Abstract
Objective
To investigate the association of grip strength with disease specific incidence and mortality and whether grip strength enhances the prediction ability of an established office based risk score.
Design
Prospective population based study.
Setting
UK Biobank.
Participants
502 293 participants (54% women) aged 40-69 years.
Main outcome measures
All cause mortality as well as incidence of and mortality from cardiovascular disease, respiratory disease, chronic obstructive pulmonary disease, and cancer (all cancer, colorectal, lung, breast, and prostate).
Results
Of the participants included in analyses, 13 322 (2.7%) died over a mean of 7.1 (range 5.3-9.9) years’ follow-up. In women and men, respectively, hazard ratios per 5 kg lower grip strength were higher (all at P<0.05) for all cause mortality (1.20, 95% confidence interval 1.17 to 1.23, and 1.16, 1.15 to 1.17) and cause specific mortality from cardiovascular disease (1.19, 1.13 to 1.25, and 1.22, 1.18 to 1.26), all respiratory disease (1.31, 1.22 to 1.40, and 1.24, 1.20 to 1.28), chronic obstructive pulmonary disease (1.24, 1.05 to 1.47, and 1.19, 1.09 to 1.30), all cancer (1.17, 1.13 to 1.21, 1.10, 1.07 to 1.13), colorectal cancer (1.17, 1.04 to 1.32, and 1.18, 1.09 to 1.27), lung cancer (1.17, 1.07 to 1.27, and 1.08, 1.03 to 1.13), and breast cancer (1.24, 1.10 to 1.39) but not prostate cancer (1.05, 0.96 to 1.15). Several of these relations had higher hazard ratios in the younger age group. Muscle weakness (defined as grip strength <26 kg for men and <16 kg for women) was associated with a higher hazard for all health outcomes, except colon cancer in women and prostate cancer and lung cancer in both men and women. The addition of handgrip strength improved the prediction ability, based on C index change, of an office based risk score (age, sex, diabetes diagnosed, body mass index, systolic blood pressure, and smoking) for all cause (0.013) and cardiovascular mortality (0.012) and incidence of cardiovascular disease (0.009).
Conclusion
Higher grip strength was associated with a range of health outcomes and improved prediction of an office based risk score. Further work on the use of grip strength in risk scores or risk screening is needed to establish its potential clinical utility.
Introduction
The main role of skeletal muscle is to control body movements through the generation of force. Skeletal muscle is also the primary protein store within the body and in chronic conditions, such as cancer, and it can provide gluconeogenic precursors that are crucial for survival as such conditions progress.1 In addition to this, skeletal muscle is the primary outlet for glucose disposal in the body and is therefore important in metabolic conditions such as diabetes.2 Muscle mass is also decreased (cachexia) in many conditions, such as cancer, respiratory disease, chronic kidney disease, and chronic infection and sepsis.3 With these broad physiological and functional roles, skeletal muscle has a critical, but often underrated, role in health.4
Many studies have shown that lower muscle function is associated with greater mortality and morbidity.5 6 7 8 9 10 11 12 13 14 For example, in 1 142 599 male adolescents (age 16-19 years) followed up over 24 years, low muscle strength was associated with higher all cause mortality and mortality from cardiovascular disease but not from cancer.8 Recent data from the Prospective Urban Rural Epidemiology (PURE) study (n=139 691 adults aged 35-70 years followed up for four years) showed that grip strength was inversely associated with all cause mortality and with non-cardiovascular and cardiovascular mortality, but no significant association was found with respiratory disease and chronic obstructive pulmonary disease.7 Moreover, lower grip strength was found to be positively associated with incident cancer in high income countries but not in middle or lower income countries. The authors of this study concluded that, although some data support an association between grip strength and mortality, further research is needed to confirm this association with other health outcomes.7
Therefore, clear evidence shows that low grip strength is associated with a range of poorer health outcomes.5 6 7 8 9 10 11 12 13 14 Furthermore, grip strength enhances prediction of mortality based on age (C index increase from 0.65 for age to 0.69 for age+grip strength) and sex (C index increase from 0.54 for sex to 0.63 for sex+grip).14 However, whether the addition of handgrip strength to a traditional office based risk score (including age, sex, smoking, blood pressure, presence of diabetes, and body mass index15) improves risk prediction is unclear. Such analysis will allow us to determine whether measurement of grip strength has clinical utility for risk prediction in settings where blood based measures are not readily available (for example, community/rural settings or low/middle income countries).
A meta-analysis (53 476 participants) published in 2010 showed that grip strength was associated with lower all cause mortality; the association seemed to be weaker in studies in which participants had an average age of 60 years or less, relative to those with a higher average age at baseline.6 The relatively small sample size in this analysis limited the ability to fully explore how associations vary with age. The Tromso study (6850 participants, age 50-80 years) found associations between low grip strength and mortality,5 but no interaction with age was observed; again, power was limited by low participant numbers in this study. The large size of the UK Biobank cohort provides the opportunity to robustly determine whether the association between grip strength and health outcomes differs by age.
The aim of this study, therefore, was to investigate the associations of grip strength with all cause mortality and disease specific incidence and mortality, how these associations vary with age, and whether the addition of grip strength improves the prediction ability of established office based risk scores, in the UK Biobank, a very large, general population cohort.
Methods
Between April 2007 and December 2010, UK Biobank recruited 502 628 participants (5.5% response rate after invitation letters sent to around 9 million people), aged 40-69 years, from the general population.16 Participants attended one of 22 assessment centres across England, Wales, and Scotland,17 18 where they completed a touch screen questionnaire, had physical measurements taken, and provided biological samples, as described in detail elsewhere.17 18 The outcomes in the study reported here were incidence of and mortality from cardiovascular disease, all respiratory disease, chronic obstructive pulmonary disease, all cancers, breast cancer, prostate cancer, colorectal cancer, and lung cancer, as well as all cause mortality, with the exposure variable being grip strength (age and sex specific quarters and 5 kg increase in grip strength). We treated sociodemographic factors (age, sex, ethnicity, and area based socioeconomic status), month of recruitment, smoking status, height, body mass index, and self reported physical activity, sedentary time, and dietary intake as potential confounders, as well as prevalent diabetes, hypertension, chronic obstructive pulmonary disease, depression, cancer, and longstanding illness at baseline (where participants with these conditions were not excluded from the analyses).
Procedures
Date of death and date and cause of hospital admissions were identified as described previously.19 We defined incident cardiovascular disease as a hospital admission or death with ICD-10 (international classification of diseases, 10th revision) codes I60, I61, I63, I64, I21, I21.4, and I21.9. We defined respiratory disease as ICD-10 codes J09-J98 and I26-I27 and chronic obstructive pulmonary disease as ICD-10 code J44. We defined all cause cancer as an ICD-10 code of C0.0-C9.9, D3.7-9, or D4.0-8 recorded on the cancer registry, death certificate, or hospital admission. We defined cause specific cancers by using the following ICD-10 codes recorded on the cancer registry, death certificate, or hospital admission: breast cancer (C50), prostate cancer (C61), lung cancer (C34), and colorectal cancer (C18, C19, and C20).
Grip strength was measured as previously described,19 and the mean of the right and left values was expressed in absolute units (kg) for subsequent analysis. Physical activity was based on self report, using the International Physical Activity Questionnaire short form,20 and total physical activity was calculated as the sum of walking and moderate and vigorous activity, measured as metabolic equivalents (MET-min/week). We derived total time spent in sedentary behaviours from the sum of self reported time spent driving, using a computer, and watching television.
Dietary information was collected via the Oxford WebQ, a web based 24 hour recall questionnaire that was developed specifically for use in large population studies.21 22 We derived area based socioeconomic status from the postcode of residence, using the Townsend score.23 We calculated age from dates of birth and baseline assessment. We categorised smoking status into never, former, and current smoking. We collected medical history (physician’s diagnosis of depression, stroke, angina, heart attack, hypertension, cancer, diabetes, hypertension, chronic obstructive pulmonary disease, or longstanding illness) from the self completed baseline assessment questionnaire. Trained nurses measured height and body weight during the initial assessment centre visit. We calculated body mass index as weight/height2 and used the World Health Organization’s criteria to classify it into categories of underweight (<18.5), normal weight (18.5 to <25), overweight (25 to <30), obese (30 to <35), obesity class 2 (35 to <40), and obesity class 3 (≥40). Trained nurses measured body composition (body fat and fat free mass) by using bio-impedance. Further details of these measurements can be found in the UK Biobank’s online protocol (http://www.ukbiobank.ac.uk).
Statistical analyses
We used multivariable cubic regression splines to visually explore non-linear associations between grip strength and health outcomes. As we found no evidence of deviation from linearity, we investigated the associations of grip strength with cause specific incidence and mortality over follow-up with Cox proportional hazard models. We reported the results as hazard ratios together with 95% confidence intervals. The models for incidence of and mortality from cardiovascular disease, respiratory disease, and cancer excluded participants with a history of cardiovascular disease (myocardial infarction, angina, or stroke), respiratory disease and chronic obstructive pulmonary disease, or cancer at baseline, respectively.
Firstly, we treated grip strength as a continuous variable and calculated hazard ratios per 5 kg decrement in grip strength for men and women separately. To enable comparability with other reports in the literature, we also calculated hazard ratios for age and sex specific quarters of grip strength (supplementary table A), with participants in the highest quarter for grip strength used as the reference group. We also did analyses using a sex specific cut-off point for clinically relevant muscle weakness (supplementary table B), as recommended by the Foundation for the National Institutes of Health Sarcopenia Project.24 We investigated potential sex interactions by fitting a multiplicative interaction term into the models between grip strength (kg) and sex; although we found no significant interactions for the effect of grip strength on health outcomes by sex, we have presented stratified analyses because levels of grip strength differ significantly between woman and men (supplementary table C).
For Cox proportional hazard analyses, we ran four models that included an increasing number of covariates: model 0 (minimally adjusted) included sociodemographic covariates (age, sex, ethnicity, Townsend index, and month of recruitment); model 1 was adjusted as in model 0 but also included height; model 2 was adjusted as in model 1 but also included prevalent morbidity (hypertension, diabetes, depression, and longstanding illness, as well as cardiovascular disease, respiratory disease, and cancer when these were not the outcome); model 3 (fully adjusted) was adjusted as in model 2 but also included lifestyle factors (smoking status, body mass index categories, total physical activity, total sedentary time, and dietary intake of alcohol, fruit and vegetables, oily fish, red meat, and processed meat). To minimise the potential contribution of reverse causality to the findings, we did a landmark analysis excluding events occurring within the two years after recruitment in model 4 (landmark analysis). This landmark analysis was adjusted as in model 3.
We then investigated whether these associations differed by age by doing a two way interaction analysis and fitting a handgrip strength*age interaction term to our model. We then repeated our Cox proportional hazard analyses (models 3 and 4) stratified by age categories (≤55, 56-65, and >65 years).
To assess the predictive ability of handgrip strength, we calculated Harrell’s C index (which estimates the probability of concordance between observed and predicted responses25) for a model including office based risk factors such as age, sex, diabetes diagnosed, body mass index (per 5 units), systolic blood pressure (per 10 mm Hg), and smoking, and we then compared the ability to predict all cause mortality and incidence of and mortality from cardiovascular disease in this model with the addition of handgrip strength (per 5 kg). To validate the predictive ability of grip strength, we divided the cohort into two subsets by using random sampling stratified by age (<60 and ≥60 years) and sex to ensure that the proportions of men and older adults were similar in both subsets. For the derivation set, we used sex adjusted models and then estimated C indices and 95% confidence intervals in the validation set.26 In addition, we compared the magnitude of the hazard for all cause mortality and incidence of and mortality from cardiovascular disease associated with a 1 SD increment in handgrip strength and other well known modifiable risk factors—systolic blood pressure and total physical activity—for which the associations were also expressed per 1 SD increment.
We checked the proportional hazard assumption by tests based on Schoenfeld residuals. We used Stata 14 statistical software for all analyses.
Patient involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Results
Of the 502 628 participants recruited to UK Biobank, 502 293 (99%) had data on grip strength. The mean follow-up period was 7.1 (range 5.3-9.9) years for all cause and cause specific mortality and 6.1 (4.4-9.0) years for cause specific incidence. Of the 502 293 participants included in the respective analyses, over the follow-up period, 28 059 (5.6%) participants developed cardiovascular disease, 10 542 (2.1%) developed respiratory disease, and 27 704 (5.5%) developed cancer. In addition, 13 322 (2.7%) participants died: 3033 (0.6%) from cardiovascular disease, 2062 (0.4%) from respiratory disease, and 5738 (1.1%) from cancer.
Table 1 summarises the main characteristics of the participants by quarters of grip strength. In summary, people in the lowest quarter for strength (Q1) were from more deprived fifths and had a higher prevalence of current smoking, obesity, and comorbidities, including cancer, diabetes, cardiovascular disease, longstanding illness, depression, and hypertension, compared with the highest grip strength group (Q4). They had a lower height but higher body mass index, waist circumference, and percentage body fat; had lower intake of alcohol, fruit, and vegetables; and had lower levels of physical activity and higher levels of television viewing in comparison with those in the lowest grip strength group. The main characteristics by age specific quarters, stratified by sex, are shown in supplementary tables D and E.
Table 1.
Characteristics of cohort by age and sex specific quarters of grip strength. Values are percentages (numbers) unless stated otherwise
| Characteristics | All (n=477 074) | Quarter of grip strength* | |||
|---|---|---|---|---|---|
| Q4 (highest) (n=113 177) | Q3 (n=120 735) | Q2 (n=121 439) | Q1 (lowest) (n=121 723) | ||
| Sociodemographics | |||||
| Women | 54.5 (260 063) | 55.5 (62 777) | 54.1 (65 338) | 54.3 (65 943) | 54.2 (66 005) |
| Mean (SD) age, years | 56.5 (8.1) | 55.7 (8.4) | 56.5 (8.1) | 56.7 (8.0) | 57.1 (7.8) |
| Mean (SD) deprivation index | −1.3 (3.1) | −1.7 (2.9) | −1.5 (2.9) | −1.3 (3.1) | −0.8 (3.3) |
| Fifths of deprivation index: | |||||
| Lowest | 20.4 (97 082) | 22.8 (25 749) | 21.5 (25 931) | 20.4 (24 714) | 17.0 (20 688) |
| Lower-middle | 20.2 (96 263) | 21.6 (24 458) | 21.2 (25 504) | 20.2 (24 477) | 17.9 (21 824) |
| Middle | 20.1 (95 940) | 20.5 (23 190) | 20.6 (24 885) | 20.3 (24 592) | 19.1 (23 273) |
| Middle-higher | 19.9 (95 121) | 19.1 (21 612) | 19.5 (23 551) | 20.0 (24 248) | 21.1 (25 710) |
| Highest | 19.4 (92 668) | 16.1 (18 168) | 17.3 (20 864) | 19.3 (23 408) | 24.8 (30 228) |
| Ethnicity†: | |||||
| White | 94.9 (451 146) | 96.3 (108 717) | 96.1 (115 725) | 95.2 (115 303) | 91.9 (111 401) |
| Mixed background | 1.4 (6851) | 1.2 (1324) | 1.2 (1496) | 1.4 (1664) | 2.0 (2367) |
| South Asian | 1.9 (8829) | 0.5 (580) | 1.0 (1217) | 1.7 (2093) | 4.1 (4939) |
| Black | 1.5 (7309) | 1.8 (2051) | 1.4 (1640) | 1.4 (1630) | 1.6 (1988) |
| Chinese | 0.3 (1412) | 0.2 (174) | 0.3 (309) | 0.3 (396) | 0.4 (533) |
| Smoking status: | |||||
| Never | 55.0 (262 252) | 54.3 (61 455) | 54.7 (65 981) | 54.4 (67 272) | 55.5 (67 544) |
| Previous | 34.7 (165 467) | 35.8 (40 562) | 35.3 (42 571) | 34.5 (41 874) | 33.2 (40 460) |
| Current | 10.3 (49 355) | 9.9 (11 160) | 10.1 (12 183) | 10.1 (12 293) | 11.3 (13 719) |
| Obesity related markers | |||||
| Mean (SD) height, m | 1.68 (0.1) | 1.70 (0.1) | 1.69 (0.1) | 1.67 (0.1) | 1.66 (0.1) |
| Mean (SD) BMI | 27.4 (4.8) | 27.5 (4.6) | 27.2 (4.6) | 27.2 (4.7) | 27.7 (5.1) |
| BMI categories: | |||||
| Underweight (<18.5) | 0.5 (2482) | 0.3 (334) | 0.4 (535) | 0.6 (671) | 0.8 (942) |
| Normal weight (18.5-<25.0) | 32.8 (156 314) | 31.1 (35 237) | 33.6 (40 584) | 34.3 (41 698) | 31.9 (38 795) |
| Overweight (25.0-<30.0) | 42.5 (202 770) | 44.0 (49 809) | 43.3 (52 314) | 42.2 (51 188) | 40.6 (49 459) |
| Obese (≥30.0) | 24.2 (115 508) | 24.6 (27 797) | 22.6 (27 302) | 23.0 (27 882) | 26.7 (32 527) |
| Mean (SD) waist circumference, cm | 90.2 (13.4) | 90.2 (13.3) | 89.7 (13.2) | 89.8 (13.4) | 91.1 (13.9) |
| Central obesity | 33.4 (159 517) | 33.4 (37 756) | 31.6 (38 140) | 32.0 (38 839) | 36.8 (44 782) |
| Mean (SD) body fat, % | 31.4 (8.5) | 31.0 (8.4) | 31.1 (8.4) | 31.4 (8.5) | 32.2 (8.7) |
| Physical activity | |||||
| Mean (SD) grip strength, kg | 30.7 (11.0) | 39.6 (10.6) | 33.3 (8.9) | 28.7 (8.0) | 21.7 (7.9) |
| Mean (SD) total physical activity, MET-min/week | 2846.3 (3045.8) | 3000.3 (3095.7) | 2898.3 (3055.7) | 2823.1 (2037.8) | 2660.6 (2982.5) |
| Mean (SD) television viewing, h/day | 2.8 (1.6) | 2.6 (1.5) | 2.7 (1.5) | 2.8 (1.6) | 3.0 (1.7) |
| Mean (SD) total discretionary sedentary behaviour, h/day | 5.0 (2.3) | 5.0 (2.2) | 5.0 (2.2) | 5.0 (2.3) | 5.1 (2.4) |
| Mean (SD) dietary intakes | |||||
| Total energy, kcal/day)‡ | 2165 (668) | 2196 (668) | 2170 (656) | 2154 (661) | 2140 (685) |
| Protein, % of TE‡ | 15.6 (3.6) | 15.6 (3.6) | 15.5 (3.6) | 15.5 (3.6) | 15.5 (3.7) |
| Total fat, % of TE‡ | 32.1 (6.7) | 32.2 (6.7) | 32.0 (6.6) | 32.0 (6.8) | 32.0 (6.8) |
| Saturated fat, % of TE‡ | 12.3 (3.3) | 12.4 (3.3) | 12.3 (3.3) | 12.3 (3.3) | 12.3 (3.4) |
| Carbohydrate, % of TE‡ | 47.2 (8.2) | 46.9 (8.1) | 47.1 (8.1) | 47.3 (8.2) | 47.7 (8.4) |
| Sugar, % of TE‡ | 22.5 (7.0) | 22.5 (6.8) | 22.5 (6.9) | 22.5 (7.0) | 22.6 (7.3) |
| Alcohol, % of TE‡ | 5.2 (6.5) | 5.3 (6.4) | 5.3 (6.5) | 5.2 (6.6) | 4.8 (6.6) |
| Fruit and vegetables, g/day | 330.0 (195.2) | 336.2 (190.7) | 332.4 (192.1) | 327.0 (191.6) | 324.7 (205.6) |
| Processed meat, portion/day | 1.9 (1.1) | 1.8 (1.0) | 1.9 (1.1) | 1.9 (1.1) | 1.9 (1.1) |
| Red meat, portion/day | 2.1 (1.4) | 2.1 (1.4) | 2.1 (1.4) | 2.1 (1.4) | 2.1 (1.5) |
| Oily fish, portion/day | 1.1 (1.0) | 1.1 (1.0) | 1.1 (1.0) | 1.1 (1.0) | 1.1 (1.1) |
| Health status | |||||
| Diabetes history | 5.2 (24 911) | 3.5 (4010) | 4.3 (5126) | 5.1 (6185) | 7.9 (9590) |
| Cancer history | 7.7 (36 564) | 7.0 (7911) | 7.4 (8925) | 7.8 (9420) | 8.5 (10 308) |
| Longstanding illness | 32.4 (154 382) | 26.1 (29 491) | 28.7 (34 610) | 31.7 (38 478) | 42.6 (51 803) |
| Cardiovascular disease | 29.4 (140 357) | 26.8 (30 296) | 27.9 (33 666) | 29.2 (35 434) | 33.7 (40 961) |
| Depression history | 5.6 (26 616) | 4.4 (5004) | 5.0 (6090) | 5.7 (6879) | 7.1 (8643) |
| Hypertension | 23.7 (113 191) | 22.8 (25 745) | 23.1 (27 836) | 23.6 (28 596) | 25.5 (31 014) |
BMI=body mass index; MET=metabolic equivalent; TE=total energy intake.
For cut-off points for age and sex specific quarters of grip strength, see supplementary table A.
1527 participants reported “other” or “mixed ethnic” background.
Data for nutrient intakes were available for a subset of 211 064 participants.
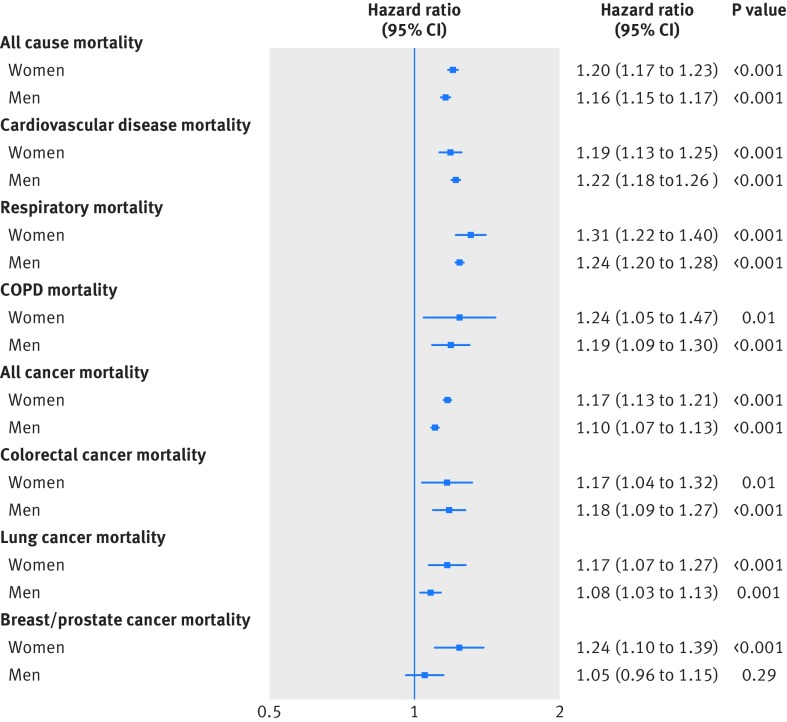
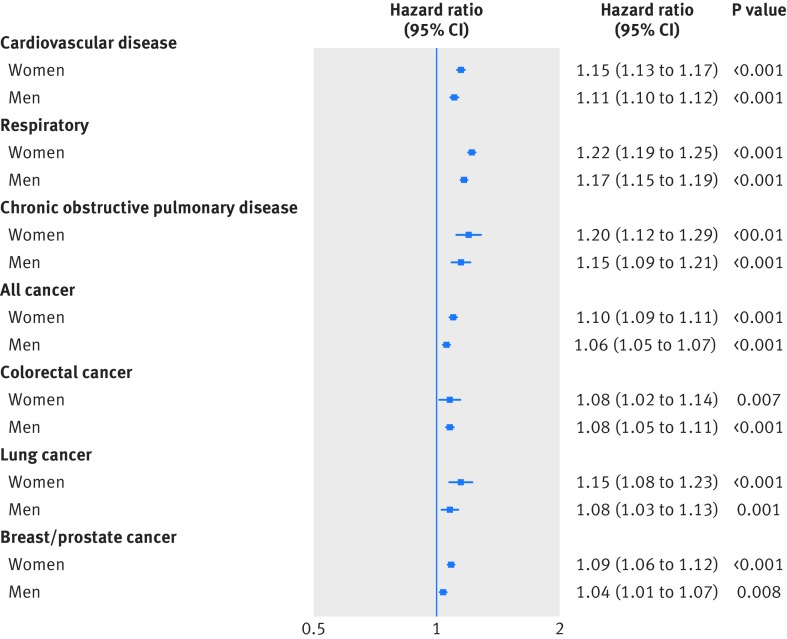
As shown in figure 1, in both men and women, a 5 kg lower grip strength was associated with a higher hazard for all cause mortality and incidence of and mortality from cardiovascular disease, all respiratory disease, chronic obstructive pulmonary disease, all cancer, and colorectal, lung, and breast cancer in model 0. The associations were similar after adjustment for height in model 1; after further adjustment, the magnitude of associations were slightly attenuated in models 2, 3, and 4 (supplementary tables F-K). These associations remained significant for all cause specific mortality (fig 1) and incidence outcomes (fig 2), in both men and women in model 4. The mortality hazards per 5 kg lower grip strength (in model 4) observed were as follows: for all respiratory disease (hazard ratio 1.31 and 1.24 for women and men, respectively), chronic obstructive pulmonary disease (1.24 and 1.19), cardiovascular disease (1.19 and 1.24), and all cause mortality (1.20 and 1.16), followed by breast cancer (1.24), colorectal cancer (1.17 and 1.18), lung cancer (1.17 and 1.08), and all cancer mortality (1.17 and 1.10). When we treated grip strength as an ordinal variable by using age specific quarters, we found similar results for all incidence and mortality outcomes in men and women, and grip strength was not significantly associated with prostate cancer mortality but was associated with incident prostate cancer (supplementary tables H-K).
Fig 1.
Hazard for all cause and cause specific mortality per 5 kg lower grip strength stratified by sex. Data presented as adjusted hazard ratio and 95% CI by 5 kg decrease in grip strength. Analyses were adjusted for age, deprivation index, ethnicity, month of recruitment, comorbidities (depression, diabetes, hypertension, longstanding illness, respiratory diseases, cancer, and cardiovascular disease), height, body mass index categories, smoking, physical activity, sedentary behaviour, and dietary intake (alcohol, fruit and vegetables, oily fish, red meat, and processed meat) and excluding events in first two years after recruitment (model 4). COPD=chronic obstructive pulmonary disease
Fig 2.
Hazard for cause specific incidence per 5 kg lower grip strength stratified by sex. Data presented as adjusted hazard ratio and 95% CI by 5 kg decrease in grip strength. Analyses were adjusted for age, deprivation index, ethnicity, month of recruitment, comorbidities (depression, diabetes, hypertension, longstanding illness, respiratory diseases, cancer, and cardiovascular disease), height, body mass index categories, smoking, physical activity, sedentary behaviour, and dietary intake (alcohol, fruit and vegetables, oily fish, red meat, and processed meat) and excluding events in first two years after recruitment (model 4)
We observed grip strength*age interactions in women for all cause mortality, all cancer mortality, cardiovascular disease incidence, chronic obstructive pulmonary disease incidence, all cancer incidence, and lung cancer incidence and in men for all cause mortality, cardiovascular disease mortality, all respiratory mortality, chronic obstructive pulmonary disease mortality, all cancer mortality, lung cancer mortality, cardiovascular disease incidence, chronic obstructive pulmonary disease incidence, all cancer incidence, lung cancer incidence, and prostate cancer incidence (supplementary tables L and M, model 3). The hazard ratios were higher in the younger age categories. These interactions remained in our landmark analysis with events in the first two years excluded (supplementary tables N and O, model 4).
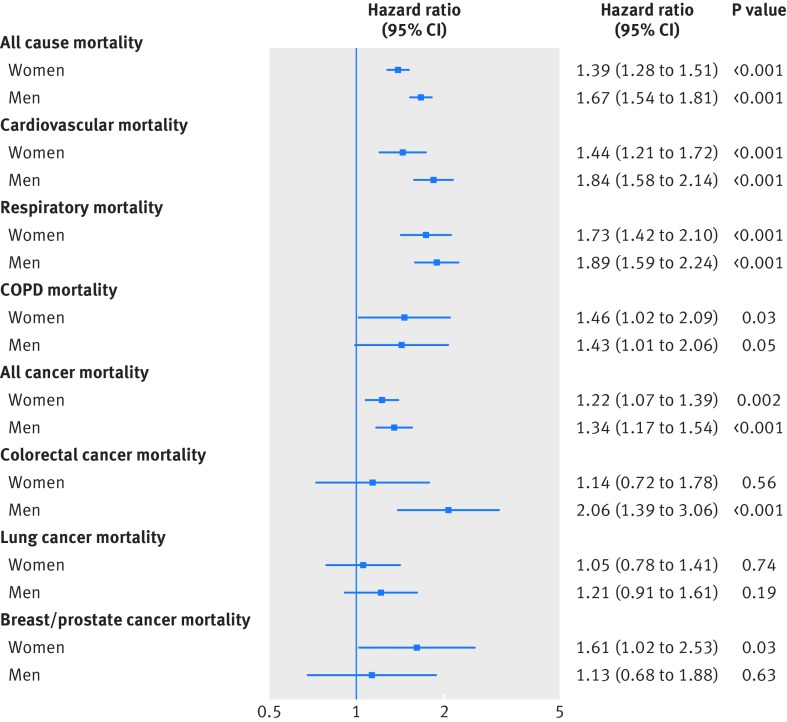
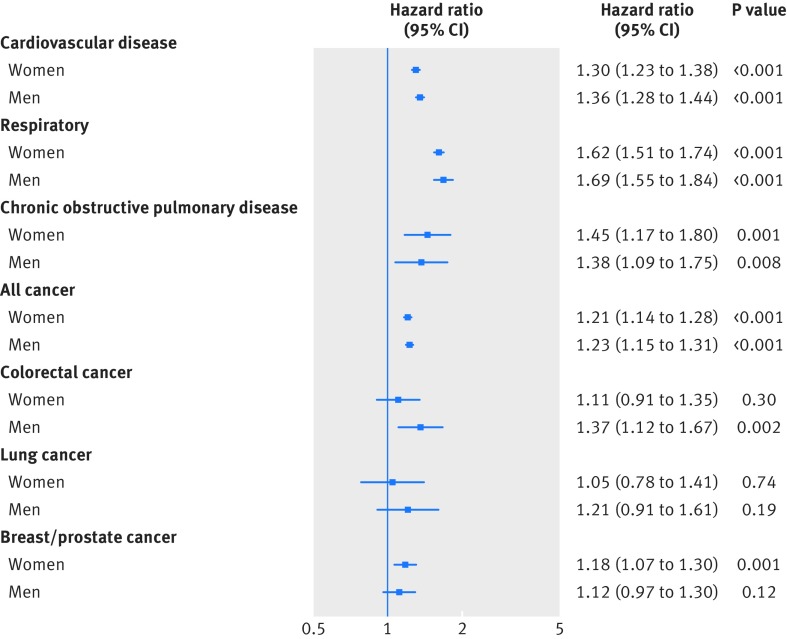
As shown in figure 3 and figure 4, and in supplementary tables P and Q, muscle weakness (defined as a grip strength ≤26.0 kg for men and ≤16.0 kg for women) was associated with an increased risk of all health outcomes, except for both incidence of and mortality from colorectal cancer in women, lung cancer in both men and women, and prostate cancer in men. Using a previously established office based (non-laboratory) risk score,15 which includes age, sex, smoking, blood pressure, diabetes, and body mass index, we investigated whether the prediction ability of this risk score was improved when handgrip strength was added into the score. Our results showed that the traditional office based score had good predictive abilities for all cause mortality and incidence of and mortality from cardiovascular disease (table 2). Adding grip strength significantly improved prediction compared with the office based score alone (changes in Harrell’s C statistics versus the conventional risk score were 0.013 for all cause mortality, 0.012 for cardiovascular disease mortality, and 0.009 for incident cardiovascular disease) (table 2). Both the risk scores performed well in the derivation and validation cohorts (table 2).
Fig 3.
Hazard for all cause and cause specific mortality in women and men by FNIH Sarcopenia cut-off points for muscle weakness. Data presented as adjusted hazard ratio and 95% CI by FNIH Sarcopenia cut-off points (reference groups ≥26.0 kg for men and ≥16.0 kg for women). Analyses were adjusted for age, deprivation index, ethnicity, month of recruitment, comorbidities (depression, diabetes, hypertension, longstanding illness, respiratory diseases, cancer, and cardiovascular disease), height, body mass index categories, smoking, physical activity, sedentary behaviour, and dietary intake (alcohol, fruit and vegetables, oily fish, red meat, and processed meat) and excluding events in first two years after recruitment (model 4). COPD=chronic obstructive pulmonary disease
Fig 4.
Hazard for cause specific incidence in women and men by FNIH Sarcopenia cut-off points for muscle weakness. Data presented as adjusted hazard ratio and 95% CI by FNIH Sarcopenia cut-off points (reference groups ≥26.0 kg for men and ≥16.0 kg for women). Analyses were adjusted for age, deprivation index, ethnicity, month of recruitment, comorbidities (depression, diabetes, hypertension, longstanding illness, respiratory diseases, cancer, and cardiovascular disease), height, body mass index categories, smoking, physical activity, sedentary behaviour, and dietary intake (alcohol, fruit and vegetables, oily fish, red meat, and processed meat) and excluding events in first two years after recruitment (model 4)
Table 2.
Improvement in risk discrimination for all cause mortality and cardiovascular disease outcomes by addition of handgrip strength to model with conventional office based risk factors
| C index (95% CI) | C index change (95% CI) | P value | |
|---|---|---|---|
| All cause mortality | |||
| Derivation cohort: | |||
| Conventional office based risk factors | 0.722 (0.717 to 0.727) | 0.013 (0.011 to 0.015) | <0.001 |
| Plus handgrip strength | 0.735 (0.730 to 0.740) | ||
| Validation cohort: | |||
| Conventional office based risk factors | 0.722 (0.717 to 0.728) | 0.012 (0.011 to 0.014) | <0.001 |
| Plus handgrip strength | 0.734 (0.729 to 0.740) | ||
| Cardiovascular disease mortality | |||
| Derivation cohort: | |||
| Conventional office based risk factors | 0.788 (0.774 to 0.801) | 0.012 (0.007 to 0.017) | <0.001 |
| Plus handgrip strength | 0.800 (0.786 to 0.913) | ||
| Validation cohort: | |||
| Conventional office based risk factors | 0.787 (0.773 to 0.800) | 0.011 (0.005 to 0.017) | <0.001 |
| Plus handgrip strength | 0.799 (0.785 to 0.812) | ||
| Cardiovascular disease incidence | |||
| Derivation cohort: | |||
| Conventional office based risk factors | 0.736 (0.730 to 0.743) | 0.009 (0.007 to 0.010) | <0.001 |
| Plus handgrip strength | 0.745 (0.739 to 0.751) | ||
| Validation cohort: | |||
| Conventional office based risk factors | 0.737 (0.730 to 0.743) | 0.009 (0.007 to 0.011) | <0.001 |
| Plus handgrip strength | 0.746 (0.740 to 0.751) | ||
Data presented as C index and 95% CI and as differences in C index versus reference model. Reference model includes information on age, sex, body mass index (5 units), systolic blood pressure (10 mm Hg), history of diabetes, and smoking. Handgrip strength was added into model as 5 kg decrease in grip strength. Analyses were performed in derivation cohort and then in randomly selected internal validation cohort (~50% of cohort balanced by sex). Then Harrell’s C statistic was calculated in both derivation cohort (n=219 087) and validation cohort (n=218 852).
In view of the significant improvement in the prediction ability between handgrip strength and all cause mortality, cardiovascular disease mortality, and incident cardiovascular disease, we did a post hoc comparison of the associated hazard of grip strength with systolic blood pressure (a robust modifiable risk factor for premature death) and total physical activity (supplementary table R). After adjustment, grip strength showed the strongest association, per 1 SD change, with all cause and cardiovascular disease mortality (hazard ratio 1.48 and 1.57 per 1 SD lower grip strength, respectively) in comparison with systolic blood pressure (1.03 and 1.26) and total physical activity (1.06 and 1.07). However, for incident cardiovascular disease, the hazard associated with a 1 SD change in strength and systolic blood pressure was of a similar magnitude (hazard ratio 1.29 and 1.30, respectively). We found no associations between total physical activity and incident cardiovascular disease (supplementary table R).
Discussion
The main finding of this study is that lower grip strength was strongly associated with a wide range of adverse health outcomes. The associations observed were consistent between sexes and remained robust after adjustment for deprivation, ethnicity, and several other health, lifestyle, and dietary factors. For several adverse health outcomes, we observed interactions with age, with the risk associated with lower grip strength modestly stronger in younger age groups. Moreover, our results provide evidence that the addition of grip strength can improve the prediction ability of an office based risk score for all cause mortality and incidence of and mortality from cardiovascular disease. These findings could have important public health implications, as grip strength in comparison with other physical measures, specifically cardiorespiratory fitness and physical activity, is easily measured, cheap, and highly reproducible in clinical practice.27 We found that grip strength had a stronger association with all cause and cardiovascular disease mortality than do systolic blood pressure or total physical activity, and that the strength of associations for grip strength with incidence of cardiovascular disease was similar to that for systolic blood pressure and stronger than for total physical activity. Grip strength may, therefore, be a useful method of identifying people with muscle weakness who are at high risk of a wide range of diseases and who might benefit from further health assessments of risk for vascular and non-vascular outcomes.
Comparisons with other studies
The finding of an inverse association between grip strength and mortality is consistent with previous studies.5 6 7 8 9 10 11 12 13 Our finding of a hazard ratio for all cause mortality of 1.16 for men and 1.20 for women, for a 5 kg lower grip strength, in our fully adjusted model, is in agreement with observations from the PURE study of 139 691 participants, which reported that a 5 kg lower grip strength was, after adjustment, associated with a hazard ratio of 1.16 for all cause mortality.7 Furthermore, our findings for all cause mortality are also comparable to those reported in the meta-analysis of Cooper et al for all cause mortality (hazard ratio for a 5 kg lower grip strength of 1.16, after adjustment for sex, height, and body mass index).6 The inverse association between grip strength and cardiovascular disease mortality seen in our study is also of a similar magnitude to findings reported previously. For example, in the PURE study a 5 kg lower grip strength was, after adjustment, associated with a hazard ratio of 1.17 for cardiovascular mortality.7 The fully adjusted hazard ratio in our study, per 5 kg lower grip strength, for cardiovascular disease mortality was 1.22 for men and 1.19 for women.
Our data on the associations between grip strength and cancer do, however, differ from previously published studies and, importantly, provide novel results for specific types of cancer. In the recent report of Strand et al,5 a 1 standard deviation lower grip strength was, after adjustment, associated with a hazard ratio of 1.05 for cancer mortality. This is similar to our finding that a 5 kg (equivalent to about 0.5 SD) lower grip strength was associated, in the fully adjusted model, with a hazard ratio of 1.10 for men and 1.17 for women. However, in the PURE study,7 a 5 kg lower grip strength was, after adjustment, associated with a hazard ratio of 0.95 for all cancer outcomes, in high income countries but not in middle or low income countries. The reason for these differential findings is not clear but, as pointed out by Leong and colleagues,7 they merit further investigation and examination of different cancer subtypes. We have extended limited evidence in this area and shown that grip strength is inversely associated with mortality from colorectal (hazard ratio 1.18 and 1.17) and lung cancer (1.08 and 1.17) in men and women, respectively, and breast cancer in women (1.24). Although we found no association between grip strength and prostate cancer mortality, a positive association existed between strength and incidence of prostate cancer. The reason for this is not clear, but it may reflect people with higher grip strength being more health conscious and more readily visiting their doctor—thus the association with incidence but not mortality. Whether this is the case remains to be established. Some of these hazard ratios are, although significant, relatively small, particularly for cancer, and whether these are clinically significant and also not the result of reverse causality (see further discussion below) remains to be established.
Clinical potential
The association between grip strength and health outcomes has been extensively studied, but whether measurement of grip strength in health screening settings has clinical utility has been unclear. It has previously been shown that grip strength enhances risk prediction for all cause mortality on top of the prediction seen with age or sex alone.13 14 Neither of the studies, however, was able to investigate whether the addition of handgrip strength to an established office based risk score (including age, sex, diabetes diagnosed, body mass index, systolic blood pressure, and smoking) improves prediction of all cause or cardiovascular disease mortality, for which several interventions to reduce risk are possible. Our data showed that handgrip strength improves the prediction ability of this model. The magnitude of improvement with the addition of grip strength (C index change=0.013) is similar to that seen when adding high density lipoprotein cholesterol and N-terminal pro b-type natriuretic peptide (C index change 0.007 for high density lipoprotein and 0.020 for N-terminal pro b-type natriuretic peptide, for a composite outcome of coronary heart disease plus stroke and heart failure) to conventional risk factor scores (age, sex, smoking, systolic blood pressure, history of diabetes, and concentration of total cholesterol).28 Thus, our findings suggest that measurement of handgrip strength—which is easily measured objectively and highly reproducible in clinical practice27—in risk screening settings where blood sampling is not possible (for example, in community settings or in low and middle income countries) may add clinical utility over existing risk prediction scores.
In addition, we have shown that, using previously derived cut-offs,24 muscle weakness is associated with an increased risk of most health outcomes studied, the only exceptions being colorectal cancer (women), lung cancer (both men and women), and prostate cancer (men). These data complement the aforementioned change in C index and support the use of these grip strength thresholds to indicate muscle weakness and thus identify high risk populations.
Whether the associations between health outcomes and grip strength are consistent across age categories has rarely been investigated. A meta-analysis including 53 476 participants from 14 studies suggested that the association between grip strength and mortality seemed to be weaker in people aged 60 years or less, relative to older participants, but an interaction with age was not formally tested owing to the low number of studies.6 Another investigation of 6850 participants found no interaction between grip strength and age for mortality, probably owing to the relatively low participant numbers. Our analysis of more than 500 000 participants provided sufficient power for us to robustly determine interactions between grip strength and age for health outcomes, showing that grip strength was significantly associated with health outcomes across the age range of the UK Biobank population, but that these associations were moderately stronger, not weaker, in the younger age groups. This possibly indicates that with age, factors, in addition to grip strength, that influence health become more important. We are, however, unable to determine the reasons underlying these differences in associations with age, and further work should investigate this.
Handgrip strength correlates strongly to leg muscle strength and provides a valid marker of overall limb muscle strength throughout the age range.29 It has been suggested that handgrip strength is a marker for nutritional status,30 and analysis from the Hertfordshire Cohort Study has shown that a healthier (“prudent diet”) pattern of eating and consumption of dietary protein, antioxidant nutrients, vitamin D, and fatty fish is associated with grip strength.31 Previous work has also shown that people who are more physically active and have lower sedentary time have a higher grip strength.32 33 34 35 Thus, lifestyle factors are clearly important in the maintenance of grip strength. Ethnicity, age, sex, height, and socioeconomic status have also been shown to be associated with grip strength,31 36 37 38 and handgrip strength has a strong genetic component with heritability shown to be 52%.39 With so many factors contributing to handgrip strength, determining the mechanisms that fully underlie the association between handgrip strength and health outcomes is not possible, but they are probably multifactorial in nature and are worthy of further investigation.
Limitations of study
The UK Biobank is not representative of the general population of the UK in several ways. It is relatively representative of the general UK population in terms of age, sex, ethnicity, and socioeconomic status but is only partially representative in terms of lifestyle. Therefore, caution is needed in generalising summary statistics to the general population, but estimates of the magnitude of the associations are, nevertheless, generalisable. Participants were more likely to be older, to be women, and to live in less socioeconomically deprived areas; were less likely to be obese, to smoke, or to drink alcohol on a daily basis; and had fewer self reported health outcomes. Rates of all cause mortality and incidence of cancer were also lower.16 40 Our study had sufficient power to allow subgroup analyses by age, which overcomes limitations from previous studies. Reverse causality is possible in any observational study; although our results were similar after a landmark analysis of events occurring from two years after recruitment, we cannot exclude the possibility of reverse causality. Similarly, residual confounding is always possible and the associations observed may not imply causality. However, given that we are largely interested in prediction and identification of people at increased risk, and not seeking to make strong causal inferences, reverse causality is not a major limitation.
Conclusions
This study has shown that grip strength is strongly and inversely associated with all cause mortality and incidence of and mortality from cardiovascular disease, respiratory disease, chronic obstructive pulmonary disease, all cancer, and subtypes of cancer, including colorectal, lung, and breast cancer, with associations being modestly stronger in the younger age groups. Our results show that adding handgrip strength to an existing office based risk score improves the prediction ability for all cause mortality and incidence of and mortality from cardiovascular disease and that muscle weakness (using previously defined grip strength cut-offs) is associated with poorer health outcomes. This indicates that the addition of the measurement of grip strength may be useful in screening for risk of cardiovascular disease in community/rural settings and in developing countries where access to biochemical measurements (such as total cholesterol) is not possible. Further work is needed to define how to use grip strength in this manner, in particular in non-British populations.
What is already known on this topic
Grip strength has previously been found to be associated with health outcomes
What this study adds
Higher grip strength was associated with a lower risk of all cause mortality and incidence of and mortality from cardiovascular disease, respiratory disease, chronic obstructive pulmonary disease, all cancer and sub-types of cancer
The associations were independent of confounders, but several of these associations were weaker in older age categories
Grip strength improved the prediction ability of an office based risk score, and muscle weakness is associated with poorer health outcomes and thus may have clinical utility
Acknowledgments
We are grateful to UK Biobank participants. This research has been conducted using the UK Biobank resource under application number 7155.
Web Extra.
Extra material supplied by the author
Supplementary tables
Contributors: The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. CCM, SG, JPP, NS, and JMRG contributed to the conception and design of the study, advised on all statistical aspects, and interpreted the data. CCM did the statistical analysis, assisted by SG, FP, AS, and PW. CCM and SG drafted the manuscript. All authors reviewed the manuscript and approved the final version to be published. CCM, SG, JPP, NS, and JMRG had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. JPP, JMRG, NS, and SG contributed equally to this work and are joint senior authors. CACM, SG, JPP, JMRG, and NS are the guarantors.
Funding: The UK Biobank was supported by the Wellcome Trust, Medical Research Council, Department of Health, Scottish government, and Northwest Regional Development Agency. It has also had funding from the Welsh Assembly government and British Heart Foundation. The research was designed, conducted, analysed, and interpreted by the authors entirely independently of the funding sources.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: UK Biobank received ethical approval from the North West Multi-centre Research Ethics Committee (REC reference: 11/NW/03820). All participants gave written informed consent before enrolment in the study, which was conducted in accordance with the principles of the Declaration of Helsinki.
Data sharing: Researchers can apply to use the UK Biobank resource and access the data used. No additional data are available.
Transparency: The manuscript’s guarantors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1. Kotler DP, Tierney AR, Wang J, Pierson RN., Jr Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr 1989;50:444-7. 10.1093/ajcn/50.3.444 [DOI] [PubMed] [Google Scholar]
- 2. Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 2006;55:1813-8. 10.2337/db05-1183 [DOI] [PubMed] [Google Scholar]
- 3. Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr 2008;27:793-9. 10.1016/j.clnu.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 4. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 2006;84:475-82. 10.1093/ajcn/84.3.475 [DOI] [PubMed] [Google Scholar]
- 5. Strand BH, Cooper R, Bergland A, et al. The association of grip strength from midlife onwards with all-cause and cause-specific mortality over 17 years of follow-up in the Tromsø Study. J Epidemiol Community Health 2016;jech-2015-206776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooper R, Kuh D, Hardy R, Mortality Review Group. FALCon and HALCyon Study Teams Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ 2010;341:c4467. 10.1136/bmj.c4467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leong DP, Teo KK, Rangarajan S, et al. Prospective Urban Rural Epidemiology (PURE) Study investigators Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015;386:266-73. 10.1016/S0140-6736(14)62000-6 [DOI] [PubMed] [Google Scholar]
- 8. Ortega FB, Silventoinen K, Tynelius P, Rasmussen F. Muscular strength in male adolescents and premature death: cohort study of one million participants. BMJ 2012;345:e7279. 10.1136/bmj.e7279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 2006;61:72-7. 10.1093/gerona/61.1.72 [DOI] [PubMed] [Google Scholar]
- 10. Celis-Morales CA, Lyall DM, Anderson J, et al. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: evidence from 498 135 UK-Biobank participants. Eur Heart J 2017;38:116-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol 2007;36:228-35. 10.1093/ije/dyl224 [DOI] [PubMed] [Google Scholar]
- 12. Rolland Y, Lauwers-Cances V, Cesari M, Vellas B, Pahor M, Grandjean H. Physical performance measures as predictors of mortality in a cohort of community-dwelling older French women. Eur J Epidemiol 2006;21:113-22. 10.1007/s10654-005-5458-x [DOI] [PubMed] [Google Scholar]
- 13. Cooper R, Strand BH, Hardy R, Patel KV, Kuh D. Physical capability in mid-life and survival over 13 years of follow-up: British birth cohort study. BMJ 2014;348:g2219. 10.1136/bmj.g2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ganna A, Ingelsson E. 5 year mortality predictors in 498,103 UK Biobank participants: a prospective population-based study. Lancet 2015;386:533-40. 10.1016/S0140-6736(15)60175-1 [DOI] [PubMed] [Google Scholar]
- 15. Ueda P, Woodward M, Lu Y, et al. Laboratory-based and office-based risk scores and charts to predict 10-year risk of cardiovascular disease in 182 countries: a pooled analysis of prospective cohorts and health surveys. Lancet Diabetes Endocrinol 2017;5:196-213. 10.1016/S2213-8587(17)30015-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Collins R. What makes UK Biobank special? Lancet 2012;379:1173-4. 10.1016/S0140-6736(12)60404-8 [DOI] [PubMed] [Google Scholar]
- 17. Palmer LJUK. UK Biobank: bank on it. Lancet 2007;369:1980-2. 10.1016/S0140-6736(07)60924-6 [DOI] [PubMed] [Google Scholar]
- 18. Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Celis-Morales CA, Petermann F, Hui L, et al. Associations between diabetes and both cardiovascular disease and all-cause mortality are modified by grip strength: Evidence from UK Biobank, a prospective population-based cohort study. Diabetes Care 2017;40:1710-8. 10.2337/dc17-0921 [DOI] [PubMed] [Google Scholar]
- 20. Guo W, Bradbury KE, Reeves GK, Key TJ. Physical activity in relation to body size and composition in women in UK Biobank. Ann Epidemiol 2015;25:406-413.e6. 10.1016/j.annepidem.2015.01.015 [DOI] [PubMed] [Google Scholar]
- 21. Liu B, Young H, Crowe FL, et al. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr 2011;14:1998-2005. 10.1017/S1368980011000942 [DOI] [PubMed] [Google Scholar]
- 22. Anderson JJ, Celis-Morales CA, Mackay DF, et al. Adiposity among 132 479 UK Biobank participants; contribution of sugar intake vs other macronutrients. Int J Epidemiol 2017;46:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Townsend P, Phillimore M, Beattie A. Health and deprivation. Inequality and the North. Health Policy (New York) 1988;10:207 10.1016/0168-8510(88)90006-1. [DOI] [Google Scholar]
- 24. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547-58. 10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87. [DOI] [PubMed] [Google Scholar]
- 26. Newson RB. Comparing the predictive power of survival models using Harrell ’ s c or Somers ’ D. Stata J 2010;10:339-58. [Google Scholar]
- 27. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011;40:423-9. 10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- 28. Willeit P, Kaptoge S, Welsh P, et al. Natriuretic Peptides Studies Collaboration Natriuretic peptides and integrated risk assessment for cardiovascular disease: an individual-participant-data meta-analysis. Lancet Diabetes Endocrinol 2016;4:840-9. 10.1016/S2213-8587(16)30196-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bohannon RW, Magasi SR, Bubela DJ, Wang YC, Gershon RC. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve 2012;46:555-8. 10.1002/mus.23350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Norman K, Stobäus N, Gonzalez MC, Schulzke JD, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr 2011;30:135-42. 10.1016/j.clnu.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 31. Robinson SM, Jameson KA, Batelaan SF, et al. Hertfordshire Cohort Study Group Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire cohort study. J Am Geriatr Soc 2008;56:84-90. 10.1111/j.1532-5415.2007.01478.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hamer M, Stamatakis E. Screen-based sedentary behavior, physical activity, and muscle strength in the English longitudinal study of ageing. PLoS One 2013;8:e66222. 10.1371/journal.pone.0066222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gianoudis J, Bailey CA, Daly RM. Associations between sedentary behaviour and body composition, muscle function and sarcopenia in community-dwelling older adults. Osteoporos Int 2015;26:571-9. 10.1007/s00198-014-2895-y [DOI] [PubMed] [Google Scholar]
- 34. Rantanen T, Era P, Heikkinen E. Physical activity and the changes in maximal isometric strength in men and women from the age of 75 to 80 years. J Am Geriatr Soc 1997;45:1439-45. 10.1111/j.1532-5415.1997.tb03193.x [DOI] [PubMed] [Google Scholar]
- 35. Gianoudis J, Bailey CA, Daly RM. Associations between sedentary behaviour and body composition, muscle function and sarcopenia in community-dwelling older adults. Osteoporos Int 2015;26:571-9. 10.1007/s00198-014-2895-y [DOI] [PubMed] [Google Scholar]
- 36. Newman AB, Haggerty CL, Goodpaster B, et al. Health Aging And Body Composition Research Group Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc 2003;51:323-30. 10.1046/j.1532-5415.2003.51105.x [DOI] [PubMed] [Google Scholar]
- 37. Ramlagan S, Peltzer K, Phaswana-Mafuya N. Hand grip strength and associated factors in non-institutionalised men and women 50 years and older in South Africa. BMC Res Notes 2014;7:8. 10.1186/1756-0500-7-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuh D, Bassey EJ, Butterworth S, Hardy R, Wadsworth ME, Musculoskeletal Study Team Grip strength, postural control, and functional leg power in a representative cohort of British men and women: associations with physical activity, health status, and socioeconomic conditions. J Gerontol A Biol Sci Med Sci 2005;60:224-31. 10.1093/gerona/60.2.224 [DOI] [PubMed] [Google Scholar]
- 39. Frederiksen H, Gaist D, Petersen HC, et al. Hand grip strength: a phenotype suitable for identifying genetic variants affecting mid- and late-life physical functioning. Genet Epidemiol 2002;23:110-22. 10.1002/gepi.1127 [DOI] [PubMed] [Google Scholar]
- 40. Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am J Epidemiol 2017;186:1026-34. 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables