Abstract
Background
Combined chemotherapy and radiation therapy are used to treat nasopharyngeal carcinoma (NPC). Previous studies have shown that induction chemotherapy, given before radiotherapy, is beneficial in patients with local lymph node metastases. The aim of this study was to evaluate regional lymph node size in patients with NPC and the efficacy of five induction chemotherapy regimens given before radiotherapy.
Material/Methods
Between December 2007 and June 2011, 190 patients were included in this study, who had regionally advanced NPC (Stages II–IV). Five induction chemotherapy regimens were given prior to radiation: 98 patients (51.6%) received the TPF regimen (docetaxel, cisplatin, and fluorouracil); 56 patients (29.5%) received PF regimen (cisplatin and fluorouracil); 26 patients (13.7%) received the TP regimen (cisplatin and docetaxel); seven patients (3.7%) received combined nimotuzumab with TPF; three patients (1.6%) received a combination of the novel modified recombinant human endostatin (Endostar) with PF. The length and width of the regional lymph nodes were measured using neck B-mode (high-resolution grey scale) ultrasonography before chemotherapy and on the second day following completion of chemotherapy. Gastrointestinal tract and bone marrow suppression were also monitored during and after chemotherapy.
Results
The TPF chemotherapy induction regimen resulted in an improved early response of lymph node size reduction, compared with the PF and TP chemotherapy induction regimens. The combined use of nimotuzumab with the TPF regimen improved efficacy by 15%. The combined use of Endostar improved the efficacy of the PF regimen by 56% (P<0.05).
Conclusions
In a retrospective study in patients with NPC, different induction chemotherapy regimens had different effects on lymph node size before radiation therapy.
MeSH Keywords: Induction Chemotherapy, Nasopharyngeal Neoplasms, Giant Lymph Node Hyperplasia
Background
Worldwide, nasopharyngeal carcinoma (NPC) is an uncommon tumor, but this tumor is prevalent in southeast Asian countries. Guangdong Province in China has a particularly high incidence of NPC, where it affects between 30–50 individuals in every 100,000 [1,2]. Due to the anatomical location of NPC, it can be difficult to detect in the early stage. Between 60–70% of patients present with stage III-IV NPC at the time of diagnosis with metastases being present in the regional lymph nodes [3].
Radiation therapy is the primary therapeutic modality for NPC due to the anatomical location of this tumor and its high radiosensitivity. Treatment of early-stage NPC with radiotherapy can result in successful control, but regionally advanced NPC with does not respond well to radiotherapy alone. Therefore, since 1998, induction chemotherapy, given before radiotherapy, has been recommended as the standard of care for advanced NPC [4].
In the past few decades, the use of advanced combined therapeutic strategies has improved the 5-year survival rate for patients with NPC from about 50% to 70% [5,6]. Among the recommended therapeutic strategies for patients with NPC, adjuvant chemotherapy is given after the initial treatment to eliminate metastatic tumor cells. When given together, combined or concurrent treatment regimens can be used, including combined chemotherapy and radiation therapy. Although concurrent chemoradiotherapy (CCRT) had shown significant improvement of locoregional control (LRC) and overall survival (OS) of head and neck cancer patients, especially for patients with late-stage NPC, there are associated treatment toxicities. To circumvent the problem of CCRT toxicity, induction chemotherapy (IC) can be used, before radiotherapy or surgery.
According to the most recent edition of the American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) staging system for NPC, the involvement of the regional lymph node and enlargement by tumor metastasis represents the N in the TNM staging system for NPC [7]. An increase in the N stage is associated with a worse patient prognosis.
In clinical practice, the involved cervical lymph nodes in patients with NPC have different degrees of reduction in size during induction chemotherapy, but it is unclear whether the reduction in the size of regional lymph nodes reflects a more effective response to chemotherapy. Previously published clinical studies have focused on the long-term efficacy of treatment in NPC, but the early lymph node changes during induction chemotherapy have not been described. Therefore, because of the limited clinical research on regional lymph node size during induction chemotherapy for NPC, and because lymph node response may be predictive of treatment response, this is an area worth studying.
The aim of this retrospective study was to evaluate regional lymph node size in patients with NPC and the efficacy of five induction chemotherapy regimens prior to radiation therapy.
Material and Methods
Patients
All patients who participated in this study signed an informed consent. The study protocol was approved by Ethical Committee of Nanfang Hospital, Southern Medical University. Between December 2007 and June 2011, a total of 217 previously untreated patients, who were diagnosed with nasopharyngeal carcinoma (NPC) in NanFang Hospital, Southern Medical University, were included in this study. All patients were given questionnaires about their symptoms and medical history before their physical examination. All patients underwent a clinical evaluation that included an electrocardiogram (ECG) examination.
When NPC was detected by nasopharyngoscope, the patient was hospitalized. Tumor tissue biopsy samples were obtained and sent for histopathological examination. The diagnosis of NPC was confirmed by computed tomography (CT), magnetic resonance imaging (MRI), chest X-ray, emission CT, and whole abdomen ultrasound, urinary tract ultrasound, and whole-body bone scan. In some cases, patients were given whole-body positron emission tomography and computed tomography (PET/CT) examination for TNM tumor staging.
Initially, 217 patients were identified with NPC. Based on the 2008 Chinese NPC staging system, 11 patients had early-stage NPC and received standard radiation therapy only. A further 16 patients with different stages of NPC had incomplete treatment. Therefore, 27 out of 217 patients diagnosed with NPC at our hospital, between December 2007 and June 2011, were excluded from the study. A total of 190 patients were included in this study, who had regionally advanced NPC (Stages II–IV), and received induction chemotherapy.
Induction chemotherapy regimens
In general, two cycles of induction chemotherapy were administered. The following chemotherapy agents were given: docetaxel (Jiangsu Hengrui Pharmaceutical Co., Ltd.) (lot number: H20020543); cisplatin (Jiangsu Haosen Pharmaceutical Co., Ltd.) (lot number: H20040812); fluorouracil (Xi’an Haixin Pharmaceutical Co., Ltd.) (lot number: H20031272). Endostar (Shandong Xian-Sheng Maide Jin Pharmaceutical Co., Ltd.) (lot number: S20050088); and nimotuzumab (100 Thai Biopharmaceuticals Co., Ltd.) (lot number: S200810001)
Five induction chemotherapy regimens were given prior to radiation: 98 patients (51.6%) received the TPF regimen (docetaxel, cisplatin, and fluorouracil); 56 patients (29.5%) received the PF regimen (cisplatin and fluorouracil); 26 patients (13.7%) received the TP regimen (cisplatin and docetaxel); seven patients (3.7%) received combined nimotuzumab with TPF; three patients (1.6%) received a combination of the novel modified recombinant human endostatin (Endostar) with PF.
Docetaxel was administered at a dose of 60–75 mg/m2 on day 1; cisplatin was administered at a dose of 75 mg/m2 on days 1–3; fluorouracil was administered at a dose of 3,750–5,000 mg on days 1–5; Endostar was administered at a dose of 7.5 mg/m2 on days 1–7; nimotuzumab was administered at a dose of 60–120 mg/m2, weekly for eight weeks, twice in combination with the TPF regimen, and six times during the course of radiation therapy. Radiation therapy was scheduled two days after induction chemotherapy. Patients with NPC who were TNM stage M1 received chemotherapy only, without radiation treatment.
Treatment efficacy as measured by lymph node size
The length and width of the regional lymph nodes were measured using neck B-mode (high-resolution grey scale) ultrasonography before chemotherapy and on the second day following completion of chemotherapy.
The early response to induction chemotherapy was measured by the reduction of the size of the regional neck lymph node, as follows:
Volume (V)=0.52×ab2, where ‘a’ was the length, and ‘b’ was the width of the lymph node. The lymph node size reduction rate was equal to (Vbefore−Vafter)/Vbefore ×100% [8].
Treatment side effects
The side effects of induction chemotherapy on the gastrointestinal (GI) system were evaluated based on the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 published by the combined Department of Health and Human Services (HHS), National Institutes of Health (NIH), National Cancer Institute (NCI), to define toxicity grades. Leukopenia was evaluated by white blood cell (WBC) counts.
Statistical analysis
One-way analysis of variance or multiple analysis using the least significant difference (LSD) method were used to analyze the cervical lymph node reduction rate and reduction in WBC counts. Kruskal-Wallis multiple independent sample nonparametric tests were used to analyze chemotherapeutic gastrointestinal side effects and the effects of chemotherapy regimens. The Mann-Whitney U test and Spearman test were used. Statistical analysis was performed using SPSS 13.0 software (IBM, USA). A P-value <0.05 was considered to be statistically significant.
Results
Patient characteristics
Between December 2007 and June 2011, a total of 217 previously untreated patients were diagnosed with nasopharyngeal carcinoma (NPC) in NanFang Hospital, Southern Medical University. Of these patients, 190 patients were eligible for inclusion in this retrospective study. The age of the patients ranged from 17–77 years, with an average age of 45.93±11.78 years, with most patients presenting between the ages of 35–55 years, and with a male to a female patient ratio of 2.19: 1.
The TNM tumor staging of the patients included in the study is shown in Table 1. Based on the 2008 Chinese NPC staging system, the number of patients with NPC stages I, II, III, and IV were 10, 52, 69 and 86, respectively. Consistent with the findings from previously published studies, most patients (71.4%) were in the late stages of the disease when NPC was initially diagnosed.
Table 1.
Clinical stage of 217 NPC patients.
| M Stage | T Stage | N Stage | Sub-total | |||
|---|---|---|---|---|---|---|
| N0 | N1 | N2 | N3 | |||
| M0 | T1 | 10 (4.6%) | 10 (4.6%) | 8 (3.7%) | 11 (5.1%) | 39 (18.0%) |
| T2 | 13 (6.0%) | 29 (13.4%) | 10 (4.6%) | 0 (0.0%) | 52 (24.0%) | |
| T3 | 6 (2.8%) | 31 (14.3%) | 14 (6.5%) | 8 (3.7%) | 59 (27.2%) | |
| T4 | 5 (2.3%) | 27 (12.4%) | 11 (5.1%) | 7 (3.2%) | 50 (23.0%) | |
| Sub-total | 34 (15.7%) | 97 (44.7%) | 43 (19.8%) | 26 (12.0%) | 200 (92.2%) | |
| M1 | T1 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.9%) | 2 (0.9%) |
| T2 | 0 (0.0%) | 1 (0.5%) | 1 (0.5%) | 1 (0.5%) | 3 (1.4%) | |
| T3 | 0 (0.0%) | 2 (0.9%) | 2 (0.9%) | 3 (1.4%) | 7 (3.2%) | |
| T4 | 0 (0.0%) | 0 (0.0%) | 4 (1.8%) | 1 (0.5%) | 5 (2.3%) | |
| Sub-total | 0 (0.0%) | 3 (1.4%) | 7 (3.2%) | 7 (3.2%) | 17 (7.8%) | |
Efficacy of the induction chemotherapy regimens
The efficacy of induction chemotherapy was measured by reduction in the size of the neck lymph nodes prior to radiation therapy. Patients who had no indication of tumor metastasis on imaging studies were not included in the analysis. All induction chemotherapy regimens resulted in a positive response and the differences between responses were statistically significant (P<0.05) (Figure 1). Two patients treated with the TPF regimen achieved 100% reduction in lymph node size. In each group, more than half of the patients achieved ≥50% reduction in lymph node size from imaging of the neck lymph nodes. The mean regional lymph node size reduction rates were 47.8±20.5%, 55.8±22.1%, and 61.0±24.5% for the TP, PF and TPF regimens, respectively (Table 2).
Figure 1.
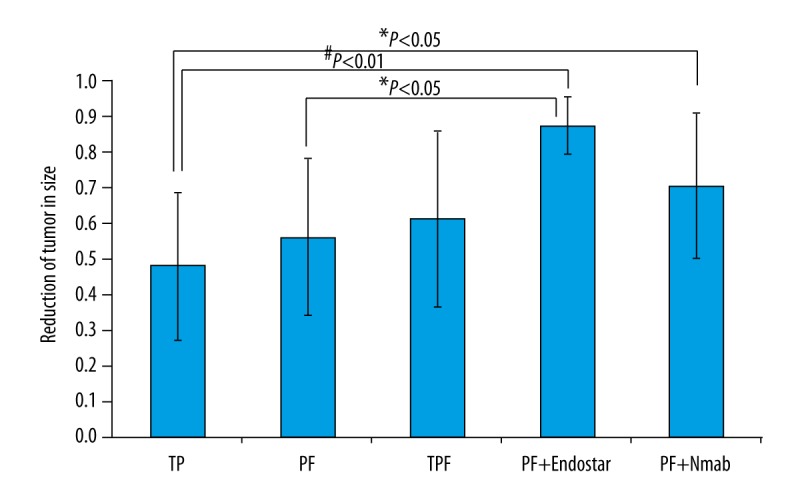
Statistical analysis on five induction chemotherapy regimens. Efficacy was measured by the reduction of tumor in size. Each group falls in normal distribution. One-way ANOVA was performed to compare two groups. Nmab – Nimotuzumab.
Table 2.
The efficacy of induction chemotherapy (IC).
| IC regimens | N | Efficacya | ||||
|---|---|---|---|---|---|---|
| Average (%) | 0–25% | 25–50% | 50–100% | 100% | ||
| TP | 13 | 47.8±20.5 | 2 | 5 | 6 | 0 |
| PF | 34 | 55.8±22.1 | 1 | 13 | 20 | 0 |
| TPF | 65 | 61.0±24.5 | 5 | 14 | 44 | 2 |
| PF + Endostar | 3 | 87.1±7.9 | 0 | 0 | 3 | 0 |
| TPF + Nimotuzumab | 6 | 70.8±20.9 | 0 | 0 | 6 | 0 |
| Statistic analysis | F=2.515 | |||||
| P=0.045* | ||||||
Reduction in the size of neck lymph node lesion followed by Fisher’s least significant difference (LSD) test.
P<0.05.
The PF chemotherapy induction regimen was more effective in reducing regional lymph node size when compared with the TP regimen. The TPF chemotherapy induction regimen was more effective in reducing regional lymph node size when compared with the PF regimen. However, there were no statistically significant differences between these three chemotherapy induction regimens. For the three patients who received the PF plus Endostar chemotherapy induction regimen, their regional neck lymph nodes were reduced in size by 79.0%, 87.5%, and 94.8%, respectively. The average reduction in lymph node size was 87.1±7.9%, which was 56% better than that of treatment with PF regimen alone (P<0.05).
For the six patients who received the TPF plus nimotuzumab chemotherapy induction regimen, they achieved a partial reduction in regional neck lymph node size of ≥50%. The average reduction in the size of the lymph nodes in this group was 70.8±20.9%, which was slightly (15%) better than with TPF induction chemotherapy alone. However, this difference was not statistically significant.
Side effects of the induction chemotherapy regimens
Bone marrow suppression was evaluated by measuring the white blood cell (WBC) counts before and after induction chemotherapy. As shown in Table 3, all induction chemotherapy regimens reduced the average WBC counts in each group. The TPF regimen (docetaxel, cisplatin, and fluorouracil) was more toxic than the TP regimen (cisplatin and docetaxel) and the PF regimen (cisplatin and fluorouracil). In addition to leukopenia, one patient in the TPF group developed a high fever. Comparison of the TP with the PF chemotherapy induction groups showed that TP induction chemotherapy was less toxic compared with PF induction chemotherapy. However, the differences in toxicity between the TPF, TP and PF chemotherapy induction regimens were not statistically significant. However, the addition of Endostar to the PF chemotherapy induction regimen did not reduce the number of WBCs. In contrast, the addition of nimotuzumab to the TPF regimen was less favorable in terms of WBC count. The side effect of induction chemotherapy on gastrointestinal (GI) system varied from grade 0 to grade 3, where most patients were either grade 1 and 2, with no patient having grade 4 GI side effects.
Table 3.
The side effect of induction chemotherapy (IC).
| IC regimen | N | WBCa | GIb | Toxicityc | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||
| TP | 26 | −0.365±1.530 | 92.46 | 1 | 9 | 15 | 3 | 0 |
| PF | 56 | −0.614±2.498 | 100.75 | 0 | 28 | 20 | 8 | 0 |
| TPF | 98 | −1.211±2.426 | 92.12 | 4 | 48 | 37 | 9 | 0 |
| PF + Endostar | 3 | −0.590±1.568 | 51.5 | 0 | 3 | 0 | 0 | 0 |
| TPF + Nimotuzumab | 7 | −1.780±2.673 | 117 | 1 | 0 | 5 | 1 | 0 |
| Statistic analysis | F=1.219 | χ2=4.810 | ||||||
| P=0.304 | P=0.307 | |||||||
The average of WBC counts before treatment was (6.8±2.0) ×1000 per ml. The change in cell counts after treatment was given and followed by Fisher’s least significant difference (LSD) test.
The mean rank of gastrointestinal (GI) side effects followed by Kruskal-Wallis one-way analysis of variance.
According to chemotherapy toxicity grading system by World Health Organizaiton (WHO).
As shown in Table 3, statistical analysis included the mean rank from all groups treated with induction chemotherapy that included paclitaxel, cisplatin, and fluorouracil combinations. PF was more toxic than TP and TPF, but the difference between TP and TPF was subtle, and the difference between the three regimens was not statistically significant. However, the addition of Endostar to the PF chemotherapy induction regimen reduced the degree of side effects. The addition of nimotuzumab to the TPF chemotherapy induction regimen increased the side effects. Furthermore, female patients with NPC were significantly less tolerant to chemotherapy compared with male patients with NPC, as shown by the Mann-Whitney U test (Z=−2.078, P=0.038). However, there was no significant correlation between side effect and age by the Spearman test (r=−0.093, P=0.205).
Discussion
This retrospective study included 190 cases of nasopharyngeal carcinoma (NPC) who were treated in a single center, and who received five regimens of induction chemotherapy prior to radiation therapy. Imaging of regional neck lymph nodes was performed using neck B-mode (high-resolution grey scale) ultrasonography to evaluate the effects of five induction chemotherapy regimens: the TPF regimen (docetaxel, cisplatin, and fluorouracil); the PF regimen (cisplatin and fluorouracil); the TP regimen (cisplatin and docetaxel); combined nimotuzumab with TPF; and a combination of the novel modified recombinant human endostatin (Endostar) with PF. Because there have been limited previous studies on lymph node reactions during chemotherapy in NPC, the aim of this study was to evaluate regional lymph node size in patients with NPC and the efficacy of five induction chemotherapy regimens.
The findings of this study showed that regional lymph node size for patients with NPC treated with the PF induction chemotherapy regimen was reduced compared with the TP induction chemotherapy regimen. The lymph node size reduction of TPF induction chemotherapy regimen was greater than for the PF induction chemotherapy regimen.
The TPF induction chemotherapy regimen of docetaxel, cisplatin, and fluorouracil is commonly used in advanced head and neck cancer [9]. Kong et al. reported that TPF-based induction chemotherapy significantly improved clinical outcome in patients with stage III or IVA/B NPC [10]. These previous findings support those of the present study, which showed a reduction in lymph node size using the TPF induction chemotherapy regimen. Also, the results of this study indicate that early lymph node responses may predict tumor prognosis. However, in this study, there was no statistically significant difference between the TPF regimen (docetaxel, cisplatin, and fluorouracil), the PF regimen (cisplatin and fluorouracil), and the TP regimen (cisplatin and docetaxel), possibly because the study size for each group was too small.
Nimotuzumab is a humanized therapeutic monoclonal antibody against epidermal growth factor receptor (EGFR), which is better tolerated than non-humanized therapeutic monoclonal antibodies [11,12]. In the present study, nimotuzumab increased the efficacy of reduction in regional lymp[h node size in patients with NPC by about 15%, but the difference was not statistically significant when compared with TPF alone (Table 2). However, the use of nimotuzumab induced side effects that included both leukopenia and gastrointestinal symptoms.
Endostar (rh-endostatin, YH-16) is a recombinant human endostatin that, when combined with chemotherapy has previously been shown to improve the treatment response in patients with advanced non-small cell lung cancer (NSCLC) [13–15]. Similar results with Endostar have also been found in the treatment of other types of solid tumor [16–18]. The findings of this present study showed that PF induction chemotherapy plus Endostar resulted in a reduction in lymph node size that was significantly better when compared with PF induction chemotherapy alone (P<0.05) (Table 2). The reasons for these findings are unclear, as the present study included small patient numbers in these induction chemotherapy regimen groups. Therefore, larger, controlled, multicenter studies are recommended to investigate the effects of induction chemotherapy regimens in patients with NPC.
The findings of this study have shown that the differences between responses in patients with NPC in lymph node size between TPF, TP, and PF induction chemotherapy regimens were not statistically significant. It is possible that the use of induction chemotherapy before treatment with radiotherapy may have a limited systemic impact. However, the addition of the recombinant human endostatin, Endostar to the PF (cisplatin and fluorouracil) induction chemotherapy regimen did not reduce the number of white blood cells (WBCs) after treatment. In contrast, nimotuzumab, a humanized therapeutic monoclonal antibody to EGFR, was less favorable when it was added to the TPF (docetaxel, cisplatin, and fluorouracil) induction chemotherapy regimen.
The TNM tumor staging system for cancer, including for NPC, is widely used to guide clinical treatment, assess the effects of treatment, and to predict patient prognosis [19]. Lymph node reactions may be useful in assessing the effectiveness of chemotherapy in NPC. However, in this study, patients did not undergo long-term follow-up, and the effect of regional lymph node responses on patient prognosis in NPC could not be evaluated. Future well-designed, large-scale, multi-center, controlled prospective clinical studies are needed on the efficacy and safety of induction chemotherapy regimens given before radiotherapy, for patients with NPC.
Conclusions
In this retrospective study, which included patients with nasopharyngeal carcinoma (NPC), different induction chemotherapy regimens had different effects on lymph node size before radiation therapy. Although this was a retrospective clinical study with small patient numbers, the study findings may provide the basis for future studies on induction chemotherapy regimens in patients with NPC.
Acknowledgements
The authors thank Shengli An from the Department of Biostatistics at Southern Medical University for his technical assistance and Dr. Suiyang Li from Canada for his critical review of the manuscript.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the National Natural Science Fund of China (grant number 81572649)
References
- 1.Chua ML, Wee JT, Hui EP, Chan AT. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–24. doi: 10.1016/S0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 2.Liu YT, Fan YY, Xu CH, et al. Habitual consumption of soy products and risk of nasopharyngeal carcinoma in Chinese adults: A case-control study. PLoS One. 2013;8(10):e77822. doi: 10.1371/journal.pone.0077822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23(27):6730–38. doi: 10.1200/JCO.2005.16.790. [DOI] [PubMed] [Google Scholar]
- 4.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16(4):1310–17. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 5.Cao SM, Yang Q, Guo L, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase III multicentre randomised controlled trial. Eur J Cancer. 2017;75:14–23. doi: 10.1016/j.ejca.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 6.Li WF, Chen L, Sun Y, Ma J. Induction chemotherapy for locoregionally advanced nasopharyngeal carcinoma. Chin J Cancer. 2016;35(1):94. doi: 10.1186/s40880-016-0157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan JJ, Ng WT, Zong JF, et al. Prognostic normogram for refining the prognostication of the proposed 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016;122(21):3307–15. doi: 10.1002/cncr.30198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shridhar R, Hoffe SE, Almhanna K, et al. Lymph node harvest in esophageal cancer after neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2013;20(9):3038–43. doi: 10.1245/s10434-013-2988-4. [DOI] [PubMed] [Google Scholar]
- 9.Strojan P, Grašič Kuhar C, Žumer B, et al. TPF induction chemotherapy and concomitant irradiation with cisplatin and cetuximab in unresectable squamous cell carcinoma of the head and neck. Head Neck. 2014;36(11):1555–61. doi: 10.1002/hed.23506. [DOI] [PubMed] [Google Scholar]
- 10.Kong L, Zhang Y, Hu C, et al. Effects of induction docetaxel, platinum, and fluorouracil chemotherapy in patients with stage III or IVA/B nasopharyngeal cancer treated with concurrent chemoradiation therapy: Final results of two parallel phase 2 clinical trials. Cancer. 2017;123(12):2258–67. doi: 10.1002/cncr.30566. [DOI] [PubMed] [Google Scholar]
- 11.Ramakrishnan MS, Eswaraiah A, Crombet T, et al. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. MAbs. 2009;1(1):41–48. doi: 10.4161/mabs.1.1.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu M, Wang X, Shen L, et al. Nimotuzumab plus paclitaxel and cisplatin as the first line treatment for advanced esophageal squamous cell cancer: A single centre prospective phase II trial. Cancer Sci. 2016;107(4):486–90. doi: 10.1111/cas.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biaoxue R, Xiguang C, Hua L, et al. Thoracic perfusion of recombinant human endostatin (Endostar) combined with chemotherapeutic agents versus chemotherapeutic agents alone for treating malignant pleural effusions: A systematic evaluation and meta-analysis. BMC Cancer. 2016;16(1):888. doi: 10.1186/s12885-016-2935-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rong B, Yang S, Li W, et al. Systematic review and meta-analysis of Endostar (rh-endostatin) combined with chemotherapy versus chemotherapy alone for treating advanced non-small cell lung cancer. World J Surg Oncol. 2012;24(10):170. doi: 10.1186/1477-7819-10-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Sun Y, Liu Y, et al. [Results of randomized, multicenter, double-blind phase III trial of rh-endostatin (YH-16) in treatment of advanced non-small cell lung cancer patients]. Zhongguo Fei Ai Za Zhi. 2005;8(4):283–90. doi: 10.3779/j.issn.1009-3419.2005.04.07. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Huang XE, Yan PW, et al. Efficacy and safety of endostar combined with chemotherapy in patients with advanced solid tumors. Asian Pac J Cancer Prev. 2010;11(4):1119–23. [PubMed] [Google Scholar]
- 17.Xu HX, Huang XE, Qian ZY, et al. Clinical observation of Endostar combined with chemotherapy in advanced colorectal cancer patients. Asian Pac J Cancer Prev. 2011;12(11):3087–90. [PubMed] [Google Scholar]
- 18.Cui C, Mao L, Chi Z, et al. A phase II, randomized, double-blind, placebo-controlled multicenter trial of Endostar in patients with metastatic melanoma. Mol Ther. 2013;21(7):1456–63. doi: 10.1038/mt.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan J, Xu Y, Qiu S, et al. A comparison between the Chinese 2008 and the 7th edition AJCC staging systems for nasopharyngeal carcinoma. Am J Clin Oncol. 2015;38(2):189–96. doi: 10.1097/COC.0b013e31828f5c96. [DOI] [PubMed] [Google Scholar]


