Hyperhemolytic group B streptococci trade capsule for increased virulence, blood–brain barrier penetration, intracellular persistence, and antibiotic evasion.
Keywords: Streptococcus, capsule, hemolysin, intracellular, antibiotics
Abstract
Group B streptococci (GBS) are encapsulated, β-hemolytic bacteria that are a common cause of infections in human newborns and certain adults. Two factors important for GBS virulence are the sialic acid capsular polysaccharide that promotes immune evasion and the hemolytic pigment that induces host cell cytotoxcity. These virulence factors are often oppositely regulated by the CovR/CovS two-component system. Clinical GBS strains exhibiting hyperhemolysis and low capsule due to pathoadaptive covR/S mutations have been isolated from patients. Given the importance of capsule to GBS virulence, we predicted that a decrease or loss of capsule would attenuate the virulence of covR/S mutants. Surprisingly, hyperhemolytic GBS with low or no capsule exhibit increased virulence, intracellular persistence, and blood–brain barrier penetration, which was independent of a Trojan horse mechanism of barrier penetration. Additionally, intracellular persistence enabled both hemolytic and hyperhemolytic GBS to evade antibiotics routinely used to treat these infections. The finding that diminished capsule expression promotes GBS virulence, intracellular persistence, and antibiotic evasion has important implications for sustained antibiotic therapy and efficacy of capsule-based vaccines.
Group B streptococci (GBS) are β-hemolytic, gram-positive bacteria that colonize the lower gastrointestinal tract of healthy adults but cause invasive infections in newborns and in elderly, immunocompromised, and diabetic adults. Although intrapartum antibiotic prophylaxis has reduced transmission of GBS to the newborn during labor and delivery, GBS infections that occur earlier in pregnancy, or in newborns later in life (late-onset disease), are not prevented by these measures [1]. Increased incidence of GBS infections in adults has been reported [2, 3], and the identification of antibiotic-resistant strains [4, 5] imposes concerns for sustained measures of treatment. As antibiotic efficiency wanes, a better understanding of GBS pathogenesis is essential for new intervention strategies.
Virulence factors play a crucial role in GBS pathogenesis [6]. The sialic acid–rich GBS capsular polysaccharide is a critical component for host defense as it protects GBS from opsonophagocytic killing [7, 8] and dampens immune responses [9]. Capsular polysaccharide protein conjugate vaccines are currently being explored in clinical trials for prevention of GBS infections [10, 11]. Despite the importance of capsule to GBS virulence, acapsular strains have been isolated from clinical settings [12–14], suggesting that loss of capsule may benefit certain aspects of GBS pathogenesis.
Another key virulence factor is the β-hemolysin/cytolysin. Hemolytic and hyperhemolytic GBS induce host cell cytolysis and are significantly more pathogenic compared to nonhemolytic strains [15, 16]. Hyperhemolytic GBS, with mutations in the transcriptional repressor of hemolysin known as CovR/CovS, have been isolated from women in preterm labor [17] and from patients with GBS-associated prosthetic joint infections, conjunctivitis, sore throat, necrotizing fasciitis, toxic shock, or GBS disease–associated isolates [12, 18–21]. The GBS pigment is hemolytic and cytolytic [17, 22] and is produced by the cyl genes [23, 24], whose transcription is regulated by the CovR/S system [25, 26].
In some GBS strains, the CovR/S system also activates expression of cps genes, which produce the capsule [25, 26]. Clinical isolates of hyperhemolytic GBS, with mutations in covR/S, were observed to exhibit significantly decreased capsule levels [18, 20]. Based on observations that (1) GBS clinical isolates with genetic mutations in covR/S have diminished capsule [18, 20]; (2) antibiotic exposure can decrease capsule size [27]; and (3) there are current clinical trials of capsule-based GBS vaccines [10, 11], we investigated how the decrease or loss of capsule affects the hypervirulence of GBS covR/S mutants. Here, we show that hyperhemolytic GBS with low to no capsule exhibit significantly increased virulence, blood–brain barrier penetration, intracellular persistence, and antibiotic evasion. These findings have significant implications for antibiotic therapy and utility of GBS capsule-based vaccines and highlight the necessity for additional interventions to prevent and treat GBS infections.
MATERIALS AND METHODS
Written informed patient consent for human blood was approved by the Seattle Children’s Research Institute institutional review board (protocol number 11117). All animal experiments were approved by the Seattle Children’s Research Institutional Animal Care and Use Committee (protocol number 13311) and performed as per the recommendations in the Guide for the Care and Use of Laboratory Animals (eighth edition).
Bacterial Strains
GBS were cultured in tryptic soy broth (TSB; Difco) in 5% CO2 at 37°C. A909 and NEM316 are wild-type (WT) GBS clinical isolates belonging to serotype Ia and III, respectively. The ΔcpsE and ΔcovR strains are isogenic to A909 [28, 29]. The ΔcovRΔcpsE and NEMΔcovR strains were derived from A909ΔcpsE and NEM316, respectively, using the plasmid pJR233csrRD::Sp as described [26, 30].
Mouse Infection With Extracellular GBS
For survival studies, 6- to 8-week-old C57BL6/J mice were injected intravenously with 1 × 108 colony-forming units (CFU) of GBS WT or mutants and survival was monitored for 14 days postinfection. NLRP3KO mice were bred in house and infected as above.
For bacterial dissemination, C57BL6/J mice were intravenously infected with 107 GBS CFU. At 48 hours postinfection, systemic organs were collected aseptically and bacterial burden in organ homogenates were determined by plating serial dilutions on TSB agar as described [31].
For enumeration of intracellular bacteria during systemic infection, C57BL6/J mice were injected intravenously with 5 × 107 GBS CFU, and peritoneal fluids and brains were collected at 48 hours postinfection as described previously [31]. Brains were processed through a 100-µM mesh to obtain single cell suspensions, which were incubated with penicillin G (5 µg/mL) and gentamycin (100 µg/mL) for 2 hours to kill extracellular bacteria. The cell suspension was then centrifuged, washed twice with phosphate-buffered saline (PBS), lysed with 0.025% Triton X-100 and serially diluted and plated for CFU enumeration.
GBS Invasion and Exit From Bone Marrow–Derived Macrophages
Murine bone marrow–derived macrophages (BMDMs) were derived from bone marrow cells of C57BL6/J mice as described previously [32]. For invasion into macrophages, approximately 5 × 105 BMDMs/well were infected with GBS (optical density at 600 nm [OD600] = 0.3) at a multiplicity of infection (MOI) of 2 for 30 minutes. The infected BMDMs were then washed with PBS, incubated for 2 hours in Roswell Park Memorial Institute medium (RPMI) containing antibiotics (5 µg/mL penicillin G and 100 µg/mL gentamycin), then lysed with 0.025% Triton-X 100 before plating for CFU enumeration. To compare exit from infected macrophages, we modified the protocol described [33]. In brief, infected BMDMs containing only intracellular GBS were incubated in antibiotic-free RPMI for 4 hours to permit exit of internalized GBS. Supernatants and cell lysates were plated for CFU enumeration.
In Vivo Infection of GBS Internalized in BMDMs
C57BL6/J mice were injected intravenously with 100 µL BMDMs containing only internalized GBS (107 CFU). At 48 hours postinfection, systemic organs were homogenized and plated for CFU enumeration. For assessment of BBB penetration by macrophages containing intracellular GBS, BMDMs from B6.SJL-Ptprca Pepcb/BoyJ mice (Jackson Laboratories) expressing CD45.1 were infected with GBS. Approximately 2.5 × 106 BMDMs containing only intracellular GBS were intravenously infected in C57BL6/J mice expressing CD45.2 leukocytes. As controls, uninfected CD45.1 BMDMs were injected into C57BL6/J mice expressing CD45.2. At 24 hours postinfection, brains were harvested and single-cell suspensions were prepared from one-half of the brain as described previously. The single cells were then stained with PE-F4/80 (BD catalog number 565410 1:200), APC-CD11b (BD catalog number 553312 1:100), APC-Fire-CD45.1 (BioLegend clone 30-F11 1:100), and AF700-CD45.2 (BioLegend clone 104 1:100) and processed using flow cytometry on an LSR II instrument (BD Biosciences). The other half of the brain was homogenized, serially diluted, and plated for CFU enumeration.
Antibiotic Sensitivity
The minimum inhibitory concentrations (MICs) for both extracellular and intracellular GBS were estimated as described [34]. For extracellular bacteria, overnight cultures of GBS were diluted 1:10 in RPMI + 10% fetal calf serum and supplemented with either ampicillin (0–2500 ng/mL) or clindamycin (0–200 ng/mL). Bacterial growth was determined by reading the OD600 after 4 and 24 hours of incubation at 37°C, and MIC was determined to be the dose of antibiotic that inhibited bacterial growth by ≥90% at both time points. Surviving GBS CFU was also enumerated after 2 hours of incubation in RPMI containing the above antibiotics to compare to intracellular bacterial CFU (see below).
For intracellular MIC, approximately 5 × 105 BMDMs per well in a 96-well plate were infected with GBS at MOI 2 for 30 minutes. Infected BMDMs were washed 3 times with PBS and extracellular bacteria were killed by incubation with penicillin G (5 µg/mL) and gentamycin (100 µg/mL) for 2 hours. After 3 additional PBS washes, the cells were incubated with ampicillin (0–2500 ng/mL) or clindamycin (0–200 ng/mL) for 2 hours. Macrophages were then washed, trypsinized, and lysed with 0.025% Triton-X. The number of surviving intracellular bacteria was determined by plating serial dilutions. Linear regression was used to evaluate the relationship between antibiotic concentration and bacterial CFU. The F-test for each regression was used to determine relationship between antibiotic concentration and bacterial CFU.
Statistical Analysis
Statistical tests are indicated and P < .05 was considered significant. These tests were performed using GraphPad Prism version 6.0a and 7.0a (GraphPad Software, www.graphpad.com).
RESULTS
Loss of Capsule Increases Virulence of Hyperhemolytic GBS
To understand how capsule expression influences the virulence of hyperhemolytic GBS, we first compared the virulence properties of acapsular covR/S mutants to encapsulated covR/S mutants. A covR/S mutation did not diminish capsule expression in the GBS WT strain A909 [16], unlike other GBS strains such as NEM316, 2603v/r, 515 [25, 26], or those isolated from infected patients [18, 20]. Thus, the acapsular A909ΔcpsE mutant was used to derive the double ΔcovRΔcpsE strain. Both ΔcovRΔcpsE and ΔcpsE were attenuated for production of the GBS sialic acid capsule (Supplementary Figure 1). The ΔcovRΔcpsE strain displayed hyperhemolysis, similar to ΔcovR (Supplementary Figure 2), indicating that loss of capsule did not alter its hemolytic activity.
We then compared the virulence properties of acapsular and hyperhemolytic ΔcovRΔcpsE to encapsulated hyperhemolytic ΔcovR, capsule-deficient ΔcpsE, and WT A909. To this end, WT C57BL6/J mice were infected intravenously with approximately 1 × 108 CFU of GBS (WT, ΔcpsE, ΔcovR, or ΔcovRΔcpsE) and survival was monitored up to 14 days. As noted previously [16], mice infected with GBSΔcovR succumbed to the infection more rapidly compared with WT GBS. Surprisingly, the ΔcovRΔcpsE strain exhibited significantly greater virulence compared to ΔcovR (Figure 1A), suggesting that loss of capsule exacerbates virulence of hyperhemolytic GBS. Although the acapsular ΔcpsE strain was significantly attenuated for virulence in the neonatal rat model [35, 36], we noted that this strain was as virulent as WT GBS in the adult murine model (Figure 1A). These results indicate that acapsular GBS are not attenuated for virulence and that acapsular and hyperhemolytic GBS are hypervirulent in the adult systemic model of infection.
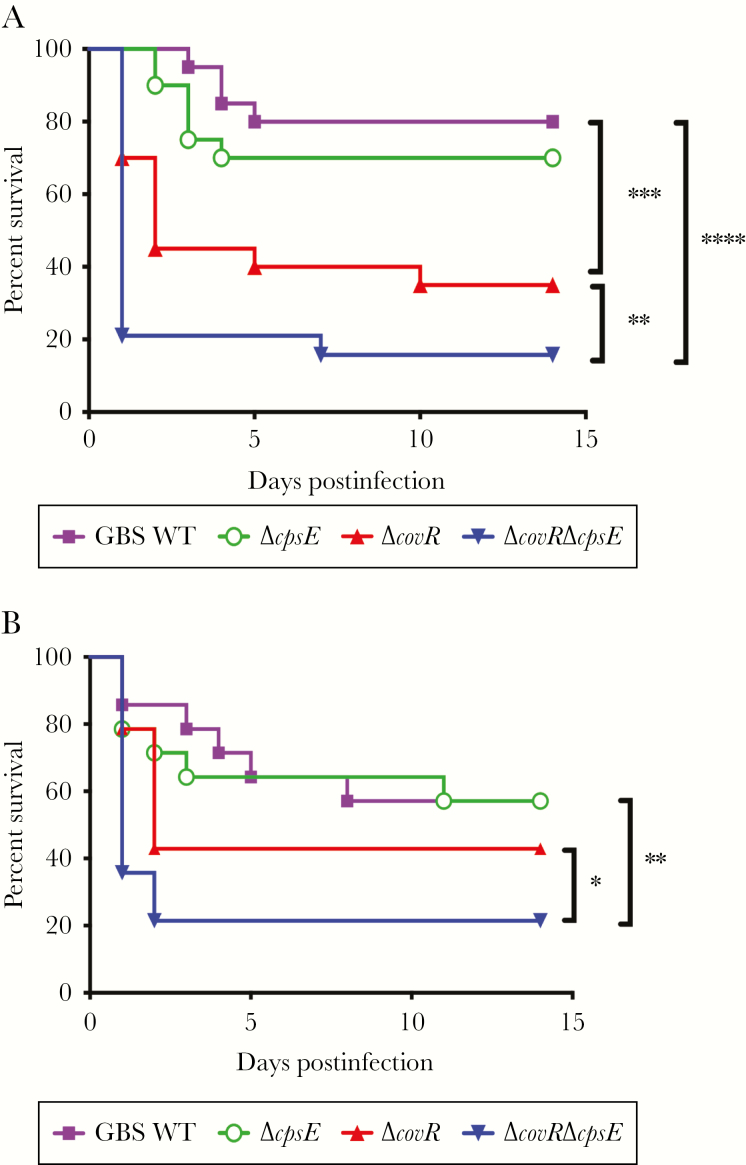
Figure 1.
Increased virulence of acapsular and hyperhemolytic group B streptococci (GBS). Wild-type (WT) C57BL6/J mice (n = 20/group, A) or NLRP3 knockout (KO) mice (n = 14/group, B) were infected intravenously with GBS (WT, ΔcovR, ΔcovRΔcpsE, or ΔcpsE). Gehan–Breslow–Wilcoxon test was used to compare Kaplan–Meier survival curves. A, **P = .009, ***P = .0009, ****P < .0001; survival of mice infected with GBS WT compared to ΔcpsE was not significantly different (P = .3). B, *P = .04, **P = .005.
We and others have previously described the importance of the NLRP3 inflammasome to GBS virulence [22, 37, 38]. To test if virulence of ΔcovRΔcpsE is linked to the NLRP3 inflammasome, NLRP3KO mice were infected with the GBS strains (WT, ΔcpsE, ΔcovR, or ΔcovRΔcpsE) and survival was monitored as indicated above. Although GBSΔcovR and ΔcovRΔcpsE exhibit increased virulence compared to WT GBS, virulence of ΔcovRΔcpsE in NLRP3KO mice was significantly higher than ΔcovR (Figure 1B). These data indicate that increased virulence of GBSΔcovRΔcpsE is observed even in the absence of the NLRP3 inflammasome.
Acapsular and Hyperhemolytic GBS Exhibit Increased Bacterial Dissemination
To determine if the absence of capsule promoted bacterial dissemination, bacterial burden was estimated at 48 hours postinfection in systemic organs such as the lung and the brain in WT C57BL6/J mice infected with the GBS strains above. A lower dose of infection (1 × 107 GBS CFU) was used to prevent induction of morbidity/mortality within the experimental end point. Bacterial burden was significantly higher in the lungs and brains of mice infected with GBSΔcovRΔcpsE compared to any other GBS strain (Figure 2A and 2B). Thus, loss of capsule exacerbated bacterial dissemination of hyperhemolytic GBS.
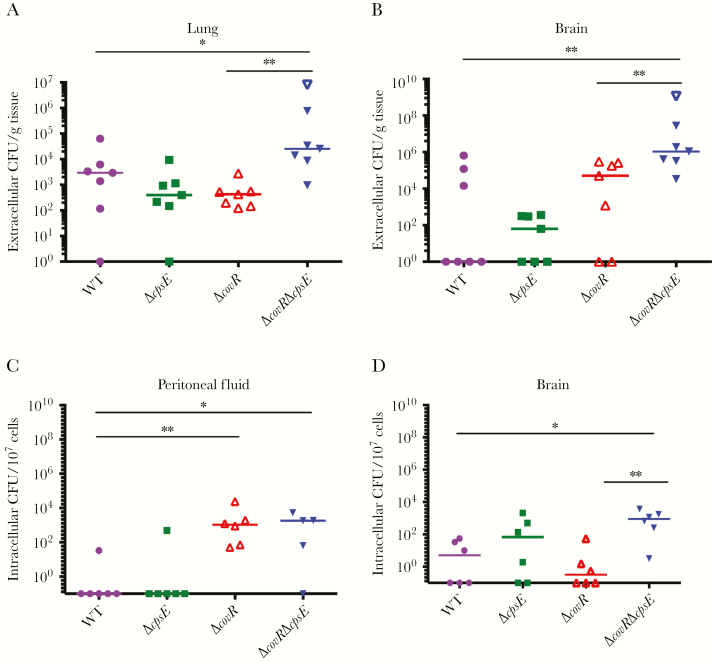
Figure 2.
Acapsular and hyperhemolytic group B streptococci (GBS) exhibit increased dissemination and intracellular persistence. A and B, Wild-type (WT) C57BL6/J mice (n = 7/group) were infected intravenously with GBS (WT, ΔcpsE, ΔcovR, or ΔcovRΔcpsE). Bacterial burden (colony-forming units [CFU]) was estimated 48 hours postinfection. Open triangle shows 1 ΔcovRΔcpsE animal that succumbed to the infection near experimental end. Medians are shown. Mann–Whitney test was used to compare differences between 2 groups. A, *P = .05, **P = .001. B, **P < .005. C and D, WT C57BL6/J mice (n = 6/group) were infected intravenously with GBS (WT, ΔcpsE, ΔcovR, or ΔcovRΔcpsE). Intracellular GBS in peritoneal fluid and brain are shown. Medians are depicted. Dunn multiple comparison test following analysis of variance. C, *P < .02, **P = .002. D, *P = .01, **P = .004.
Increased Virulence of Acapsular and Hyperhemolytic GBS Is Not due to Increased Resistance to Opsonophagocytic Killing
We next examined if the increased virulence of ΔcovRΔcpsE could be attributed to increased resistance to opsonophagocytic killing. Neutrophils isolated from adult human blood were incubated with opsonized GBS (WT, ΔcpsE, ΔcovR, or ΔcovRΔcpsE) for 1 hour and survival index was calculated as described previously [28, 29]. Consistent with previous findings [7, 8, 39], the acapsular ΔcpsE strain was significantly more sensitive to opsonophagocytic killing compared to WT GBS (Supplementary Figure 3). Hyperhemolytic GBSΔcovR and ΔcovRΔcpsE both exhibited similar levels of resistance to opsonophagocytic killing that was less than WT GBS but greater than ΔcpsE (Supplementary Figure 3). Thus, increased virulence of acapsular ΔcovRΔcpsE compared to ΔcovR could not be attributed to increased resistance to opsonophagocytic killing.
Acapsular GBS Exhibit Increased Intracellular Persistence In Vivo
We next wondered if the increased virulence of acapsular ΔcovRΔcpsE is related to intracellular persistence. Previous studies have shown that acapsular GBS efficiently invade eukaryotic cells in vitro [36, 40, 41]. To test if acaspular GBS persisted intracellularly in vivo, WT C57BL6/J mice were infected with the GBS strains (WT, ΔcpsE, ΔcovR, ΔcovRΔcpsE), and peritoneal fluid, lungs, spleen, and brain were harvested at 48 hours postinfection. Single-cell suspensions generated from these organs were treated with antibiotics to kill extracellular bacteria, after which the cells were lysed and intracellular GBS were enumerated. Whereas both GBS ΔcovR strains exhibited increased intracellular persistence in peritoneal fluid compared to WT and ΔcpsE, only the acapsular ΔcovRΔcpsE strain showed significantly increased intracellular persistence in the brains of the infected mice (Figure 2C and 2D). No significant load of intracellular bacteria was detected in the spleens and lungs. Similar results of increased dissemination and intracellular persistence in the brain were also observed with an independent ΔcovRΔcpsE mutant and these were diminished in the complemented ΔcovRΔcpsE/pCpsECovR (see Supplementary Figures 4 and 5 for in vitro and in vivo complementation, respectively).
Internalized Acapsular GBS Are Released From Infected Macrophages
We then compared the ability of acapsular GBS to invade and subsequently exit from infected macrophages. To compare the invasive ability, BMDMs were exposed to GBS (WT, ΔcpsE, ΔcovR, ΔcovRΔcpsE) for 30 minutes, after which extracellular bacteria were removed using washes and antibiotic treatment. Then, infected BMDMs were lysed and plated to enumerate intracellular bacteria. Consistent with the in vivo findings, we observed that acapsular GBS (ΔcpsE, ΔcovRΔcpsE) more efficiently invaded macrophages compared to WT or ΔcovR, respectively (Figure 3A). To compare exit of GBS from infected macrophages, BMDMs containing only intracellular GBS were incubated in antibiotic-free media for 4 hours, after which the supernatants and cell lysates were plated to enumerate extracellular and intracellular CFU, respectively. The number of bacteria released from infected macrophages was significantly higher for acapsular GBSΔcpsE and ΔcovRΔcpsE, as evidenced by higher CFU and ratio of extracellular/intracellular CFU (Figure 3B and 3C). These data demonstrate that acapsular GBS are able to invade and eventually exit from the infected macrophage.
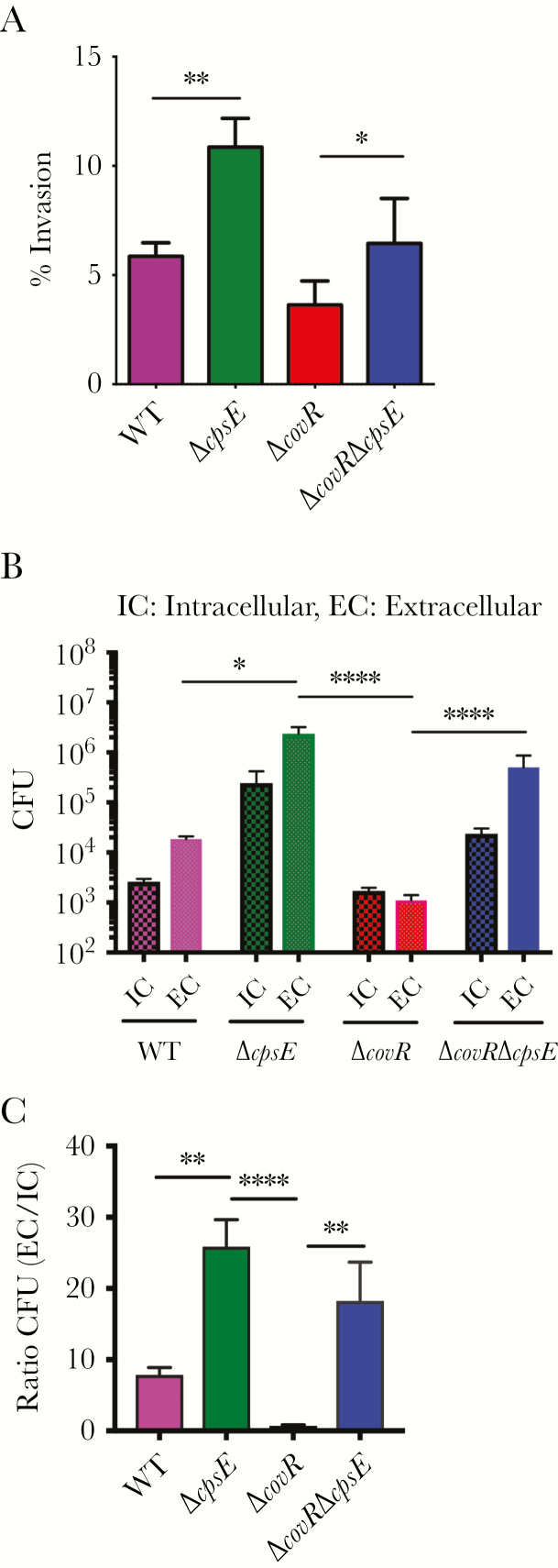
Figure 3.
Acapsular group B streptococci (GBS) exhibit increased invasion and exit from infected macrophages. A, Percent invasion of GBS in bone marrow–derived macrophages (BMDMs) after 30 minutes. Data shown are the mean of 3 independent experiments (*P = .04, **P = .006, Student t test, error bars ± standard error of the mean). B and C, BMDMs containing only intracellular GBS were incubated in antibiotic-free media for 4 hours and colony-forming units (CFU) were estimated in supernatants (ie, extracellular [EC]) and cell lysates (ie, intracellular [IC]). B, The IC and EC CFU were normalized to 105 CFU of the initial inoculum of each strain. C, Ratio of EC and IC CFU for each GBS strain. Data represent the mean of 4 independent experiments (*P < .05, **P < .006, ****P < .0001, Dunn multiple comparison test following analysis of variance).
Intracellular Acapsular GBS Exhibit Increased Blood–Brain Barrier Penetration
The increase in intracellular persistence of acapsular GBS prompted us to assess whether internalized GBS can potentially hide within macrophages for dissemination (often referred to as a “Trojan horse” mechanism). WT C57BL6/J mice were infected with BMDMs containing approximately 107 CFU of internalized GBS. At 48 hours postinfection, systemic organs were harvested and bacterial dissemination was estimated. The acaspular ΔcovRΔcpsE exhibited increased dissemination into the lungs and particularly into the brains of the infected mice (Figure 4A and 4B).
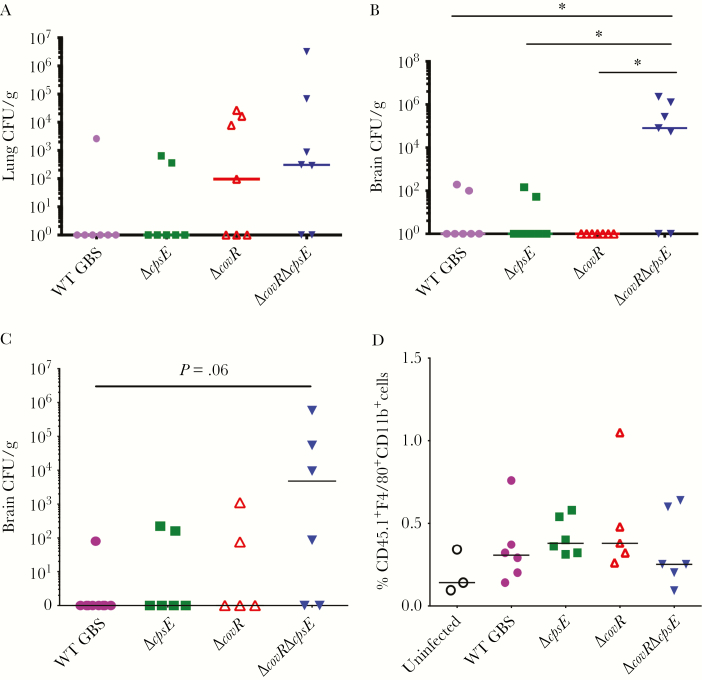
Figure 4.
Intracellular acapsular and hyperhemolytic group B streptococci (GBS) exhibit increased blood–brain barrier penetration. A and B, Bone marrow–derived macrophages (BMDMs) containing 107 intracellular GBS colony-forming units (CFU) (wild-type [WT], ΔcpsE, ΔcovR, or ΔcovRΔcpsE) were intravenously injected into WT C57BL6/J mice (n = 7/group). At 48 hours postinfection, lungs (A) and brains (B) were harvested for bacterial enumeration. *P ≤ .01, Dunn multiple comparison test following analysis of variance (ANOVA). C and D, WT C57BL6/J mice (n = 6/group) expressing CD45.2 were infected intravenously with 2.5 × 106 BMDMs from CD45.1 mice containing 105–6 intracellular GBS (WT, ΔcpsE, ΔcovR, or ΔcovRΔcpsE). Uninfected refers to mice injected with CD45.1 macrophages not containing intracellular GBS. The number of CD45.1 BMDMs was kept constant between the groups to examine blood–brain barrier penetration of macrophages. At 24 hours postinfection, brains were processed for enumeration of GBS CFU (C) and for CD45.1 macrophages (D). Dunn multiple comparison test following ANOVA was used to estimate statistical significance.
To determine if macrophages containing internalized GBS themselves penetrated the blood–brain barrier, we performed adoptive transfer experiments using the CD45 marker to distinguish between donor and recipient macrophages. CD45, also known as the leukocyte common antigen, is a general marker of leukocytes commonly used in flow cytometry to differentiate immune cells from other cell types. CD45 can have multiple alleles (eg, CD45.1 or CD45.2 in mice), which can be distinguished using specific antibodies. Adoptive transfer experiments were performed wherein WT C57BL6/J mice expressing CD45.2 lymphocytes were injected with CD45.1 BMDMs containing internalized GBS CFU. As recipient WT C57BL6/J mice expressed CD45.2, we were able to differentiate between endogenous and donor macrophages via the CD45 allelic variant. At 48 hours postinfection, brains were harvested and flow cytometry was performed to determine if CD45.1 macrophages were present in the brains of the infected mice. Bacterial dissemination was estimated in parallel. Although the ΔcovRΔcpsE mutant showed increased presence in the brains (Figure 4C), no significant differences in the numbers of CD45.1 macrophages were observed between the various groups (Figure 4D). These data indicate that while the acapsular ΔcovRΔcpsE shows increased intracellular persistence and trafficking to the brain, this is not due to its ability to disseminate via macrophage trafficking.
Intracellular GBS Evade the Action of Antibiotics Used to Treat GBS in Clinical Settings
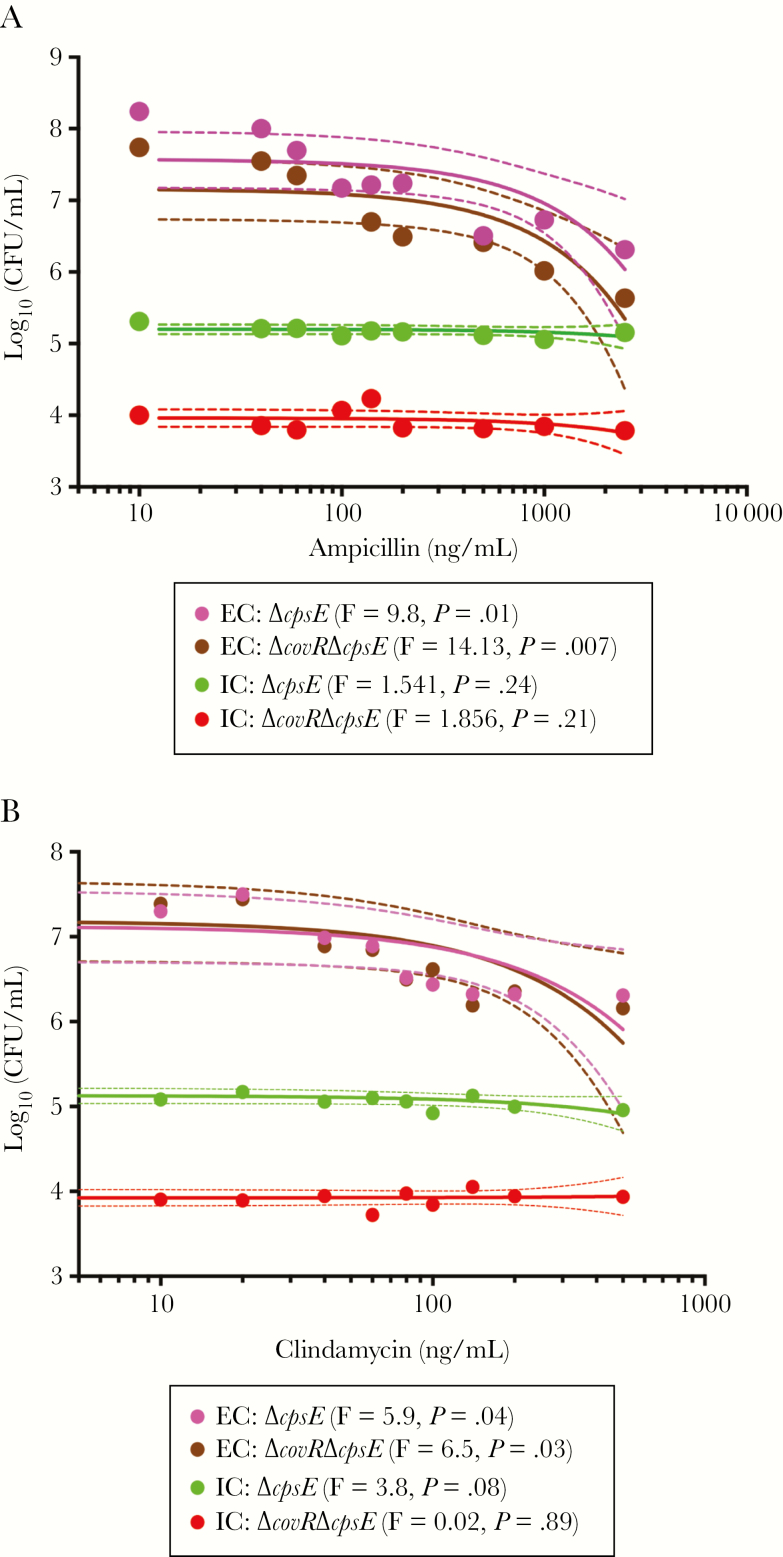
Currently, the antibiotics commonly used to treat GBS in clinical settings are ampicillin or clindamycin [42]. Therefore, we examined if intracellular persistence enabled GBS evade the action of these antibiotics. We compared the MICs for both extracellular and intracellular GBS using methods described previously [34]. For extracellular GBS, the MIC was 500 ng/mL ampicillin and 100 ng/mL clindamycin during a 24-hour growth period in RPMI for all strains (WT A909, ΔcovR, ΔcpsE, ΔcovRΔcpsE including NEM316, NEM316ΔcovR; see next section). We then examined if BMDMs containing intracellular and acapsular GBS (ΔcpsE, ΔcovRΔcpsE) exhibited similar or altered antibiotic sensitivity. Extracellular GBS or BMDMs containing only intracellular GBS were incubated with ampicillin or clindamycin at various concentrations for 2 hours and the number of surviving GBS was determined. Ampicillin or clindamycin did not significantly decrease the numbers of intracellular GBS but did significantly decrease the numbers of extracellular GBS (Figure 5A and 5B). These data suggest that intracellular GBS are protected from the action of antibiotics such as ampicillin and clindamycin and, consequently, increased intracellular persistence can exacerbate GBS infections.
Figure 5.
Intracellular group B streptococci (GBS) are protected from antibiotics. Bone marrow–derived macrophages (BMDMs) containing intracellular acaspular GBS (ΔcpsE, ΔcovRΔcpsE) were exposed to increasing concentrations of ampicillin (A) or clindamycin (B) for 2 hours. The infected BMDMs were lysed to determine the bactericidal effects of antibiotics on intracellular GBS (n = 2). As controls, extracellular GBS (GBS in RPMI medium) were also exposed to increasing concentrations of these antibiotics and colony-forming units (CFU) were enumerated after 2 hours. Linear regression was used to evaluate the relationship between antibiotic concentration and bacterial CFU (colored line represents regression and dashed lines represent 95% confidence intervals). The F-test for each regression comparing antibiotic concentration and bacterial CFU was significant (P < .05) for extracellular bacteria (EC) and nonsignificant (P > .05) for intracellular bacteria (IC).
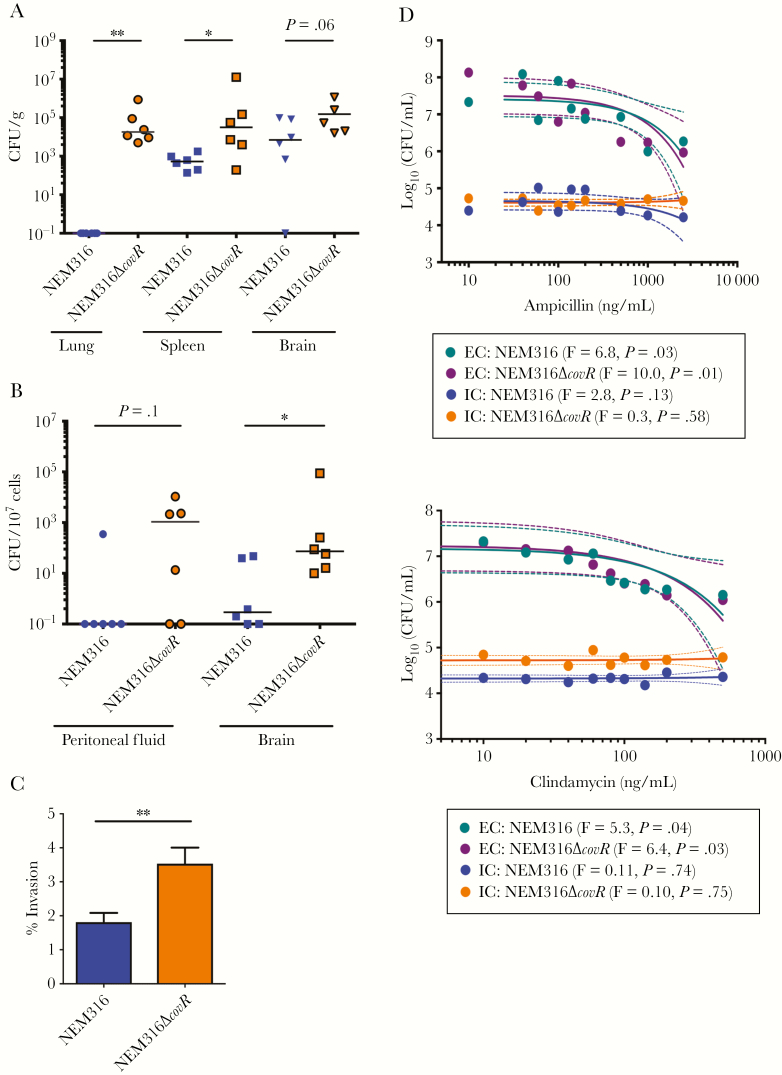
The results above indicate that loss of capsule exacerbates virulence of hyperhemolytic GBS, which can be attributed to increased invasion and exit that promotes dissemination, blood–brain barrier penetration, and antibiotic evasion. To date, clinical GBS covR/S mutants isolated from patients exhibit a significant decrease, but not loss, of capsule [18, 20]. Therefore, we examined the effect of decreased capsule using the NEM316ΔcovR strain, which was previously shown to exhibit decreased capsule and increased hemolysis compared to WT NEM316 [25]. Consistent with our observations with acapsular and hyperhemolytic GBSΔcovRΔcpsE, the NEM316ΔcovR strain exhibited increased dissemination, intracellular persistence, invasion, and antibiotic evasion compared to NEM316 (Figure 6A–D). Thus, either diminished or loss of capsule expression enables intracellular persistence, which exacerbates virulence of hyperhemolytic GBS and permits antibiotic evasion. These findings emphasize the importance of continued surveillance of GBS infections in the era of antibiotics and implementation of capsule-based vaccines.
Figure 6.
Hyperhemolytic group B streptococci (GBS) with decreased capsule also exhibit increased dissemination, intracellular persistence, and antibiotic evasion. A, Wild-type (WT) C57BL6/J mice (n = 6/group) were infected intravenously with NEM316 (WT) or NEM316ΔcovR in bone marrow derived macrophages (BMDM). At 48 hours postinfection, total (A) and intracellular (B) bacterial burden was estimated. Mann–Whitney test was used to compare differences between the 2 groups. A, *P = .04, **P = .002. B, *P = .02. C, Percent invasion of GBS NEM316 and NEM316ΔcovR. Data shown is the mean of 3 independent experiments. **P = .007, Student t test, error bars ± standard error of the mean. D, Bone marrow–derived macrophages (BMDMs) containing intracellular GBS NEM316 and NEM316ΔcovR were exposed to increasing concentrations of ampicillin (top panel) or clindamycin (bottom panel) for 2 hours. Infected BMDMs were lysed to determine bactericidal effects of the antibiotics to intracellular GBS. As controls, extracellular GBS were also exposed to increasing concentrations of these antibiotics and colony-forming units (CFU) were enumerated after 2 hours. Linear regression was used to evaluate the relationship between antibiotic concentration and bacterial CFU (colored line represents regression and dashed line represents 95% confidence intervals). The F-test for each regression comparing antibiotic concentration and bacterial CFU was significant (P < .05) for extracellular bacteria (EC) and nonsignificant (P > .05) for intracellular bacteria (IC).
DISCUSSION
GBS remain an important cause of neonatal and adult infections. Current measures for treatment of GBS infections rely on antibiotic therapy that is administered during labor and delivery or upon GBS diagnosis. However, GBS infections in adults are on the rise [2, 3] and intrapartum antibiotic prophylaxis does not prevent all occurrences of GBS disease [42, 43].
Owing to the importance of the sialic acid capsule to GBS pathogenesis [7, 8], capsule polysaccharide protein conjugates are being explored as vaccine candidates and are in clinical trials [10, 11]. However, acapsular strains have been isolated from patients with infections [12, 13]. Also, hyperpigmented GBS with reduced capsule were isolated from cervical/vaginal swabs and from patients with throat infections, prosthetic joint infections, conjunctivitis, necrotizing fasciitis, or toxic shock [18, 20]. Although the recent advances in capsule-based vaccine trials are exciting, it must be acknowledged that the capsule-based vaccines will not protect against infections caused by acapsular strains. Moreover, use of capsule conjugate vaccines may lead to a selection pressure that could permit expansion of acapsular GBS isolates. Effects on changes in capsular serotype have been observed following the implementation of conjugate capsule vaccines against Streptococcus pneumoniae [44], and higher prevalence of acapsular strains was reported in certain cases [45]. A better understanding of the pathogenesis of acapsular GBS strains is critical for the development of interventions in the case of insufficient vaccine protection.
In this study, we aimed to understand how changes in capsule expression altered the virulence properties of hyperhemolytic GBS, which we have previously observed to be hypervirulent [16]. We show that hyperhemolytic GBS with decrease or loss of capsule exhibit increased virulence compared to encapsulated hyperhemolytic strains and this could be correlated with increased internalization and persistence. Increased internalization enables bacteria to evade host defenses and permits antibiotic evasion.
Intracellular persistence has been described for pathogens such as Staphylococcus aureus [34], wherein intracellular survival enabled antibiotic evasion and was indicated to promote recurrent infections. Although relatively infrequent, recurrent infections of GBS have been observed and include the manifestation of sepsis [46], meningitis in twin infants [47], and persistent colonization in women even after intrapartum antibiotic prophylaxis [48]. Also, addition of antibiotics increased phagocytic uptake of certain GBS strains and, interestingly, antibiotic treatment correlated with decreased GBS capsule size [27]. These observations suggest that GBS likely trades the benefits of capsule for increased intracellular survival and pathogenesis. Whether intracellular persistence facilitates antibiotic evasion leading to recurrent GBS infections requires further study. Nevertheless, treatment of the elderly adult with necrotizing fasciitis and toxic shock, from whom the hyperhemolytic GBS covR mutant with low capsule was isolated, required 3 repeated debridements and antimicrobial therapy for 6 weeks [20]. Thus, treatment of infections by GBS exhibiting increased intracellular persistence may require sustained antibiotic regimens, which could lead to the emergence of new antibiotic-resistant strains. Collectively, these observations reinforce the importance of developing additional preventive and therapeutic measures for GBS infections.
In summary, we report that a decrease or loss of capsule enhances intracellular persistence and exit leading to increased GBS dissemination and blood–brain barrier penetration. Intracellular persistence also diminishes GBS sensitivity to antibiotics, and the extended use of antibiotics and/or capsule-based vaccines could promote emergence of acaspular strains that are hypervirulent. These findings have important implications toward continued strategies for prevention of GBS infections in humans.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Devin Margolies and Erika Wulfert for assistance with animal infections and Dr Claire Poyart for GBS NEM316.
Author contributions. C. G., J. V., M. C., and L. R. designed the research. C. G., S. M., J. V., M. C., B. A., L. N., A. A., P. Q., and J. B. performed the experiments. C. G., S. M., J. V., M. C., L. N., A. A., and L. R. analyzed the results and C. G., J. V., and L. R. wrote the manuscript. All authors reviewed the final draft of the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or other funders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the NIH (grant numbers R01AI112619, R01AI33976, R01AI100989, and R21AI125907 to L. R.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Schrag SJ, Zywicki S, Farley MM et al. . Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med 2000; 342:15–20. [DOI] [PubMed] [Google Scholar]
- 2. Sunkara B, Bheemreddy S, Lorber B et al. . Group B Streptococcus infections in non-pregnant adults: the role of immunosuppression. Int J Infect Dis 2012; 16:e182–6. [DOI] [PubMed] [Google Scholar]
- 3. Skoff TH, Farley MM, Petit S et al. . Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clin Infect Dis 2009; 49:85–92. [DOI] [PubMed] [Google Scholar]
- 4. Teatero S, Ferrieri P, Martin I, Demczuk W, McGeer A, Fittipaldi N. Serotype distribution, population structure, and antimicrobial resistance of group B Streptococcus strains recovered from colonized pregnant women. J Clin Microbiol 2017; 55:412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castor ML, Whitney CG, Como-Sabetti K et al. . Antibiotic resistance patterns in invasive group B streptococcal isolates. Infect Dis Obstet Gynecol 2008; 2008:727505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Landwehr-Kenzel S, Henneke P. Interaction of Streptococcus agalactiae and cellular innate immunity in colonization and disease. Front Immunol 2014; 5:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takahashi S, Aoyagi Y, Adderson EE, Okuwaki Y, Bohnsack JF. Capsular sialic acid limits C5a production on type III group B streptococci. Infect Immun 1999; 67:1866–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marques MB, Kasper DL, Pangburn MK, Wessels MR. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect Immun 1992; 60:3986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carlin AF, Lewis AL, Varki A, Nizet V. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J Bacteriol 2007; 189:1231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heath PT. Status of vaccine research and development of vaccines for GBS. Vaccine 2016; 34:2876–9. [DOI] [PubMed] [Google Scholar]
- 11. Kobayashi M, Schrag SJ, Alderson MR et al. . WHO consultation on group B Streptococcus vaccine development: report from a meeting held on 27–28 April 2016. Vaccine 2016. doi:10.1016/j.vaccine.2016.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Almeida A, Villain A, Joubrel C et al. . Whole-genome comparison uncovers genomic mutations between group B streptococci sampled from infected newborns and their mothers. J Bacteriol 2015; 197:3354–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosini R, Campisi E, De Chiara M et al. . DEVANI Consortium Genomic analysis reveals the molecular basis for capsule loss in the group B Streptococcus population. PLoS One 2015; 10:e0125985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Creti R, Imperi M, Pataracchia M, Alfarone G, Recchia S, Baldassarri L. Identification and molecular characterization of a S. agalactiae strain lacking the capsular locus. Eur J Clin Microbiol Infect Dis 2012; 31:233–5. [DOI] [PubMed] [Google Scholar]
- 15. Liu GY, Doran KS, Lawrence T et al. . Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc Natl Acad Sci U S A 2004; 101:14491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lembo A, Gurney MA, Burnside K et al. . Regulation of CovR expression in group B Streptococcus impacts blood-brain barrier penetration. Mol Microbiol 2010; 77:431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whidbey C, Harrell MI, Burnside K et al. . A hemolytic pigment of group B Streptococcus allows bacterial penetration of human placenta. J Exp Med 2013; 210:1265–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lupo A, Ruppen C, Hemphill A, Spellerberg B, Sendi P. Phenotypic and molecular characterization of hyperpigmented group B streptococci. Int J Med Microbiol 2014; 304:717–24. [DOI] [PubMed] [Google Scholar]
- 19. Whidbey C, Burnside K, Martinez RM et al. . A hyperhemolytic/hyperpigmented group B Streptococcus strain with a CovR mutation isolated from an adolescent patient with sore throat. Clin Res Infect Dis 2015; 2. [PMC free article] [PubMed] [Google Scholar]
- 20. Sendi P, Johansson L, Dahesh S et al. . Bacterial phenotype variants in group B streptococcal toxic shock syndrome. Emerg Infect Dis 2009; 15:223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Almeida A, Rosinski-Chupin I, Plainvert C et al. . Parallel evolution of group B Streptococcus hypervirulent clonal complex 17 unveils new pathoadaptive mutations. mSystems 2017; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whidbey C, Vornhagen J, Gendrin C et al. . A streptococcal lipid toxin induces membrane permeabilization and pyroptosis leading to fetal injury. EMBO Mol Med 2015; 7:488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spellerberg B, Martin S, Brandt C, Lütticken R. The cyl genes of Streptococcus agalactiae are involved in the production of pigment. FEMS Microbiol Lett 2000; 188:125–8. [DOI] [PubMed] [Google Scholar]
- 24. Pritzlaff CA, Chang JC, Kuo SP, Tamura GS, Rubens CE, Nizet V. Genetic basis for the beta-haemolytic/cytolytic activity of group B Streptococcus. Mol Microbiol 2001; 39:236–47. [DOI] [PubMed] [Google Scholar]
- 25. Lamy MC, Zouine M, Fert J et al. . CovS/CovR of group B Streptococcus: a two-component global regulatory system involved in virulence. Mol Microbiol 2004; 54:1250–68. [DOI] [PubMed] [Google Scholar]
- 26. Jiang SM, Cieslewicz MJ, Kasper DL, Wessels MR. Regulation of virulence by a two-component system in group B Streptococcus. J Bacteriol 2005; 187:1105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Korir ML, Laut C, Rogers LM, Plemmons JA, Aronoff DM, Manning SD. Differing mechanisms of surviving phagosomal stress among group B Streptococcus strains of varying genotypes. Virulence 2016:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones AL, Needham RH, Clancy A, Knoll KM, Rubens CE. Penicillin-binding proteins in Streptococcus agalactiae: a novel mechanism for evasion of immune clearance. Mol Microbiol 2003; 47:247–56. [DOI] [PubMed] [Google Scholar]
- 29. Rajagopal L, Vo A, Silvestroni A, Rubens CE. Regulation of cytotoxin expression by converging eukaryotic-type and two-component signalling mechanisms in Streptococcus agalactiae. Mol Microbiol 2006; 62:941–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajagopal L, Clancy A, Rubens CE. A eukaryotic type serine/threonine kinase and phosphatase in Streptococcus agalactiae reversibly phosphorylate an inorganic pyrophosphatase and affect growth, cell segregation, and virulence. J Biol Chem 2003; 278:14429–41. [DOI] [PubMed] [Google Scholar]
- 31. Gendrin C, Vornhagen J, Ngo L et al. . Mast cell degranulation by a hemolytic lipid toxin decreases GBS colonization and infection. Sci Adv 2015; 1:e1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kusuzaki K, Kageyama N, Shinjo H et al. . Development of bone canaliculi during bone repair. Bone 2000; 27:655–9. [DOI] [PubMed] [Google Scholar]
- 33. Thulin P, Johansson L, Low DE et al. . Viable group A streptococci in macrophages during acute soft tissue infection. PLoS Med 2006; 3:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lehar SM, Pillow T, Xu M et al. . Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature 2015; 527:323–8. [DOI] [PubMed] [Google Scholar]
- 35. Wessels MR, Rubens CE, Benedí VJ, Kasper DL. Definition of a bacterial virulence factor: sialylation of the group B streptococcal capsule. Proc Natl Acad Sci U S A 1989; 86:8983–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones AL, Mertz RH, Carl DJ, Rubens CE. A streptococcal penicillin-binding protein is critical for resisting innate airway defenses in the neonatal lung. J Immunol 2007; 179:3196–202. [DOI] [PubMed] [Google Scholar]
- 37. Costa A, Gupta R, Signorino G et al. . Activation of the NLRP3 inflammasome by group B streptococci. J Immunol 2012; 188:1953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gupta R, Ghosh S, Monks B et al. . RNA and β-hemolysin of group B Streptococcus induce interleukin-1β (IL-1β) by activating NLRP3 inflammasomes in mouse macrophages. J Biol Chem 2014; 289:13701–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci U S A 1995; 92:11490–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hulse ML, Smith S, Chi EY, Pham A, Rubens CE. Effect of type III group B streptococcal capsular polysaccharide on invasion of respiratory epithelial cells. Infect Immun 1993; 61:4835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nizet V, Kim KS, Stins M et al. . Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun 1997; 65:5074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Verani JR, McGee L, Schrag SJ; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC) Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep 2010; 59:1–36. [PubMed] [Google Scholar]
- 43. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep 2002; 51:1–22. [PubMed] [Google Scholar]
- 44. Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 2011; 378:1962–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haas W, Hesje CK, Sanfilippo CM, Morris TW. High proportion of nontypeable Streptococcus pneumoniae isolates among sporadic, nonoutbreak cases of bacterial conjunctivitis. Curr Eye Res 2011; 36:1078–85. [DOI] [PubMed] [Google Scholar]
- 46. Ekelund K, Konradsen HB. Invasive group B streptococcal disease in infants: a 19-year nationwide study. Serotype distribution, incidence and recurrent infection. Epidemiol Infect 2004; 132:1083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moylett EH, Fernandez M, Rench MA, Hickman ME, Baker CJ. A 5-year review of recurrent group B streptococcal disease: lessons from twin infants. Clin Infect Dis 2000; 30:282–7. [DOI] [PubMed] [Google Scholar]
- 48. Manning SD, Lewis MA, Springman AC, Lehotzky E, Whittam TS, Davies HD. Genotypic diversity and serotype distribution of group B Streptococcus isolated from women before and after delivery. Clin Infect Dis 2008; 46:1829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.