Abstract
Introduction
Cholangiopancreatoscopy with SpyGlass™ Direct Visualization System (SGDVS) is being used in specialized centers for improving the sensitivity of endoscopic retrograde cholangiopancreatography (ERCP) in patients with indeterminate pancreatobiliary strictures (PBS). The aims of this study were to report our initial experience with SGDVS in the evaluation of indeterminate PBS, and discuss the improvements of ERCP brought by this technique in our center.
Methods
The usefulness of SGDVS in patients with indeterminate PBS (defined after nondiagnostic previous ERCP with brush cytology) was evaluated in a prospective observational cohort study conducted at a single tertiary biliopancreatic unit. The accuracy of diagnosis by the SGDVS visual findings, SGDVS-guided biopsy, technical success, image quality, change in patient management after the procedure, and complication rate were assessed.
Results
In our single-center cohort, there were 13 SGDVS procedures for evaluating indeterminate PBS. Technical success, defined by the ability to progress with the SpyScope to the target lesion, was achieved in all the cases. The diagnostic accuracy of visual findings (87.5%) was superior to SGDVS-guided biopsy (55%). In 11 (85%) procedures, the image quality was considered good. The procedure permitted exclusion of malignancy and avoiding surgery in 9 patients (69%). There were no complications during the procedures. However, in the post-procedure monitoring, 3 patients developed acute pancreatitis (19%) and 2 patients developed acute cholangitis (13%).
Conclusion
The SGDVS can be considered useful in the context of indeterminate PBS. The intervention is associated with high procedural success and alters clinical outcome compared to conventional approaches.
Keywords: Endoscopic retrograde cholangiopancreatography, Biliary stricture, Cholangioscopy, Pancreatoscopy
Resumo
Introdução
A colangiopancreatoscopia com o Sistema de Visualização Direta SpyGlass™ (SVDSG) tem vindo a ser utilizada em centros de refer'ncia para aumentar a sensibilidade da colangiopancreatografia retrógrada endoscópica (CPRE) em doentes com estenoses pancreatobiliares (EPB) indeterminadas. Os objetivos deste estudo são relatar a experi'ncia inicial com SGDVS na avaliação de EPB indeterminadas e discutir as vantagens associadas a esta técnica como adjuvante à CPRE no nosso centro.
Métodos
A utilidade do SVDSG em doentes com EPB indeterminadas (definida após a CPRE anterior não diagnóstica com citologia esfoliativa) foi avaliada num estudo de coorte observacional prospectivo realizado numa unidade biliopancreática de referenciação terciária. Foi avaliada a acuidade diagnóstica dos achados visuais do SVDSG, bem como da biópsia guiada pelo SVDSG, o sucesso técnico, a qualidade da imagem, o impacto no manejo do doente após o procedimento e a taxa de complicações.
Resultados
Foram incluídos nesta coorte 13 procedimentos de SVDSG para avaliação de EPB indeterminadas. O sucesso técnico, definido pela capacidade de avançar o SpyScope até à lesão alvo, foi alcançado em todos os casos. A precisão diagnóstica dos achados visuais (87.5%) foi superior à biópsia guiada por SVDSG (55%). Em 11 (85%) procedimentos, a qualidade da imagem foi considerada boa. O procedimento permitiu a exclusão de malignidade e consequente cirurgia em 9 doentes (69%). Não houve complicações durante os procedimentos. No entanto, na monitorização pós-procedimento, tr's doentes desenvolveram pancreatite aguda (19%) e dois pacientes desenvolveram colangite aguda (13%), em ambas as situações sem critérios de gravidade.
Conclusão
O SVDSG pode ser considerado como uma ferramente útil no contexto do EPB indeterminadas. A intervenção está associada a um alto sucesso técnico, alterando de forma mais significativa a estratégia clínica em comparação com as abordagens convencionais.
Palavras Chave: Colangiopancreatografia retrógrada endoscópica, Estenose biliar, Colangioscopia, Pancreatoscopia
Introduction
Pancreatobiliary strictures (PBS) are a common finding in clinical practice. While many of these strictures are due to malignancies of the biliary tract and pancreas, up to 30% of biliary strictures are benign [1]. The major etiology of a malignant PBS includes a primary tumor or local extension, such as cholangiocarcinoma or pancreatic head cancer [2]. For further chemotherapy or radiotherapy, tissue sampling at endoscopic retrograde cholangiopancreatography (ERCP) by forceps biopsy, brush cytology, or endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is important; however, these techniques are associated with a high degree of interoperator variability and typically have poor sensitivity and negative predictive value [3, 4].
In specialized centers, intraductal ultrasonography and cholangioscopy are being used to direct biopsy sampling and to reduce the number of false negative results of fluoroscopic guided sampling. In this context, single-operator cholangioscopy with SpyGlass™ Direct Visualization System (SGDVS) has been evaluated in several clinical trials [5, 6, 7, 8, 9, 10, 11, 12]. In a meta-analysis of 10 studies, the pooled sensitivity and specificity of SpyGlass cholangioscopy-guided biopsies in diagnosing malignant biliary strictures was 60.1 and 98.0%, respectively; however, the sensitivity increases to 84.5% based on the visual cholangioscopic impression [11].
Direct visualization of the biliary or pancreatic ducts with targeted biopsies improves diagnostic accuracy of ERCP, reducing the number of procedures and possibly the costs associated with the workup in these patients. In a work by Njei et al. [13], SGDVS with targeted biopsies was the most cost-effective diagnostic strategy for the detection of CCA in patients with primary sclerosing cholangitis among the various ERCP-based modalities.
The aims of this paper are (1) to report our initial experience with SGDVS in the evaluation of indeterminate PBS, and (2) to discuss the improvements to ERCP brought by this technique in our center.
Materials and Methods
Study Design
This study was designed as a prospective observational cohort study conducted at a single tertiary biliopancreatic unit with an overall annual volume of over 400 ERCPs. Institutional review board approval and informed consent were obtained for the purpose of this study.
Patients
Eligibility for study entry related to the need of further evaluation of a PBS of uncertain clinical significance (“indeterminate stricture”) in a multidisciplinary team consultation. All consecutive eligible patients in whom the use of the SGDVS platform was considered were registered between July and December 2015. Patient demographics, anatomical target (intrahepatic, perihilar, common bile duct, pancreatic duct), visual impression of stenosis, probable related adverse events, and change in clinical orientation after the procedure were recorded. Patients were followed up for at least 6 months.
Procedures
All interventions were carried out by 2 (P.P. and FVB) experienced endoscopists, who visualized and performed 5–10 SpyGlass procedures to obtain and build up the necessary skills and technical experience with the platform.
All procedures were performed under general anesthesia with orotracheal intubation. Before the procedure, patients received antibiotic prophylaxis (ciprofloxacin), and in the end 100 mg of rectal indomethacin were administered to prevent postprocedure pancreatitis. Biliary sphincterotomy, if not yet performed, was carried out just before the SGDVS examination.
Each procedure was performed with an Olympus TJF-160V or TJF-Q180V duodenoscope (Olympus Medical Systems, Tokyo, Japan) along with the SGDVS and accessories (Boston Scientific Corp, Natick, MA, USA). After initial fluoroscopic PBS characterization, a guidewire was introduced into the target duct, and the single-use 10-Fr multichanneled sheath (SpyScope disposable access and delivery catheter) was advanced proximal to the stricture. On withdrawal, careful examination of the biliary or pancreatic mucosa was performed and every ductal lesion was visually characterized. Thereafter, and if possible, biopsy specimens (at least 4) were obtained using the SpyBite forceps under SGDVS guidance. An experienced pathologist evaluated all samples for adequacy. In all the cases, the stenosis accommodated the SpyScope, so there was no need to perform balloon dilatation prior to the cholangioscopy or pancreatoscopy. At the end of the procedure, a stent was inserted to ensure drainage.
Outcome and Definitions
The main study outcome was to assess the accuracy of diagnosis by the SGDVS visual findings and SGDVS-guided biopsy findings in comparison with the final diagnosis. The secondary end points included technical success, image quality, change in patient management after the procedure and complication rate.
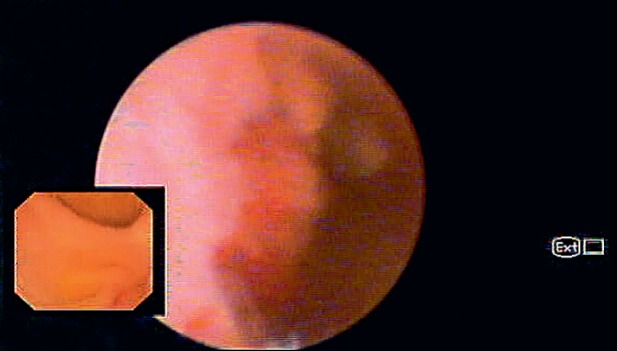
Biliary lesions were defined as malignant or benign based on the presence or absence of the following visual findings: (1) dilated tortuous vessels (tumor vessels), (2) intraductal mass, and (3) irregular mucosal nodularity (Fig. 1). There was a case of pancreatoscopy that was considered benign because the patient had fibrotic scars suggestive of chronic pancreatitis. The final diagnosis of malignancy was made based on positive histopathology of SGDVS-guided biopsy or other tissue sampling procedures such as brush cytology, endobiliary biopsies, EUS-FNA and surgical specimens, or progression of disease consistent with malignancy during the follow-up. The final diagnosis of a benign lesion was made in the case of negative histopathology of tissue sampling and an uneventful clinical follow-up for at least 6 months after the SGDVS procedure.
Fig. 1.
Biliary stricture with irregular mucosal nodularity suggestive of malignancy.
Results
Between July 2015 and December 2015, 16 SGDVS procedures were performed in our institution. In 13 cases (81%), the indication was evaluation of indeterminate PBS (12 biliary and 1 pancreatic) after multidisciplinary consultation in the oncologic pancreatobiliary group. Technical success, defined by the ability to progress with the SpyScope to the target lesion, was achieved in all the cases. Regarding the location of the lesions, 7 were perihilar, 5 in the distal common bile duct, and 1 in the pancreatic duct. The image quality was considered moderate or good in 11 (85%) cases. In 2 cases, the image was considered poor mainly due to the presence of debris despite irrigation that interfered with visualization. The baseline characteristics of patients are summarized in Table 1.
Table 1.
Patient characteristics and procedures performed with the SpyGlass™ system
| Sex | |
| Male | 10 (77) |
| Female | 3 (23) |
| Age, years | 49 ± 16 |
| Procedure/indication | |
| Cholangioscopy | |
| Indeterminate biliary strictures | 12 (92) |
| Pancreatoscopy | |
| Indeterminate pancreatic stricture | 1 (8) |
| Quality of visualization | |
| Moderate/excellent | 11 (85) |
| Poor | 2 (15) |
| Technical success rate | 13 (100) |
Data are presented as n (%) or mean ± SD. Biliary and pancreatic strictures were considered indeterminate if they remained without diagnosis after conventional ERCP with negative or inconclusive cytology and/or biopsy. Cholangioscopy and pancreatoscopy were considered technically successful if the endoscopist was able to progress with the SpyScope® to the lesion, with successful biopsies in the case of undetermined strictures.
In 4 (31%) patients, the endoscopic appearance of the biliary strictures was considered malignant; the findings were described as intraductal mass in 2 patients and mucosa nodularity in the other 2. The final diagnosis of malignancy was confirmed by surgical specimens in 2 patients, SGDVS-guided biopsy in 1 patient, and clinical follow-up in 1 patient. Upon visual impression, there were 9 (69%) lesions (8 biliary and 1 pancreatic) considered benign. None of these patients was submitted to surgery, so the final diagnosis of benign lesions was established by SGDVS-guided biopsy in 5 patients and clinical follow-up in the other 4.
SGDVS-guided biopsies using the SpyBite biopsy forceps were taken in 11 patients (85%). In 2 cases, it was not possible to obtain biopsies because of difficulty passing the biopsy forceps through the SpyScope due to angulation.
The median number of biopsy samples collected was 3 (range 1–6). The samples obtained using the SpyBite forceps were considered adequate for histological assessment in 8 cases (62%); in other 3 cases, findings were considered inconclusive by inadequate sample size.
In all the 4 patients with a final diagnosis of malignancy, the lesions were considered malignant according to the SGDVS visual findings. In 3 of these patients, it was possible to establish a histological diagnosis. In 1 patient with clinical suspicion of perihilar cholangiocarcinoma, it was not possible to establish a histological diagnosis by SGDVS directed biopsies or brush cytology. The patient was considered not suitable for surgery, and on follow-up the progression of the lesion was consistent with malignancy.
The diagnostic accuracy of the biopsies was 55%. Comparing the diagnostic impression given by endoscopic features with histological findings, there was an 87.5% accuracy. Procedure-related findings are summarized in Table 2.
Table 2.
Endoscopic findings and histopathological results of indeterminate strictures
| Diagnostic impression (cholangioscopy) | |
| Benign | 9 (69.3) |
| Malignant | 4 (30.8) |
| Endoscopic appearance (cholangioscopy) | |
| Fibrous appearance | 3 (23.1) |
| Polypoid/tumoral lesion | 4 (30.8) |
| Inflammatory changes | 4 (30.8) |
| Normal | 2 (15.4) |
| Histological findings | |
| Inconclusive | 5 (45.5) |
| Benign | 5 (45.5) |
| Malignant | 1 (9) |
Data are presented as n (%). Diagnostic accuracy (biopsies): 55%. Diagnostic accuracy (operator visual impression): 85%.
There were no complications during the procedures. However, in the post-procedure monitoring, 3 patients developed acute pancreatitis (19%) and 2 patients developed acute cholangitis (13%). All adverse events were considered mild.
Discussion
Indeterminate PBS are frequently a diagnostic dilemma in which the underlying etiology is difficult to determine even after extensive preoperative evaluation. Endoscopic evaluation of biliary strictures with tissue sampling by ERCP and/or endoscopic ultrasonography remains a mainstay in the evaluation of these lesions. Increasingly, intraductal ultrasonography and cholangioscopy are being utilized to direct biopsy sampling. In addition, emerging technologies, including confocal laser endomicroscopy, fluorescent in situ hybridization, and bile duct fluid biomarkers, offer the possibility of an increased ability to differentiate benign and malignant lesions preoperatively. Of these, cholangioscopy with SGDVS has been regarded as a promising tool, since it enables direct visualization of the biliary and pancreatic ducts, directing biopsies, and using a technology that is operator friendly.
In our center, the SGDVS became available in July 2015, and 16 procedures have been performed since then. The main indication was evaluation of indeterminate PBS. The diagnosis of indeterminate PBS was established in a multidisciplinary team consultation, which is a referral center in northern Portugal for treating patients with pancreatobiliary pathology.
We achieved technical success, defined as the ability to visualize the indeterminate lesion, in all the cases. The good system maneuverability and the dedicated irrigation system allowed a good visualization of the target lesion in most patients. The low technical success of the older systems of cholangioscopy may be due to the lack of irrigation channel, the need for 2 endoscopists (mother-baby cholangioscopy), and the system rigidity that limited overcoming seep angulations. Our technical success rate is consistent with the first reported experience by Chen and Pleskow [14] who described a rate of procedural success of 91%, providing proof of concept that this technique is generally applicable and overcomes some of the limitations of the older systems of cholangioscopy. The image quality was considered suboptimal in 2 procedures. This may be due to the difficulty in removing mucus and debris in mucin-producing lesions and the limited resolution of the 6,000-pixel image bundle produced by the SpyGlass probe. The introduction of the digital version of the SGDVS, which enables enhanced visualization and has a wider field of view, may improve image quality of the system in the future [15].
In our cohort, the accuracy of visual impression was superior to the diagnostic accuracy of SGDVS-guided biopsies. The visual impression permits looking for certain features of cancer, enhancing diagnostic sensitivity; however, a diagnosis solely based on visual impression seems to be associated with a loss of specificity [16]. Using the cholangioscopic criteria [17] for labelling a stricture as malignant, we correctly identified all the patients with malignant disease and were able to exclude all patients with benign strictures; however, we could only provide an adequate histological specimen for diagnosis in 8 cases (62%).
The main difficulties in obtaining a histological diagnosis were the inability to pass the Spybite forceps in an angulated SpyScope and instability of the image caused by blood and mucus adhering to the optical fiber. Moreover, the SpyBite has an outer diameter of 1 mm and allows for a 4.1-mm jaw opening at 55°, which results in small sample sizes, which makes histological examination difficult.
Our modest diagnostic accuracy of SGDVS-guided biopsies (55%) is similar to the results of a recent meta-analysis in which SpyGlass cholangioscopy with SpyBite biopsies produced results comparable to those of a combination of standard brushings and forceps biopsies in the diagnosis of malignant biliary strictures. Ten studies involving 456 patients revealed a sensitivity and specificity of SGDVS-guided biopsies of 60.1 and 98.0%, respectively [11]. Preliminary data suggest that the diagnostic outcomes of SGDVS-guided biopsies in indeterminate PBS can be significantly improved by using rapid onsite evaluation (ROSE) [18]. In our unit, we are performing ROSE for improving the diagnostic accuracy of EUS-FNA, and plan to use this technique in future SGDVS procedures.
Regarding management after the procedure, all patients with a suspected malignant stricture were considered for surgery or palliative treatment. The diagnosis of malignancy was confirmed in the 2 patients with visual impression but no histological diagnosis. After multidisciplinary team discussion, all patients with benign strictures were considered for clinical follow-up, and no surgery was performed. The clinical impact of SpyGlass in our patients was particularly relevant in excluding malignancy and avoiding unnecessary surgery.
The last component of usefulness involves adverse events and safety related to the SGDVS procedure. A 2011 study by Sethi et al. [19] reported on adverse events associated with cholangioscopy and pancreatoscopy. These authors found an overall higher rate of adverse events when cholangioscopy or pancreatoscopy was carried out compared with when ERCP without these additional procedures was performed (7 vs. 2.9%, respectively). Of interest, the development of procedure-related cholangitis was specifically associated with cholangioscopy or pancreatoscopy. Addressing all possible adverse events, we found procedure-related complications in 5 patients (38%). All adverse events were considered minor, and the patients had a clinically uneventful course. In fact, the number of complications is greater than previously reported in the literature. This may be due to the learning curve related to the procedure, which is reflected in a longer duration of the examinations compared to conventional ERCP, and an increase in papilla trauma resulting from repeated cannulation. In addition, a longer duration of the procedure with prolonged insufflation and vigorous irrigation of the bile duct may increase the risk of cholangitis [19]. As such, antibiotic prophylaxis, orotracheal intubation, and rectal indomethacin should always be considered for prevention of complications.
In conclusion, SGDVS is an effective adjunctive tool with ERCP for the evaluation of PBS. The procedure is associated with a high technical success rate and alters clinical outcome in difficult cases with an acceptable safety profile. Given the recent introduction of an upgraded digitalized version of SpyGlass (SpyDS), it is expected that this technological update will improve the visualization increasing the diagnostic accuracy in indeterminate PBS.
Statement of Ethics
This study did not require informed consent or review/approval by the appropriate ethics committee.
Disclosure Statement
The authors declare not to have any financial support or relationships that may pose conflict of interest.
References
- 1.Tummala P, Munigala S, Eloubeidi MA, Agarwal B. Patients with obstructive jaundice and biliary stricture +/− mass lesion on imaging: prevalence of malignancy and potential role of EUS-FNA. J Clin Gastroenterol. 2013;47:532–537. doi: 10.1097/MCG.0b013e3182745d9f. [DOI] [PubMed] [Google Scholar]
- 2.Larghi A, Tringali A, Lecca PG, Giordano M, Costamagna G. Management of hilar biliary strictures. Am J Gastroenterol. 2008;103:458–473. doi: 10.1111/j.1572-0241.2007.01645.x. [DOI] [PubMed] [Google Scholar]
- 3.Burnett AS, Calvert TJ, Chokshi RJ. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-years review of the literature. J Surg Res. 2013;184:304–311. doi: 10.1016/j.jss.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Burnett AS, Bailey J, Oliver JB, Ahlawat S, Chokshi RJ. Sensitivity of alternative testing for pancreaticobiliary cancer: a 10-years review of the literature. J Surg Res. 2014;190:535–547. doi: 10.1016/j.jss.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Ramchandani M, Reddy DN, Gupta R, Lakhtakia S, Tandan M, Darisetty S, et al. Role of single-operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: a single-center, prospective study. Gastrointest Endosc. 2011;74:511–519. doi: 10.1016/j.gie.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 6.Kalaitzakis E, Webster GJ, Oppong KW, Kallis Y, Vlavianos P, Huggett M. Diagnostic and therapeutic utility of single-operator peroral cholangioscopy for indeterminate biliary lesions and bile duct stones. Eur J Gastroenterol Hepatol. 2012;24:656–664. doi: 10.1097/MEG.0b013e3283526fa1. [DOI] [PubMed] [Google Scholar]
- 7.Manta R, Frazzoni M, Conigliaro R, Maccio L, Melotti G, Dabizzi E. SpyGlass single-operator peroral cholangioscopy in the evaluation of indeterminate biliary lesions: a single-center, prospective, cohort study. Surg Endosc. 2013;27:1569–1572. doi: 10.1007/s00464-012-2628-2. [DOI] [PubMed] [Google Scholar]
- 8.Woo YS, Lee JK, Oh SH, Kim MJ, Jung JG, Lee KH, et al. Role of SpyGlass peroral cholangioscopy in the evaluation of indeterminate biliary lesions. Dig Dis Sci. 2014;59:2565–2570. doi: 10.1007/s10620-014-3171-x. [DOI] [PubMed] [Google Scholar]
- 9.Siiki A, et al. Spyglass single-operator peroral cholangioscopy seems promising in the evaluation of primary sclerosing cholangitis-related biliary strictures. Scand J Gastroenterol. 2014;49:1385–1390. doi: 10.3109/00365521.2014.940376. [DOI] [PubMed] [Google Scholar]
- 10.Siiki A, Rinta-Kiikka I, Koivisto T, Vasama K, Sand J, Laukkarinen J. Diagnostic and therapeutic utility of SpyGlass® peroral cholangioscopy in intraductal biliary disease: single-center, retrospective, cohort study. Dig Endosc. 2015;27:479–485. doi: 10.1111/den.12405. [DOI] [PubMed] [Google Scholar]
- 11.Navaneethan U, Hasan MK, Lourdusamy V, Njei B, Varadarajulu S, Hawes RH. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc. 2015;82:608–614. doi: 10.1016/j.gie.2015.04.030. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laleman W, Verraes K, Van Steenbergen W, Cassiman D, Nevens F, Van der Merwe S, et al. Usefulness of the single-operator cholangioscopy system SpyGlass in biliary disease: a single-center prospective cohort study and aggregated review. Surg Endosc. 2017;31:2223–2232. doi: 10.1007/s00464-016-5221-2. [DOI] [PubMed] [Google Scholar]
- 13.Njei B, McCarty TR, Varadarajulu S, Navaneethan U. Cost utility of ERCP-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Gastrointest Endosc. 2017;85:773–781. doi: 10.1016/j.gie.2016.08.020. e10. [DOI] [PubMed] [Google Scholar]
- 14.Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video) Gastrointest Endosc. 2007;65:832–841. doi: 10.1016/j.gie.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Shah RJ, Raijman I, Brauer B, Gumustop B, Pleskow DK. Performance of a fully disposable, digital, single-operator cholangiopancreatoscope. Endoscopy. 2017;49:651–658. doi: 10.1055/s-0043-106295. [DOI] [PubMed] [Google Scholar]
- 16.Chen YK, Parsi MA, Binmoeller KF, Hawes RH, Pleskow DK, Slivka A, et al. Single-operator cholangioscopy in patients requiring evaluation of bile duct disease or therapy of biliary stones (with videos) Gastrointest Endosc. 2011;74:805–814. doi: 10.1016/j.gie.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Itoi T, Neuhaus H, Chen YK. Diagnostic value of image-enhanced video cholangiopancreatoscopy. Gastrointest Endosc Clin N Am. 2009;19:557–566. doi: 10.1016/j.giec.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Varadarajulu S, Bang JY, Hasan MK, Navaneethan U, Hawes R, Hebert-Magee S. Improving the diagnostic yield of single-operator cholangioscopy-guided biopsy of indeterminate biliary strictures: ROSE to the rescue? Gastrointest Endosc. 2016;84:681–687. doi: 10.1016/j.gie.2016.03.1497. [DOI] [PubMed] [Google Scholar]
- 19.Sethi A, Chen YK, Austin GL, Brown WR, Brauer BC, Fukami NN, et al. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: a single-center experience. Gastrointest Endosc. 2011;73:251–256. doi: 10.1016/j.gie.2010.08.058. [DOI] [PubMed] [Google Scholar]