Initiation of antiretroviral therapy early after HIV infection disproportionately decreases HIV antibodies in CSF compared with blood, suggesting that early treatment may reduce the magnitude of the central nervous system HIV reservoir to a greater extent than the systemic reservoir.
Keywords: antibodies, anti-retroviral therapy, cerebrospinal fluid, central nervous system, early infection, HIV-1, persistence, serology
Abstract
Background
Despite effective antiretroviral therapy (ART), human immunodeficiency virus (HIV) likely persists in the central nervous system (CNS) in treated individuals. We examined anti-HIV antibodies in cerebrospinal fluid (CSF) and blood as markers of persistence.
Methods
Human immunodeficiency virus antibodies were measured in paired CSF and serum before and after long-term treatment of chronic (n = 10) and early infection (n = 12), along with untreated early infection (n = 10).
Results
Treatment of chronic infection resulted in small reductions of anti-HIV antibodies in CSF and serum despite >10 years of suppressive ART. In untreated early infection, anti-HIV antibodies emerged in blood by day 30, whereas CSF antibodies reached similar levels 2 weeks later. Compared with long-term treatment of chronic infection, early ART initiation reduced CSF antibodies by 43-fold (P > .0001) and blood antibodies by 7-fold (P = .0003). Two individuals receiving pre-exposure prophylaxis and then ART early after infection failed to develop antibodies in CSF or blood, whereas CSF antibodies were markedly reduced in the Berlin patient.
Conclusions
To the extent that differential CSF and blood antibodies indicate HIV persistence, these data suggest a relative delay in establishment of the CNS compared with the systemic HIV reservoir that provides an opportunity for early treatment to have a greater impact on the magnitude of long-term CNS infection.
(See the Editorial Commentary by Clifford, on pages 1017–9.)
Treatment with antiretroviral therapy (ART) is highly effective in suppressing human immunodeficiency virus (HIV) replication, not only systemically but in the central nervous system (CNS), resulting in HIV ribonucleic acid (RNA) levels in blood and cerebrospinal fluid (CSF) below clinical detection [1]. This suppression of HIV replication is associated with a marked reduction in mortality and morbidity, including those related to CNS injury [2]. However, ART fails to eliminate HIV, and when treatment is interrupted, active replication and viremia resume and are followed by a rise in CSF HIV RNA as well [3]. In addition, even well treated persons living with HIV frequently exhibit elevated levels of immune activation and inflammation systemically [4–7] and in the CNS [8]. Increasing evidence suggests that persistent HIV infection may contribute to immune activation [9] and consequently to a spectrum of “nonacquired immune deficiency syndrome” systemic morbidity and perhaps continued CNS injury detected in neuropsychological test impairment [10].
Most studies of the HIV reservoir have focused on cells of the lymphatic system, particularly memory T cells that maintain the principal systemic reservoir [11]. Various methods provide direct or partial measurements of the systemic lymphocyte reservoir. By contrast, the CNS reservoir is much more difficult to assess. The cells supporting this reservoir are less clear, with tissue myeloid cells being a major candidate, although astrocytes and CNS-resident memory CD4+ T cells may be a source [12]. The deep and inaccessible location of these cells in the CNS presents a formidable technical obstacle to measurement, as well as for evaluating cure strategies [13].
Although it is well accepted that the quantity and avidity of antibodies produced against a foreign agent are antigen-dependent [14], very little is known about antibody dynamics in relation to the level of antigen produced during acute and chronic infection or after treatment. In primary HIV infection, anti-HIV antibodies first appear 3 weeks after detection of viremia and p24 antigen expression and continue to rise relatively linearly over the next year [15]. Detailed quantitative studies of serum anti-HIV antibody profiles against a panel of defined viral antigens using the luciferase immunoprecipitation systems technology have detected novel profiles in elite HIV controllers [16], HIV-treated patients [17], and HIV-infected subjects with multiple reservoir measurements [18]. Particularly encouraging was the finding that the Berlin patient, an HIV-infected subject who received a stem cell transplant resulting in a functional cure [19, 20], showed a serum antibody profile approaching the uninfected controls with low antibodies against HIV p24, integrase, and reverse transcriptase [17]. Studies with a detuned enzyme-linked immunosorbent assay demonstrated that HIV antibodies decreased in treated patients and correlated with a lower level of viral replication [21]. Despite direct evidence that anti-HIV antibodies are proportional to the dynamic level of HIV antigens, antibody profiles may reflect the relative level of continued antigen expression and present a broad, albeit imprecise, method for assessing the HIV reservoir and treatment outcomes [16–18]. Antibody measurements might also be useful for assessing the CNS reservoir, because CSF antibodies may be dissociated from those of blood and require persistent local antigenic stimulation for maintenance [22].
In this study, we hypothesized that CSF antibodies serve as a parallel index of active CNS infection during early and chronic infection and of persistent CNS infection during ART. More specifically, we hypothesized that the emergence of CNS antibodies during early infection would allow us to assess the rate the CNS reservoir is established and maintained, compared with the periphery.
METHODS
Study Design and Characteristics of the Participants
This retrospective study analyzed archived, cross-sectional, and longitudinal CSF and blood samples collected from participants enrolled in Institutional Review Board-approved protocols with informed consent at 3 clinical sites: University of Gothenburg, University of California San Francisco, and Yale University. As summarized in Table 1, 33 HIV-infected and 12 uninfected individuals were evaluated. The chronic group included 10 HIV-infected individuals studied before and after >10 years of suppressive ART. Although the precise timing of infection was uncertain, their low blood CD4+ T-cell counts at baseline (median 157 per µL) and clinical information suggests that they had been infected for several years before ART initiation. Their median duration of treatment was 12.5 years, their plasma and CSF HIV RNA levels were 5.36 and 3.88 log10 copies/mL before treatment, and <1.69 log10 copies/mL in both fluids after treatment.
Table 1.
Subject Characteristics (At First Visit)
| Subject Groups | N | Site (SF/GOT) Ratio | Gender (M/F) Ratio | Age (Years) | Time Since Infection (Years) | CD4 (Cells/μL) | Plasma HIV RNA | CSF HIV RNA | CSF WBC (per μL) | CSF Neopterin (nMol/L) | CSF/Blood Albumin Ratio Mean (SD) | CSF NFL (Log10 ng/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Log10 Copies/mL) | ||||||||||||
| Mean (SD) | Median (IQR) | |||||||||||
| Chronic infection | 10 | 10:0 | 7:3 | 40.2 ± 8.3 | ? | 137.6 ± 98.7 | 4.38 ± 0.49 | 3.55 ± 0.56 | 1.5 (2.8–11.3) | 24.9 ± 10.5 | 6.1 ± 2.2 | 2.84 ± 0.29 |
| Primary infection | 4.8 ± 1.9 | 2.78 + 0.14 | ||||||||||
| No treatment | 12 | 8:3:1* | 12:0 | 40.1 ± 10.0 | 0.09 ± 0.02 | 608.8 ± 152.0 | 4.17 ± 1.50 | 2.80 ± 1.17 | 3.5 | 10.2 ± 9.2 | 5.0 ± 2.0 | 2.74 ± 0.28 |
| Treatment | 8 | 2:6 | 7:1 | 48.3 ± 9.7 | 0.14 ± 0.10 | 807.8 ± 413.4 | 1.63 ± 0.22 | 1.66 ± 0.05 | 0 | 5.8 ± 1.9 | 5.8 ± 1.9 | 2.64 ± 0.20 |
| Hyperacute | 2 | 2:0 | 2:0 | 42.0 ± 17.0 | 0.20 ± 0.04 | 1059 ± 636.4 | 1.59 ± 0 | 1.59 ± 0 | 5.5 (0–12.5) | 11.1 ± | 4.6 ± 0.9 | 3.48 ± 0.54 |
| Berlin | 1 | 1:0 | 1:0 | 50 | 16.31 | 364 | 5.46 ± 0.44 | 4.91 ± 0.47 | 0 | 4.5 | 9.7 | 4.5 |
| HIV negative | 12 | 12:0 | 7:5 | 48.8 ± 19.6 | NA | NA | NA | NA | 0 | NA | 7.1 ± 4.0 | 2.56 ± 0.26 |
Abbreviations: CSF, cerebrospinal fluid; GOT, Gothenburg; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable; NFL, neurofilament light; RNA, ribonucleic acid; SD, standard deviation; SF, San Francisco; WBC, white blood cells.
*Yale.
The early infection groups were diagnosed within 20–132 estimated days of exposure [23], and they have been included in studies characterizing primary HIV infection of the CNS [24, 25]. The treated subgroup initiated ART within 1–3 weeks of diagnosis, except for 2 who started ART later at 147 and 256 days after infection. Baseline blood CD4+ T-cell counts were higher in the untreated group (604 cells/µL) compared with the treated group (480 cells/µL). Samples were analyzed before treatment and in several individuals at varying intervals after treatment. Of note, only CSF, but not serum, from several of the untreated early infected participants were tested.
In addition, 2 individuals were included who coincidentally initiated pre-exposure prophylaxis (PrEP) within an estimated 10 and 12 days after infection and subsequently transitioned to ART at 18 and 24 days postinfection, and we refer to them as hyperacute [26]. Cerebrospinal fluid and serum from the Berlin patient, sampled 4 years after stem cell transplant were analyzed to serve as a comparative benchmark for the effects of functional cure [17, 19, 20]. Cerebrospinal fluid and serum from 12 HIV uninfected volunteers served as controls.
Luciferase Immunoprecipitation Systems Human Immunodeficiency Virus Antibody Measurement
Luciferase immunoprecipitation systems use recombinant chimeric fusion proteins with luciferase producing light and specific antigens for quantitative detection of antibodies. In the current study, HIV antigens for p24, matrix, gp41, gp120, reverse transcriptase, integrase, and protease were used for antibody profiling [17, 18, 27]. Serum and CSF samples were stored frozen at −80°C until use. For antibody testing, 1 µL of serum and 10 µL of CSF were used, respectively, producing approximately the same dynamic range of antibody detection. All assays were performed with a master plate of serum and CSF samples, and data were presented as light units (LU) [17]. For analysis, the antibody values against the 7 antigens were combined and presented as total HIV antibody levels. Antibody levels against the individual HIV proteins are provided as Supplementary Information.
Virological and Other Clinical Methods
Plasma and CSF HIV-1 RNA levels were measured at the local site laboratories using Cobas TaqMan assay version 1 or 2 (Hoffman La-Roche, Basel, Switzerland) or Abbott RealTime HIV-1 assay (Abbott Laboratories, IL), with 20 and 40 copies/mL, respectively, as lower limits of quantification. Both plasma and CSF HIV levels were measured at the same time points assessed for antibodies. All participants with chronic and acute HIV infection treated with ART, for more than 40 days, showed CSF HIV viral loads <50. Local laboratories also generated the background data including CSF cell counts, differential, and albumin and blood CD4+ T-cell counts and albumin [8]. As shown in Table 1, the CSF/blood albumin ratio in most of the HIV-infected subjects were normal, suggesting that there was no breakdown of the blood-brain barrier.
Data Analysis
Antibody levels in the chronic HIV infection group are reported as the geometric mean level (GML) and 95% confidence interval. Cutoff limits for determining positive antibodies in the HIV-infected samples were based on the mean plus 5 standard deviations of the serum and CSF values derived from the 12 uninfected controls. The Wilcoxon rank non-parametric test was used to study statistically significant antibody level changes in the paired samples. Additional analysis of antibodies against the 7 individual HIV proteins in the HIV participants are shown in the Supplementary Figures. A heatmap (Supplementary Figure 8) provides an overview of the serum and CSF antibody responses against the 7 different HIV antigens responses in the different groups and impact of treatment. The heatmap was generated essentially as previously described [28], in which a cutoff value (10000 LU) based on receiver operator characteristic was used for data transformation.
RESULTS
Long-Term Treatment of Chronic Human Immunodeficiency Virus (HIV) Infection Participants Results in a Small Decrease in Serum and Cerebrospinal Fluid HIV Antibody Levels
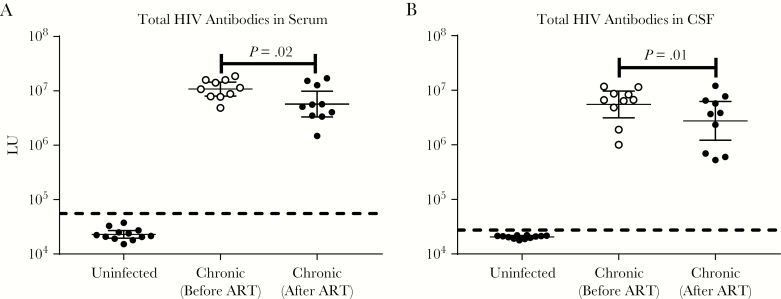
Using a panel of 7 HIV antigens, serum antibody levels were determined in the uninfected HIV controls and chronic HIV-infected subjects before and after >10 years of ART. To generate an overview for each subject, the antibody levels against the 7 antigens were combined into the total HIV antibody levels. As shown in Figure 1A, the GML of HIV serum antibodies observed in the pre- and posttreatment samples were 10810000 LU and 5736000 LU, respectively, which were more than 250-fold higher than the uninfected controls (22980 LU). Comparing the paired pre- and posttreatment serum sample from each subject revealed a significant decrease in antibody levels (Wilcoxon rank analysis; P = .02) with treatment (Figure 1A). Further analysis revealed that 6 of 10 subjects showed little or no change in overall antibody levels, whereas 4 subjects showed a pronounced 3- to 6-fold antibody decrease with treatment (Figure 1A). Consistent with these findings, there was a corresponding significant (P < .03 to P < .001) decline in the serum antibody responses against 6 of 7 HIV antigens, in which gp120 and integrase showed the greatest drop in serum antibody levels between pre- and posttreatment (Supplementary Figure 1). The 1 exception was serum antibodies to p24, which did not decrease significantly with treatment (Supplementary Figure 1). Overall, these results show that long-term ART in this group causes only a modest drop in serum HIV antibodies although with some variability amongst the individual subjects.
Figure 1.
Serum and cerebrospinal fluid (CSF) human immunodeficiency virus (HIV) antibody levels in patients with chronic HIV infection before and after long-term treatment. Antibody measurements were made in (A) serum and (B) CSF samples taken from before and after long-term treatment of subjects with chronic HIV infection and uninfected controls. The y axis reflects the antibody levels in light units determined by luciferase immunoprecipitation systems. The total HIV antibody levels were derived from the sum of antibody values against 7 antigens. The geometric mean antibody levels and 95% confidence interval are shown for each group. The geometric mean level in the chronic HIV-infected participants after treatment was further used as reference for Figure 2. The black dotted line is the cutoff value, based on the uninfected controls, for determining seropositivity of the combined antibody total for serum and CSF. Wilcoxon rank statistical analysis was used to statistically evaluate differences between the paired before and after treatment samples. Abbreviations: ART, antiretroviral therapy.
Cerebrospinal fluid humoral responses against the 7 HIV antigens were also measured at the same time points. The combined CSF antibody profile revealed that the GML in the pretreatment and posttreatment samples was 5503000 LU and 2753867 LU, respectively (Figure 1B). Although well above the uninfected volunteers (20460 LU), these CSF HIV antibody levels from before and after ART showed a significant (P = .01) decrease. Except for the p24, all other CSF antibody levels against individual antigens were significantly lower (P = .04 to .002) after treatment (Figure 1B and Supplementary Figure 2). Comparison of the serum and CSF profiles for each HIV antigen showed that they tracked each other well; correlation coefficients for integrase, protease, p24, matrix, reverse transcriptase, gp120, and gp41 were 0.87, 0.84, 0.82, 0.69, 0.60, 0.58, and 0.43, respectively. These results highlight how anti-HIV antibodies levels between the systemic blood and CSF of chronic-infection subjects align closely and that long-term ART associates with a significant, although modest, decrease in anti-HIV antibody levels in both compartments.
Robust Increases in Serum and Cerebrospinal Fluid Human Immunodeficiency Virus (HIV) Antibody Levels Over Time in Untreated Early HIV Infection
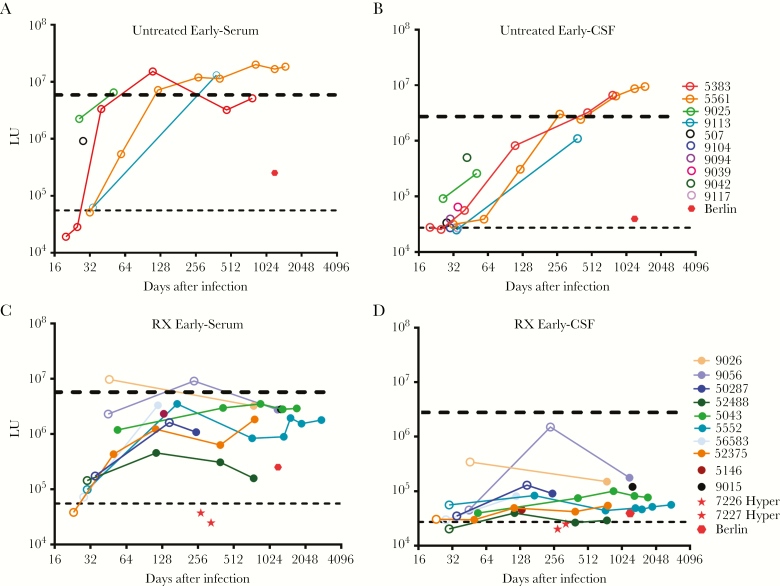
To understand temporal changes in the HIV antibody profile during the first year of infection in the absence of treatment, longitudinal serum and CSF samples were examined from early HIV-infected individuals. Analysis of the first available serum samples from 26 to 46 days after infection revealed measurable HIV antibody responses (Figure 2A). Two participants (5383 and 5561) lacked detectable antibodies between days 20 to 32, but analysis at day 40 and beyond revealed high levels of HIV antibodies. Examination of additional longitudinal time points from participants of early, untreated HIV-infected subjects showed that the antibody levels then rose substantially during the next 300 days to levels of >1000000 LU, approximating the levels observed in the chronically infected subjects. Inspection of the humoral responses against the different HIV antigens revealed that the individual antibody levels increased similarly against the 7 HIV proteins (Supplementary Figure 3).
Figure 2.
Serum and cerebrospinal fluid (CSF) antibody levels in untreated and treated early human immunodeficiency virus (HIV) infection. Untreated subjects with early HIV infection were evaluated for (A) serum and (B) CSF antibodies in paired cross-sectional and longitudinal samples. Similarly, treated subjects with early HIV infection were evaluated for (C) serum and (D) CSF antibodies in paired cross-sectional and longitudinal samples. Each colored dot reflects an individual subject. An open circle reflects the absence of antiretroviral therapy at that time point. The black dotted line is the cutoff value for determining seropositivity and is based on the uninfected controls of the combined antibody total for serum and CSF. As a reference, the blue dotted line represents the geometric mean antibody levels found in the corresponding serum and CSF of the treated chronically infected subjects. The serum and CSF antibody values for the Berlin patient (red solid dot) are also shown. Abbreviations: LU, light units.
The corresponding CSF antibody profiles from the untreated early subjects also showed very low levels of CSF HIV antibodies at the earliest time, with all but 1 above the healthy control cutoff value (Figure 2B). However, CSF antibodies rose rapidly rose more than 100-fold between days 32 and 64 and continued to increase through 18 months, eventually attaining levels of >1000000 LU observed in the CSF of the chronically infected participants (Figure 2B). Neither in these individuals nor in the chronic group was there a correlation between the changes in HIV antibodies and the CSF-plasma albumin ratio, a marker of the blood-brain barrier disruption [29]. Compared with the serum profile, the CSF antibodies at the early time points were mainly directed against matrix, reverse transcriptase, gp41, and gp120 (Supplementary Figure 4). In general, the CSF antibodies were lower than those of serum during the initial weeks of infection but rose over the next 6–18 months to levels equivalent to the chronic group.
Attenuated Antihuman Immunodeficiency Virus Antibodies in Serum and Cerebrospinal Fluid With Treated Early Infection
To understand the impact of ART administered early, longitudinal samples were examined from individuals who initiated treatment early between 10 and 256 days after initial HIV infection. As in the untreated individuals, these subjects showed initial low serum antibody levels at the earliest sample before 40 days. With treatment, the subsequent antibody trajectories appeared to be attenuated (Figure 2C), reaching levels well below the treated chronic infection and the untreated early infection groups, with some approaching serum antibody levels near those of the Berlin patient [17].
Direct comparison of serum antibody levels from the last time point available after at least 240 days of infection from the treated early were reduced 7-fold (P = .0003) compared with with the long-term treated chronic subjects, highlighting the impact of early treatment (Supplementary Figure 5). Further analysis revealed that the subject who started treatment before day 40 developed high levels of serum antibodies to p24, gp41, and gp120 but completely lacked antibodies to integrase and protease (Supplementary Figure 6). Finally, a substantial drop in antibodies was noted in 2 cases (ie, 50287 and 9056) who initiated ART later at 148 and 256 days after infection.
The blunting effect of early ART on antibody responses was even greater in CSF than serum (Figure 2D). Strikingly, the very low levels of HIV antibody levels noted at the earliest time points of 14–62 days remained stable during the ensuing period from 100 to 2000 days, clearly diverging from the increase seen in their untreated counterparts. The CSF values observed in these early-treated subjects were less than 10-fold higher than the cutoff derived from the uninfected subjects, close to or in the same range as those of the Berlin patient or nearing the uninfected controls (Figure 2D). In one individual (52488), the antibody levels reverted close to the seronegative cutoff value at 2.5 years after infection. Analysis of CSF antibody profile against the individual proteins revealed that most of the observed immunoreactivity was contributed by p24 antibodies (Supplementary Figures 7 and 8). The 2 hyperacute individuals who started PrEP very early after infection without seroconversion before treatment remained antibody-negative months later. (Figure 2C and D). Unlike the chronically infected group, comparison of the serum and CSF antibody levels in these treated individuals showed a marked discordance in values, suggesting that these 2 compartments did not equilibrate over time (Supplementary Figure 8). More importantly, the CSF antibodies from the last time point available after at least 240 days of infection from the early treated subjects showed a 43-fold lower level (P > .0001) compared with the long-term ART-treated chronic subjects, emphasizing the important impact of early treatment on the CNS response (Supplementary Figure 9). Compared with blood, these CSF results also support the relative independence and greater reduction of the CNS response to early treatment.
DISCUSSION
The impact of ART on serum and CSF HIV antibodies was investigated after initiation of treatment during chronic or early infection. A major finding was that early initiation of treatment after infection had a markedly greater impact in selectively sparing the CNS from acquiring HIV antibodies compared with the blood. To the extent that the serum and CSF HIV antibody levels reflect responses to continued expression of viral antigens, these results suggest that early treatment can abort or reduce the magnitude of the CNS HIV reservoir to a greater degree than its systemic counterpart.
Individuals with chronic HIV infection years after sustained treatment showed only modest, largely parallel decreases in HIV antibody levels. Although there was considerable heterogeneity among the chronically infected subjects, these findings are in general agreement with recent studies showing a similar decrease in serum antibodies with ART [21, 30]. More importantly, our study demonstrates that the serum and CSF antibodies seen in the long-term treated chronically infected subjects remain about the same as the untreated early infected subjects 1 year after infection. A likely explanation for limited antibody decay is the continued expression of HIV antigens within lymphoid tissue despite viral “suppression” in plasma [31], which is consistent with the frequency of low-level viremia detected by sensitive measures [32] and the capacity to detect persistent HIV outgrowth in suppressed patients [33]. We speculate that the large reservoir, established both systemically and in the CNS of subjects with chronic infection despite therapy, continues to produce HIV proteins, which in turn provides continued antigenic stimulation of the corresponding B cells.
Analysis of matching serum and CSF from longitudinal samples from untreated early infection revealed that HIV antibodies were first detectable in serum and then subsequently appeared in CSF. These results are in line with the known delay of detectable HIV RNA and cellular immune responses in the CSF compared with the systemic blood during early stage acute infection [34, 35]. In the untreated group, there was no enrichment in antibodies against the different HIV proteins in serum and CSF, in which the different antibodies generally tracked each other, including seropositive responses against integrase and protease. In the absence of treatment, these subjects at 1 year attained HIV antibodies in both blood and CSF comparable with the chronic HIV group.
In contrast, early ART dramatically blunted the CSF HIV antibody response. Early treated individuals exhibited very low antibodies over the extended period of observation. The 2 hyperacute individuals treated during very early infection at Fiebig stage 1, while antigen positive and antibody negative on days 10 and 12 after infection, remained seronegative when studied 10–11 months later. Those diagnosed in the days and early weeks later exhibited low antibodies at these time points. Early treatment attenuates further antibody increases in both fluids, but it is more strikingly in the CSF. This suggests that not only are the CSF antibodies slower to develop in the CNS, but that local antigen expression is also delayed, and that with early treatment the development and perhaps “maturation” of CNS infection and reservoir is likewise blunted. Hence, the attenuation of the CSF antibody response in early treatment may well be an indicator of the long-term impact of early treatment on the CNS reservoir.
The finding that the 2 treated hyperacute subjects never generated HIV antibodies in serum or CSF is also consistent with other studies showing that individuals who start therapy very early in acute infection have low or no HIV antibodies in serum and occasionally serorevert using standard antibody tests [36, 37]. However, as part of another study, these 2 hyperacute subjects underwent treatment interruption and experienced recurrence of plasma HIV viremia [26]. Although CSF was not collected to evaluate viral RNA in the CNS, this provides a cautionary note that the absence of HIV antibodies in the systemic reservoir for a prolonged period does not signify a cure.
The serum antibody findings appear consonant with observations comparing the impact of early versus delayed ART on HIV deoxyribonucleic acid (DNA) levels [38]. Antiretroviral therapy administration in early infection caused a larger decrease in cell-associated HIV-DNA than seen with treatment initiated during the chronic phase [39, 40]. The finding that early ART causes a modest reduction in serum HIV antibodies is consistent with these molecular findings. The blunted serum antibody profile observed in many of the treated early-infected subjects, stabilizing at intermediate values between chronic and uninfected participants, likely reflects their lower systemic reservoir. Antiretroviral therapy administered early may be more effective at blocking HIV replication in a limited pool of infected cells. This is reflected by the antibody profile of some early (<day 40) treated participants who showed low levels of serum antibodies to integrase and protease (Supplemental Figure 7) but modest antibodies to structural HIV proteins resembling the antibody profile in super-elite controllers [16]. The relative stable HIV antibody levels observed in the different ART-treated early patients depended on how soon after infection therapy was initiated and highlights the potential benefit of very early treatment.
CONCLUSIONS
Despite the small number of participants and retrospective nature of our study, the results have important implications for understanding and preventing CNS infection. First, the observation that high levels of HIV antibodies exist in the blood several weeks after initial infection, but are reduced in the CSF, supports the notion of independent HIV replication within the CNS that is delayed compared with systemic infection. The markedly lower HIV antibody levels in CSF compared with serum in the early treated subjects, even years later, points to the stability and durability of ART protection. These results also suggest that in these subjects, it is unlikely that there is systemic contamination of the CNS with serum antibodies. Although several other biomarkers are useful for studying HIV infection of the CNS [13], none appear to clearly suggest a relationship with the CNS reservoir as HIV antibodies. Additional studies of the analysis of CSF antibodies with detailed HIV reservoir measurements and long-term neurological morbidity assessment should further clarify their clinical importance.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was funded by the Division of Intramural Research, National Institute of Dental and Craniofacial Research, the National Institutes of Health (grants R01 NS094067, P01 MH094177, P01 DA026134, R01 MH62701, R01MH081772), the Delaney AIDS Research Enterprise (grants AI096109 and A127966), amfAR Institute for HIV Cure Research (grant amfAR 109301), the University of California San Francisco/Gladstone Institute of Virology and Immunology Center for AIDS Research (grant P30 AI027763), and Sahlgrenska Academy at the University of Gothenburg (grant ALFGBG-430271).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Price RW, Spudich S. Antiretroviral therapy and central nervous system HIV type 1 infection. J Infect Dis 2008; 197:S294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. d’Arminio Monforte A, Cinque P, Mocroft A et al. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann Neurol 2004; 55:320–8. [DOI] [PubMed] [Google Scholar]
- 3. Price RW, Deeks SG. Antiretroviral drug treatment interruption in human immunodeficiency virus-infected adults: clinical and pathogenetic implications for the central nervous system. J Neurovirol 2004; 10:44–51. [DOI] [PubMed] [Google Scholar]
- 4. Hunt PW, Martin JN, Sinclair E et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003; 187:1534–43. [DOI] [PubMed] [Google Scholar]
- 5. French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis 2009; 200:1212–5. [DOI] [PubMed] [Google Scholar]
- 6. Neuhaus J, Jacobs DR Jr, Baker JV et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010; 201:1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sandler NG, Wand H, Roque A et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edén A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslén M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis 2007; 196:1779–83. [DOI] [PubMed] [Google Scholar]
- 9. Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edén A, Marcotte TD, Heaton RK et al. increased intrathecal immune activation in virally suppressed HIV-1 infected patients with neurocognitive impairment. PLoS One 2016; 11:e0157160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barouch DH, Deeks SG. Immunologic strategies for HIV-1 remission and eradication. Science 2014; 345:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joseph SB, Arrildt KT, Sturdevant CB, Swanstrom R. HIV-1 target cells in the CNS. J Neurovirol 2015; 21:276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hellmuth J, Valcour V, Spudich S. CNS reservoirs for HIV: implications for eradication. J Virus Erad 2015; 1:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wabl M, Steinberg C. Affinity maturation and class switching. Curr Opin Immunol 1996; 8:89–92. [DOI] [PubMed] [Google Scholar]
- 15. Cohen MS, Gay CL, Busch MP, Hecht FM. The detection of acute HIV infection. J Infect Dis 2010; 202:S270–7. [DOI] [PubMed] [Google Scholar]
- 16. Mendoza D, Johnson SA, Peterson BA et al. Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood 2012; 119:4645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burbelo PD, Bayat A, Rhodes CS et al. HIV antibody characterization as a method to quantify reservoir size during curative interventions. J Infect Dis 2014; 209:1613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee SA, Bacchetti P, Chomont N et al. Anti-HIV antibody responses and the HIV reservoir size during antiretroviral therapy. PLoS One 2016; 11:e0160192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hütter G, Nowak D, Mossner M et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009; 360:692–8. [DOI] [PubMed] [Google Scholar]
- 20. Yukl SA, Boritz E, Busch M et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog 2013; 9:e1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keating SM, Pilcher CD, Jain V et al. HIV antibody level as a marker of HIV persistence and low-level viral replication. J Infect Dis 2017; 216:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hauser SL. The Charcot Lecture | beating MS: a story of B cells, with twists and turns. Mult Scler 2015; 21:8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suh J, Sinclair E, Peterson J et al. Progressive increase in central nervous system immune activation in untreated primary HIV-1 infection. J Neuroinflammation 2014; 11:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rahimy E, Li FY, Hagberg L et al. Blood-brain barrier disruption is initiated during primary hiv infection and not rapidly altered by antiretroviral therapy. J Infect Dis 2017; 215:1132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spudich S, Gisslen M, Hagberg L et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis 2011; 204:753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Henrich TJ, Hatano H, Bacon O et al. Extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection leads to prolonged, antiretroviral-free remission but is insufficiant to acheive cure. PLoS Med 2017; 14:e1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burbelo PD, Ching KH, Mattson TL, Light JS, Bishop LR, Kovacs JA. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems). Biochem Biophys Res Commun 2007; 352:889–95. [DOI] [PubMed] [Google Scholar]
- 28. Burbelo PD, Bren KE, Ching KH et al. LIPS arrays for simultaneous detection of antibodies against partial and whole proteomes of HCV, HIV and EBV. Mol Biosyst 2011; 7:1453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anesten B, Yilmaz A, Hagberg L et al. Blood-brain barrier integrity, intrathecal immunoactivation, and neuronal injury in HIV. Neurol Neuroimmunol Neuroinflamm 2016; 3:e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang W, Morshed MM, Noyan K, Russom A, Sönnerborg A, Neogi U. Quantitative humoral profiling of the HIV-1 proteome in elite controllers and patients with very long-term efficient antiretroviral therapy. Sci Rep 2017; 7:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang L, Ramratnam B, Tenner-Racz K et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med 1999; 340: 1605–13. [DOI] [PubMed] [Google Scholar]
- 32. Ostrowski SR, Katzenstein TL, Thim PT, Pedersen BK, Gerstoft J, Ullum H. Low-level viremia and proviral DNA impede immune reconstitution in HIV-1-infected patients receiving highly active antiretroviral therapy. J Infect Dis 2005; 191:348–57. [DOI] [PubMed] [Google Scholar]
- 33. Metcalf Pate KA, Pohlmeyer CW, Walker-Sperling VE et al. A murine viral outgrowth assay to detect residual HIV Type 1 in patients with undetectable viral loads. J Infect Dis 2015; 212:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kessing CF, Spudich S, Valcour V et al. High number of activated CD8+ T cells targeting HIV antigens are present in cerebrospinal fluid in acute HIV infection. J Acquir Immune Defic Syndr 2017; 75:108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Valcour V, Chalermchai T, Sailasuta N et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 2012; 206:275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kassutto S, Johnston MN, Rosenberg ES. Incomplete HIV type 1 antibody evolution and seroreversion in acutely infected individuals treated with early antiretroviral therapy. Clin Infect Dis 2005; 40:868–73. [DOI] [PubMed] [Google Scholar]
- 37. de Souza MS, Pinyakorn S, Akapirat S et al. Initiation of antiretroviral therapy during acute HIV-1 infection leads to a high rate of nonreactive HIV serology. Clin Infect Dis 2016; 63:555–61. [DOI] [PubMed] [Google Scholar]
- 38. Ananworanich J, Chomont N, Eller LA et al. HIV DNA set point is rapidly established in acute HIV infection and dramatically reduced by early ART. EBioMedicine 2016; 11:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buzon MJ, Martin-Gayo E, Pereyra F et al. Long-term antiretroviral treatment initiated at primary HIV-1 infection affects the size, composition, and decay kinetics of the reservoir of HIV-1-infected CD4 T cells. J Virol 2014; 88:10056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laanani M, Ghosn J, Essat A et al. Impact of the timing of initiation of antiretroviral therapy during primary HIV-1 infection on the decay of cell-associated HIV-DNA. Clin Infect Dis 2015; 60:1715–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.