Abstract
Background: Bladder cancer mortality rates have been elevated in northern New England for at least five decades. Incidence rates in Maine, New Hampshire, and Vermont are about 20% higher than the United States overall. We explored reasons for this excess, focusing on arsenic in drinking water from private wells, which are particularly prevalent in the region.
Methods: In a population-based case-control study in these three states, 1213 bladder cancer case patients and 1418 control subjects provided information on suspected risk factors. Log transformed arsenic concentrations were estimated by linear regression based on measurements in water samples from current and past homes. All statistical tests were two-sided.
Results: Bladder cancer risk increased with increasing water intake ( Ptrend = .003). This trend was statistically significant among participants with a history of private well use ( Ptrend = .01). Among private well users, this trend was apparent if well water was derived exclusively from shallow dug wells (which are vulnerable to contamination from manmade sources, Ptrend = .002) but not if well water was supplied only by deeper drilled wells ( Ptrend = .48). If dug wells were used pre-1960, when arsenical pesticides were widely used in the region, heavier water consumers (>2.2 L/day) had double the risk of light users (<1.1 L/day, Ptrend = .01). Among all participants, cumulative arsenic exposure from all water sources, lagged 40 years, yielded a positive risk gradient ( Ptrend = .004); among the highest-exposed participants (97.5th percentile), risk was twice that of the lowest-exposure quartile (odds ratio = 2.24, 95% confidence interval = 1.29 to 3.89).
Conclusions: Our findings support an association between low-to-moderate levels of arsenic in drinking water and bladder cancer risk in New England. In addition, historical consumption of water from private wells, particularly dug wells in an era when arsenical pesticides were widely used, was associated with increased bladder cancer risk and may have contributed to the New England excess.
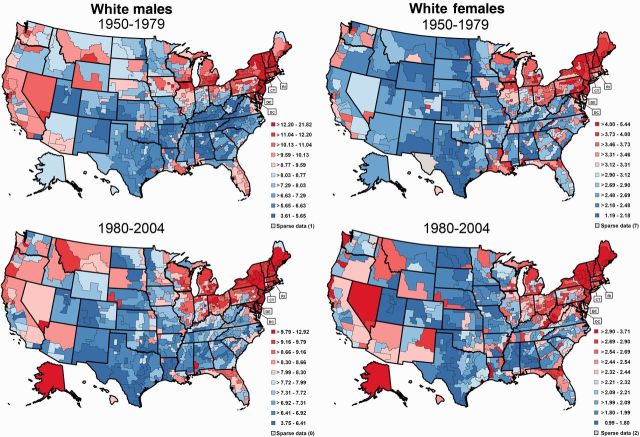
Bladder cancer mortality rates have been elevated in northern New England for at least five decades ( Figure 1 ) ( 1 ). Incidence rates for Maine, New Hampshire, and Vermont show a similar pattern with rates between 2001 and 2004 about 20% higher than that for the United States overall. In 2001 to 2004, the age-adjusted incidence rate in these three states combined was 38.8/100 000 compared with 32.0/100 000 in the United States. This elevation is particularly provocative because bladder cancer rates in the United States tend to be higher in urban compared with rural areas ( 2 , 3 ). The persistent excess in New England has been observed in both sexes, suggesting the role of a shared environmental etiologic factor. Historically, a number of exposures have been hypothesized to be responsible, including established factors such as smoking and occupation ( 4 , 5 ) and suspected risk factors that are prevalent in the region (French/French Canadian ancestry [ 4 ], consumption of shellfish [ 6 ], and bracken fern [fiddlehead greens] [ 7 ], and use of wood burning stoves [exposure to polycyclic aromatic hydrocarbons] [ 5 , 8 ]). More recently, it was recognized that a unique feature of this region is the high proportion of the population using private wells for drinking water ( 9 , 10 ). Because of the region’s geology, well water in northern New England often contains low-to-moderate levels of arsenic (generally <100 µg/L) ( 9 ). Additionally, extensive use of arsenical pesticides from the 1920s to 1960s on blueberry, apple, and potato crops was a manmade source of arsenic in the region ( 11 ). Arsenic is an established cause of bladder cancer, largely based on observations in highly exposed populations (≥100 μg/L) ( 5 , 12 ); however, emerging evidence from two studies, including one in New Hampshire, suggests that low-to-moderate levels may also increase bladder cancer risk ( 13 , 14 ).
Figure 1.
Bladder cancer mortality rates (age-adjusted 2000 US population per 100 000) among white men and women by state economic area and time period (1950-1979 and 1980-2004).
To explore possible reasons for the excess incidence of bladder cancer in northern New England, we conducted a large, comprehensive, population-based case-control study in Maine, New Hampshire, and Vermont. We examined the role of known and suspected bladder cancer risk factors with a focus on private well water consumption and arsenic levels in drinking water.
Methods
Study Population
As described elsewhere ( 15 ), case patients included all patients with histologically confirmed carcinoma of the urinary bladder (including carcinoma in situ) newly diagnosed between 2001 and 2004 among residents of Maine, New Hampshire, and Vermont, age 30 to 79 years. Patients were ascertained through hospital pathology departments and hospital and state cancer registries.
We interviewed 1213 bladder cancer patients (64.6% of 1878 eligible). The median time from diagnosis to interview was 5.9 months. Based on a review of diagnostic slides by the study’s expert pathologist (AS) ( 16 ), 20 case patients with no evidence of bladder cancer were excluded. We also excluded 44 case patients with missing information on arsenic exposure and 70 nonwhite case patients because only about 5% of the population in these states is nonwhite and the excess risk in the region is apparent only in whites ( 1 ); 1079 case patients remained.
Control subjects were selected randomly from state Department of Motor Vehicle (DMV) records (age 30-64 years) and Centers for Medicare and Medicaid Services (CMS) beneficiary records (age 65-79 years), frequency matched to case patients by state, sex, and five-year age group at diagnosis. We interviewed 1418 (594 DMV, 824 CMS) control subjects (64.8% of eligible DMV and 64.7% of eligible CMS control subjects). We excluded 47 control subjects missing information on arsenic exposure and 84 nonwhite control subjects; 1287 control subjects remained.
All participants gave written consent. The study protocol was approved by the institutional review boards of the National Cancer Institute, the US Geological Survey, Westat, Inc., Geisel School of Medicine at Dartmouth, and the departments of health for the states of Maine, New Hampshire, and Vermont.
Interviewed case patients and control subjects were comparable with respect to age at diagnosis/interview, sex, state, and race/ethnicity ( Supplementary Table 1 , available online).
Risk Factors
Information on ancestry, smoking, occupation, and use of wood-burning stoves was obtained by in-person interview. Shellfish and bracken fern intakes were assessed through a self-administered dietary history questionnaire ( 17 ) on usual adult diet over the five years preceding the interview. We queried participants about the amount of water they had typically consumed over their lifetime, including water alone and water from beverages and foods made with water, and used this information to calculate their total water intake (L/day), hereafter referred to as “drinking water” intake. The lifetime arsenic exposure assessment is described in the Supplementary Material (available online). We focus here on four arsenic exposure metrics: 1) average drinking water arsenic concentration (µg/L), calculated by summing the concentration for each year and dividing by the number of years with an assigned arsenic value; 2) average daily arsenic intake (µg/day), calculated by multiplying the average concentration by the drinking water intake (L/day); 3) cumulative intake (mg), calculated by multiplying the average daily intake by the number of days with an assigned arsenic level; and 4) number of years drinking from a source containing more than 10 μg/L arsenic (current regulatory limit). We were able to assign an arsenic level for at least 85% of the lifetime exposure-years for 88.2% of the case patients and 89.5% of the control subjects.
Statistical Analysis
We computed odds ratios (ORs) and 95% confidence intervals (CIs) for each risk factor using unconditional logistic regression models, adjusting for age, sex, Hispanic ethnicity, state of residence, smoking, education, employment in a high-risk occupation ( 18 ), and exposure to disinfection byproducts (represented by total trihalomethanes [THMs]) ( 19 ), which are possible bladder carcinogens that occur only in chlorinated public water supplies ( 20 ). For arsenic metrics 1-3, we also conducted analyses lagged 10, 20, 30, 40, and 50 years ( Supplementary Table 2 , available online). The Odds ratios peaked with a 40-year lag, and thus we also present results lagged 40 years.
To test for linear trend, we used the Wald test, treating the median value for each category among control subjects as continuous. To test for heterogeneity between trends, we included an interaction term in the model and conducted a likelihood ratio test of the statistical significance of the interaction. Statistical tests were two-sided, with type I error α of .05.
We computed population-attributable risks (PARs) using the method of Bruzzi et al. ( 21 ) and their two-sided 95% confidence intervals by the method of Benichou and Gail ( 22 ). The PARs were adjusted for the same potential confounding variables as the odds ratios.
Results
Of the known and suspected factors that are unrelated to drinking water ( Table 1 ), statistically significantly increased risk was observed only for smoking and occupation, both well-established bladder cancer risk factors ( 5 ). The PARs for smoking (men: 55.4%, women: 50.8%) were comparable with those observed in a recent nationwide cohort study (men: 50%, women: 52%) ( 23 ). The PARs for high-risk occupation (men: 26.2%, women: 13.7%) were also consistent with previous studies including a national, population-based case-control study (men: 21-25%, women: 11%) ( 5 , 24 , 25 ). Because the PARs for these risk factors were similar to those observed elsewhere in the country, neither is likely to have played an important role in the New England bladder cancer excess.
Table 1.
ORs * and 95% CIs for bladder cancer according to risk factors unrelated to drinking water, suspected of contributing to the bladder cancer excess in Maine, New Hampshire, and Vermont, 2001–2004
| Risk factor | Case patients † | Control subjects † | OR (95% CI) |
|---|---|---|---|
| Any French/French Canadian ancestry ‡ | |||
| No | 793 | 950 | 1.00 (Referent) |
| Yes | 330 | 384 | 0.99 (0.82 to 1.20) |
| Shellfish consumption (how often ate shellfish) | |||
| 1–6 times/y | 303 | 348 | 1.00 (Referent) |
| 7–11 times/y | 222 | 281 | 0.90 (0.70 to 1.16) |
| 1 time/mo | 161 | 199 | 0.96 (0.73 to 1.27) |
| 2–3 times/mo | 193 | 236 | 0.94 (0.72 to 1.22) |
| 1 time/wk | 64 | 82 | 0.90 (0.61 to 1.33) |
| 2+ times/wk | 27 | 26 | 1.14 (0.63 to 2.06) |
| Bracken fern (fiddle head greens) consumption (average monthly servings) | |||
| Did not eat | 822 | 943 | 1.00 (Referent) |
| ≤0.15 | 86 | 128 | 0.82 (0.60 to 1.12) |
| >0.15–0.57 | 66 | 64 | 1.40 (0.95 to 2.06) |
| >0.57–4.29 | 73 | 115 | 0.69 (0.50 to 0.97) |
| >4.29 | 72 | 78 | 1.18 (0.82 to 1.69) |
| Cumulative wood stove use (cord-years) | |||
| None | 240 | 239 | 1.00 (Referent) |
| >0–78 | 281 | 352 | 0.86 (0.67 to 1.11) |
| >78–172 | 140 | 178 | 0.81 (0.60 to 1.10) |
| >172–396 | 88 | 138 | 0.75 (0.53 to 1.06) |
| >396 | 24 | 35 | 0.88 (0.49 to 1.58) |
| Smoking status § | |||
| Never smoker | 171 | 444 | 1.00 (Referent) |
| Occasional smoker | 21 | 39 | 1.46 (0.82 to 2.60) |
| Former smoker | 578 | 664 | 2.22 (1.79 to 2.77) |
| Current smoker | 352 | 186 | 5.20 (4.00 to 6.75) |
| High-risk occupation ‖ | |||
| Never | 276 | 456 | 1.00 (Referent) |
| Ever | 837 | 869 | 1.50 (1.24 to 1.82) |
*Odds ratios are adjusted for age (<55, 55–64, 65–74, ≥75 years), sex, Hispanic ethnicity (yes or no), state of residence (Maine, New Hampshire, or Vermont), smoking status (never, occasional, former or current smokers), and high-risk occupation (yes or no). CI = confidence interval; OR = odds ratio.
†The following number of case patients and control subjects were excluded because of missing information: shellfish consumption, 152 case patients/162 control subjects; bracken fern consumption, 4 case patients/6 control subjects; wood stove use (cord-years), 350 case patients/392 control subjects; smoking, 1 case patient/1 control subject; high-risk occupation (never worked, 10 case patients/9 control subjects).
‡Any French birth place/ethnicity or born in Quebec and both parents born in Canada.
§“Never smokers” were defined as subjects who smoked fewer than 100 cigarettes over their lifetime. “Occasional smokers” smoked more than 100 cigarettes but never consumed cigarettes regularly (ie, at least 1 cigarette per day for at least 6 months). “Regular smokers” smoked at least 1 cigarette per day for 6 months or more. Regular smokers were further categorized as “former smokers” (ie, quit smoking one year or more before the diagnosis date for case patients or selection date for control subjects) or “current smokers” (ie, smoking at the time of interview or quit within one year of the date of diagnosis/selection).
‖High-risk occupation was defined as a priori “suspect occupation” with an odd ratio in this study of 1.1 or higher based on 15 or more exposed individuals ( 18 ).
We observed a statistically significant trend in risk with increasing drinking water intake (L/day) from all sources ( Ptrend = .003) ( Table 2 ). Compared with participants in the lowest quartile (≤1.1 L/day), the odds ratios were 1.07 (95% CI = 0.83 to 1.38), 1.17 (95% CI = 0.91 to 1.50), 1.22 (95% CI = 0.94 to 1.59), and 1.86 (95% CI = 1.23 to 2.81) for water intake categories corresponding to the 25th, 50th, 75th and 95th percentiles among control subjects, respectively.
Table 2.
ORs * and 95% CIs for bladder cancer according to drinking water intake and private well type, Maine, New Hampshire, and Vermont, 2001–2004
| Drinking water intake from all sources, L/d † | Case patients | Control subjects | OR (95% CI) | Case patients | Control subjects | OR (95% CI) |
|---|---|---|---|---|---|---|
| Overall | ||||||
| ≤1.1 | 226 | 327 | 1.00 (Referent) | |||
| >1.1–1.5 | 246 | 328 | 1.07 (0.83 to 1.38) | |||
| >1.5–2.2 | 282 | 325 | 1.17 (0.91 to 1.50) | |||
| >2.2–3.8 | 243 | 254 | 1.22 (0.94 to 1.59) | |||
| >3.8 | 82 | 53 | 1.86 (1.23 to 2.81) | |||
| P trend ‡ = .003 | ||||||
| Never-used private well | Ever-used private well § | |||||
| ≤1.1 | 61 | 62 | 1.00 (Referent) | 165 | 265 | 1.00 (Referent) |
| >1.1–1.5 | 68 | 82 | 0.85 (0.50 to 1.43) | 178 | 246 | 1.15 (0.85 to 1.54) |
| >1.5–2.2 | 65 | 92 | 0.70 (0.42 to 1.17) | 217 | 233 | 1.38 (1.03 to 1.84) |
| >2.2–3.8 | 63 | 48 | 1.16 (0.66 to 2.03) | 180 | 206 | 1.25 (0.93 to 1.69) |
| >3.8 | 17 | 9 | 1.94 (0.76 to 4.98) | 65 | 44 | 1.84 (1.15 to 2.93) |
| P trend ‡ = .13 | P trend ‡ = .01 | |||||
| P heterogeneity ‖ = .71 | ||||||
| Ever-used drilled well and never-used dug well § | Ever-used dug well and never-used drilled well § | |||||
| ≤1.1 | 89 | 144 | 1.00 (Referent) | 21 | 34 | 1.00 (Referent) |
| >1.1–1.5 | 86 | 105 | 1.25 (0.82 to 1.90) | 15 | 38 | 0.58 (0.22 to 1.48) |
| >1.5–2.2 | 108 | 117 | 1.42 (0.95 to 2.13) | 19 | 24 | 1.01 (0.40 to 2.59) |
| >2.2–3.8 | 69 | 110 | 0.85 (0.55 to 1.31) | 33 | 18 | 2.28 (0.91 to 5.71) |
| >3.8 | 29 | 16 | 2.03 (0.96 to 4.30) | 12 | 5 | 4.01 (1.06 to 15.14) |
| P trend ‡ = .48 | P trend ‡ = .002 | |||||
| P heterogeneity ‖ = .01 | ||||||
| Any dug well use before 1960§¶ | Dug well use only after 1960 § | |||||
| ≤1.1 | 39 | 64 | 1.00 (Referent) | 37 | 57 | 1.00 (Referent) |
| >1.1–1.5 | 57 | 84 | 1.19 (0.68 to 2.07) | 35 | 57 | 1.00 (0.50 to 2.00) |
| >1.5–2.2 | 59 | 60 | 1.67 (0.94 to 2.97) | 50 | 56 | 1.06 (0.55 to 2.05) |
| >2.2–3.8 | 62 | 51 | 1.93 (1.08 to 3.44) | 49 | 45 | 1.91 (0.97 to 3.76) |
| >3.8 | 20 | 14 | 2.27 (0.97 to 5.32) | 16 | 14 | 1.28 (0.49 to 3.39) |
| P trend ‡ = .01 | P trend ‡ = .15 | |||||
| P heterogeneity ‖ = .51 | ||||||
*Odds ratios are adjusted for age (<55, 55–64, 65–74, ≥75 years), sex, Hispanic ethnicity (yes or no), state of residence (Maine, New Hampshire, or Vermont), smoking status (never, occasional, former or current smokers), high-risk occupation (yes or no), and exposure to disinfection byproducts as represented by total trihalomethanes (THM; ≤15.7, >15.7-26.8, >26.8-37.1, >37.1-45.7, >45.7 µg/L, corresponding to categories of average concentration at or below the 50th, 75th, 90th, and 95th percentiles, respectively). CI = confidence interval; OR = odds ratio.
†Total amount typically consumed over the participant's lifetime including water alone and water from beverages and foods made with water. The cutpoints were quartiles among all control subjects in the study, with a further split of the highest quartile at the 95th percentile.
‡ Ptrend based on a two-sided Wald test for linear trend.
§Private well use only in three study states.
‖ Ptrend based on a two-sided likelihood ratio test of interaction.
¶Adjusted also for duration of drilled well use (<15, 15-29, 30+ years).
Because of the high prevalence of private well use in northern New England (46.0% among control subjects in this study compared with 15% in the total US [ 26 ]), we examined the association between water intake and bladder cancer risk according to whether subjects ever used private wells as their primary drinking water source ( Table 2 ). The trend in risk with water intake was statistically significant among private well users ( Ptrend = .01) despite the absence of an increased risk among those who had ever used a private well compared with those who had never used a private well (OR = 0.88, 95% CI = 0.72 to 1.09) (data not shown). Duration of private well use was not associated with risk (data not shown). Among those who never used private wells, no trend was apparent although risk was elevated in the highest water intake category (OR = 1.94, 95% CI = 0.76 to 4.98) ( Table 2 ).
We further examined the association with water intake by type of private well, both in subjects whose private well use was limited to dug wells and subjects whose private well use was limited to drilled wells. We found a strong, statistically significant trend in risk with increasing water intake with exclusive use of dug wells ( Ptrend = .002), with a quadrupling of risk in the highest water intake category (OR = 4.01, 95% CI = 1.06 to 15.14) ( Table 2 ). No trend with water intake was present among those with exclusive use of drilled wells ( P = .48) although heavy water drinkers had a statistically marginally significant doubling of risk. The trends in water intake among exclusive dug well users compared with exclusive drilled well users were statistically significantly different ( Pheterogeneity = .01). Among heavy water drinkers (>2.2 L/day), the odds ratio for exclusive use of a dug well was 1.87 (95% CI = 1.05 to 3.33) (data not shown).
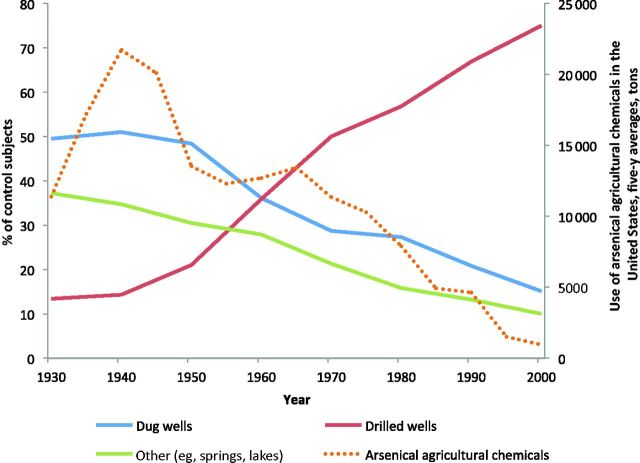
We explored the potential influence on risk of arsenical pesticide use as a possible source of arsenic contamination of dug wells in this region during the past century ( 11 ). Although information on historical arsenical pesticide application rates in New England is limited, reports indicate that use was highest between 1920 and 1960 ( Figure 2 ) ( 11 ). Among those who used dug wells before 1960, we observed a statistically significant trend in risk with increasing water intake ( Ptrend = .01), with a two-fold risk if consumption exceeded 2.2 L/day ( Table 2 ). No association was apparent among subjects using dug wells only after 1960 ( Ptrend = .15). We then developed a crude proxy for exposure to arsenical pesticides using information on historic (1935-1977) acreage planted with blueberries, apples, and potatoes in New England by census tract ( 27 ), census tract locations of participants’ past residences, and number of years of pre-1960 dug well use by home (see Supplementary Material ). We observed no effect of water intake among participants in the lowest quartile of cumulative acres per square mile (0-6.1), but a statistically significant trend in risk with increasing water intake was apparent among subjects above the lowest quartile (>6.1 acres per square mile, Ptrend = .004), with water intake odds ratios of 1.00, 1.08 (95% CI = 0.56 to 2.07), 1.53 (95% CI = 0.76 to 3.06), and 2.30 (95% CI = 1.20 to 4.40) ( Supplementary Table 3 , available online).
Figure 2.
Change in prevalence of residential well use among control subjects by well type in Maine, New Hampshire, and Vermont and use of arsenical agricultural chemicals in the United States (1930-2000). Source: US Department of Agriculture. 1935-1997. Census of Agriculture. Vol. 1, Geographic Area Series. Table 1 , County Summary Highlights. National Agriculture Statistics Service, Washington, DC. The use of arsenical agricultural chemicals is expected to be similar in New England ( 11 ).
Table 3 shows the bladder cancer risk associated with the primary arsenic exposure metrics. For lifetime exposure unlagged, no evidence of an association with average arsenic concentration in drinking water was apparent. Risk increased slightly, but was not statistically significant, with increasing average daily and cumulative arsenic intakes unlagged, with a 50% to 60% increased risk among subjects in the 97.5% percentile of each metric. Lagging exposure 40 years yielded stronger associations for all three metrics. For average arsenic concentration, the odds ratio was 1.49 (95% CI = 0.85 to 2.61) in the highest exposure category (>8.7 μg/L), with no trend in risk with increasing exposure. For average daily and cumulative arsenic intakes lagged 40 years, trends with increasing exposure were statistically significant ( Ptrend = .01 and .004, respectively). For the latter, the highest-exposed participants (>124.8 mg) had twice the risk of those in the lowest-exposure quartile (<3.5 mg) (OR = 2.24, 95% CI = 1.29 to 3.89). Restricting these analyses to participants with greater than 85% coverage had little impact on risk estimates. The PAR for cumulative arsenic intake above the lowest quartile, lagged 40 years, was 13.8% (95%CI = 0 to 29.2%). For those drinking from a source containing more than 10 μg/L for 40 years or more, we observed a modestly increased risk (OR = 1.47, 95% CI = 0.50 to 4.26) based on small numbers (8 case patients, 7 control subjects), with no evidence of a trend for duration ( Table 3 ). Our evaluation of additional arsenic exposure metrics, including limiting exposure to current homes with measured values and maximum level of exposure, did not suggest increased risk. We observed no pattern of risk by tumor stage and grade for any exposure metric (data not shown).
Table 3.
ORs * and 95% CIs for Bladder Cancer According to Lifetime Arsenic Exposure, Maine, New Hampshire and Vermont, 2001–2004
| Unlagged |
Lagged 40 y |
||||||
|---|---|---|---|---|---|---|---|
| Arsenic exposure | Case patients | Control subjects | OR (95% CI) | Arsenic exposure | Case patients | Control subjects | OR (95% CI) |
| Average arsenic concentration, μg/L † | Average arsenic concentration, μg/L † | ||||||
| ≤0.5 | 303 | 325 | 1.00 (Referent) | ≤0.4 | 280 | 314 | 1.00 (Referent) |
| >0.5–1.0 | 226 | 318 | 0.77 (0.60 to 0.98) | >0.4–0.7 | 260 | 309 | 0.91 (0.71 to 1.17) |
| >1.0–2.1 | 281 | 323 | 0.97 (0.76 to 1.24) | >0.7–1.6 | 233 | 304 | 0.93 (0.72 to 1.20) |
| >2.1–7.0 | 225 | 259 | 0.98 (0.74 to 1.28) | >1.6–5.7 | 220 | 248 | 1.06 (0.81 to 1.40) |
| >7.0–10.4 | 18 | 30 | 0.64 (0.33 to 1.23) | >5.7–8.7 | 26 | 33 | 0.92 (0.51 to 1.66) |
| >10.4 | 26 | 32 | 1.10 (0.61 to 2.00) | >8.7 | 37 | 29 | 1.49 (0.85 to 2.61) |
| P trend ‡ = .82 | P trend ‡ = .16 | ||||||
| Average daily arsenic intake, µg/d † | Average daily arsenic intake, µg/d † | ||||||
| ≤0.7 | 244 | 327 | 1.00 (Referent) | ≤0.5 | 250 | 315 | 1.00 (Referent) |
| >0.7–1.6 | 270 | 323 | 1.05 (0.82 to 1.35) | >0.5–1.0 | 250 | 311 | 0.98 (0.76 to 1.27) |
| >1.6–3.6 | 292 | 324 | 1.16 (0.91 to 1.49) | >1.0–2.5 | 266 | 309 | 1.15 (0.89 to 1.48) |
| >3.6–13.2 | 213 | 251 | 1.16 (0.88 to 1.52) | >2.5–8.5 | 210 | 243 | 1.16 (0.88 to 1.53) |
| >13.2–19.8 | 28 | 33 | 0.95 (0.53 to 1.69) | >8.5–13.5 | 37 | 30 | 1.69 (0.96 to 2.96) |
| >19.8 | 32 | 29 | 1.53 (0.86 to 2.74) | >13.5 | 43 | 29 | 1.81 (1.05 to 3.12) |
| P trend ‡ = .28 | P trend ‡ = .01 | ||||||
| Cumulative arsenic intake, mg † | Cumulative arsenic intake, mg † | ||||||
| ≤15.7 | 228 | 327 | 1.00 (Referent) | ≤3.5 | 233 | 313 | 1.00 (Referent) |
| >15.7–34.5 | 288 | 321 | 1.18 (0.92 to 1.52) | >3.5–8.8 | 269 | 308 | 1.13 (0.87 to 1.47) |
| >34.5–77.0 | 263 | 321 | 1.13 (0.88 to 1.46) | >8.8–22.4 | 260 | 311 | 1.21 (0.92 to 1.58) |
| >77.0–291.0 | 235 | 257 | 1.32 (1.00 to 1.73) | >22.4–83.5 | 213 | 247 | 1.28 (0.95 to 1.72) |
| >291.0–483.6 | 33 | 32 | 1.30 (0.74 to 2.28) | >83.5–124.8 | 34 | 29 | 1.72 (0.96 to 3.10) |
| >483.6 | 32 | 29 | 1.60 (0.90 to 2.87) | >124.8 | 47 | 29 | 2.24 (1.29 to 3.89) |
| P trend ‡ = .12 | P trend ‡ = .004 | ||||||
| No. of y drinking water with >10 μg/L arsenic | |||||||
| Zero years ≥1 µg/L§ | 198 | 212 | 1.00 (Referent) | ||||
| <10 | 793 | 962 | 0.90 (0.71 to 1.14) | ||||
| 10–24 | 68 | 83 | 0.92 (0.61 to 1.38) | ||||
| 25–39 | 12 | 23 | 0.60 (0.27 to 1.32) | ||||
| 40+ | 8 | 7 | 1.47 (0.50 to 4.26) | ||||
| P trend ‡ = .85 | |||||||
*Odds ratios are adjusted for age (<55, 55–64, 65–74, ≥75 years), sex, Hispanic ethnicity (yes or no), state of residence (Maine, New Hampshire, or Vermont), smoking status (never, occasional, former or current smokers), high-risk occupation (yes or no), and exposure to disinfection byproducts as represented by total trihalomethanes (THM; ≤15.7, >15.7-26.8, >26.8-37.1, >37.1-45.7, >45.7 µg/L, corresponding to categories of average concentration at or below the 50th, 75th, 90th, and 95th percentiles, respectively). CI = confidence interval; OR = odds ratio.
†The cutpoints were quartiles among all control subjects in the study, with a further split of the highest quartile at the 95th and 97.5th percentiles. Average arsenic concentration (µg/L) was calculated by summing the weighted arsenic concentrations for each year and dividing by the total number of years with an assigned arsenic value. Average daily arsenic (µg/day) was calculated by multiplying each participant’s average arsenic concentration by the amount of daily drinking water intake recorded during the interview. Cumulative arsenic (mg) exposure was calculated by multiplying each participant’s average arsenic concentration by the amount of daily drinking water intake and total number of days with an assigned arsenic value.
‡ Ptrend based on a two-sided Wald test for linear trend.
§Reference category includes only subjects with no water source containing arsenic ≥1 μg/L.
Discussion
The primary purpose of our study was to identify the factors responsible for the elevated incidence of bladder cancer in northern New England. We examined several a priori suspect exposures including arsenic in drinking water, smoking, occupation, French/Canadian ancestry, shellfish and bracken fern consumption, and use of wood-burning stoves. Factors unrelated to drinking water do not appear to have played an important role because they were either not associated with risk or the PARs (ie, smoking and occupation) were similar to those in national studies. We observed a trend in risk with increasing daily water intake, suggesting the possible presence of a bladder carcinogen in the drinking water. We explored whether this trend might be attributable to arsenic in private wells, which are highly prevalent in the region. We observed a strong association with water intake among participants who derived all of their private well water from dug wells and among those who used dug wells before 1960. This is noteworthy as arsenical pesticides were widely used in this region during the first half of the 20 th century ( Figure 2 ) and dug wells were potentially vulnerable to contamination from arsenic leaching from the treated soils ( 28 ). That the water intake/bladder cancer association was most pronounced among people who drank from dug wells during the period of heavy arsenical pesticide use and absent in areas with lowest use points to arsenic as a possible link between water intake and bladder cancer risk.
We observed statistically significant and consistent trends in risk with average daily arsenic intake and cumulative arsenic intake, lagged 40 years. Four previous studies have suggested an association between low-to-moderate arsenic exposure and bladder cancer risk ( 13 , 14 , 29 , 30 ). To our knowledge, this is the first large-scale study to report a statistically significant exposure-response relationship for arsenic in drinking water and bladder cancer in a low to moderately exposed population. Our 40-year lag period for cumulative arsenic is remarkably consistent with the observed elevated risk among heavily-exposed subjects in Chile who ceased arsenic exposure more than 40 years before diagnosis ( 31 , 32 ). Such Chileans experienced a seven-fold risk compared with those with little or no exposure. In our study, average arsenic concentration lagged 40 years appeared unrelated to risk, although those with exposures in the top 97.5th percentile experienced a 50% elevated risk. The contrast in our findings for cumulative arsenic intake and average arsenic concentration underscores the importance of incorporating water intake when estimating an individual’s total arsenic exposure in low to moderately exposed populations such as that in northern New England.
Arsenic levels in drinking water were estimated based on an extensive exposure assessment effort involving drinking water sampling and statistical modeling ( 33 ). Despite our best efforts, estimates were accompanied by substantial uncertainty due, in part, to the large spatial variability of arsenic in groundwater over short distances ( 33 ) and our limited ability to account for potentially important temporal variability. For example, the overall agreement between a dichotomous classification of 2 or fewer or more than 2 µg/L arsenic in over 1400 drilled wells based on predicted and observed values in our study did not exceed 60%, and the specificity and sensitivity were only 57% and 77%, respectively ( 33 ). These uncertainties can lead to nondifferential exposure misclassification and an underestimation of risk ( 34 ). Importantly, our exposure assessment was unable to account accurately for the potential effect of historical arsenical pesticide use on arsenic levels in dug wells. To our knowledge, no data are available on arsenic levels in dug wells over 50 years ago, and prediction modeling is difficult because of uncertainties related to changes in land use, pesticide application rates, and the complex mechanisms governing arsenic fate and transport. Although applied arsenic has been shown to be adsorbed strongly in the soil ( 35 ), excess arsenic could have been transported to the water table if the sorption capacity of the soil was exceeded because of pH changes and anoxic conditions associated with agricultural practices ( 36–40 ).
It is noteworthy that the longstanding excess bladder cancer mortality rates in this region date back to the 1950s or earlier ( Figure 1 ), when arsenical pesticides were still widely used and dug wells were commonplace ( 11 ). Well use patterns in northern New England have changed markedly over the past century. Although deep-drilled wells now far outnumber dug wells, the reverse was true during the first half of the 20th century ( Figure 2 ). If ingestion of arsenic-contaminated water from dug wells during this time period was indeed causing bladder cancer, given the widespread use of private wells in this area relative to the rest of the country, this may have contributed to the historical bladder cancer excess in this region.
Ingestion of water from drilled wells containing low-to-moderate (and sometimes high) levels of predominantly naturally occurring arsenic originating from minerals in the bedrock may have also contributed to the bladder cancer excess. Heavy consumers of water who drew all of their private well water from drilled wells experienced a doubling of risk, with no clear trend in risk with increasing water intake. Recent sampling demonstrates that drilled wells currently are the predominant source of elevated arsenic in this region’s drinking water supplies ( 9 , 33 , 39 ); future research should focus on the health effects of the low-to-moderate levels found in these wells.
It is possible that a historical drinking water contaminant other than, or in interaction with, arsenic explains the associations observed here. We measured levels of two other suspect bladder carcinogens, nitrate and gross alpha emissions, in private wells of current homes and found that more than 50% were undetectable. Among those with detectable levels, concentrations were similar in case patients and control subjects, providing no evidence of increased risk for either contaminant. Thus, arsenic appears to be the only recognized bladder carcinogen ( 5 ) known to be present in the well water of northern New England ( 9 ).
The most important limitation of our study is the imprecision of the arsenic exposure assessment, hampering our ability to detect an effect for average arsenic concentration in our study population and, thus, to accurately quantify the contribution of arsenic exposure to the New England bladder cancer excess. Therefore, we believe our PAR estimate of 13.8% for cumulative arsenic lagged 40 years is likely to be an underestimate because nondifferential misclassification of exposure results in underestimation of risk. In addition, our data on water intake and the proportion from the home tap are self-reported and recall bias is a concern in case-control studies. To address this concern, we queried subjects regarding whether they made a major change in their total water intake during their adult life. When we excluded subjects reporting any major change in water intake, the odds ratios by water intake were not affected (ORs = 1.00, 1.25, 1.31, 1.32, and 2.27 for water intakes of ≤ 1.1, >1.1-1.5, >1.5-2.2, >2.2-3.8, and >3.8 L/day, respectively), suggesting that recall bias is not a major concern. Additionally, we asked subjects if they were aware of any news reports of health risks that may be related to drinking water containing arsenic. The trend in risk with increasing water intake was stronger among subjects who reported no knowledge that drinking water could be injurious to health (unaware = 1.00, 0.89, 1.37, 1.39, and 2.18; aware = 1.00, 1.29, 1.02, 1.13, and 1.56 for water intakes of ≤ 1.1, >1.1-1.5, >1.5-2.2, >2.2-3.8, and >3.8 L/day, respectively). If recall bias was seriously impacting our findings, it is unlikely that unaware subjects would have a more pronounced water intake effect than aware subjects. Lastly, our response rate of 65% in both case patients and control subjects raises the question of whether selection bias could have threatened the generalizability of our findings. To address this concern, we compared respondents and nonrespondents with respect to the opportunity for exposure based on current residence at the time of the interview among subjects age 30 to 64 years (we did not have comparable data for the older control subjects). This comparison was based on the percent of subjects with a current residence outside a designated census place, which is typically a town boundary and is directly related to having a private well. For case patients, 58.7% of respondents and 62.0% of nonrespondents lived outside a designated census place and were thus likely to be private well users. For control subjects, 64.8% of respondents and 60.1% of nonrespondents lived outside a designated census place. The similarity between respondents and nonrespondents provides evidence that selection bias is likely not a serious concern in this study and that our findings are likely generalizable to the general populations of Maine, New Hampshire, and Vermont.
To our knowledge, our study is the largest case-control study to date to evaluate bladder cancer risk associated with exposure to low-to-moderate levels of arsenic. Additional strengths are the population-based design and use of histologically confirmed incident bladder cancer case patients. Risk estimates were controlled for confounding by smoking and other bladder cancer risk factors, including exposure to THMs from drinking water.
In our large, population-based case-control study, we observed an association between lifetime cumulative arsenic exposure from drinking water and bladder cancer risk in New England. In addition, historical consumption of water from private wells, particularly from dug wells, was associated with increased bladder cancer risk and may have contributed to the longstanding bladder cancer excess in northern New England. Although challenges in estimation of historical arsenic levels in private wells precluded us from definitively indicting or exonerating arsenic as the responsible agent, our findings are consistent with a potential role for the widespread application of arsenical pesticides in agricultural areas of the region during the first half of the 20th century. Although the likelihood of exposure from dug wells has diminished in recent years because arsenical pesticides are no longer used and dug wells are rarely used, possible current exposure to arsenic in drinking water through use of deep domestic-supply wells drilled into fractured bedrock is a potential public health concern.
Funding
Supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics (contract number N02-CP-01037).
Notes
None of the authors has conflicts of interest that are relevant to the subject matter or materials discussed in the manuscript. The authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
We thank Anna McIntosh, Paul Hurwitz, Linda Lannom, Patricia Clark, Matt Airola, Abigal Flory, and Vanessa Olivo (Westat, Rockville, MD) for their support in study and data management, and Mary McAdams (IMS, Calverton, MD) for her programming support. We would like to acknowledge Dr. Michael Jones (Maine Medical Center), Sue Ledoux and Dawn Nicolaides (Maine Cancer Registry), Kimberley Walsh and Christina Robinson (Dartmouth Medical School), Dr. Masatoshi Kida (University of Vermont), William Apao and Carolyn Greene (Vermont Cancer Registry) for their contributions during the fieldwork and data collection phases. We also thank all fieldwork staff, interviewers, and data abstractors for their dedicated work, our study participants for agreeing to be part of this study, and Drs. Stephen Norton (University of Maine) and Andrew Smith (Maine Department of Health and Human Services) for their thoughtful suggestions. We thank Dr. Susan Devesa and David Check (National Cancer Institute) for their help with Figure 1 . Lastly, we would like to acknowledge Dr. Kenneth P. Cantor (Cantor Environmental, LLC, Silver Spring, MD, formerly Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD) for his valuable contribution to the study, particularly to the environmental exposure assessment.
Supplementary Material
References
- 1. U.S. National Cancer Institute, Cancer Mortality Maps . http://ratecalc.cancer.gov/ .
- 2. Howe HL. Urban-rural gradients in cancer incidence and mortality in the United States . Springfield, IL: : North American Association of Central Cancer Registries, Inc; .; 2005. . [Google Scholar]
- 3. Colli J, Lee BR, Thomas R. Population densities in relation to bladder cancer mortality rates in America from 1950 to 1994 . Int Urol Nephrol . 2012. ; 44 ( 2 ): 443 – 449 . [DOI] [PubMed] [Google Scholar]
- 4. Brown LM, Zahm SH, Hoover RN , et al. . High bladder cancer mortality in rural New England (United States): an etiologic study . Cancer Causes Control. 1995. ; 6 ( 4 ): 361 – 368 . [DOI] [PubMed] [Google Scholar]
- 5. Silverman D, Devesa SS, Moore LE , et al. . Bladder Cancer . In: Schottenfeld D, Fraumeni JF. (eds). Cancer Epidemiology and Prevention . 3rd Edition ed.Oxford: : Oxford University Press; ; 2006. . [Google Scholar]
- 6. Ling MP, Liao CM. Risk characterization and exposure assessment in arseniasis-endemic areas of Taiwan . Environ Int. 2007. ; 33 ( 1 ): 98 – 107 . [DOI] [PubMed] [Google Scholar]
- 7. Corteggio A, Urraro C, Roperto S , et al. . Phosphatidylinositol-3-kinase-AKT pathway, phospho-JUN and phospho-JNK expression in spontaneously arising bovine urinary bladder tumours . J Comp Pathol. 2010. ; 143 ( 2-3 ): 173 – 178 . [DOI] [PubMed] [Google Scholar]
- 8. Gustafson P, Ostman C, Sallsten G. Indoor levels of polycyclic aromatic hydrocarbons in homes with or without wood burning for heating . Environ Sci Technol. 2008. ; 42 ( 14 ): 5074 – 5080 . [DOI] [PubMed] [Google Scholar]
- 9. Ayotte JD, Montgomery DL, Flanagan SM , et al. . Arsenic in groundwater in eastern New England: occurrence, controls, and human health implications . Environ Sci Technol. 2003. ; 37 ( 10 ): 2075 – 2083 . [DOI] [PubMed] [Google Scholar]
- 10. Ayotte JD, Baris D, Cantor KP , et al. . Bladder cancer mortality and private well use in New England: an ecological study . J Epidemiol Community Health. 2006. ; 60 ( 2 ): 168 – 172 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D’Angelo D, Norton SA, Loiselle MC. Historical uses and fate of arsenic in Maine . Water Research Institute, Sawyer Environmental Research Center: University of Maine; ; 1996. . [Google Scholar]
- 12. IARC . IARC monographs on the evaluation of carcinogenic risks to humans: Arsenic, metals, fibres, and dusts . Lyon, France: : World Health Organization, International Agency for Research on Cancer; ; 2012. , 11 – 465 . [PMC free article] [PubMed] [Google Scholar]
- 13. Karagas MR, Tosteson TD, Morris JS , et al. . Incidence of transitional cell carcinoma of the bladder and arsenic exposure in New Hampshire . Cancer Causes Control. 2004. ; 15 ( 5 ): 465 – 472 . [DOI] [PubMed] [Google Scholar]
- 14. Bates MN, Smith AH, Cantor KP. Case-control study of bladder cancer and arsenic in drinking water . Am J Epidemiol. 1995. ; 141 ( 6 ): 523 – 530 . [DOI] [PubMed] [Google Scholar]
- 15. Baris D, Karagas MR, Verrill C , et al. . A case-control study of smoking and bladder cancer risk: emergent patterns over time . J Natl Cancer Inst. 2009. ; 101 ( 22 ): 1553 – 1561 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schned AR, Lenz P, Moore LE , et al. . Analysis of the Distribution and Temporal Trends of Grade and Stage in Urothelial Bladder Cancer in Northern New England from 1994 to 2004 . ISRN Pathol. 2012. ; 2012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu JW, Cross AJ, Baris D , et al. . Dietary intake of meat, fruits, vegetables, and selective micronutrients and risk of bladder cancer in the New England region of the United States . Br J Cancer. 2012. ; 106 ( 11 ): 1891 – 1898 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colt JS, Karagas MR, Schwenn M , et al. . Occupation and bladder cancer in a population-based case-control study in Northern New England . Occup Environ Med. 2011. ; 68 ( 4 ): 239 – 249 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beane Freeman L. Personal communication. 2015. .
- 20. Villanueva CM, Cantor KP, Grimalt JO , et al. . Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools . Am J Epidemiol. 2007. ; 165 ( 2 ): 148 – 156 . [DOI] [PubMed] [Google Scholar]
- 21. Bruzzi P, Green SB, Byar DP , et al. . Estimating the population attributable risk for multiple risk factors using case-control data . Am J Epidemiol. 1985. ; 122 ( 5 ): 904 – 914 . [DOI] [PubMed] [Google Scholar]
- 22. Benichou J, Gail MH. Variance calculations and confidence intervals for estimates of the attributable risk based on logistic models . Biometrics. 1990. ; 46 ( 4 ): 991 – 1003 . [PubMed] [Google Scholar]
- 23. Freedman ND, Silverman DT, Hollenbeck AR , et al. . Association between smoking and risk of bladder cancer among men and women . JAMA. 2011. ; 306 ( 7 ): 737 – 745 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Silverman DT, Levin LI, Hoover RN , et al. . Occupational risks of bladder cancer in the United States: I. White men . J Natl Cancer Inst. 1989. ; 81 ( 19 ): 1472 – 1480 . [DOI] [PubMed] [Google Scholar]
- 25. Silverman DT, Levin LI, Hoover RN. Occupational risks of bladder cancer among white women in the United States . Am J Epidemiol. 1990. ; 132 ( 3 ): 453 – 461 . [DOI] [PubMed] [Google Scholar]
- 26. U.S. Census, Historical census of housing tables: source of water . https://http://www.census.gov/hhes/www/housing/census/historic/water.html .
- 27. Robinson GR, Jr, Ayotte JD. The influence of geology and land use on arsenic in stream sediments and ground waters in New England, USA . Applied Geochemistry. 2006. ; 21 ( 9 ): 1482 – 1497 . [Google Scholar]
- 28. Ayuso RA, Foley NK, Robinson GRJ , et al. . Containing arsenic-enriched groundwater tracing lead isotopic compositions of common arsenical pesticides in a coastal Maine watershed. In: Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy . Amherst, MA, 2010. : Abstract 11. University of MA.
- 29. Kurttio P, Pukkala E, Kahelin H , et al. . Arsenic concentrations in well water and risk of bladder and kidney cancer in Finland . Environ Health Perspect. 1999. ; 107 ( 9 ): 705 – 710 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang YK, Huang YL, Hsueh YM , et al. . Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: a twelve-year follow-up study . Cancer Causes Control. 2008. ; 19 ( 8 ): 829 – 839 . [DOI] [PubMed] [Google Scholar]
- 31. Steinmaus CM, Ferreccio C, Romo JA , et al. . Drinking water arsenic in northern chile: high cancer risks 40 years after exposure cessation . Cancer Epidemiol Biomarkers Prev. 2013. ; 22 ( 4 ): 623 – 630 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steinmaus C, Ferreccio C, Acevedo J , et al. . Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure . Cancer Epidemiol Biomarkers Prev. 2014. ; 23 ( 8 ): 1529 – 1538 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nuckols JR, Freeman LE, Lubin JH , et al. . Estimating water supply arsenic levels in the New England Bladder Cancer Study . Environ Health Perspect. 2011. ; 119 ( 9 ): 1279 – 1285 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cantor KP, Lubin JH. Arsenic, internal cancers, and issues in inference from studies of low-level exposures in human populations . Toxicol Appl Pharmacol. 2007. ; 222 ( 3 ): 252 – 257 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Robinson GR, Jr, Larkins P, Boughton CJ , et al. . Assessment of contamination from arsenical pesticide use on orchards in the Great Valley region, Virginia and West Virginia, USA . J Environ Qual. 2007. ; 36 ( 3 ): 654 – 663 . [DOI] [PubMed] [Google Scholar]
- 36. Stollenwerk KG. Geochemical processes controlling transport of arsenic in groundwater: a review of adsorption . In: Welch AH, Stollenwerk KG , (eds). Arsenic in Ground Water: Geochemistry and Occurrence . Boston: : Kluwer Academic Publishers; ; 2003. , 67 – 100 . [Google Scholar]
- 37. Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters . Applied Geochemistry. 2002. ; 17 ( 5 ): 517 – 568 . [Google Scholar]
- 38. Garbarino JR, Bednar AJ, Rutherford DW , et al. . Environmental fate of roxarsone in poultry litter. I. Degradation of roxarsone during composting . Environ Sci Technol. 2003. ; 37 ( 8 ): 1509 – 1514 . [DOI] [PubMed] [Google Scholar]
- 39. Peters SC. Arsenic in groundwaters in the Northern Appalachian Mountain belt: a review of patterns and processes . J Contam Hydrol. 2008. ; 99 ( 1-4 ): 8 – 21 . [DOI] [PubMed] [Google Scholar]
- 40. Rutherford DW, Bednar AJ, Garbarino JR , et al. . Environmental fate of roxarsone in poultry litter. Part II. Mobility of arsenic in soils amended with poultry litter . Environ Sci Technol. 2003. ; 37 ( 8 ): 1515 – 1520 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.