Abstract
Background: Although 20% to 30% of melanomas are histopathologically ‘nevus associated,’ the majority of melanomas arise de novo, ie, in clinically normal skin with no associated nevus. We examined whether these forms of melanoma differed in their associations with clinical and histopathologic features and patient survival.
Methods: We analyzed two prospective cohorts from our institution with protocol-driven follow-up information (NYU1, n = 1024; NYU2, n = 1125). We used univariate and multivariable analyses to examine associations between de novo vs nevus-associated melanoma classification and age, anatomic site, tumor thickness, tumor ulceration, mitotic index, histological subtype, clinical stage, and survival. We tested the associations identified in NYU1 using NYU2 as a replication cohort. All tests of statistical significance were two-sided.
Results: In NYU1, de novo melanomas were associated with tumor thickness greater than 1.0 mm (odds ratio [OR] = 1.96, 95% confidence interval [CI] = 1.43 to 2.70, P < .001), ulceration (OR = 1.65, 95% CI = 1.10 to 2.54, P = .02), nodular subtype (OR = 3.26, 95% CI = 1.70 to 7.11, P = .001), greater than stage I (OR = 2.35, 95% CI = 1.65 to 3.40, P < .001), older age (OR = 1.64, 95% CI = 1.18 to 2.30, P = .004), and shorter overall survival (HR = 1.63, 95% CI = 1.22 to 2.18, P < .001). In NYU2, de novo melanoma was again statistically significantly associated with thickness greater than 1.0 mm (OR = 2.24, 95% CI = 1.72 to 2.93, P < .001), ulceration (OR = 2.88, 95% CI = 1.95 to 4.37, P < .001), nodular subtype (OR = 2.41, 95% CI = 1.75 to 3.37, P < .001), greater than stage I (OR = 2.42, 95% CI = 1.80 to 3.29, P < .001), older age (OR = 1.68, 95% CI = 1.31 to 2.17, P < .001), and shorter overall survival (HR = 2.52, 95% CI = 1.78 to 3.56, P < .001). In multivariable analysis, de novo classification was an independent, poor prognostic indicator in NYU2 (HR = 1.70, 95% CI = 1.19 to 2.44, P = .004). Male patients had a statistically significantly worse survival than female patients if their melanoma was de novo (NYU1, P < .001; NYU2, P < .001); unexpectedly, there was no sex difference in survival among patients with nevus-associated tumors.
Conclusions: These data suggest that de novo melanomas are more aggressive than nevus-associated melanomas. This classification scheme may also provide a useful framework for investigations into sex differences in melanoma outcomes.
Cutaneous melanoma is a malignant, melanocytic tumor that is often found in patients with increased numbers of melanocytic nevi, which are benign neoplasms. Whether nevi, especially clinically atypical nevi, are melanoma precursors is controversial ( 1 ). Pathology-based studies have found that 20% to 30% of melanomas contain nevus cells in histologic continuity with melanoma ( 2–9 ), suggesting direct transformation of a nevus into melanoma. Current models of melanoma pathogenesis often indicate a progression from normal melanocytes to melanoma, with nevi representing an intermediate step for certain melanoma subtypes ( 10 , 11 ). The majority of melanomas (70%–80%), however, arise de novo, ie, with no associated nevus, and the majority of melanoma patients lack clinically atypical nevi or increased numbers of nevi ( 12 , 13 ). In addition, the lifetime risk of an individual nevus transforming into melanoma has been estimated to be far less than one in 1000 ( 14 ).
At present, little is known about the host and/or tumor factors that cause melanoma to arise in normal skin vs in association with a nevus. The divergent pathway model of melanoma pathogenesis, based primarily on epidemiologic studies identifying differences in the sun exposure patterns and mole phenotypes of patients with different subtypes of melanoma ( 15–18 ), provides a conceptual framework regarding differences in nevus-associated and de novo melanoma. For example, melanomas removed from ‘nevus-prone’ patients, ie, those with increased numbers of nevi, were more frequently found in physical association with a nevus than melanomas arising in patients with few nevi (ie, ‘nevus-resistant’ patients) ( 19 ).
Whether the presence of an associated nevus has any prognostic significance for patient outcomes is uncertain. There is no consensus among the published studies, which are generally limited by small sample sizes, patient selection criteria, retrospective designs, and/or varying quality of the follow-up data ( 2–9 , 19–23 ). In the current study, we sought to examine differences between nevus-associated and de novo melanomas in two large cohorts of prospectively followed patients. We aimed to determine whether nevus-associated and de novo melanomas differ in their associations with histopathologic features and whether de novo vs nevus-associated classification is an independent prognostic variable for melanoma patient survival.
Methods
Patients
We studied two cohorts of melanoma patients with protocol-driven follow-up prospectively enrolled and treated at New York University Medical Center. The first cohort, NYU1, was comprised of patients with primary cutaneous melanoma enrolled between 1972 and 1982, with follow-up until 1993. Clinical and pathological data were collected in 415 fields, from which 11 variables were examined including age, sex, mole phenotype, primary tumor thickness, ulceration status, tumor anatomic site, tumor mitoses, histopathological subtype, histopathological association of melanoma with a nevus, clinical stage, and overall survival. Cause of death and patient status were determined at the date of last follow-up. Patient nevus phenotype was based on the number of nevi assessed by physician exam. Patients were classified as having none, few ( 1–25 ), some (26–100), or many (>100) melanocytic nevi, defined as melanocytic lesions larger than 2 mm in size. For analytical purposes, we adopted the mole phenotype scheme proposed by Whiteman et al. ( 17 ) and grouped patients as nevus-prone (ie, having ‘some’ or ‘many’ nevi) or nevus-resistant (ie, having ‘none’ or ‘few’ nevi). There were 1134 patients with available data. We excluded 86 patients where the de novo/nevus-associated classification was missing. Among the remaining patients: 20 had a second primary tumor and were excluded; three were excluded as their time to death information was missing; and three were excluded because of a time to death being 0 or less than 0.1 years. This process yielded a total of 1024 patients for the analyses.
The second cohort, NYU2, included patients prospectively enrolled in the NYU Interdisciplinary Melanoma Cooperative Group registry ( 24 ) between 2002 and 2009, with follow-up until 2013. Clinical and pathological variables were chosen in order to replicate the findings from the NYU1 cohort. Ten relevant variables were examined: age, sex, primary tumor thickness, ulceration status, tumor anatomic site, tumor mitoses, tumor histological subtype, histopathological association of melanoma with a nevus, clinical stage, and overall survival. Survival data was collected as of last patient follow-up. Patient nevus phenotype was not available for this cohort. There were 1164 patients with available data from this cohort. We excluded 30 patients missing the de novo/nevus-associated classification and nine patients with time to death of less than 0.1 year, so the analyses are based on 1125 patients. All patients were restaged according to the American Joint Committee on Cancer Melanoma staging system, 7th edition. Written informed consent was obtained from all patients in the NYU2 cohort (NYU IRB study #10362); such consent was not obtained from patients in the NYU1 cohort. Informed consent procedures/requirements were not in place during the time period of their enrollment, and by current standards the analysis of the de-identified data in the NYU1 patient cohort would not be considered human subjects research.
Statistical Analysis
Univariate analyses using chi square for categorical data and the Mann-Whitney test for continuous data were performed for all variables to determine if they were associated with de novo or nevus-associated melanoma. Patients lacking a de novo or nevus association classification (n = 86 for NYU1; n = 30 for NYU2) were excluded from these analyses. Univariate survival analysis was performed using Cox PH models and log-rank analyses to evaluate which variables were associated with survival. Multivariable logistic analysis was performed to determine which variables were statistically significantly associated with de novo vs nevus-associated subtype in the presence of other covariates. Finally, multivariable Cox PH regression analysis was used to construct survival models using all univariate variables and de novo/nevus-associated status. We used the cox.zph test in R to verify the proportional hazards assumption for using Cox PH models and concluded that the Cox PH models can be used in both NYU1 and NYU2. All analyses were performed using R. All tests of statistical significance were two-sided, and a P of less than .05 was considered statistically significant.
We chose to compare the two datasets separately rather than merging the data into one large analysis for three reasons: Firstly, there is a 20-year gap between the time when the last patient was enrolled in NYU1 and the first patient was enrolled in NYU2; secondly, analyzing the datasets separately is a more conservative approach. The sample size for each dataset is smaller than the combined dataset, so associations between the variables under study need to have higher effect size to achieve statistical significance; finally, analyzing these datasets separately allows us to test the reproducibility of our observations in the two independent datasets, providing increased confidence in the robustness of the associations we identified.
Results
Table 1 describes the baseline characteristics of patients in both cohorts. In the NYU1 cohort, 48.8% of patients were male and the median age was 53 years. The median tumor thickness was 1.3 mm, 21.8% of tumors were ulcerated, 51.7% had a mitotic index > 1, 10.7% were of the nodular histotype, and 19.3% of melanomas were nevus-associated. In the NYU2 cohort, 54.8% of patients were male, and the median age was 59 years. The median tumor thickness was 0.9mm, 18.4% of tumors were ulcerated, 60.0% had a mitotic index of 1 or greater, 26.1% were of the nodular histotype, and 31.0% were nevus-associated. In univariate ( Table 2 ) and multivariable survival analyses ( Table 3 ; Supplementary Table 1 , available online), well-established prognostic factors for melanoma survival (ie, age, tumor thickness, ulceration, mitotic index, and anatomic site on the trunk) were found to be statistically significantly associated with survival in both NYU1 and NYU2, demonstrating that these cohorts are typical of melanoma cohorts studied elsewhere.
Table 1.
Patient demographic and tumor characteristics
| Characteristics |
NYU1 (1972-1982)
|
NYU2 (2002-2009)
|
||
|---|---|---|---|---|
| Nevus-assoc | De novo | Nevus-assoc | De novo | |
| (n = 198, 19.3%) | (n = 826, 80.7%) | (n = 349, 31.0%) | (n = 776, 69.0%) | |
| Age, y | ||||
| Median (range) | 46.0 (19–88) | 54.0 (9–91) | 55.0 (19–91) | 61.0 (6–97) |
| Missing, No. (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sex, No. (%) | ||||
| Male | 92 (46.5) | 408 (49.4) | 201 (57.6) | 415 (53.5) |
| Female | 106 (53.5) | 418 (50.6) | 148 (42.4) | 361 (46.5) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Primary tumor thickness, mm | ||||
| No. (range) | 1.0 (0.1–9.8) | 1.4 (0.1–15) | 0.7 (0.1–30) | 1.0 (0.12–30) |
| 0–1.00, No. (%) | 105 (53.0) | 297 (36.0) | 238 (68.2) | 381 (49.1) |
| 1.00–2.00, No. (%) | 51 (25.8) | 215 (26.0) | 70 (20.1) | 169 (21.8) |
| 2.00–4.00, No. (%) | 24 (12.1) | 170 (20.6) | 26 (7.4) | 138 (17.8) |
| >4.00, No. (%) | 12 (6.1) | 98 (11.9) | 14 (4.0) | 88 (11.3) |
| Missing, No. (%) | 6 (3.0) | 46 (5.6) | 1 (0.3) | 0 (0.0) |
| Primary tumor ulceration status, No. (%) | ||||
| Absent | 164 (82.8) | 613 (74.2) | 317 (90.8) | 600 (77.3) |
| Present | 31 (15.7) | 192 (23.2) | 32 (9.2) | 175 (22.6) |
| Missing | 3 (1.5) | 21 (2.5) | 0 (0.0) | 1 (0.1) |
| Primary tumor anatomic site, No. (%) | ||||
| Axial | 89 (44.9) | 293 (35.5) | 182 (52.1) | 253 (32.6) |
| Head/Neck | 31 (15.7) | 119 (14.4) | 35 (10.0) | 149 (19.2) |
| Extremity | 75 (37.9) | 410 (49.6) | 132 (37.8) | 374 (48.2) |
| Missing | 3 (1.5) | 4 (0.5) | 0 (0.0) | 0 (0.0) |
| Primary tumor mitosis, No. (%) | ||||
| Absent | 74 (37.4) | 298 (36.1) | 168 (48.1) | 271 (34.9) |
| Present | 100 (50.5) | 429 (51.9) | 175 (50.1) | 500 (64.4) |
| Missing | 24 (12.1) | 99 (12.0) | 6 (1.7) | 5 (0.6) |
| Primary tumor histologic subtype, No. (%) | ||||
| Superficial | 164 (82.8) | 555 (67.2) | 249 (71.3) | 419 (54.0) |
| Nodular | 9 (4.5) | 101 (12.2) | 58 (16.6) | 236 (30.4) |
| Acral | 4 (2.0) | 19 (2.3) | 2 (0.6) | 32 (4.1) |
| Lentigo | 7 (3.5) | 41 (5.0) | 11 (3.2) | 33 (4.3) |
| Others | 4 (2.0) | 34 (4.1) | 24 (6.9) | 49 (6.3) |
| Missing | 10 (5.1) | 76 (9.2) | 5 (1.4) | 7 (0.9) |
| AJCC * stage at pathological diagnosis, No. (%) | ||||
| I | 147 (74.2) | 450 (54.5) | 281 (80.5) | 489 (63.0) |
| II | 34 (17.2) | 224 (27.1) | 30 (8.6) | 173 (22.3) |
| III/IV | 12 (6.1) | 108 (13.1) | 38 (10.9) | 114 (14.7) |
| Missing | 5 (2.5) | 44 (5.3) | 0 (0.0) | 0 (0.0) |
*AJCC = American Joint Committee on Cancer.
Table 2.
Univariate survival analysis
|
NYU1
|
NYU2
|
||||||
|---|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | HR (95% CI), IQR | P * | HR (95% CI) | HR (95% CI), IQR | P * | |
| Sex | |||||||
| Female vs Male | 0.65 (0.53 to 0.79) | <.001 | 0.64 (0.50 to 0.83) | .001 | |||
| Age, y | 1.04 (1.03 to 1.05) | 2.84 (2.35 to 3.42) | <.001 | 1.04 (1.03 to 1.05) | 2.61 (2.11 to 3.23) | <.001 | |
| Primary tumor thickness, mm | 1.31 (1.27 to 1.36) | 1.57 (1.48 to 1.67) | <.001 | 1.13 (1.11 to 1.15) | 1.23 (1.19 to 1.26) | <.001 | |
| Primary tumor ulceration | |||||||
| Present vs absent | 2.46 (1.99 to 3.04) | <.001 | 4.56 (3.56 to 5.85) | .001 | |||
| Primary tumor mitotic index | |||||||
| Present vs absent | 1.99 (1.57 to 2.54) | <.001 | 3.45 (2.46 to 4.83) | .001 | |||
| Primary tumor histological type | |||||||
| Nodular vs superficial spreading/other | 1.68 (1.26 to 2.24) | <.001 | 2.95 (2.30 to 3.78) | .001 | |||
| AJCC stage at pathological diagnosis | |||||||
| III/IV vs I/II | 3.50 (2.74 to 4.47) | <.001 | 4.87 (3.76 to 6.30) | .001 | |||
| Primary tumor anatomic site | |||||||
| Extremity vs axial/head & neck | 0.69 (0.56 to 0.84) | <.001 | 0.72 (0.56 to 0.93) | .01 | |||
| De novo/nevus-associated | |||||||
| De novo vs nevus-associated | 1.63 (1.22 to 2.18) | <.001 | 2.52 (1.78 to 3.56) | .001 | |||
*Based on two-sided Wald test for univariate Cox PH model. Hazard ratio per interquartile range for continuous covariates (age and tumor thickness). AJCC = American Joint Committee on Cancer; CI = confidence interval; HR = hazard ratio; IQR = interquartile range.
Table 3.
Multivariable survival analysis of traditional prognostic indicators
|
NYU1
|
NYU2
|
|||||
|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | HR (95% CI), IQR | P * | HR (95% CI) | HR (95% CI), IQR | P * |
| Age, y | 1.04 (1.03 to 1.05) | 2.51 (2.04 to 3.08) | <.001 | 1.04 (1.03 to 1.05) | 2.49 (2.00 to 3.09) | <.001 |
| Primary tumor thickness, mm | 1.22 (1.16 to 1.28) | 1.40 (1.29 to 1.51) | <.001 | 1.09 (1.07 to 1.12) | 1.16 (1.11 to 1.20) | <.001 |
| Primary tumor ulceration | ||||||
| Present vs absent | 1.50 (1.15 to 1.96) | .003 | 2.49 (1.89 to 3.28) | <.001 | ||
| Primary tumor mitotic index, | ||||||
| Present vs absent | 1.32 (1.01 to 1.73) | .04 | 1.97 (1.37 to 2.83) | <.001 | ||
| Primary tumor anatomic site | ||||||
| Extremity vs axial/head and neck | 0.57 (0.46 to 0.72) | <.001 | 0.67 (0.52 to 0.87) | .003 | ||
*Based on two-sided Wald test for Cox PH model. Hazard ratio per interquartile range for continuous covariates (age and tumor thickness). CI = confidence interval; HR = hazard ratio; IQR = interquartile range.
To investigate whether melanomas arising in association with a melanocytic nevus had a different outcome than those arising without an associated nevus (ie, de novo) we first tested the potential association of ‘nevus-associated’ or de novo classification with other histopathological variables and then examined survival outcomes. We found that de novo melanomas were statistically significantly associated with several variables conferring poor outcomes ( Table 4 ). For example, in univariate analysis of the NYU1 cohort, de novo melanomas were associated with tumor thickness greater than 1.0 mm (odds ratio [OR] = 1.96, 95% confidence interval [CI] = 1.43 to 2.70, P < .001), ulceration (OR = 1.65, 95% CI = 1.10 to 2.54, P = .02), nodular subtype (OR = 3.26, 95% CI = 1.70 to 7.11, P = .001), greater than stage I (OR = 2.35, 95% CI = 1.65 to 3.40, P < .001), older age (OR = 1.64, 95% CI = 1.18 to 2.30, P = .004). These observations were also found in the NYU2 replication cohort, where de novo melanomas were associated with thickness greater than 1.0 mm (OR = 2.24, 95% CI = 1.72 to 2.93, P < .001), ulceration (OR = 2.88, 95% CI = 1.95 to 4.37, P < .001), nodular subtype (OR = 2.41, 95% CI = 1.75 to 3.37, P < .001), greater than stage I (OR = 2.42, 95% CI = 1.80 to 3.29, P < .001), older age (OR = 1.68, 95% CI = 1.31 to 2.17, P < .001). In both cohorts, de novo melanomas were statistically significantly associated with anatomic location on the extremities (NYU1 HR = 1.66, 95% CI = 1.18 to 2.34, P = .01; NYU2, HR = 3.05, CI = 2.03 to 4.67, P <.001), a location that, in several survival models, has been associated with better outcomes than location on the trunk ( 25–29 ). The presence of tumor mitoses was statistically significantly associated with de novo classification in NYU2 only. Incidentally, de novo melanomas were statistically significantly associated with the nevus-resistant mole phenotype in NYU1 (OR = 1.80, 95% CI = 1.28 to 2.51, P < .001); nevus phenotype was not available for NYU2.
Table 4.
Univariate analysis of factors associated with de novo melanoma
| Characteristics |
NYU1
|
NYU2
|
||
|---|---|---|---|---|
| OR (95% CI) | P * | OR (95% CI) | P * | |
| Age, y | <.001 | <.001 | ||
| Sex | ||||
| Male | 1.00 (Referent) | .51 | 1.00 (Referent) | .22 |
| Female | 0.89 (0.65 to 1.21) | 1.18 (0.92 to 1.53) | ||
| Primary tumor thickness, mm | ||||
| 0–1.00 | 1.00 (Referent) | <.001 | 1.00 (Referent) | <.001 |
| 1.00–2.00 | 1.49 (1.02 to 2.18) | 1.51 (1.09 to 2.09) | ||
| 2.00–4.00 | 2.49 (1.56 to 4.12) | 3.30 (2.13 to 5.27) | ||
| >4 | 2.85 (1.56 to 5.69) | 3.89 (2.23 to 7.29) | ||
| Primary tumor ulceration status | ||||
| Absent | 1.00 (Referent) | .02 | 1.00 (Referent) | <.001 |
| Present | 1.65 (1.10 to 2.54) | 2.88 (1.95 to 4.37) | ||
| Primary tumor anatomic site | ||||
| Axial | 1.00 (Referent) | .01 | 1.00 (Referent) | <.001 |
| Head/neck | 1.16 (0.74 to 1.87) | 3.05 (2.03 to 4.67) | ||
| Extremity | 1.66 (1.18 to 2.34) | 2.04 (1.55 to 2.68) | ||
| Primary tumor histologic subtype | ||||
| Superficial | 1.00 (Referent) | .003 | 1.00 (Referent) | <.001 |
| Nodular | 3.26 (1.70 to 7.11) | 2.41 (1.75 to 3.37) | ||
| Acral | 1.36 (0.50 to 4.87) | 8.86 (2.65 to 59.17) | ||
| Lentigo | 1.70 (0.79 to 4.24) | 1.76 (0.90 to 3.74) | ||
| Others | 2.43 (0.95 to 8.41) | 1.21 (0.73 to 2.05) | ||
| AJCC stage at pathological diagnosis | ||||
| I | 1.00 (Referent) | <.001 | 1.00 (Referent) | <.001 |
| II | 2.14 (1.44 to 3.26) | 3.30 (2.21 to 5.08) | ||
| III/IV | 2.90 (1.61 to 5.72) | 1.72 (1.17 to 2.58) | ||
| Primary tumor mitosis | ||||
| Absent | 1.00 (Referent) | .78 | 1.00 (Referent) | <.001 |
| Present | 1.07 (0.76 to 1.49) | 1.68 (1.31 to 2.17) | ||
*Based on two-sided Chi-square test (categorical variables) or Wilcoxon test (continuous variables). AJCC = American Joint Committee on Cancer; CI = confidence interval; OR = odds ratio.
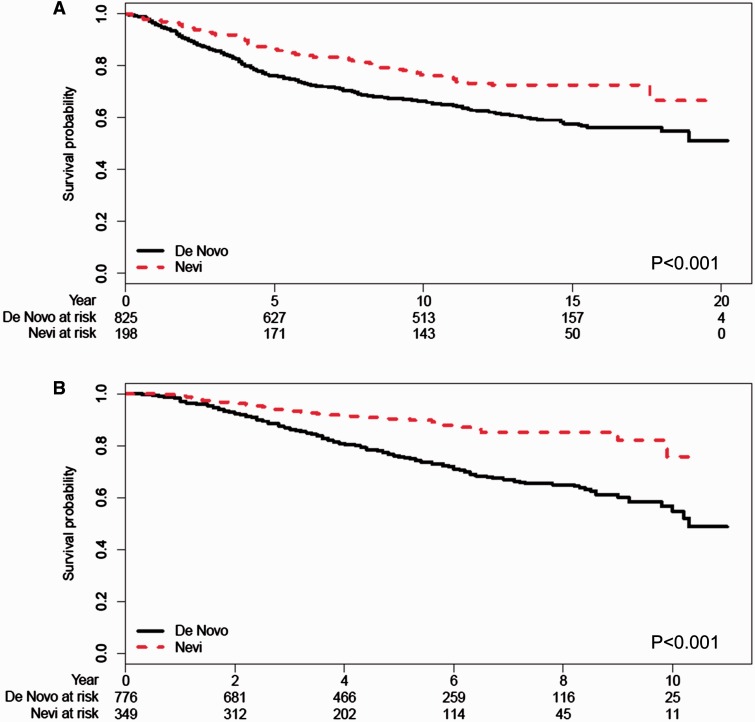
Next we examined whether melanomas arising de novo had a worse survival outcome compared with nevus-associated melanomas. In both the NYU1 and NYU2 cohorts, we found that patients with de novo melanomas had shorter overall survivals than patients whose melanomas arose in association with a melanocytic nevus ( Figure 1 ). In a univariate proportional hazards analysis for survival, we found that de novo classification was statistically significantly associated with worse survival in both the NYU1 (HR = 1.63, 95% CI = 1.22 to 2.18, P < .001) and NYU2 cohorts (HR = 2.52, 95% CI = 1.78 to 3.56, P < .001) ( Table 2 ). In multivariable analysis including tumor thickness, ulceration, mitotic index, and anatomic site, de novo classification was an independent predictor of poor survival outcome in the NYU2 cohort (HR = 1.70, 95% CI = 1.19 to 2.44, P = .004); there was a trend in the same direction in the NYU1 cohort (HR = 1.27, 95% CI = 0.93 to 1.75, P = .14) ( Table 5 ; Supplementary Table 2 , available online).
Figure 1.
Overall survival stratified by de novo ( solid lines ) or nevus-associated ( dashed lines ) melanoma classification. A) NYU1 cohort. B) NYU2 cohort. Tables of the numbers of patients at risk at different time points are given below each graph. P values are calculated based on two-sided log-rank test.
Table 5.
Multivariable survival analysis including de novo vs nevus-associated classification
|
NYU1
|
NYU2
|
|||||
|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | HR (95% CI), IQR | P * | HR (95% CI) | HR (95% CI), IQR | P * |
| Age, y | 1.04 (1.03 to 1.05) | 2.53 (2.04 to 3.13) | <.001 | 1.03 (1.03 to 1.04) | 2.34 (1.88 to 2.91) | <.001 |
| Primary tumor thickness, mm | 1.22 (1.16 to 1.28) | 1.39 (1.28 to 1.51) | <.001 | 1.10 (1.07 to 1.12) | 1.17 (1.12 to 1.21) | <.001 |
| Primary tumor ulceration | ||||||
| Present vs absent | 1.49 (1.14 to 1.95) | .003 | 2.32 (1.75 to 3.07) | <.001 | ||
| Primary tumor mitotic index | ||||||
| Present vs absent | 1.32 (1.01 to 1.73) | .04 | 1.87 (1.30 to 2.69) | <.001 | ||
| Primary tumor anatomic site | ||||||
| Extremity vs axial/head and neck | 0.57 (0.45 to 0.72) | <.001 | 0.65 (0.50 to 0.85) | .001 | ||
| De novo/nevus-associated | ||||||
| De novo vs nevus-associated | 1.27 (0.93 to 1.75) | .14 | 1.70 (1.19 to 2.44) | .004 | ||
*Based on two-sided Wald test for Cox PH model. Hazard ratio per interquartile range for continuous covariates (age and tumor thickness). CI = confidence interval; HR = hazard ratio; IQR = interquartile range.
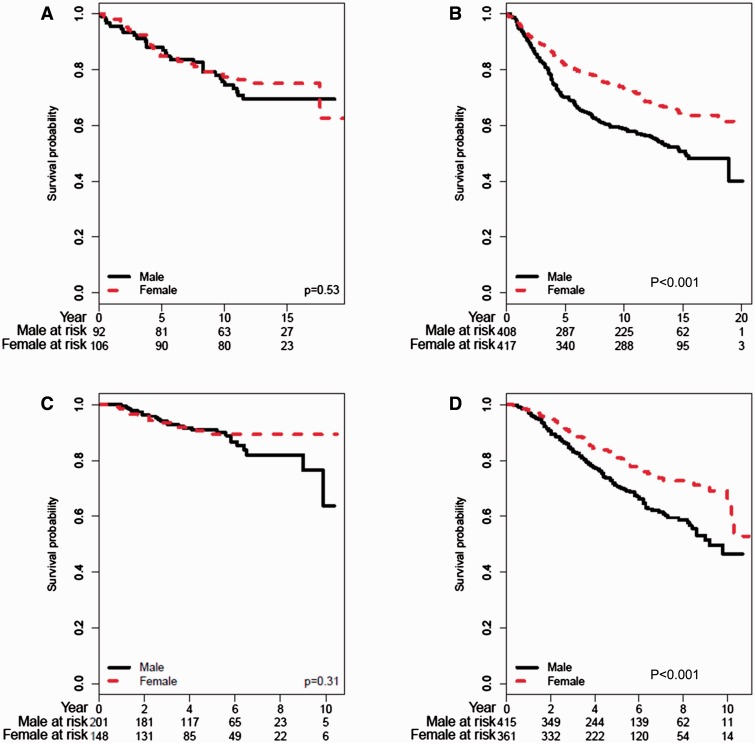
We also examined whether there were differences in the associations between traditional prognostic variables and survival for patients classified with either de novo or nevus-associated melanomas. In both cohorts, we found that increasing tumor thickness, ulceration, and clinical stage were prognostic indicators of short survival irrespective of tumor association with a nevus ( Supplementary Figures 1, 2, and 5 , respectively, available online). Unexpectedly, the associations between survival and sex differed for patients with de novo vs nevus-associated melanomas. In both cohorts, male patients had a statistically significantly worse survival than female patients if their melanoma was de novo (NYU1, P < .001; NYU2, P < .001), but there was no difference in survival if their tumor was nevus-associated ( Figure 2 ).
Figure 2.
Overall survival analyses for nevus-associated and de novo melanoma patients stratified by sex ( solid lines = male; dashed lines = female). A) NYU1 = nevus-associated patients. B) NYU1 = de novo patients. C) NYU2 = nevus-associated patients. D) NYU2 = de novo patients. Tables of the numbers of patients at risk at different time points are given below each graph. P values are calculated based on two-sided log-rank test.
Finally, to address potential confounding because of the positive association between de novo melanomas and nodular melanomas, which are known to be an aggressive melanoma subtype, we repeated the analysis excluding patients diagnosed with nodular melanomas. In both the NYU1 and NYU2 cohorts, all associations remained statistically significant except for the association of ulceration with de novo melanoma in the NYU1 cohort. As expected, ulceration was found more often in de novo melanomas than nevus-associated melanomas; however, the association did not reach statistical significance ( P = .19) ( Supplementary Table 3 , available online).
Discussion
We found that de novo melanomas were statistically significantly associated with older age at diagnosis, fewer nevi, anatomic location on the extremities, thicker tumors, ulceration, nodular subtype, higher stage, and shorter overall survival in both the NYU1 and NYU2 cohorts. The two cohorts were recruited decades apart and histopathologic slides were interpreted by different pathologists, suggesting that these are robust associations. In addition, these findings indicate that de novo melanomas may represent a more aggressive form of melanoma than nevus-associated melanomas, as the de novo classification was an independent prognostic indicator of short survival in multivariable analysis in the NYU2 cohort and it trended in the same direction in the NYU1 cohort.
Although several studies have attempted to determine if differences in etiology and prognosis exist between de novo and nevus-associated melanomas, to our knowledge no prior studies have definitively found de novo melanomas to be a prognostic indicator for shorter survival. Most of the prior studies have used Breslow thickness as a surrogate marker for patient outcome instead of survival, and the results have largely been inconclusive. Two studies found that nevus-associated melanomas were thicker than de novo melanomas, two studies found they were thinner, and two studies found no difference ( 20 , 22 , 23 , 30–32 ). Possible reasons for these discrepancies include the possibility that advanced melanomas may obliterate underlying nevus cells as they progress and/or inconsistencies in the measurement of thickness because of nonstandardized measures of evaluation of the associated nevus component.
Few studies have specifically analyzed survival outcomes with respect to nevus-associated and de novo classification of melanomas. A recent study by Lin et al. did not find statistically significant differences between de novo and nevus-associated melanoma with respect to tumor thickness and survival; however, their patient group was assembled from a retrospective chart review of consecutively seen patients who underwent sentinel lymph node biopsy ( 21 ). The median tumor thickness of their group was 1.7 mm, much higher than the tumor thicknesses of either the NYU1 or NYU2 cohorts. Interestingly, they did make similar observations to ours with respect to the patient and tumor characteristics associated with either nevus-associated or de novo melanoma classification (eg, patient age, anatomic site of the melanoma, histopathologic subtype, and ulceration). A small, retrospective study by Kaddu et al. also failed to find a difference in survival between patients classified with nevus-associated and de novo melanomas ( 30 ). In contrast to our prospectively recruited cohort design, both of these studies are retrospective investigations, limiting their ability to conduct a statistically robust survival analysis. A prospective study conducted by investigators from our institution was published in 1983. These investigators performed a preliminary analysis of 557 patients from the NYU1 dataset. They found poorer disease-free survival for de novo melanomas; however, the impact of this finding was limited by the shorter follow-up time and small number of patient recurrences. Importantly, no multivariable survival analysis was described ( 20 ).
Unexpectedly, we found that the association between sex and survival was dependent upon whether a patient’s melanoma was de novo or nevus-associated. In both cohorts, men had a statistically significantly worse survival than women among patients with de novo melanomas; however, there was no difference in survival between men and women who were diagnosed with nevus-associated melanoma. Several melanoma studies have found that male patients have worse survival than female patients, which suggests that important sex-associated biological differences exist ( 33–40 ). Additionally, recent analyses of Surveillance, Epidemiology, and End Results data demonstrate that men have poorer survival than women for most cancer types ( 41 ). Related hypotheses potentially worth exploring include sex differences in immune function, the potential effects of male vs female hormones on tumor cells and/or immune function, and the possible differences in how these (or other) factors may interact with a patient’s underlying susceptibility to developing nevus-associated or de novo melanoma tumors. It is also possible that nevus-associated melanomas are intrinsically less aggressive because of their genotypic or phenotypic characteristics so that potential sex-related differences in host responses to these tumors are not manifested. Of note, we performed a formal statistical test of the interaction of sex and de novo vs nevus-associated melanomas on survival; however, the interaction term did not achieve statistical significance (data not shown).
These findings suggest there are potentially important differences in the biology of nevus-associated and de novo melanomas. Our results are largely consistent with the two-pathway (ie, divergent pathway) model for the development of melanoma on sun-exposed skin (reviewed in [ 16 ]). This model reconciles epidemiologic differences in the sun exposure patterns and anatomic distribution of melanomas by incorporating a concept related to an individual’s propensity to develop melanocytic nevi (ie, nevus-prone vs nevus-resistant). In this model, nevus-prone patients have increased numbers of melanocytic nevi and develop melanomas at younger ages that are more likely to arise in association with a melanocytic nevus, on axial locations, of the superficial spreading subtype, and with frequent BRAF mutations. Conversely, nevus-resistant patients have fewer nevi and develop melanomas at older ages that are more likely to arise de novo, be of nodular subtype, and be associated with NRAS mutations. These clinical features are also shared by the recently described ‘high-mitotic-rate melanomas,’ which, notably, are statistically significantly more likely to arise de novo than in association with a nevus ( 42 ). Other potential biological differences include sex-specific variations in the host response to de novo melanoma. Moving forward, it may be useful to use the nevus-associated vs de novo classification in analyses of melanoma risk factors, tumor biology, and response to therapy.
Strengths of our study include the analysis of two large, prospectively ascertained patient cohorts, enrolled decades apart by different investigators at a single institution. We had multiple clinical and histopathologic features available for analysis, along with lengthy survival data. This enabled us to use a second patient cohort to test associations identified in the initial cohort. Nearly all the associations were strongly statistically significant in both multivariable and univariate analyses in both cohorts. One weakness of our study is that the initial cohort was enrolled prior to the advent of sentinel node biopsy. For this reason, we were not able to include an analysis of sentinel node biopsy results in this analysis.
In summary, de novo melanoma classification was associated with several adverse histopathologic features in primary cutaneous melanoma and appears to be an independent predictor of poor outcome in multivariable analysis. De novo melanomas are more likely to possess molecular characteristics associated with poor survival compared with nevus-associated melanomas and may differ in their molecular pathogenesis as suggested by the divergent pathway model. As sex-specific survival differences were only observed among the patients with de novo melanomas, the de novo vs nevus-associated melanoma classification scheme may be helpful for investigations into sex-specific differences in melanoma survival.
Funding
Supported in part by the NYU CTSA grant UL1TR000038 from the National Center for the Advancement of Translational Science (NCATS), NIH to RMC and LAP; and NIH/NCI Cancer Center Support Grant 2P30 CA16087 to YS.
Notes
The study funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript. All writers and contributors who participated in the preparation of the manuscript are listed as authors. The authors have no conflict of interest to declare.
Supplementary Material
References
- 1. Goldstein AM, Tucker MA . Dysplastic nevi and melanoma . Cancer Epidemiol Biomarkers Prev . 2013. ; 22 ( 4 ): 528 - 532 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stolz W, Schmoeckel C, Landthaler M , et al. . Association of early malignant melanoma with nevocytic nevi . Cancer. 1989. ; 63 ( 3 ): 550 - 555 . [DOI] [PubMed] [Google Scholar]
- 3. Black WC. Residual dysplastic and other nevi in superficial spreading melanoma. Clinical correlations and association with sun damage . Cancer. 1988. ; 62 ( 1 ): 163 - 173 . [DOI] [PubMed] [Google Scholar]
- 4. Clark WH, Jr, Elder DE, Guerry Dt , et al. . A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma . Hum Pathol. 1984. ; 15 ( 12 ): 1147 - 1165 . [DOI] [PubMed] [Google Scholar]
- 5. Cook MG, Robertson I. Melanocytic dysplasia and melanoma . Histopathology. 1985. ; 9 ( 6 ): 647 - 658 . [DOI] [PubMed] [Google Scholar]
- 6. Harley S, Walsh N. A new look at nevus-associated melanomas . Am J Dermatopathol. 1996. ; 18 ( 2 ): 137 - 141 . [DOI] [PubMed] [Google Scholar]
- 7. Hastrup N, Osterlind A, Drzewiecki KT , et al. . The presence of dysplastic nevus remnants in malignant melanomas. A population-based study of 551 malignant melanomas . Am J Dermatopathol. 1991. ; 13 ( 4 ): 378 - 385 . [DOI] [PubMed] [Google Scholar]
- 8. Massi D, Carli P, Franchi A , et al. . Naevus-associated melanomas: cause or chance? Melanoma Res. 1999. ; 9 ( 1 ): 85 - 91 . [DOI] [PubMed] [Google Scholar]
- 9. Sagebiel RW. Melanocytic nevi in histologic association with primary cutaneous melanoma of superficial spreading and nodular types: effect of tumor thickness . J Invest Dermatol. 1993. ; 100 ( 3 ): 322S - 325S . [DOI] [PubMed] [Google Scholar]
- 10. Shain AH, Yeh I, Kovalyshyn I , et al. . The Genetic Evolution of Melanoma from Precursor Lesions . N Engl J Med. 2015. ; 373 ( 20 ): 1926 - 1936 . [DOI] [PubMed] [Google Scholar]
- 11. Bastian BC. The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia . Annu Rev Pathol. 2014. ; 9 : 239 - 271 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tucker MA, Halpern A, Holly EA , et al. . Clinically recognized dysplastic nevi. A central risk factor for cutaneous melanoma . JAMA. 1997. ; 277 ( 18 ): 1439 - 1444 . [PubMed] [Google Scholar]
- 13. Lazovich D, Vogel RI, Berwick M , et al. . Indoor tanning and risk of melanoma: a case-control study in a highly exposed population . Cancer Epidemiol Biomarkers Prev. 2010. ; 19 ( 6 ): 1557 - 1568 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsao H, Bevona C, Goggins W , et al. . The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: a population-based estimate . Arch Dermatol. 2003. ; 139 ( 3 ): 282 - 288 . [DOI] [PubMed] [Google Scholar]
- 15. Kvaskoff M, Pandeya N, Green AC , et al. . Site-specific determinants of cutaneous melanoma: a case-case comparison of patients with tumors arising on the head or trunk . Cancer Epidemiol Biomarkers Prev. 2013. ; 22 ( 12 ): 2222 - 2231 . [DOI] [PubMed] [Google Scholar]
- 16. Whiteman DC, Pavan WJ, Bastian BC. The melanomas: a synthesis of epidemiological, clinical, histopathological, genetic, and biological aspects, supporting distinct subtypes, causal pathways, and cells of origin . Pigment Cell Melanoma Res. 2011. ; 24 ( 5 ): 879 - 897 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whiteman DC, Watt P, Purdie DM , et al. . Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma . J Natl Cancer Inst. 2003. ; 95 ( 11 ): 806 - 812 . [DOI] [PubMed] [Google Scholar]
- 18. Whiteman DC, Stickley M, Watt P , et al. . Anatomic site, sun exposure, and risk of cutaneous melanoma . J Clin Oncol. 2006. ; 24 ( 19 ): 3172 - 3177 . [DOI] [PubMed] [Google Scholar]
- 19. Purdue MP, From L, Armstrong BK , et al. . Etiologic and other factors predicting nevus-associated cutaneous malignant melanoma . Cancer Epidemiol Biomarkers Prev. 2005. ; 14 ( 8 ): 2015 - 2022 . [DOI] [PubMed] [Google Scholar]
- 20. Friedman RJ, Rigel DS, Kopf AW , et al. . Favorable prognosis for malignant melanomas associated with acquired melanocytic nevi . Arch Dermatol. 1983. ; 119 ( 6 ): 455 - 462 . [PubMed] [Google Scholar]
- 21. Lin WM, Luo S, Muzikansky A , et al. . Outcome of patients with de novo versus nevus-associated melanoma . J Am Acad Dermatol. 2015. ; 72 ( 1 ): 54 - 58 . [DOI] [PubMed] [Google Scholar]
- 22. Weatherhead SC, Haniffa M, Lawrence CM. Melanomas arising from naevi and de novo melanomas--does origin matter? Br J Dermatol. 2007. ; 156 ( 1 ): 72 - 76 . [DOI] [PubMed] [Google Scholar]
- 23. Garcia-Cruz A, Florez A, de la Torre-Fraga C , et al. . Observational cross-sectional study comparing Breslow thickness of melanoma arising from naevi and melanoma de novo . Br J Dermatol. 2009. ; 161 ( 3 ): 700 - 702 . [DOI] [PubMed] [Google Scholar]
- 24. Wich LG, Hamilton HK, Shapiro RL , et al. . Developing a multidisciplinary prospective melanoma biospecimen repository to advance translational research . Am J Transl Res . 2009. ; 1 ( 1 ): 35 - 43 . [PMC free article] [PubMed] [Google Scholar]
- 25. Thompson JF, Soong SJ, Balch CM , et al. . Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database . J Clin Oncol. 2011. ; 29 ( 16 ): 2199 - 2205 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garbe C, Buttner P, Bertz J , et al. . Primary cutaneous melanoma. Prognostic classification of anatomic location . Cancer. 1995. ; 75 ( 10 ): 2492 - 2498 . [DOI] [PubMed] [Google Scholar]
- 27. Clark WH, Jr, Elder DE, Guerry Dt , et al. . Model predicting survival in stage I melanoma based on tumor progression . J Natl Cancer Inst. 1989. ; 81 ( 24 ): 1893 - 1904 . [DOI] [PubMed] [Google Scholar]
- 28. Balch CM, Soong SJ, Gershenwald JE , et al. . Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system . J Clin Oncol . 2001. ; 19 ( 16 ): 3622 - 3634 . [DOI] [PubMed] [Google Scholar]
- 29. Hemo Y, Gutman M, Klausner JM. Anatomic site of primary melanoma is associated with depth of invasion . Arch Surg. 1999. ; 134 ( 2 ): 148 - 150 . [DOI] [PubMed] [Google Scholar]
- 30. Kaddu S, Smolle J, Zenahlik P , et al. . Melanoma with benign melanocytic naevus components: reappraisal of clinicopathological features and prognosis . Melanoma Res. 2002. ; 12 ( 3 ): 271 - 278 . [DOI] [PubMed] [Google Scholar]
- 31. Jones WM, Williams WJ, Roberts MM , et al. . Malignant melanoma of the skin: prognostic value of clinical features and the role of treatment in 111 cases . Br J Cancer. 1968. ; 22 ( 3 ): 437 - 451 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. MacKie RM. Malignant melanoma: clinical variants and prognostic indicators . Clin Exp Dermatol. 2000. ; 25 ( 6 ): 471 - 475 . [DOI] [PubMed] [Google Scholar]
- 33. Joosse A, van der Ploeg AP, Haydu LE , et al. . Sex differences in melanoma survival are not related to mitotic rate of the primary tumor . Ann Surg Oncol. 2015. ; 22 ( 5 ): 1598 - 1603 . [DOI] [PubMed] [Google Scholar]
- 34. Joosse A, Collette S, Suciu S , et al. . Superior outcome of women with stage I/II cutaneous melanoma: pooled analysis of four European Organisation for Research and Treatment of Cancer phase III trials . J Clin Oncol. 2012. ; 30 ( 18 ): 2240 - 2247 . [DOI] [PubMed] [Google Scholar]
- 35. Joosse A, Collette S, Suciu S , et al. . Sex is an independent prognostic indicator for survival and relapse/progression-free survival in metastasized stage III to IV melanoma: a pooled analysis of five European organisation for research and treatment of cancer randomized controlled trials . J Clin Oncol. 2013. ; 31 ( 18 ): 2337 - 2346 . [DOI] [PubMed] [Google Scholar]
- 36. Joosse A, de Vries E, Eckel R , et al. . Gender differences in melanoma survival: female patients have a decreased risk of metastasis . J Invest Dermatol. 2011. ; 131 ( 3 ): 719 - 726 . [DOI] [PubMed] [Google Scholar]
- 37. Lasithiotakis K, Leiter U, Meier F , et al. . Age and gender are significant independent predictors of survival in primary cutaneous melanoma . Cancer. 2008. ; 112 ( 8 ): 1795 - 1804 . [DOI] [PubMed] [Google Scholar]
- 38. Mervic L, Leiter U, Meier F , et al. . Sex differences in survival of cutaneous melanoma are age dependent: an analysis of 7338 patients . Melanoma Res. 2011. ; 21 ( 3 ): 244 - 252 . [DOI] [PubMed] [Google Scholar]
- 39. Sanlorenzo M, Ribero S, Osella-Abate S , et al. . Prognostic differences across sexes in melanoma patients: what has changed from the past? Melanoma Res. 2014. ; 24 ( 6 ): 568 - 576 . [DOI] [PubMed] [Google Scholar]
- 40. de Vries E, Nijsten TE, Visser O , et al. . Superior survival of females among 10,538 Dutch melanoma patients is independent of Breslow thickness, histologic type and tumor site . Ann Oncol. 2008. ; 19 ( 3 ): 583 - 589 . [DOI] [PubMed] [Google Scholar]
- 41. Cook MB, McGlynn KA, Devesa SS , et al. . Sex disparities in cancer mortality and survival . Cancer Epidemiol Biomarkers Prev. 2011. ; 20 ( 8 ): 1629 - 1637 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shen S, Wolfe R, McLean CA , et al. . Characteristics and associations of high-mitotic-rate melanoma . JAMA Dermatol. 2014. ; 150 ( 10 ): 1048 - 1055 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.