 7.39 | 1.95–28.03 | .003 | ||
| P. ovale spp. | Negative | 1.00 | … | … | |
| Positive | 4.16 | 1.27–13.57 | .02 | ||
| Hookworm | Negative | 1.00 | … | … | |
| Positive | 2.33 | .99–5.48 | .053 | ||
| Houshold owns ≥1 ITN | No | 1.00 | … | … | |
| Yes | 0.40 | .20–.78 | .007 | ||
| P. ovale spp. | Lake | Albert | 1.00 | … | … |
| Victoria | 5.24 | 1.28–21.49 | .02 | ||
| P. falciparum | Negative | 1.00 | … | … | |
| Positive | 6.55 | .87–49.10 | .07 | ||
| P. malariae | Negative | 1.00 | … | … | |
| Positive | 2.98 | 1.30–6.84 | .01 | ||
| Multispecies | Age, y | <2 | 1.00 | … | … |
| 2–4 | 4.64 | 2.28–9.46 | <.0001 | ||
| 4–6 | 8.04 | 3.55–18.22 | <.0001 | ||
| Village | Bugoigo | 1.00 | … | … | |
| Walukuba | 2.83 | .84–9.53 | .09 | ||
| Piida | 1.49 | .40–5.57 | .56 | ||
| Bugoto | 3.42 | 1.10–10.63 | .03 | ||
| Bukoba | 11.66 | 3.68–36.92 | <.0001 | ||
| Lwanika | 1.93 | .52–7.18 | .33 | ||
| Household owns | No | 1.00 | … | … | |
| ≥1 ITN | Yes | 0.46 | .26–.84 | .009 |
Abbreviations: CI, confidence interval; ITN, insecticide-treated bednet; P. falciparum, Plasmodium falciparum; P. malariae, Plasmodium malariae; P. ovale, Plasmodium ovale.
A similar analysis determined whether there were risk factors associated with multispecies versus single-species malaria infections. In univariable analysis, multispecies infections were associated with age group, lake system, village, hookworm infection, and being inside the house at night. Owning >1 ITNs and sleeping under a bednet were associated with single-species rather than multispecies malaria infections (Supplementary Table 3). The final multivariable model included age group, village, and owning >1 bednets and incorporated random effects to control for clustering at household level (Table 1).
The associations observed among the different malaria species infections (Table 1) suggested that the different species were not randomly distributed. To investigate this in further detail, a multiple lottery-kind analysis was carried out [35]. The numbers of individuals infected with 2 or 3 species were greater than expected, and the number of single-species infections was smaller than expected (Table 2). Overall there was strong evidence of a departure from a random distribution of malaria parasites among infected children (Χ2 = 33.92; P < .0001).
Table 2.
Multiple-Kind Lottery Model Analysis of the Distribution of Multispecies Infections in Children
| Species combination | No. observed | No. expected | Χ 2 |
|---|---|---|---|
| 1 Plasmodium species | 796 | 841.84 | 2.50 |
| 2 Plasmodium species | 101 | 88.89 | 1.65 |
| 3 Plasmodium species | 9 | 1.87 | 27.21 |
| Not infected | 303 | 276.40 | 2.56 |
| Total | 1209 | 1209 | 33.92a |
aDegrees of freedom = 3; P < .0001.
Clinical Measures of Malaria
Associations with clinical measures of malaria were then investigated in children. Among parasitemics, no difference in parasitamia between single-species and multispecies infections was detected at either Lake Albert (Wilcoxon’s W = −0.602; P = 0.182; N = 521) or Lake Victoria (Wilcoxon’s W = 1.33; P = .547; N = 369). In multivariable models, infection with P. falciparum was associated with moderate anemia and splenomegaly. In addition, high P. falciparum infection levels were associated with fever. Infection with P. malariae was associated with an enlarged spleen, and multispecies malaria infections were more strongly associated with spleen enlargement than single-species malaria infections (Table 3).
Table 3.
Multivariable Analysis of Risk Factors for Various Clinical Indicators of Malaria in Children
| Morbidity indicator | Variable | Category | Odds ratio | 95% CI | P value | P value a | P value b |
|---|---|---|---|---|---|---|---|
| Moderate or severe anemia | P. falciparum | Negative | 1.00 | ... | ... | ... | ... |
| (≤10g/dL) | Low | 1.31 | .70–2.46 | .40 | .13 | .18 | |
| Moderate | 3.58 | 1.91–6.73 | <.0001 | .90 | .002 | ||
| High | 5.66 | 3.04–1.56 | <.0001 | .20 | .02 | ||
| Age, y | <2 | 1.00 | ... | ... | ... | ... | |
| 2–4 | 0.19 | .08–.36 | <.0001 | ... | ... | ||
| 4–6 | 0.48 | .08–.41 | .14 | ... | ... | ||
| Village | Bugoigo | 1.00 | ... | ... | ... | ... | |
| Walukuba | 0.77 | .45–1.33 | .35 | ... | ... | ||
| Piida | 0.31 | .16–.57 | <.0001 | ... | ... | ||
| Bugoto | 0.19 | .10–.33 | <.0001 | ... | ... | ||
| Bukoba | 0.28 | .16–.50 | <.0001 | ... | ... | ||
| Lwanika | 0.17 | .08–.41 | <.0001 | ... | ... | ||
| Household | No | 1.00 | ... | ... | ... | ... | |
| owns ≥1 animal | Yes | 0.63 | .45–.88 | <.007 | ... | ... | |
| S. mansoni | Negative | 1.00 | ... | ... | ... | ... | |
| (by ELISA) | Positive | 0.69 | .47–1.00 | .050 | ... | ... | |
| Fever | P. falciparum | Negative | 1.00 | ... | ... | ... | ... |
| Low | 0.58 | .25–1.37 | .22 | ... | ... | ||
| Moderate | 0.65 | .29–1.44 | .29 | ... | ... | ||
| High | 2.31 | 1.22–4.37 | .01 | ... | ... | ||
| Age, y | <2 | 1.00 | ... | ... | ... | ... | |
| 2–4 | 1.21 | .74–1.99 | .44 | ... | ... | ||
| 4–6 | 0.43 | .18–1.06 | .07 | ... | ... | ||
| Lake | Albert | 1.00 | ... | ... | ... | ... | |
| Victoria | 2.25 | 1.29–3.92 | .008 | ... | ... | ||
| Sleep under a | No | 1.00 | ... | ... | ... | ... | |
| bednet | Yes | 0.53 | .33–.84 | .008 | ... | ... | |
| Enlarged spleen | P. falciparum | Negative | 1.00 | ... | ... | ... | ... |
| Low | 2.44 | 1.61–3.68 | <.0001 | ... | ... | ||
| Medium | 3.94 | 2.58–6.02 | <.0001 | ... | ... | ||
| High | 4.92 | 3.18–7.62 | <.0001 | ... | ... | ||
| P. malariae | Negative | 1.00 | ... | ... | ... | ... | |
| Positive | 1.81 | 1.08–3.05 | .03 | ... | ... | ||
| Village | Bugoigo | 1.00 | ... | ... | ... | ... | |
| Walukuba | 0.75 | .46–1.24 | .27 | ... | ... | ||
| Piida | 0.53 | .31–.92 | .03 | ... | ... | ||
| Bugoto | 1.13 | .70–1.80 | .62 | ... | ... | ||
| Bukoba | 1.27 | .77–2.07 | .35 | ... | ... | ||
| Lwanika | 1.63 | .92–2.89 | .09 | ... | ... | ||
| S. mansoni | Negative | 1.00 | ... | ... | ... | ... | |
| (ELISA) | Positive | 0.72 | .54–.98 | .04 | ... | ... | |
| Enlarged spleen | No. Plasmodium | 1 | 1.00 | ... | ... | ... | ... |
| (multispecies model) | species | >1 | 1.69 | 1.04–2.74 | .03 | ... | ... |
| Village | Bugoigo | 1.00 | ... | ... | ... | ... | |
| Walukuba | 0.84 | .47–1.48 | .55 | ... | ... | ||
| Piida | 0.51 | .27–.94 | .03 | ... | ... | ||
| Bugoto | 1.06 | .63–1.79 | .83 | ... | ... | ||
| Bukoba | 1.21 | .71–2.08 | .48 | ... | ... | ||
| Lwanika | 1.08 | .57–2.03 | .82 | ... | ... | ||
| S. mansoni | Negative | 1.00 | ... | ... | ... | ... | |
| (ELISA) | Positive | 0.63 | .45–.88 | .006 | ... | ... |
Abbreviations: CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; P. falciparum, Plasmodium falciparum; P. malariae, Plasmodium malariae; S. mansoni, Schistosoma mansoni.
a P value for interaction with age category 2–4 years.
b P value for interaction with age category 4–6 years
Longitudinal Infection Dynamics
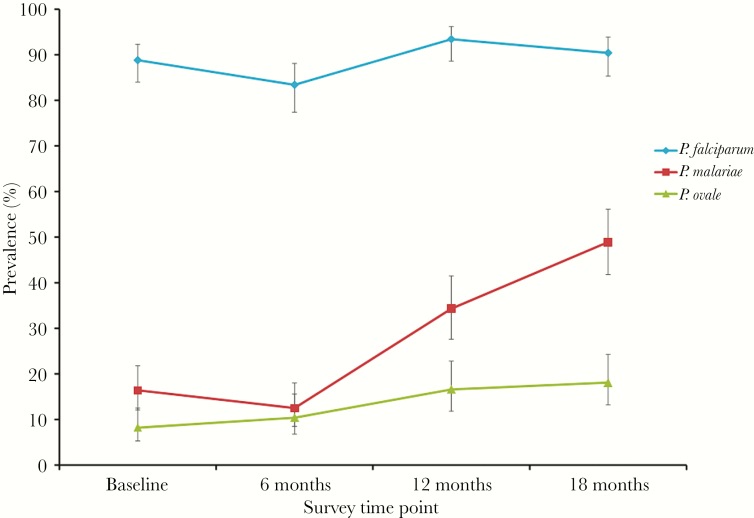
To investigate the temporal dynamics of multispecies malaria infections over the course of the study, the point prevalence of the different Plasmodium species infections was determined at 6, 12, and 18 months in children from Bukoba village, where prevalence of nonfalciparum malaria infection was highest [27]. There was a consistent rise in P. malariae prevalence (Figure 2), whereas P. falciparum prevalance remained largely static and there was a minor upward trend in P. ovale spp. prevalence. In the longitudinal multivariable analysis of risk factors, previous P. falciparum infection was associated with current P. falciparum infection at each time point, whereas mixed P. falciparum/P. malariae infections were associated with the study time point, the child’s age, and previous P. malariae infection (Table 4), demonstrating that the rise in P. malariae prevalence was not only due to increasing age of the child. This was supported by stratification of the prevalence of P. malariae infection by age group for the different study sites (Supplementary Table 4). Mixed P. falciparum/P. ovale spp. infections were associated with each time point.
Figure 2.
Plasmodium infection prevalence in children in Bukoba village at different survey time points. Infection status was determined by real-time polymerase chain reaction performed on DNA extracted from dried blood spots. Error bars represent 95% confidence intervals. Abbreviations: P. falciparum, Plasmodium falciparum; P. malariae, Plasmodium malariae; P. ovale, Plasmodium ovale.
Table 4.
Longitudinal Analysis of Risk Factors for Infection With Plasmodium falciparum (Pf ) Only, P. falciparum and Plasmodium malariae (Pf+Pm), or P. falciparum and Plasmodium ovale (Pf+Po) Among Children in Bukoba Village
| Species | Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|---|
| Pf only | Previous Pf infection | 3.01 | 1.46–5.83 | .002 |
| Pf + Pm | Time point | 2.07 | 1.58–2.84 | <.0001 |
| Age | 1.19 | 1.03–1.41 | .03 | |
| Previous Pm infection | 2.29 | 1.21–3.88 | .0001 | |
| Pf+Po | Time point | 1.42 | 1.16–1.76 | .0009 |
Abbreviations: CI, confidence interval; Pf, Plasmodium falciparum; Pm, Plasmodium malariae; Po, Plasmodium ovale.
Geospatial Analysis
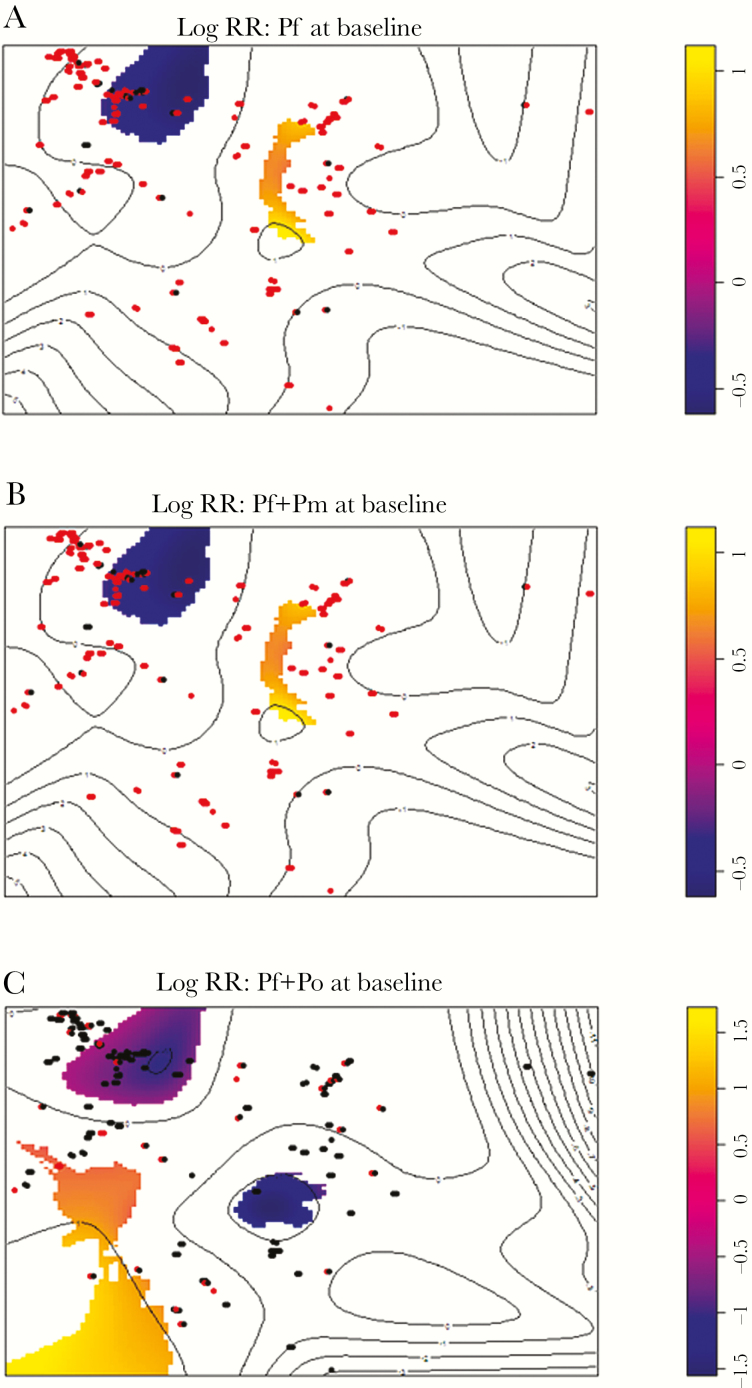
At baseline, there was no obvious visual pattern of infections of any Plasmodium species, and global tests for any clustering were nonsignificant (P > .05) (Supplementary Figure 1). However, upon examination of maps of significant log relative risk (Figure 3), the area in the northwest of the village appeared to have fewer P. falciparum, P. falciparum/P. malariae, and P. falciparum/P. ovale spp. cases than expected by chance (P = .03).
Figure 3.
Areas of Bukoba with significant log-transformed relative risk of malaria at baseline, determined using a Monte Carlo simulation envelope approach for Plasmodium falciparum infections (A), P. falciparum and Plasmodium malariae infections (B), and P. falciparum and Plasmodium ovale (C) infections. Each individual, infected (red) nor not-infected (black), is represented by a dot corresponding to their household. Yellow areas indicate more infections than expected, whereas purple areas indicate fewer infections than expected. Abbreviations: Pf, Plasmodium falciparum; Pm, Plasmodium malariae; Po, Plasmodium ovale; RR, relative risk.
DISCUSSION
Our analysis of the SIMI dried blood spot archive has provided a much deeper insight into the complex dynamics and significance of multispecies Plasmodium infections in children living in lakeshore communities in Uganda. One of the major risk factors identified for P. malariae and mixed-species infections was host age: infection with any Plasmodium species was much more common in children than in their mothers, and older children were more likely to be infected with each of the Plasmodium species than younger children. This age-prevalence pattern is well established for P. falciparum infections, and similar results have been reported for P. malariae and/or mixed-species infections in sub-Saharan Africa and Papua New Guinea [8, 11, 37, 38], likely reflecting age-related exposure with partial immunity. The fact that no association was found between P. ovale spp. infection and age in our study most likely reflects the low prevalence of P. ovale spp. infections in the baseline survey and that our diagnostic approach could not differentiate the 2 subspecies of P. ovale curtisi and P. ovale wallikeri that have been reported sympatric within this part of Uganda [39].
Infection prevalence by village varied for all Plasmodium species and was more common in children along Lake Victoria than along Lake Albert. Children in Bukoba were more at risk of mixed-species malaria infections than elsewhere. Similarly, in Malawi, Bruce et al demonstrated variations in prevalences of different Plasmodium species infections among villages [8]. All of our survey villages were located in regions of very high malaria endemicity (entomological inoculation rate > 100 per year) [40], although there is a growing appreciation of local heterogeneities even in high transmission areas, with environmental factors, household factors (eg, inclusive of domestic control measures), and insecticide resistance in Anopheles being implicated [41, 42]. Although there was no difference in bednet ownership and use, household construction, and so on among villages (Supplementary Table 1 and data not shown), there are climatic factors that differ between lakes that may influence local anopheline biology [42, 43], with potential (un)favorable local microhabitats alluded to in Figure 3.
We found a strong association between P. falciparum infection and P. malariae and P. ovale species infections, and the vast majority of P. malariae and P. ovale spp. infections existed as coinfections with P. falciparum. Consistent with this, multiple lottery-kind analysis revealed nonrandom distributions of Plasmodium species. Other studies have reported a frequency of P. falciparum and P. malariae coinfections higher than would be expected [38, 44–47], but this literature can be somewhat inconsistent (eg, in Papua New Guinea [48] and in Malawi [8]). Our findings here suggest that there may be common exposures and/or susceptibilities to different Plasmodium species, an obvious example of which could be shared Anopheles vectors. It is not yet known which vectors play a role in natural transmission of P. malariae and P. ovale spp. in Uganda [3, 4].
We did not observe any protective effect of mixed-species versus single-species Plasmodium infections on any of the clinical indicators of malaria, contrasting with other reports [8, 24], although this might benefit from additional assessments over a longer duration and ascertainment of any other underlying clinical states such as any hemoglobinopathies. Nonetheless, there was an association between mixed Plasmodium with P. malariae infection and splenomegaly, which, to our knowledge, is the first time this observation has been made and adds to the growing body of evidence supporting the clinical significance in children of nonfalciparum malaria within mixed-species Plasmodium infections.
Despite repeated artemisinin combination therapy (ACT) treatments, the dramatic rise in P. malariae prevalence seen here is most worrying, notwithstanding an upward trend in P. ovale spp. prevalence and consistently high P. falciparum prevalence. The rise in P. malariae may be partly explained by the increasing age of the children, even though the association between survey time point and mixed P. falciparum/P. malariae infections was maintained upon controlling for child age. A 4-year longitudinal study of Plasmodium infection in children in rural Burkina Faso found a 15-fold increase in P. malariae prevalence and a 4-fold increase in P. ovale spp. prevalence between 2007 and 2010 [22], an indirect consequence perhaps of drug-induced selection. The latter may also be responsible here in Bukoba because, over the 18-month period, 41% of the children (N = 248) received 4 ACT treatments, 27% received 3 treatments, and, based on reporting by mothers, 68% of children received further antimalarial treatment between surveys (unpublished data). Consistent with this hypothesis, we have previously demonstrated the persistence of P. malariae infections after ACT treatment in the SIMI cohort, most likely due to recrudescence of parasitemia after treatment rather than relapse per se [27].
In certain settings, it has been argued that P. malariae may have a relapsing, hepatic hypnozoite stage analogous to P. vivax and P. ovale spp. or that there is sequestration of quiescent blood-stage form analogous to an arrested lymphatic stage observed in rodent Plasmodium species [49]. This argument is based on historical case reports describing an ability of parasites to persist for decades and on a contemporary evaluation of imported cases of P. malariae infection in China, Sweden, and the United Kingdom [50]. The latter study demonstrated a delay in onset to symptoms that ranged from 1 day to 1 year or more and was associated with reported chemoprophylactic use by travelers. Thus the dramatic rise in P. malariae prevalence is perhaps a combination of the long-term persistence of P. malariae parasite processes and drug-induced selection, alongside implementation of more sensitive methods of molecular diagnosis that go beyond the detection thresholds of expert microscopy.
In conclusion, our findings highlight the cryptic burden of nonfalciparum malaria infections and indicate that there is a potential for emergence of P. malariae (and P. ovale spp.) infections in the face of frontline treatment for P. falciparum. With efforts increasingly directed toward elimination of falciparum malaria, we encourage better surveillance of nonfalciparum Plasmodium infections in the future, particularly in children, with more sensitive DNA detection methods and improved field-based diagnostics.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank technicians of the Vector Control Division, Ugandan Ministry of Health, for excellent support in the field. We are very grateful to all mothers and children who participated in the SIMI study.
Financial support. This work was supported by the Wellcome Trust (grant 085440) and by the Higher Education Funding Council for England.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Temporal dynamics of Plasmodium falciparum, P. malariae and P. ovale infection in a high-endemic setting in Uganda. British Society of Parasitology Spring Meeting, Bristol, United Kingdom, April 2013.
References
- 1. World Health Organization. 2017 world malaria report. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 2. Bauffe F, Desplans J, Fraisier C, Parzy D. Real-time PCR assay for discrimination of Plasmodium ovale curtisi and Plasmodium ovale wallikeri in the Ivory Coast and in the Comoros Islands. Malar J 2012; 11:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collins WE, Jeffery GM. Plasmodium ovale: parasite and disease. Clin Microbiol Rev 2005; 18:570–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collins WE, Jeffery GM. Plasmodium malariae: parasite and disease. Clin Microbiol Rev 2007; 20:579–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guerra CA, Howes RE, Patil AP et al. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis 2010; 4:e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mueller I, Zimmerman PA, Reeder JC. Plasmodium malariae and Plasmodium ovale—the “bashful” malaria parasites. Trends Parasitol 2007; 23:278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amanfo SA, Mduluza T, Midzi N, Cavanagh DR, Mutapi F. Seroepidemiology of Plasmodium species infections in Zimbabwean population. Malar J 2016; 15:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruce MC, Macheso A, Kelly-Hope LA, Nkhoma S, McConnachie A, Molyneux ME. Effect of transmission setting and mixed species infections on clinical measures of malaria in Malawi. PLoS One 2008; 3:e2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Daniels RF, Deme AB, Gomis JF et al. Evidence of non-Plasmodium falciparum malaria infection in Kédougou, Sénégal. Malar J 2017; 16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doderer-Lang C, Atchade PS, Meckert L et al. The ears of the African elephant: unexpected high seroprevalence of Plasmodium ovale and Plasmodium malariae in healthy populations in Western Africa. Malar J 2014; 13:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sitali L, Chipeta J, Miller JM et al. Patterns of mixed Plasmodium species infections among children six years and under in selected malaria hyper-endemic communities of Zambia: population-based survey observations. BMC Infect Dis 2015; 15:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roh ME, Oyet C, Orikiriza P et al. Asymptomatic Plasmodium infections in children in low malaria transmission setting, Southwestern Uganda(1). Emerg Infect Dis 2016; 22:1494–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Badiane AS, Diongue K, Diallo S et al. Acute kidney injury associated with Plasmodium malariae infection. Malar J 2014; 13:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lemmerer R, Unger M, Voßen M et al. Case report: spontaneous rupture of spleen in patient with Plasmodium ovale malaria. Wien Klin Wochenschr 2016; 128:74–7. [DOI] [PubMed] [Google Scholar]
- 15. Strydom KA, Ismail F, Frean J. Plasmodium ovale: a case of not-so-benign tertian malaria. Malar J 2014; 13:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomar LR, Giri S, Bauddh NK, Jhamb R. Complicated malaria: a rare presentation of Plasmodium ovale. Trop Doct 2015; 45:140–2. [DOI] [PubMed] [Google Scholar]
- 17. Roucher C, Rogier C, Sokhna C, Tall A, Trape JF. A 20-year longitudinal study of Plasmodium ovale and Plasmodium malariae prevalence and morbidity in a West African population. PLoS One 2014; 9:e87169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Douglas NM, Lampah DA, Kenangalem E et al. Major burden of severe anemia from non-falciparum malaria species in Southern Papua: a hospital-based surveillance study. PLoS Med 2013; 10:e1001575; discussion e1001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Langford S, Douglas NM, Lampah DA et al. Plasmodium malariae infection associated with a high burden of anemia: a hospital-based surveillance study. PLoS Negl Trop Dis 2015; 9:e0004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bruce MC, Day KP. Cross-species regulation of Plasmodium parasitemia in semi-immune children from Papua New Guinea. Trends Parasitol 2003; 19:271–7. [DOI] [PubMed] [Google Scholar]
- 21. Bousema JT, Drakeley CJ, Mens PF et al. Increased Plasmodium falciparum gametocyte production in mixed infections with P. malariae. Am J Trop Med Hyg 2008; 78:442–8. [PubMed] [Google Scholar]
- 22. Gnémé A, Guelbéogo WM, Riehle MM et al. Plasmodium species occurrence, temporal distribution and interaction in a child-aged population in rural Burkina Faso. Malar J 2013; 12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McKenzie FE, Jeffery GM, Collins WE. Plasmodium malariae infection boosts Plasmodium falciparum gametocyte production. Am J Trop Med Hyg 2002; 67:411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Black J, Hommel M, Snounou G, Pinder M. Mixed infections with Plasmodium falciparum and P malariae and fever in malaria. Lancet 1994; 343:1095. [DOI] [PubMed] [Google Scholar]
- 25. May J, Falusi AG, Mockenhaupt FP et al. Impact of subpatent multi-species and multi-clonal plasmodial infections on anaemia in children from Nigeria. Trans R Soc Trop Med Hyg 2000; 94:399–403. [DOI] [PubMed] [Google Scholar]
- 26. Betson M, Sousa-Figueiredo JC, Rowell C, Kabatereine NB, Stothard JR. Intestinal schistosomiasis in mothers and young children in Uganda: investigation of field-applicable markers of bowel morbidity. Am J Trop Med Hyg 2010; 83:1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Betson M, Sousa-Figueiredo JC, Atuhaire A et al. Detection of persistent Plasmodium spp. infections in Ugandan children after artemether-lumefantrine treatment. Parasitology 2014; 141:1880–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stothard JR, Sousa-Figueiredo JC, Betson M, Seto EY, Kabatereine NB. Investigating the spatial micro-epidemiology of diseases within a point-prevalence sample: a field applicable method for rapid mapping of households using low-cost GPS-dataloggers. Trans R Soc Trop Med Hyg 2011; 105:500–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sousa-Figueiredo JC, Oguttu D, Adriko M et al. Investigating portable fluorescent microscopy (CyScope) as an alternative rapid diagnostic test for malaria in children and women of child-bearing age. Malar J 2010; 9:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sousa-Figueiredo JC, Betson M, Kabatereine NB, Stothard JR. The urine circulating cathodic antigen (CCA) dipstick: a valid substitute for microscopy for mapping and point-of-care diagnosis of intestinal schistosomiasis. PLoS Negl Trop Dis 2013; 7:e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sousa-Figueiredo JC, Betson M, Atuhaire A et al. Performance and safety of praziquantel for treatment of intestinal schistosomiasis in infants and preschool children. PLoS Negl Trop Dis 2012; 6:e1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dlamini SV, Beshir K, Sutherland CJ. Markers of anti-malarial drug resistance in Plasmodium falciparum isolates from Swaziland: identification of pfmdr1-86F in natural parasite isolates. Malar J 2010; 9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sutherland CJ, Fifer H, Pearce RJ et al. Novel pfdhps haplotypes among imported cases of Plasmodium falciparum malaria in the United Kingdom. Antimicrob Agents Chemother 2009; 53:3405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shokoples SE, Ndao M, Kowalewska-Grochowska K, Yanow SK. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol 2009; 47:975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Janovy J, Clopton RE, Clopton DA, Snyder SD, Efting A, Krebs L. Species density distributions as null models for ecologically significant interactions of parasite species in an assemblage. Ecol Model 1995; 77:189–96. [Google Scholar]
- 36. Kelsall JE, Diggle PJ. Non-parametric estimation of spatial variation in relative risk. Stat Med 1995; 14:2335–42. [DOI] [PubMed] [Google Scholar]
- 37. Bruce MC, Donnelly CA, Packer M et al. Age- and species-specific duration of infection in asymptomatic malaria infections in Papua New Guinea. Parasitology 2000; 121 (Pt 3):247–56. [DOI] [PubMed] [Google Scholar]
- 38. Mueller I, Widmer S, Michel D et al. High sensitivity detection of Plasmodium species reveals positive correlations between infections of different species, shifts in age distribution and reduced local variation in Papua New Guinea. Malar J 2009; 8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oguike MC, Betson M, Burke M et al. Plasmodium ovale curtisi and Plasmodium ovale wallikeri circulate simultaneously in African communities. Int J Parasitol 2011; 41:677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yeka A, Gasasira A, Mpimbaza A et al. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Trop 2012; 121:184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baidjoe AY, Stevenson J, Knight P et al. Factors associated with high heterogeneity of malaria at fine spatial scale in the Western Kenyan highlands. Malar J 2016; 15:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Opondo KO, Weetman D, Jawara M et al. Does insecticide resistance contribute to heterogeneities in malaria transmission in the Gambia?Malar J 2016; 15:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Uganda National Meterological Authority https://www.unma.go.ug. Accessed 5 April 2017.
- 44. Arez AP, Pinto J, Pålsson K, Snounou G, Jaenson TG, do Rosário VE. Transmission of mixed Plasmodium species and Plasmodium falciparum genotypes. Am J Trop Med Hyg 2003; 68:161–8. [PubMed] [Google Scholar]
- 45. McKenzie FE, Bossert WH. Mixed-species Plasmodium infections of humans. J Parasitol 1997; 83:593–600. [PMC free article] [PubMed] [Google Scholar]
- 46. Pinto J, Sousa CA, Gil V et al. Mixed-species malaria infections in the human population of São Tomé island, west Africa. Trans R Soc Trop Med Hyg 2000; 94:256–7. [DOI] [PubMed] [Google Scholar]
- 47. Smith T, Genton B, Baea K, Gibson N, Narara A, Alpers MP. Prospective risk of morbidity in relation to malaria infection in an area of high endemicity of multiple species of Plasmodium. Am J Trop Med Hyg 2001; 64:262–7. [DOI] [PubMed] [Google Scholar]
- 48. Mehlotra RK, Lorry K, Kastens W et al. Random distribution of mixed species malaria infections in Papua New Guinea. Am J Trop Med Hyg 2000; 62:225–31. [DOI] [PubMed] [Google Scholar]
- 49. Sutherland CJ. Persistent parasitism: the adaptive biology of malariae and ovale malaria. Trends Parasitol 2016; 32:808–19. [DOI] [PubMed] [Google Scholar]
- 50. Teo BH, Lansdell P, Smith V et al. Delayed onset of symptoms and atovaquone-proguanil chemoprophylaxis breakthrough by Plasmodium malariae in the absence of mutation at codon 268 of pmcytb. PLoS Negl Trop Dis 2015; 9:e0004068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.