Abstract
Objective. To compare the relative human abuse potential after insufflation of manipulated morphine abuse-deterrent, extended-release injection-molded tablets (morphine-ADER-IMT) with that of marketed morphine ER tablets.
Methods. A randomized, double-blind, double-dummy, active- and placebo-controlled five-way crossover study was performed with adult volunteers who were experienced, nondependent, recreational opioid users. After intranasal (IN) administration of manipulated high-volume (HV) morphine-ADER-IMT (60 mg), participants were randomized (1:1:1:1) to receive IN manipulated low-volume (LV) morphine ER (60 mg), IN manipulated LV morphine-ADER-IMT, intact oral morphine-ADER-IMT (60 mg), and placebo in crossover fashion. Pharmacodynamic and pharmacokinetic assessments included peak effect of drug liking (Emax; primary endpoint) using drug liking visual analog scale (VAS) score, Emax using overall drug liking, and take drug again (TDA) VASs scores, and mean abuse quotient (AQ), a pharmacokinetic parameter associated with drug liking.
Results. Forty-six participants completed the study. After insufflation of HV morphine-ADER-IMT and LV morphine-ADER-IMT, drug liking Emax was significantly lower (P < 0.0001) compared with IN morphine ER. Overall drug liking and TDA Emax values were significantly lower (P < 0.0001) after insufflation of HV morphine-ADER-IMT and LV morphine-ADER-IMT compared with IN morphine ER. Mean AQ was lower after insufflation of HV (9.2) and LV (2.3) morphine-ADER-IMT or ingestion of oral morphine-ADER-IMT (5.5) compared with insufflation of LV morphine ER (37.2).
Conclusions. All drug liking, take drug again, and abuse quotient endpoints support a significantly lower abuse potential with insufflation of manipulated morphine-ADER-IMT compared with manipulated and insufflated non-AD ER morphine.
Keywords: Abuse-Deterrent, Abuse Potential, Extended-Release, Human Abuse Potential, Intranasal, Morphine
Introduction
Extended-release (ER) opioids have a significant risk of abuse and overdose [1]. Because ER formulations typically contain more active drug than immediate-release (IR) formulations, they are attractive drugs for abuse if they can be transformed or manipulated to have a faster release than intended by the manufacturer. Many abusers of ER opioids manipulate tablets in an effort to defeat the ER properties and also to facilitate routes known to have faster absorption than oral administration, such as intranasal (IN) or intravenous (IV) routes of administration [2]. The risk of misuse and abuse of prescription opioids extends beyond the patients receiving opioids for their chronic pain and includes their friends and family, as 70% of individuals who use prescription opioids for nonmedical purposes received their drugs from friends or family [3]. While abuse-deterrent products can help to reduce abuse through alternate routes of administration (IN, IV), there is currently no technology that can address the most common form of abuse, taking multiple tablets orally.
Rapid absorption resulting in high drug levels heightens the positive subjective effects of opioids such as “drug liking” [4]. Abuse of opioids by the IN route (ie, insufflation, snorting) is common, with varying frequency of occurrence depending on the type of opioid [5]. After starting with ingestion of opioids, more experienced abusers often progress to snorting [6,7]. Manipulation of opioid formulations (i.e., tablets) for IN administration requires the tablets to be reduced to particle sizes that are capable of being insufflated. Particle size affects the rate of absorption and maximum plasma concentration that can be achieved after dosing (in part because of limited retention and solubilization of drug needed to allow nasal absorption) [8,9], the severity of nasal irritation produced by insufflation, and the ease of administration and subsequently the attractiveness of a formulation for abuse by the IN route [10]. For example, a study comparing the manipulation of an oxymorphone formulation designed to be crush resistant with that of oxymorphone ER tablets estimated that particles 285.9 µM or less were optimal for insufflation [11]. Particle sizes well below 500 µM are believed to be suitable for insufflation [12]. However, insufflation of smaller particles that are suitable to IN administration does not assure that the insufflated material will be absorbed. Furthermore, a large fraction may be transported to the pharynx and swallowed, especially the larger particle sizes that take longer to solubilize. This is important because, after oral administration, morphine (and other drugs), undergo first-pass metabolism in the liver, which results in a reduction of morphine blood levels. In contrast, drug absorbed into the blood stream via the intranasal route avoids first-pass metabolism by the liver. As a result, the same amount of morphine is effectively more potent when absorbed intranasally than when taken orally.
Morphine abuse-deterrent, extended-release injection-molded tablets (morphine-ADER-IMT) are a unique AD, ER morphine product candidate incorporating a proprietary technology (Guardian, Egalet Corporation, Wayne, PA, USA) that is a polymer matrix tablet with a novel manufacturing process, plastic injection molding. This technology results in ER tablets with physical and chemical features that resist both common and more rigorous methods of manipulation, which limits particle size reduction and extraction of the active pharmaceutical ingredient (API) [13]. Using a recently developed instrument, Assessing Labor, Effort and Resources Required for Tampering (ALERRT; PinneyAssociates, Bethesda, MD, USA), morphine-ADER-IMT was shown to be extremely difficult to manipulate [14]. Morphine-ADER-IMT tablets are extremely hard and, despite a high level of effort with a variety of tools and multistep manipulation procedures used in attempts to reduce particle size, no pulverized material was produced after manipulation [14].
This study compared the relative human abuse potential of morphine-ADER-IMT with that of a currently marketed formulation of morphine sulfate extended-release tablets (morphine ER; MS Contin, Purdue Pharma LP, Stamford, CT, USA) and placebo after manipulation and insufflation.
Methods
Study Design and Participants
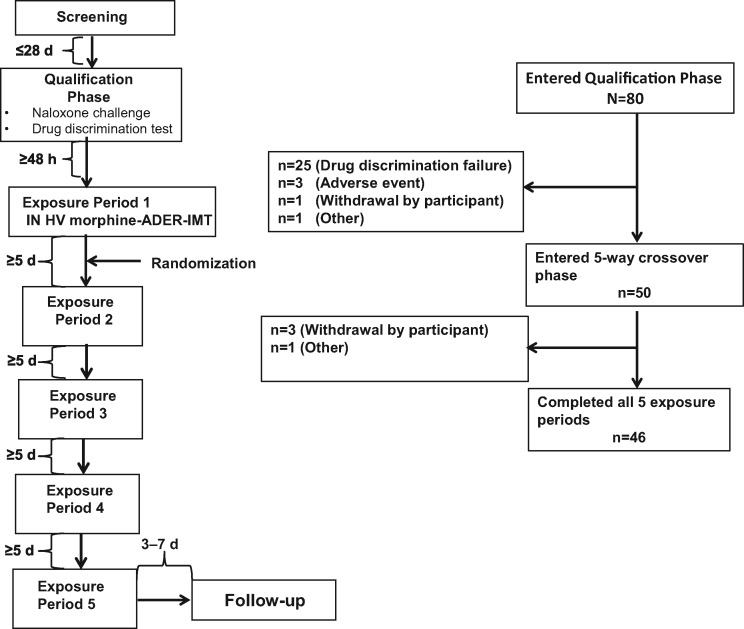
The study was a single-center (United States), randomized, double-blind, double-dummy, active- and placebo-controlled five-way crossover study (Figure 1). Adult volunteers age 18 to 55 years who were experienced, nondependent, recreational opioid users with previous experience of IN opioid administration (≥3 occasions within the year before screening) were eligible for inclusion in the study. A recreational user was defined as a user with a history of nonmedical use of opioids, with 10 or more occasions within the past year and one or more in the 12 weeks before screening. The site attempted to include more female and nonwhite study participants, but most of the participants who volunteered were white males. Key exclusion criteria included a history of substance and/or alcohol dependence (excluding caffeine and nicotine), as assessed using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria: any condition in which an opioid is contraindicated (e.g., significant respiratory depression, acute or severe bronchial asthma or hypercarbia, suspected paralytic ileus) and a history of sleep apnea in the past five years that has not resolved or been corrected.
Figure 1.
Study design and participant disposition. HV = high-volume; IN = intranasal.
Study Phases and Drug Exposures
Participants meeting the inclusion and exclusion criteria during the screening phase entered a qualification phase that consisted of a naloxone challenge test to exclude opioid-dependent participants and a drug discrimination test to exclude participants who could not tolerate 30 mg IR morphine (manipulated) or distinguish its positive subjective effects from placebo when administered intranasally. In the naloxone challenge test, participants received an initial IV bolus of naloxone 0.2 mg followed by an additional IV bolus of 0.6 mg if no signs of opioid withdrawal were observed within the first 30 seconds after naloxone administration. For the drug discrimination test, participants received IN placebo or morphine (30 mg IR, manipulated) in a randomized, double-blinded, double-dummy manner separated by a washout period of approximately 24 hours.
The study included the following five drug exposure groups: IN high-volume morphine-ADER-IMT (60 mg manipulated tablet), IN low-volume morphine-ADER-IMT (60 mg manipulated tablet), IN low-volume morphine ER (60 mg manipulated tablet), oral morphine-ADER-IMT (60 mg intact tablet), and placebo.
Study Drug Preparation
Significant time and effort were needed to prepare morphine-ADER-IMT for insufflation because of the hardness of the tablets and the difficulty in achieving particle size reduction. Preparation of IN high-volume morphine-ADER-IMT required a two-step manipulation process involving mechanical manipulation with a household tool, followed by the use of an electrical instrument for several minutes in a further attempt to reduce particle size. Preparation of IN low-volume morphine-ADER-IMT required a three-step process involving mechanical manipulation with a household tool, followed by the use of an electrical instrument to further attempt to reduce particle size for several minutes. The outcome of the two-step manipulation used to prepare IN high-volume morphine-ADER-IMT is shown in a video file in the Supplementary Data. After completion of the manipulation with the electrical instrument, the resultant material was passed through a 1,000 µM sieve to obtain smaller particle sizes amenable for insufflation. A 500 µM sieve was initially used to isolate particle sizes amenable to insufflation but the yield was extremely low. Preparation of IN morphine ER required only a one-step process of grinding the tablet into a powder using a mortar and pestle (Supplementary Data). Preparation of the placebo matched that of the corresponding active drug treatment using a double-dummy design.
At the beginning of the drug exposure phase, all participants received IN high-volume morphine-ADER-IMT during the first exposure period. The study was begun with insufflation of high-volume morphine-ADER-IMT to prevent sequence effects and because the particle size and powder volume were substantially larger than those of the other arms. Although this arm of the study was not blinded to study personnel (but was blinded to the participants), blinding for the other arms of the study could be more easily maintained without having to perform treatment placebo bulking and other complicated maneuvers. Following insufflation of high-volume morphine-ADER-IMT, participants were then randomized in a 1:1:1:1 ratio to the remaining low-volume exposure groups, with each participant receiving all study drugs in crossover fashion and each exposure period separated by a five-day washout period. Participants were instructed to complete insufflation within five minutes, with an additional 10 minutes allowed if needed. All participants completed insufflation of the manipulated products within the 15-minute time frame. Participants were confined to the clinic from the time of dosing until completion of the 24-hour postdose assessment during the drug exposure phase. Participants returned to the clinic for a follow-up visit seven to 14 days after the final exposure period for physical and laboratory examinations and to report any adverse events (AEs).
Assessments
Pharmacodynamic Endpoints
The primary pharmacodynamic (PD) endpoint was the peak effect (Emax) of drug liking using the 0 to 100 bipolar drug liking visual analog scale (VAS; 0 = strong disliking, 50 = neither like nor dislike, 100 = strong liking). Secondary PD endpoints included the Emax of overall drug liking using the 0 to 100 bipolar overall drug liking VAS (0 = strong disliking, 50 = neither like nor dislike, 100 = strong liking); the Emax of the take drug again VAS using the 0 to 100 bipolar take drug again VAS (0 = definitely would not, 50 = do not care, 100 = definitely would); response to the drug effects questionnaire (DEQ) using a 0 to 100 unipolar VAS (0 = not at all, 100 = extremely) for each statement; Emax and time to Emax for changes in pupil diameter as measured with a NeurOptics VIP-200 Pupillometer (NeurOptics, Irvine, CA, USA); ease of snorting assessment using a 0 to 100 bipolar VAS (0 = very difficult, 50 = neither easy nor difficult, 100 = very easy); pleasantness of snorting assessment using a 0 to 100 bipolar VAS (0 = very unpleasant, 50 = neither pleasant nor unpleasant, 100 = very pleasant); and nasal effects assessment: responses to six questions concerning nasal sensations using a four-point rating scale (0 = none, 1 = mild, 2 = moderate, 3 = severe). Most PD endpoints were measured predose, at 15, 30, and 45 minutes postdose, and at 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 hours postdose. The overall drug liking and take drug again assessments were evaluated at 12 and 24 hours postdose. The ease of snorting and pleasantness of snorting assessments were measured within five minutes of completing insufflation, and the nasal effects assessment was performed predose, at 5, 15, 30, and 45 minutes postdose, and at 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 hours postdose.
Pharmacokinetic Endpoints
Blood samples were collected predose, at 15, 30, and 45 minutes postdose, and at 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 12, and 24 hours postdose. Multiple pharmacokinetic (PK) parameters were calculated for plasma morphine, including maximum plasma concentration (Cmax), time to Cmax (tmax), area under the plasma concentration vs time curve extrapolated to infinity (AUC0–∞), apparent first-order terminal elimination half-life (t1/2), and abuse quotient (AQ; Cmax/tmax). The AQ reflects the rate and extent of the increase in plasma morphine concentration from dosing to tmax and is a PK parameter that has been associated with drug liking and abuse potential [4,15].
Safety
Adverse events were assessed in the qualification phase, in the exposure phases, and at the follow-up visit for all participants who received one or more doses of study drug during the exposure phase (safety population). AEs were considered treatment emergent if they occurred after the first dosing in the drug discrimination test and from the drug exposure phase through the follow-up visit.
Statistical Analyses
PD (completer population) and PK (PK population) outcomes were assessed for participants who received all five drug exposures. PD and PK outcomes were analyzed using a linear mixed-effects model with fixed effects for sequence, period, and treatment, and random effect for participant nested in sequence. PD outcomes were summarized using descriptive statistics (mean, SD, median).
Results
Participants
Forty-six of 80 participants (57.5%) who entered the qualification phase of the study completed all five drug exposure arms (Figure 1). The primary reason for discontinuation during the qualification phase was failure of the drug discrimination test. Four participants discontinued during the drug exposure phase, primarily because participants chose to withdraw from the study (3/4, 75.0%). Baseline demographics were similar among participants randomized to each of the study treatment sequences. Of the 46 participants who completed all exposure periods, the majority were white (96%) and male (78%), with a mean age of 28.1 years (SD = 8.1 years), weight of 162 lbs (SD = 26 lbs), and BMI of 24.0 kg/m2 (SD = 2.9 kg/m2) (Table 1).
Table 1.
Demographics and characteristics of the completer population
| Treatment sequence |
|||||
|---|---|---|---|---|---|
| ABCED | ACDBE | ADECB | AEBDC | Overall | |
| (N = 13) | (N = 12) | (N = 10) | (N = 11) | (N = 46) | |
| Male, N (%) | 9 (69.2) | 9 (75.0) | 9 (90.0) | 9 (81.8) | 36 (78.3) |
| Female, N (%) | 4 (30.8) | 3 (25.0) | 1 (10.0) | 2 (18.2) | 10 (21.7) |
| Race, N (%) | |||||
| White | 13 (100) | 10 (83.3) | 10 (100) | 11 (100) | 44 (95.7) |
| Black | 0 | 2 (16.7) | 0 | 0 | 2 (4.3) |
| Age, y, mean (SD) | 28.9 (9.6) | 30.3 (10.7) | 27.1 (4.9) | 25.6 (5.1) | 28.1 (8.1) |
| Weight, lb, mean (SD) | 164.5 (28.4) | 161.3 (17.4) | 160.9 (21.0) | 160.0 (36.4) | 161.8 (26.0) |
| Body mass index, kg/m2, mean (SD) | 24.7 (3.0) | 24.8 (2.5) | 23.2 (2.8) | 23.1 (3.3) | 24.0 (2.9) |
A = intranasal (IN) high-volume (HV) morphine-abuse-deterrent, extended-release injection-molded (ADER-IMT) tablets (60 mg manipulated tablet); B = IN low-volume (LV) morphine-ADER-IMT (60 mg manipulated/sieved tablet); C = IN morphine ER (60 mg manipulated tablet); D = oral morphine-ADER-IMT (60 mg; intact); E = placebo.
Pharmacodynamic Endpoints
Drug Liking
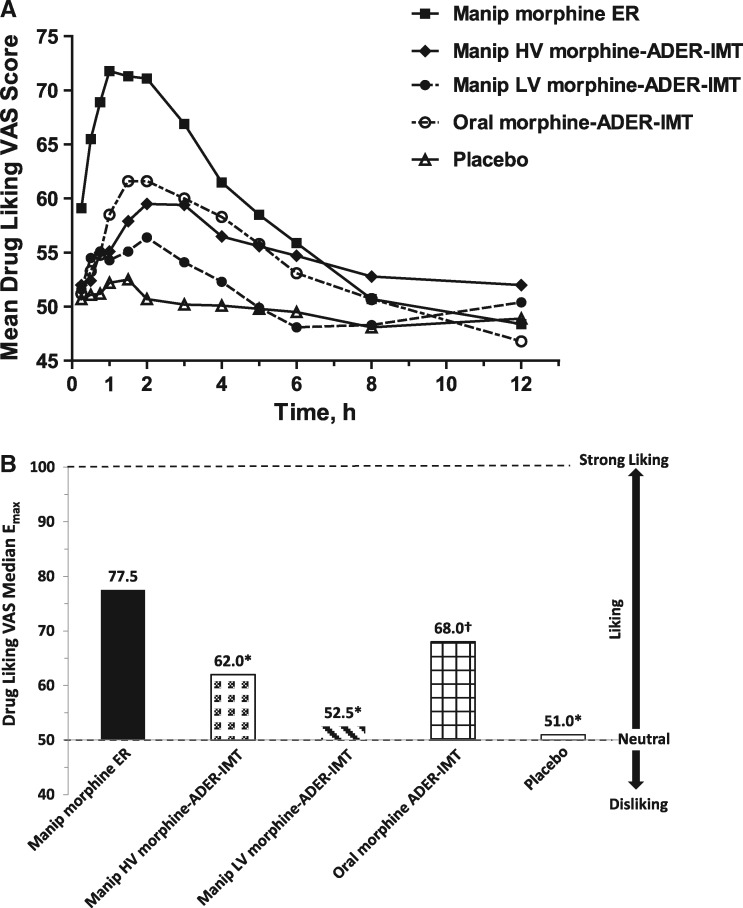
The time course for drug liking VAS scores is shown in Figure 2A. Mean drug liking VAS scores were lower after insufflation of manipulated morphine-ADER-IMT (both high and low volume) compared with IN morphine ER from 15 minutes to six hours after drug exposure. Peak drug liking (Emax) values were significantly lower after insufflation of high-volume morphine-ADER-IMT (62.0, P < 0.0001), low-volume morphine-ADER-IMT (52.5, P < 0.0001), and ingestion of oral morphine-ADER-IMT (68.0, P = 0.0001) compared with IN morphine ER (77.5) (Figure 2B).
Figure 2.
Drug liking VAS scores. (A) Mean VAS scores for drug liking over time. (B) Peak drug liking (Emax) for drug liking VAS scores. *P < 0.0001, relative to manipulated morphine ER. †P = 0.0001, relative to manipulated morphine ER. ADER-IMT = abuse-deterrent, extended-release injection-molded tablets; Emax = peak effect; ER = extended-release; HV = high-volume; LV = low-volume; manip = manipulated; VAS = visual analog scale.
Overall Drug Liking and Take Drug Again
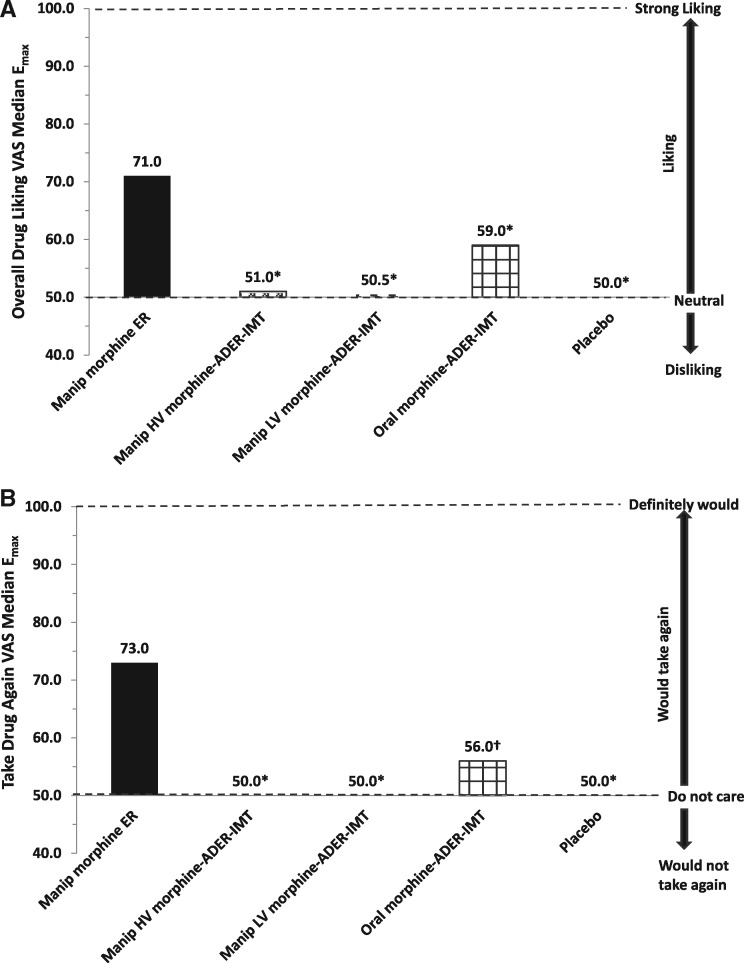
Median Emax values on the overall drug liking VAS were significantly lower following insufflation of high-volume morphine-ADER-IMT (51.0, P < 0.0001), low-volume morphine-ADER-IMT (50.5, P < 0.0001), and ingestion of oral morphine-ADER-IMT (59.0, P < 0.0001) compared with IN morphine ER (71.0) (Figure 3A). Median Emax values on the take drug again VAS were significantly lower following insufflation of high-volume morphine-ADER-IMT (50.0, P < 0.0001), low-volume morphine-ADER-IMT (50.0, P < 0.0001), and ingestion of oral morphine-ADER-IMT (56.0, P = 0.0003) compared with IN morphine ER (73.0) (Figure 3B).
Figure 3.
Overall drug liking and take drug again VAS scores. (A) Emax for overall drug liking VAS. (B) Emax for take drug again VAS. *P < 0.0001, relative to manipulated morphine ER. †P = 0.0003, relative to manipulated morphine ER. ADER-IMT = abuse-deterrent, extended-release injection-molded tablets; Emax = peak effect; ER = extended-release; HV = high-volume; LV = low-volume; manip = manipulated; VAS = visual analog scale.
Drug Effects
Median Emax values on the DEQ “I can feel a drug effect,” “I can feel good drug effects,” and “I am feeling high” statements were all significantly lower (P < 0.0001) after insufflation of high-volume morphine-ADER-IMT, low-volume morphine-ADER-IMT, and ingestion of oral morphine-ADER-IMT compared with IN morphine ER (Table 2).
Table 2.
Responses to drug effects questionnaire*
| Median drug effects questionnaire VAS score, Emax | Placebo (N = 46) | Manipulated morphine ER (N = 46) | Manipulated HV morphine-ADER-IMT (N = 46) | P values† | Manipulated LV morphine-ADER-IMT (N = 46) | P values† | Oral morphine-ADER-IMT (N = 46) | P values† |
|---|---|---|---|---|---|---|---|---|
| I can feel a drug effect | 1.0 | 64.0 | 23.5 | <0.0001 | 6.5 | <0.0001 | 38.0 | <0.0001 |
| I can feel good drug effects | 0 | 62.0 | 17.0 | <0.0001 | 4.5 | <0.0001 | 32.5 | <0.0001 |
| I can feel bad drug effects | 0 | 7.5 | 1.0 | 0.0017 | 0 | 0.0001 | 3.0 | 0.0092 |
| I am feeling high | 0 | 65.5 | 20.0 | <0.0001 | 5.0 | <0.0001 | 34.0 | <0.0001 |
| I am feeling sick | 0 | 1.0 | 0.5 | 0.018 | 0 | <0.0001 | 0 | NS |
| I am feeling nauseous | 0 | 1.0 | 0 | 0.0001 | 0 | <0.0001 | 0 | NS |
| I am feeling sleepy | 0 | 41.0 | 10.0 | <0.0001 | 1.0 | <0.0001 | 26.5 | NS |
| I am feeling dizzy | 0 | 9.0 | 0 | <0.0001 | 0 | <0.0001 | 0 | 0.0021 |
ADER-IMT = abuse-deterrent, extended-release injection-molded tablets; Emax = peak effect; ER = extended-release; HV = high-volume; IN = intranasal; LV = low-volume; NS = not significant; VAS = visual analog scale.
0–100 unipolar VAS (0 = not at all; 100 = extremely) for each statement.
Relative to IN manipulated morphine ER.
Pupillary Miosis
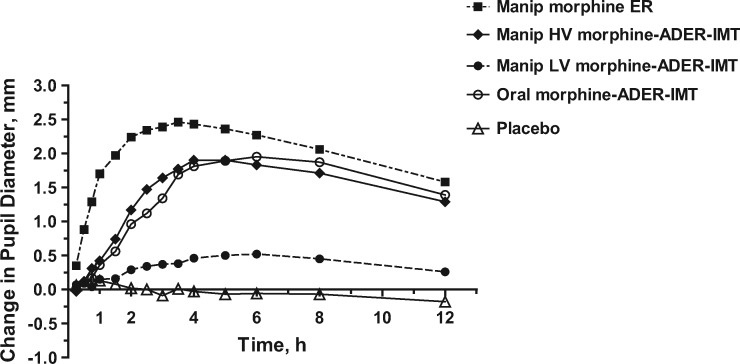
Peak pupillary miosis was observed from two to six hours after insufflation of morphine ER and approximately four to six hours after insufflation of high-volume morphine-ADER-IMT (Figure 4). Peak changes in pupillary diameter after insufflation of high-volume morphine-ADER-IMT were approximately 80% of those measured after insufflation of morphine ER, whereas low levels of pupillary miosis were observed after insufflation of low-volume morphine-ADER-IMT.
Figure 4.
Mean change in pupil diameter over time. ADER-IMT = abuse-deterrent, extended-release injection-molded tablets; ER = extended-release; HV = high-volume; LV = low-volume; manip = manipulated.
Nasal Effects
Insufflation of high-volume morphine-ADER-IMT was “difficult” (median rating of 9.5) and “unpleasant to snort” (median rating of 32.0) (Supplementary Data, Figure 1, A and B). In contrast, insufflation of morphine ER (81.5) and low-volume morphine-ADER-IMT (77.0) were “easy” to snort and were rated as “neither pleasant nor unpleasant to snort” with rating scores of 59.0 for morphine ER and 50.5 for low-volume morphine-ADER-IMT. The difficulty and unpleasantness of snorting high-volume morphine-ADER-IMT were reflected in the following comments by some study participants: “They’re like giant crystals”; “Did you guys crush this up or did you forget that part?”; “Well if the point is to make it where you can’t snort it, you guys did a good job.” Additional participant comments are available online in the audio Supplementary Data. Median Emax ratings on the nasal effects assessment were higher for all statements except “rate nasal burning at this moment” after insufflation of high-volume morphine-ADER-IMT compared with insufflation of low-volume morphine-ADER-IMT and morphine ER, with higher values indicative of greater unpleasantness of nasal sensations (data not shown). Five minutes after insufflation, median Emax ratings for “need to blow your nose at this moment” and “rate any nasal congestion at this moment” were assessed as moderate (median = 2.0) after insufflation of high-volume morphine-ADER-IMT (data not shown). In contrast, Emax ratings after insufflation of morphine ER and low-volume morphine-ADER-IMT were reported as mild (median = 1.0).
Pharmacokinetic Endpoints
Time Course
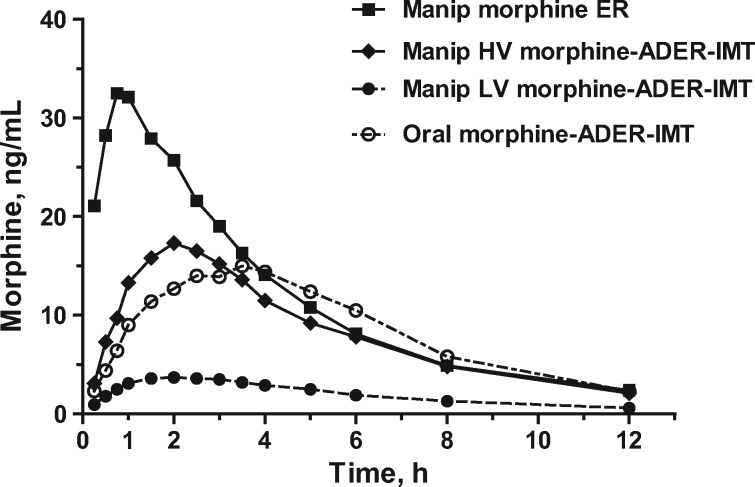
Peak morphine levels occurred between one-half to one hour after insufflation of morphine ER and were approximately double those measured following insufflation of high-volume morphine-ADER-IMT or ingestion of oral morphine-ADER-IMT and approximately 10 times higher than morphine levels after insufflation of low-volume morphine-ADER-IMT (Figure 5). Peak morphine levels were observed approximately two hours and approximately three and a half to four hours after insufflation of high-volume morphine-ADER-IMT and ingestion of oral morphine-ADER-IMT, respectively. Morphine levels remained low throughout the time course following insufflation of low-volume morphine-ADER-IMT.
Figure 5.
Mean morphine concentrations over time. ADER-IMT = abuse-deterrent, extended-release injection-molded tablets; ER = extended-release; HV = high-volume; LV = low-volume; manip = manipulated.
Pharmacokinetic Parameters
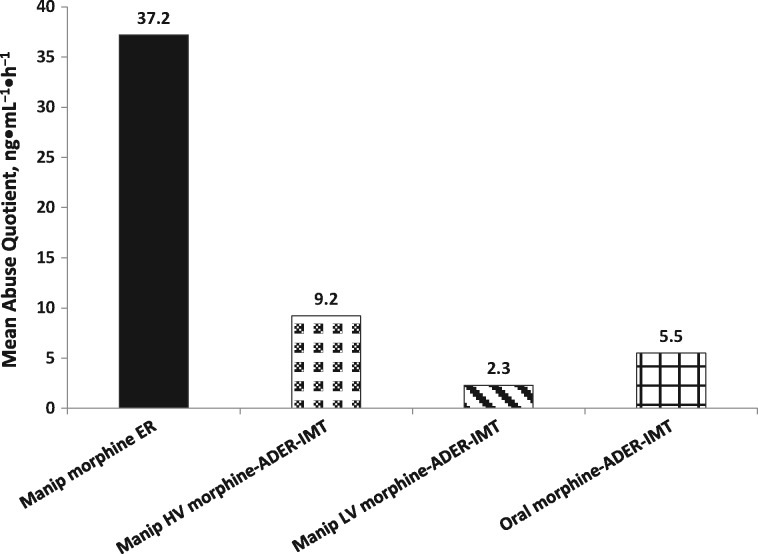
Morphine Cmax and AUC0–∞ values were highest and tmax values shortest after insufflation of morphine ER compared with all morphine-ADER-IMT exposures (Table 3). The mean AQ was approximately four to 16 times higher for IN morphine ER (37.2) compared with IN high-volume morphine-ADER-IMT (9.2), IN low-volume morphine-ADER-IMT (2.3), and oral morphine-ADER-IMT (5.5) (Figure 6).
Table 3.
Morphine pharmacokinetic parameters
| Parameter, mean (SD) | Manipulated morphine ER | Manipulated HV morphine-ADER-IMT | Manipulated LV morphine-ADER-IMT | Oral morphine-ADER-IMT |
|---|---|---|---|---|
| N = 37 | N = 45 | N = 46 | N = 39 | |
| Cmax, ng/mL | 36.3 (12.9) | 19.0 (9.6) | 4.5 (2.3) | 17.2 (4.3) |
| N = 37 | N = 45 | N = 46 | N = 39 | |
| tmax, h | 1.3 (0.6) | 2.4 (0.8) | 2.7 (1.3) | 3.5 (1.1) |
| N = 32 | N = 43 | N = 30 | N = 28 | |
| AUC0–∞, h•ng/mL | 181.6 (49.7) | 125.2 (63.6) | 29.7 (11.1) | 149.0 (25.5) |
| N = 17 | N = 41 | N = 29 | N = 6 | |
| t1/2, h | 7.5 (3.1) | 5.8 (2.3) | 3.0 (1.2) | 7.2 (1.0) |
| Abuse quotient, Cmax/tmax | 37.2 (23.3) | 9.2 (6.1) | 2.3 (2.4) | 5.5 (2.6) |
ADER-IMT = abuse-deterrent, extended-release injection-molded tablets; AUC0–∞ = area under the plasma concentration vs time curve extrapolated to infinity; Cmax = maximum plasma concentration; ER = extended-release; HV = high-volume; LV = low-volume; t1/2 = apparent first-order terminal elimination half-life; tmax = time to Cmax.
Figure 6.
Mean abuse quotient (Cmax/tmax). ADER-IMT = abuse-deterrent, extended-release injection-molded tablets; Cmax = maximum plasma concentration; ER = extended-release; HV = high-volume; LV = low-volume; manip = manipulated; tmax = time to Cmax.
Safety
Treatment-emergent AEs that were considered related to study drug were similar among all active drug exposures and were typical of morphine analgesics (e.g., gastrointestinal symptoms, pruritus) (Table 4), except for nasal events. No serious AEs occurred in this study.
Table 4.
Most frequent* adverse events related to study drug (safety population)
| Placebo | Manipulated morphine ER | Manipulated HV morphine-ADER-IMT | Manipulated LV morphine-ADER-IMT | Oral morphine-ADER-IMT | |
|---|---|---|---|---|---|
| Preferred term,† N (%) | (N = 48) | (N = 47) | (N = 50) | (N = 47) | (N = 49) |
| Participants with ≥1 | 5 (10.4) | 28 (59.6) | 15 (30.0) | 15 (31.9) | 20 (40.8) |
| TEAE | |||||
| Nausea | 0 | 9 (19.2) | 5 (10.0) | 1 (2.1) | 8 (16.3) |
| Vomiting | 0 | 7 (14.9) | 1 (2.0) | 0 | 7 (14.3) |
| Pruritus generalized | 0 | 6 (12.8) | 4 (8.0) | 0 | 2 (4.1) |
| Pruritus | 0 | 5 (10.6) | 0 | 0 | 2 (4.1) |
| Headache | 0 | 3 (6.4) | 5 (10.0) | 1 (2.1) | 6 (12.2) |
ADER-IMT = abuse-deterrent, extended-release injection-molded tablets; ER = extended-release; HV = high-volume; LV = low-volume; TEAE = treatment-emergent adverse event.
Incidence ≥10% overall.
Medical Dictionary for Regulatory Activities, version 17.0.
Discussion
The key findings of this study demonstrate a lower abuse potential for morphine-ADER-IMT compared with morphine ER when manipulated and insufflated under the conditions employed in this study. First, it took more time, effort, and tools to prepare morphine-ADER-IMT for nasal administration compared with pulverizing morphine ER by a one-step process with a mortar and pestle. Even with the greater levels of effort needed for manipulation of morphine-ADER-IMT, peak drug liking was significantly lower after insufflation of low- or high-volume morphine-ADER-IMT compared with IN morphine ER. Additionally, median Emax values on the overall drug liking VAS and the take drug again VAS were significantly lower after insufflation of low- or high-volume morphine-ADER-IMT compared with IN morphine ER and were similar to placebo. Finally, the mean AQ was much lower after insufflation of low- or high-volume morphine-ADER-IMT compared with IN morphine ER. Based on the mean AQ, the predicted abuse potential for IN morphine ER is greater than that for IN morphine-ADER-IMT, and this is consistent with the responses for the PD endpoints of drug liking.
The magnitude of the differences in the Emax values for drug liking between IN morphine-ADER-IMT and IN morphine ER reflects a clinically meaningful difference in IN abuse potential between morphine-ADER-IMT and the comparator in this study. The differences in median Emax values for drug liking between IN low- or high-volume morphine-ADER-IMT treatments and IN morphine ER ranged from 15.5 (high-volume morphine-ADER-IMT) to 25.0 (low-volume morphine-ADER-IMT) on the drug liking VAS. These differences are well above the range (8 to 10 mm difference) believed to be clinically important in abuse potential studies [16].
The inability to reduce the particle size of morphine-ADER-IMT may negatively impact the likelihood of IN administration of morphine-ADER-IMT by opioid users and make it a less attractive opioid for misuse and abuse. For individuals seeking to misuse or abuse morphine-ADER-IMT, the inability to reduce the particle size creates a tradeoff between the difficulty in particle size reduction and snorting large particles that are unpleasant vs filtering to get smaller particle sizes but losing much of the yield of the API, as evidenced by the PK data. The mean Cmax value after IN treatment with high-volume morphine-ADER-IMT was 4.2 times higher than the value following IN treatment with low-volume morphine-ADER-IMT. Among the 17 items in the Opioid Attractiveness Scale, which measures the attractiveness of opioid formulations to potential abusers, are the “ability to change into another form for recreational use,” “solubility in water, vinegar, alcohol, etc,” and “ability to use in different ways (snort, smoke, eat, IV, etc) to get different highs” [17]. Thus, given the hardness of the tablets and the high level of effort needed to reduce the particle size of morphine-ADER-IMT, it is likely to have low attractiveness to individuals who intentionally abuse prescription opioids because particle size reduction is the first step toward manipulation of an opioid product for alternative routes of abuse (eg, IN, intravenous).
Study Strengths and Limitations
A strength of this study was that the manipulations of the test drugs were rigorous, based on the findings from the Category 1 AD studies for morphine-ADER-IMT designed to identify optimized methods of manipulation, and are consistent with real-world attempts of manipulation with several types of tools and procedures commonly used by opioid abusers. In addition, the study population is the recognized and accepted population for assessing human abuse potential, and the 60 mg dose that was used is commonly abused and known to produce strong drug liking if absorbed. It is also the highest proposed dosage strength for morphine-ADER-IMT. A limitation of this study is that the study population consisted of mostly males. Another possible limitation is potential bias of having all participants exposed to high-volume morphine-ADER-IMT first and prior to randomization. This was necessary because the high volume of material made it impractical for this treatment arm to be blinded. Finally, although a number of tools and methodologies were used for manipulation, the real-world impact of morphine-ADER-IMT on ER morphine abuse is unknown. In this regard, the negative statements from some of the participants in the study, who are experienced in the misuse of opioids, may at least partly reflect how the abuser community would view a product like morphine-ADER-IMT.
In conclusion, more effort was required to physically manipulate morphine-ADER-IMT compared with morphine ER to prepare for insufflation. After manipulation, participants who snorted high-volume morphine-ADER-IMT (which included all particle sizes) and low-volume morphine-ADER-IMT (which was the result of sieving, leaving only small particle sizes that are easier to insufflate) reported significantly reduced maximum drug liking, overall drug liking, and take drug again scores compared with scores after snorting manipulated morphine ER. Overall drug liking and take drug again scores for both IN high- and low-volume morphine-ADER-IMT were similar to placebo. The results of this study demonstrate that morphine-ADER-IMT would be an important new AD, ER morphine product with lower potential for IN abuse than currently available non-AD, ER morphine products.
Supplementary Material
Acknowledgments
The authors would like to thank Patrick Little, PhD, of Complete Publication Solutions, a CHC Group Company (North Wales, PA, USA), for providing medical writing support, which was funded by Egalet Corporation (Wayne, PA, USA).
Supplementary Data
Supplementary Data may be found online at http://painmedicine.oxfordjournals.org.
References
- 1. ER/LA Opioid Analgesics REMS. http://www.er-la-opioidrems.com/IwgUI/rems/home.action. Published 2015. Accessed August 2015).
- 2. Butler SF, Cassidy TA, Chilcoat H, et al. Abuse rates and routes of administration of reformulated extended-release oxycodone: Initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment. J Pain 2013;14: 351–8. [DOI] [PubMed] [Google Scholar]
- 3. Bannwarth B. Will abuse-deterrent formulations of opioid analgesics be successful in achieving their purpose? Drugs 2012;72:1713–23. [DOI] [PubMed] [Google Scholar]
- 4. Perrino PJ, Colucci SV, Apseloff G, Harris SC.. Pharmacokinetics, tolerability, and safety of intranasal administration of reformulated OxyContin tablets compared with original OxyContin tablets in healthy adults. Clin Drug Investig 2013;33:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butler SF, Budman SH, Licari A, et al. National addictions vigilance intervention and prevention program (NAVIPPRO): A real-time, product-specific, public health surveillance system for monitoring prescription drug abuse. Pharmacoepidemiol Drug Saf 2008;17:1142–54. [DOI] [PubMed] [Google Scholar]
- 6. Hays LR. A profile of OxyContin addiction. J Addict Dis 2004;23:1–9. [DOI] [PubMed] [Google Scholar]
- 7. Katz N, Dart RC, Bailey E, et al. Tampering with prescription opioids: Nature and extent of the problem, health consequences, and solutions. Am J Drug Alcohol Abuse 2011;37:205–17. [DOI] [PubMed] [Google Scholar]
- 8. Ozsoy Y, Gungor S, Cevher E.. Nasal delivery of high molecular weight drugs. Molecules 2009;14: 3754–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lindhardt K, Gizurarson S, Stefansson SB, Olafsson DR, Bechgaard E.. Electroencephalographic effects and serum concentrations after intranasal and intravenous administration of diazepam to healthy volunteers. Br J Clin Pharmacol 2001;52:521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris SC, Perrino PJ, Smith I, et al. Abuse potential, pharmacokinetics, pharmacodynamics, and safety of intranasally administered crushed oxycodone HCl abuse-deterrent controlled-release tablets in recreational opioid users. J Clin Pharmacol 2014;54:468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vosburg SK, Jones JD, Manubay JM, et al. Assessment of a formulation designed to be crush-resistant in prescription opioid abusers. Drug Alcohol Depend 2012;126:206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bartholomaus J, Schwier S, Brett M, et al. New abuse deterrent formulation (ADF) technology for immediate-release opioids. Drug Dev Deliv 2013;13: 76–81. [Google Scholar]
- 13. Cone EJ, Buchhalter AR, Lindhardt K, Elhauge T, Skak N. Crushing and extraction resistance of EG-001, an abuse-deterrent ER morphine in clinical development [abstract 32]. Presented at PAINWeek; 2014; Las Vegas, NV.
- 14. Cone EJ, Buchhalter AR, Lindhardt K, Elhauge T, Dayno JM.. Extreme work requirement of EG-001, an abuse-deterrent extended-release morphine product, as demonstrated with the ALERRT visual analog scales. Postgrad Med 2015;127:S26. [Google Scholar]
- 15. Webster LR, Bath B, Medve RA.. Opioid formulations in development designed to curtail abuse: Who is the target? Expert Opin Investig Drugs 2009;18: 255–63. [DOI] [PubMed] [Google Scholar]
- 16. Eaton TA, Comer SD, Revicki DA, et al. Determining the clinically important difference in visual analog scale scores in abuse liability studies evaluating novel opioid formulations. Qual Life Res 2012;21: 975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Butler SF, Benoit C, Budman SH, et al. Development and validation of an opioid attractiveness scale: A novel measure of the attractiveness of opioid products to potential abusers. Harm Reduct J 2006;3:5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.