Abstract
Background
Extraordinary progress has been made in our understanding of common variants in many diseases, including melanoma. Because the contribution of rare coding variants is not as well characterized, we performed an exome-wide, gene-based association study of familial cutaneous melanoma (CM) and ocular melanoma (OM).
Methods
Using 11 990 jointly processed individual DNA samples, whole-exome sequencing was performed, followed by large-scale joint variant calling using GATK (Genome Analysis ToolKit). PLINK/SEQ was used for statistical analysis of genetic variation. Four models were used to estimate the association among different types of variants. In vitro functional validation was performed using three human melanoma cell lines in 2D and 3D proliferation assays. In vivo tumor growth was assessed using xenografts of human melanoma A375 melanoma cells in nude mice (eight mice per group). All statistical tests were two-sided.
Results
Strong signals were detected for CDKN2A (Pmin = 6.16 × 10-8) in the CM cohort (n = 273) and BAP1 (Pmin = 3.83 × 10‐6) in the OM (n = 99) cohort. Eleven genes that exhibited borderline association (P < 10‐4) were independently validated using The Cancer Genome Atlas melanoma cohort (379 CM, 47 OM) and a matched set of 3563 European controls with CDKN2A (P = .009), BAP1 (P = .03), and EBF3 (P = 4.75 × 10‐4), a candidate risk locus, all showing evidence of replication. EBF3 was then evaluated using germline data from a set of 132 familial melanoma cases and 4769 controls of UK origin (joint P = 1.37 × 10‐5). Somatically, loss of EBF3 expression correlated with progression, poorer outcome, and high MITF tumors. Functionally, induction of EBF3 in melanoma cells reduced cell growth in vitro, retarded tumor formation in vivo, and reduced MITF levels.
Conclusions
The results of this large rare variant germline association study further define the mutational landscape of hereditary melanoma and implicate EBF3 as a possible CM predisposition gene.
In 2016, an estimated 76 380 Americans developed cutaneous melanoma (CM) and 10 130 succumbed to this disease, making CM the fifth and seventh most common cancer among men and women, respectively (1). Ocular melanoma (OM) is much rarer, with only 2500 estimated cases annually in the United States (2). There is evidence for a strong genetic influence on melanoma risk. Ten percent of CM patients report a family history of melanoma (3,4), which confers a twofold risk of melanoma among first-degree relatives (5) and approximately fivefold if two or more first-degree relatives are affected. Twin studies have estimated the heritability of melanoma to be 58%, which is statistically significantly higher than the 33% for cancers overall (6).
Prior to the advent of high-density human genomic maps, linkage efforts implicated familial melanoma loci on 1p36 (7) and 9p21 (8); interval gene screening revealed deleterious germline alterations of CDKN2A in a subset of 9p21-linked families. Subsequent linkage efforts have produced additional candidate loci on chromosomes 1p36 (7), 9q21 (9), 5q31 (10), and 1p22 (11) without isolation of specific disease-causing mutations. Rare germline mutations in CDK4, BAP1, MITF, TERT (promoter), POT1, ACD, and TERF2IP have also been reported in both OM and CM families though, collectively, they account for less than 5% of all hereditary melanoma cases (12,13). Finally, a rare functional polymorphism in MITF (pE318K) has been shown to double melanoma risk and alter MITF sumoylation (14).
Common variant association studies (ie, genome-wide association studies [GWAS]) recently culminated in an analysis of 15 990 CM cases and 26 409 controls, which substantiated 20 genome-wide statistically significant loci (15). The general synthesis from GWAS and other candidate association studies is that loci associated with pigmentation (MC1R, TYR, ASIP, OCA2, and SLC45A2), nevus count (CDKN2A-MTAP, PLA2G6, and TERT), DNA repair (PARP1 and ATM), and telomere length (TERT, OBFC1) represent core drivers of a risk phenotype that has been delineated by epidemiologic studies (16–23).
The systematic pursuit of rare disease–causing variants is just emerging. Given the high cost of sequencing in the past, linkage analysis provided a robust method to leverage recombination for positional information. For relatively common end points, linkage has been largely unsuccessful because, beyond rare examples of single pedigrees that are sufficiently large and carry a near-fully penetrant mutation to generate statistically significant linkage, the polygenic nature of common disease precludes gene localization. To overcome the fact that rare variants are distributed across many genes, we and others have proposed methodologies for gene discovery through rare variant association studies (RVAS) (24,25). One common approach is to group the individual variants into sets (eg, gene-based association) and compare the aggregate frequency distribution in cases vs controls. Using this framework, we set out to comprehensively map the mutational landscape of cutaneous and ocular melanoma by performing whole-exome sequencing followed by a gene-based association study of melanoma cases and principal component analysis (PCA)–matched European noncancer controls.
Methods
Patient Cohorts
Details on the primary discovery and two validation sets are given in the Supplementary Methods (available online). The primary discovery set is registered in dbGAP under Study Accession phs000823.v1.p1. All patients provided written informed consent, and the study was approved by the respective institution’s review board.
Exome Sequencing, Variant Processing, and Calling
Whole-exome libraries were prepared using a modified version of Agilent's Exome Capture kit and protocol, automated on the Agilent Bravo and Hamilton Starlet, followed by sequencing on the Illumina HiSeq-2000. We used an aggregated set of samples consented for joint variant calling, resulting in 37 607 samples (germline from 292 CM patients, 101 OM patients, 397 The Cancer Genome Atlas [TCGA] CM patients, 47 TCGA OM patients, 24 612 controls, and 12 158 other individuals included for joint variant calling only). All samples were aligned on the reference genome with BWA (26) and the best-practices GATK/Picard Pipeline, followed by joint variant calling with all samples processed as a single batch using GATK v. 3.1-144 Haplotype Caller (27–29). The resulting data set had 7 094 027 distinct variants. Haplotype Caller, which was used for the ExAC database (30), was also used to detect indels. Selected mutations in CDKN2A and BAP1 were confirmed with Sanger sequencing.
We performed PCA on common (MAF [Minor Allele Frequency] > 5%) autosomal independent single nucleotide polymorphisms (SNPs) to filter out all non-European samples with Eigenstrat (31). Relatedness analysis among Europeans was conducted with PLINK (32), as suggested in the PLINK best practices (33). We used VEP (34) for functional annotation of the DNA variants. Common and rare variants analyses were conducted using PLINK/SEQ, which allows indexing of the large data sets. A burden test (Fisher’s test with aggregated allele counts per gene) was used for rare protein-truncating variants. Additionally, the variable threshold (VT) and C-alpha tests were chosen as an adaptive burden test and variance-component test, respectively, to complement each other and to boost the power of rare missense and protein-truncating variation association detection (35). See the Supplementary Methods (available online).
EBF3 Validation
Methods for the EBF3 validation and expression analysis can be found in the Supplementary Methods (available online).
Statistical Methods
Gene-based association was performed using three distinct, but related, analytical frameworks. In the first analysis, a burden test was applied to all rare (MAF < 1%) protein-truncating (PT) variants because the functional impact is presumed to be severe and most directly inferred. Then, to expand on all rare variants (missense and PTV), a second analysis using both the C-alpha and VT tests was employed. A third analysis applied the burden test to examine “ultra-rare” (MAF < 0.0001; ExAC database http://exac.broadinstitute.org/gene/) variants as these may represent the most highly penetrant alleles. In the case of a single-model association test, the null statistic was represented by the uniform distribution of P values. Because four different test statistics (ie, VT, C-alpha, burden of PTVs, and burden of ExAC filtered variants) were applied and the lowest P value was chosen, the null distribution was constructed by choosing the minimum P value (Pmin) from four null single-statistic models (four sets of uniform P values). This process simulates the procedure of selecting the best P value out of four different test statistics that was used for gene-association testing, thus making it a more conservative approach. Genome-wide statistical significance was adjusted by Bonferroni correction (ie, .05/17 337 genes tested, ie, P < 2.88×10‐6). Joint association statistic for EBF3 was estimated using a two-sided Mantel-Haenszel chi-square test.
P values in mouse experiments were calculated using the Student’s t test. The burden of somatic deletions in EBF3 was tested with the Wilcoxon test. Survival correlation with EBF3 expression level was assessed using a log-rank test. All statistical tests were two-sided.
Results
Cohort and Overview
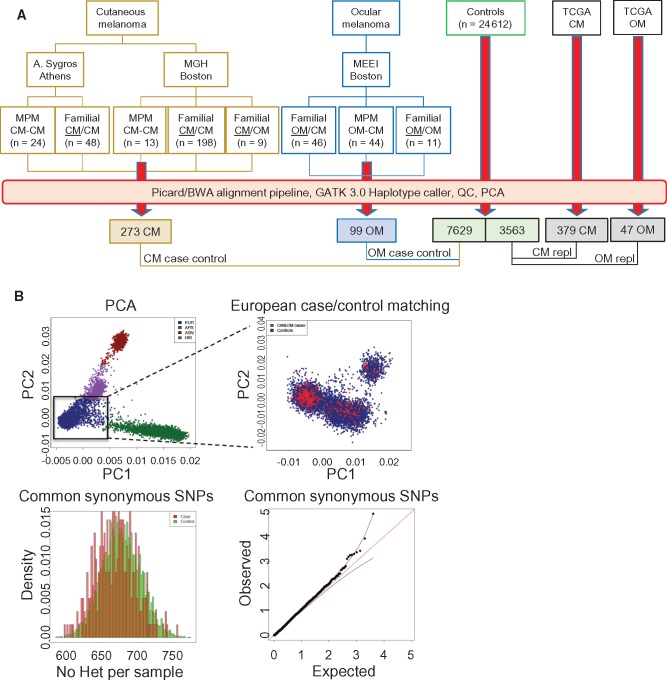
For the discovery set, individuals with familial CM/OM or multiple primary CM/OM were identified and their germline DNA were subjected to whole-exome sequencing. A total of 273 CM (M/F 128/145) (Figure 1A), 99 OM (M/F 46/53), and 7629 (M/F 5451/2178) European noncancer controls passed quality control and were included in the subsequent analysis (19 CM and 2 OM cases failed quality control). The first replication cohort included 379 CM (18 samples were eliminated in case-control matching procedure) and 47 OM cases from TCGA and 3563 European noncancer controls. These additional samples were jointly processed through the same alignment and variant calling pipeline as the initial discovery set and subjected to the same quality control standards. To ensure ancestral matching, we performed principal component analysis (PCA) (Figure 1B) between the cases and control cohorts in both the primary and TCGA replication cohorts. Our analysis was then restricted to a European cluster of samples only. Examination of common synonymous variants (MAF > 5%) revealed a null distribution of the test statistic between cases and controls. There was an average of 25 633 SNPs per sample, which is within the expected range for a typical European germline exome (28). In total, 11 990 samples were jointly processed and PCA-matched for analysis.
Figure 1.
Study cohorts. A) Cutaneous melanoma and ocular melanoma cases used in analysis. Genetically enriched probands as defined in the Supplementary Methods (available online). B) Principal component analysis using the principal component analysis module in PLINK; cases showing close matching with European controls. C) Histogram of common synonymous single nucleotide polymorphisms between cases and controls and observed:expected ratios. CM = cutaneous melanoma; MPM = multiple primary melanoma; OM = ocular melanoma; PCA = principal component analysis; repl = replication study; SNPs = single nucleotide polymorphisms; TCGA = The Cancer Genome Atlas.
For EBF3, a second replication was performed using allele counts derived from 133 familial melanoma cases (ie, 77 cases [66 families] from Leeds, UK, and 56 cases [nine families] from Sydney, Australia) and 4769 noncancer controls from the UK10K population project; whole genotypes were not available for the UK replication cohort and thus were not matched by PCA.
Mutational Landscape of Cutaneous and Ocular Melanoma
We first interrogated our melanoma cases for rare PT mutations among known melanoma predisposition genes and their associated complexes or pathways (ie, RB, telomerase/shelterin, BAP1) (Supplementary Table 1 and Supplementary Table 2, available online). As expected, the strongest associations were for CDKN2A (Pmin = 6.16×10‐8 statistically significant with CM) and BAP1 (Pmin = .005 for CM and Pmin = 3.83×10‐6 for OM) (Supplementary Table 4, available online). We detected five rare POT1 mutations (Pmin = .002), including novel nonsense (p. Arg363X) and splice donor (chr7:124475332 C/T) mutations and a previously reported p.D224N variant (Supplementary Table 1 and Supplementary Table 2, available online). For MC1R, we examined red hair color (RHC) variants and observed 184 alternative alleles among the CM cohort (n = 273) and 2891 in controls (n = 7629, cumulative allele odds ratio [OR] = 1.80, 95% confidence interval [CI] = 1.53 to 2.10, P = 5.00 × 10‐12) (Supplementary Table 3, available online).
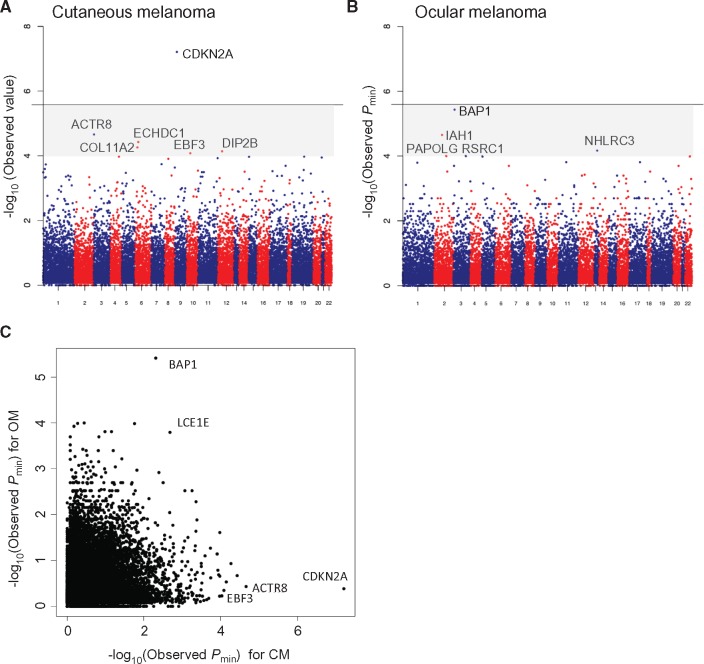
The top CM risk candidates that emerged from each of the three analytical frameworks are shown in Supplementary Table 4 (full rankings and details shown in Supplementary Tables 5–7, available online). Figure 2A shows the Pmin Manhattan plot for all 17 337 genes that were analyzed for association. CDKN2A exhibited the strongest association (Pmin = 6.16×10‐8) (Supplementary Table 4, available online) and was the only locus to reach genome-wide statistical significance. Other candidates that were near, but not reaching, genome-wide statistical significance were considered novel and that were subjected for further study included ACTR8 (Pmin = 2.18×10‐5, C-alpha), ECHD1 (Pmin = 3.73×10‐5, C-alpha), COL11A2 (Pmin = 5.42×10‐5, PTV burden), DIP2B (Pmin = 7.15 × 10-5, C-alpha), and EBF3 (Pmin = 8.22×10‐5, C-alpha). Similar analyses for OM are also shown in Supplementary Table 4 (full rankings and details shown in Supplementary Tables 8, 9, and 10, available online). BAP1 was the leading risk gene (Pmin = 3.83×10‐6) (Figure 2B;Supplementary Table 4, available online) though it did not quite reach genome-wide statistical significance. Other borderline candidates that were subjected to further analysis include IAH1 (Pmin = 2.27×10‐5, C-alpha), NHLRC3 (Pmin = 6.88x×10‐5, C-alpha), RSRC1 (Pmin = 1×10‐4, C-alpha), PAPOLG (Pmin = 1×10‐4, C-alpha).
Figure 2.
Mutational landscape of melanoma. Manhattan plot showing gene-based associations across all loci for (A) cutaneous melanoma and (B) ocular melanoma. Genome-wide statistical significance is indicated by a solid line. Genes that show near statistically significant associations fall within the shaded region (Pmin < 10-4). (C) Calculated -log10 (Pmin values) for genes in both cutaneous melanoma (CM) and ocular melanoma (OM) analyses. BAP1 and CDKN2A clearly show strong preferential associations with OM and CM, respectively. CM = cutaneous melanoma; OM = ocular melanoma.
All CM and OM loci with an association level of a P value of less than 1.00×10‐4 (six CM genes, five OM genes) were then subjected to replication using the TCGA cohort as outlined above (Table 1; Supplementary Figure 1, available online). For CM, CDKN2A (P = 9.31×10‐3, PTV burden) and EBF3 (P = 4.75×10‐4, C-alpha) both remained statistically significant while, for OM, BAP1 also remained statistically significant (P = 2.64×10‐2, PTV burden). As EBF3 represents a potentially novel risk gene, we performed a second replication with this gene by interrogating whole-exome and genome sequence data for 133 familial melanoma cases from 81 melanoma-prone kindreds. We identified a Leeds family with a p.N455S mutation that was detected in one of two affected members and a Sydney family with a p.G21S variant that was present in two of six affected members. Similar evaluation of 4769 individuals from the UK10K cohort revealed 21 EBF3 mutation carriers in this control set. In aggregate (Table 1), a joint burden test across all EBF3 cohorts resulted in a statistically significant association between EBF3 and cutaneous melanoma (9/784 cases and 42/15 961 controls, OR = 4.95, 95% CI = 2.35 to 10.41, P = 1.37×10‐5, Mantel-Haenszel chi-square test); to remove possible bias due to relatedness of carriers, we censored one of the two p.G21S carriers in the Sydney family while accounting for all noncarriers (ie, three mutations out of 133 cases were identified in the UK replication, but two carriers out of 132 individuals used for the Mantel-Haenszel test). Taken together, these results confirm the known contribution of CDKN2A and BAP1 as strong risk loci for CM and OM, respectively, and nominate EBF3 as a novel risk candidate for CM. In addition, the recovery of these known hereditary melanoma loci further verified the technical and methodological pipeline used in this RVAS.
Table 1.
Melanoma case/control and replication
| Primary cohort |
TCGA replication cohort |
UK EBF3 replication |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Method* | No. variants CM cases (n = 273) | No. variants controls (n = 7629) | Pmin | No. variants TCGA CM (n = 379) | No. variants controls (n = 3563) | P | No. variants Sanger CM (n = 132)† | No. variants UK10K (n = 4769) | Joint OR‡ (95% CI) |
| CDKN2A | PTV burden | 5§ | 0 | 6.16 × 10-8‖ | 2 | 0 | .009¶ | – | – | – |
| ACTR8 | C-alpha | 7 | 56 | 2.18 × 10-5 | 5 | 26 | .14 | – | – | – |
| ECHDC1 | C-alpha | 6 | 32 | 3.73 × 10-5 | 1 | 14 | .83 | – | – | – |
| COL11A2 | PTV burden | 4 | 3 | 5.42 × 10-5 | 0 | 4 | .60 | – | – | – |
| DIP2B | C-alpha | 21 | 272 | 7.15 × 10-5 | 4 | 78 | .20 | – | – | – |
| EBF3 | C-alpha | 4 | 14 | 8.22 × 10-5 | 3 | 7 | 4.75 × 10-4 | 2 | 21 | 4.95 (2.35 to 10.41) |
| No. variants OM cases (n = 99) | No. variants controls (n = 7629) | Pmin | No. Variants TCGA OM (n = 47) | No. variants controls (n = 3563) | P | |||||
| BAP1 | PTV burden | 4 | 3 | 3.83 × 10-6 | 1 | 1 | .03 | |||
| IAH1 | C-alpha | 6 | 68 | 2.27 × 10-5 | 0 | 31 | – | |||
| NHLRC3 | C-alpha | 10 | 183 | 6.88 × 10-5 | 0 | 84 | – | |||
| RSRC1 | C-alpha | 5 | 44 | 1.00 × 10-4 | 0 | 18 | – | |||
| PAPOLG | C-alpha | 6 | 47 | 1.00 × 10-4 | 0 | 21 | – | |||
This column represents the statistical test that was used to compute P value; all listed tests were two-sided.
Melanoma kindreds of British ancestry were included to approximate the U.K.10K control collection. The UK replication included 77 cases (66 families) from Leeds, UK, and 56 cases (nine families) from Sydney, Australia. A p.N455S mutation was found in one Leeds family (one of two cases positive for mutation), and a p.G21S mutation was identified in one Sydney family (two of six cases positive for mutation).
Mantel-Haenszel chi-square test P value = 1.37 × 10-5. In the joint burden analysis, we undertook a more conservative stance and censored the second carrier from the Sydney family while including all the noncarriers. Thus, three mutations were detected in 133 total cases, but only two mutation carriers from 132 cases were used in the calculation. CI = confidence interval; CM = cutaneous melanoma; OM = ocular melanoma; TCGA = The Cancer Genome Atlas.
Values in table indicate the number of rare (MAF < 0.01) variants.
Genome-wide statistically significant.
Identical test used in both primary and TCGA replication cohorts for each gene (eg, PTV burden for CDKN2A in both primary and TCGA replication cohorts).
Although the study was not designed to compare genes that selectively confer risk for either or both CM and OM, we did compare the Pmin for each gene relative to their melanoma type (Figure 2C). There was some evidence that LCE1E can confer risk for both CM and OM (Pmin = 1.59×10‐4 for OM and 2.56×10‐3 for CM) though neither reached genome-wide statistical significance.
Functional Validation of EBF3
Among the secondary candidates, none has been previously linked to cancer predisposition. Because EBF3 ranked in three of four framework analyses, was replicated in the TCGA and European cohorts, and has been reported to possess tumor suppressive activity in a number of nonmelanoma cancers (36,37), we decided to examine EBF3 in greater detail. Among melanoma cases, there were four mutations in our discovery set (one p.Q137R, one p.A409T, and two p.N484S) (Supplementary Figure 2, available online), three mutations in the TCGA cohort (one p.P594L and two p.G459C), and two variants in the UK-descent melanoma panel (p. N455S and p. G21S). Among the EBF3 variants in our cases, two have been previously observed, p.N484S (MAF = 3.00×10‐5, European/Non-Finnish) and p.G21S (MAF = 2.21×10‐4, European/Non-Finnish) albeit at a much lower rate. The p.Q137R mutation falls within the DNA binding domain, the p.A409T mutation lies in the third helix of the HLHLH domain, and the p.N484S alteration sits in and the C-terminal Pro/Ser/Thr-rich (PST) region (Supplementary Figure 2, available online). Because EBF3 have had minimal connection to melanoma biology, we set out to perform proof-of-concept validation for EBF3 as a tumor suppressor using available somatic data and empirical cell-based assays.
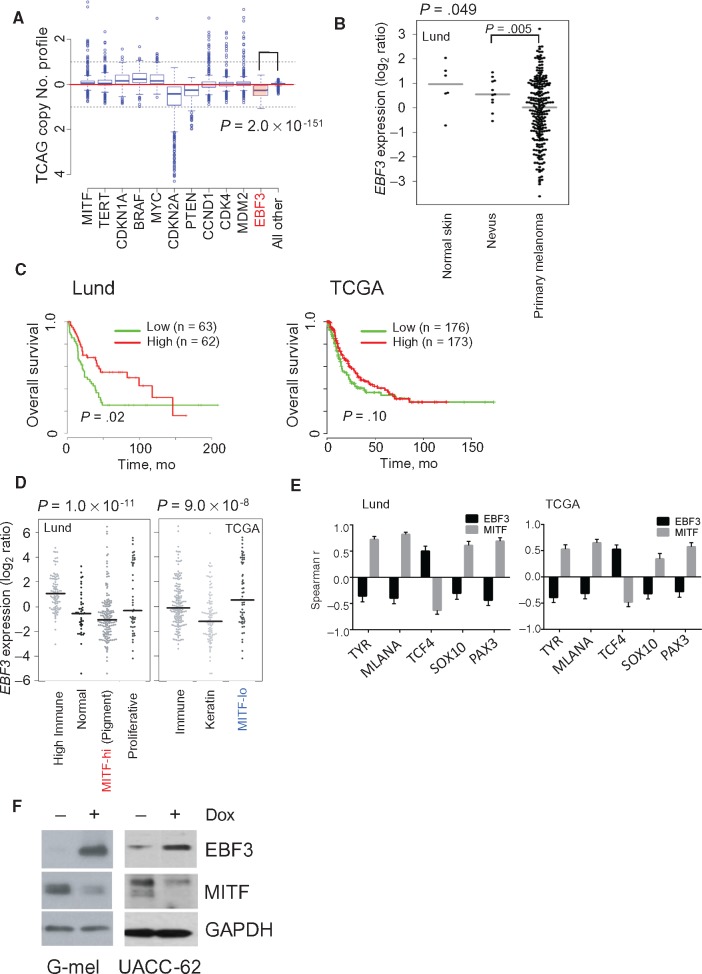
Cancer susceptibility loci (eg, CDKN2A and BAP1) often function as tumor suppressor genes and thus exhibit structural damage and/or expression loss during malignant progression. To this end, we surveyed the TCGA melanoma tumors for evidence of EBF3 somatic copy-number loss. As shown in Figure 3A, EBF3 harbored statistically significantly more deletions than other genes (P = 2.00×10‐151, Wilcoxon test), which may, in some cases, reflect loss of the entire 10q arm. There is evidence of concurrent shallow deletions of EBF3 and PTEN (also on 10q) though deep deletions of EBF3 are uncommon and independent of PTEN while expression levels of the two genes appear to be also uncorrelated (Supplementary Figure 3, available online). On a mutation level, there were 28 missense alterations identified in the TCGA melanoma cohort with no loss-of-function variants (Supplementary Table 11, available online).
Figure 3.
EBF3 validation. A) Copy number profile of EBF3 (shaded red box) and various melanoma drivers among The Cancer Genome Atlas (TCGA) tumor specimens. A two-sided Wilcoxon test was used to compute P values. B)EBF3 expression in normal skin, benign nevi, and primary melanoma. P values were calculated using a two-sided Wilcoxon test. C) Lund University Medical Center and TCGA patient survival with metastatic melanoma with high and low EBF3 expression. Two-sided Cox proportional model was used to estimate significance. D)EBF3 expression in different molecular subtypes of melanoma. E) Spearman correlations for both EBF3 and MITF and melanocyte lineage genes in the Lund and TCGA data sets (error bars indicate 95% confidence interval). F) Induction of EBF3 in G-mel and UACC-62 cells using a Tet-responsive promoter. Dox = doxycycline; TCGA = The Cancer Genome Atlas.
With regards to expression, primary melanomas exhibited lower EBF3 RNA levels when compared with either normal skin or benign nevi (melanoma vs nevus, P = .005, analysis of variance) (Figure 3B), suggesting that loss of EBF3 may contribute to melanoma progression. Diminished EBF3 expression was also associated with heightened tumor aggressiveness. Using microarray data from a panel of 125 stage III melanoma tumor specimens obtained at Lund University and RNA-seq data from 470 TCGA tumor specimens, we found that lower EBF3 levels showed a statistically significant correlation with poorer overall survival in the Lund data set (P = .02, log-rank test) (Figure 3C) and a marginal trend toward a worsened outcome among the TCGA tumors (P = .10, log-rank test).
To better understand the molecular context of EBF3 function, we examined EBF3 levels across known molecular subtypes of melanoma. Strikingly, EBF3 appears to be inversely correlated with the MITF-anchored classes; that is, EBF3 expression was lowest in the MITF-hi (“pigmentation”) subtype from the Lund cohort and highest in the “MITF-lo” subtype from the TCGA set (Figure 3D). Given this intriguing relationship, we searched for interactions between levels of EBF3 and melanocyte lineage genes. There was a statistically significant inverse relationship between EBF3 and MITF expression levels in both the Lund (Spearman r = –0.36, 95% CI = –0.47 to –0.24, P < .0001) and TCGA (Spearman r = –0.30, 95% CI = –0.40 to –0.18, P < 10‐4) (Supplementary Table 12, available online) tumor sets. Moreover, examination of several known MITF targets (TYR, MLANA) (Figure 3E) and upstream regulators of MITF (TCF4, SOX10, PAX3) all revealed a consistent pattern whereby lineage genes that positively correlated with MITF (ie, TYR, MLANA, SOX10, and PAX3) were negatively correlated with EBF3 while TCF4, a known negative regulator of MITF, was positively correlated with EBF3. These results reveal a reciprocal relationship between EBF3 and MITF and raise the possibility that EBF3 may antagonize MITF. To test this hypothesis, we induced EBF3 in two MITF-expressing melanoma lines (G-mel and UACC-62) using a Tet-responsive system and found a concomitant reduction of MITF in both cell lines (Figure 3F).
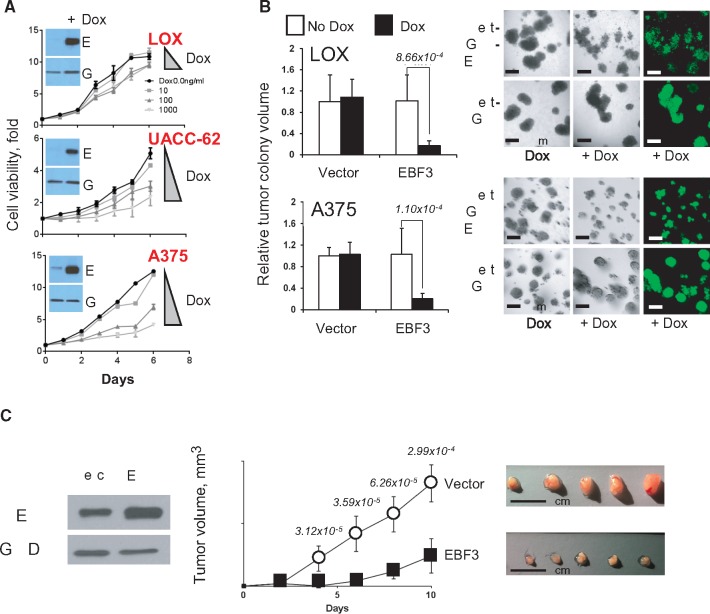
We next sought to phenotypically credential EBF3 in cellular and mouse experiments. As shown in Figure 4A, there was a dose-dependent suppression of cellular proliferation with escalating levels of EBF3 in three distinct melanoma cell lines; exposure of cells with the Tet-GFP control vector to doxycycline had no measurable effect on survival (data not shown). A375 and LOX cells were then subjected to a 3D matrigel spheroid formation assay, and for both lines there was a statistically significant reduction in colony volume with the introduction of EBF3 (Figure 4B). Finally, overexpression of EBF3 in A375 melanoma cells led to statistically significant suppression of tumor growth in nude mice (Figure 4C). Taken together, these results suggest that EBF3 is a veritable tumor suppressor in melanoma, a function consistent with the role as a predisposition gene.
Figure 4.
Functional accreditation of EBF3. A) Cell viability assay demonstrating growth arrest of human melanoma cell lines with EBF3 upregulation by dose-dependent induction with doxcycyline; experiments were performed in triplicates (relative mean viability +/- SD) and independently replicated using three different cell lines (LOX, UACC 62, and A375). B) 3D spheroid formation is dramatically reduced by the induction of EBF3 (relative mean tumor colony size +/- SD). C) Constitutive overexpression of EBF3 in A375 cells led to a profound suppression of tumor growth in nu/nu mice (n = 8 in each arm, mean tumor volume +/- SD). P values generated from two-sided t tests are shown above each time point. Five representative dissected tumors from each arm are also shown. Dox = doxycycline; Vec = vector.
Discussion
The contribution of rare coding mutations to disease risk, such as melanoma, remains a bourgeoning but largely unexplored domain in human genomics. To the best of our knowledge, this is the first exome-based, rare variant association study in melanoma. Several technical advancements, which were introduced to permit the robust assembly of the large-exome sequence data set, include joint variant calling and an advanced quality check protocol.
Our results indicate that in the landscape of hereditary melanoma, CDKN2A and BAP1 exhibit the strongest association with CM and OM, respectively. By design, we did not exclude these samples from analysis but blindly included the cases in the entire pipeline as “positive” controls; we were reassured that our methodology did recover these genes. There were additional risk loci that approached genome-wide statistical significance, one of which, EBF3, was further replicated in the TCGA and European melanoma cohorts.
We propose that EBF3 has many of the features of the melanoma predisposition gene based on multiple lines of investigation: 1) the enrichment for germline EBF3 variants among individuals with melanoma across disparate cohorts, 2) the presence of deletions at the EBF3 locus in tumor specimens, 3) the increased clinical aggressiveness associated with EBF3 loss, 4) the reciprocal interaction between EBF3 and the known lineage oncogene MITF, and 5) the direct inhibitory effects of EBF3 on cellular growth and tumor formation. In two families where multiple affected cases were available for analysis, EBF3 mutations were identified only in a fraction of the cases (one of two and two of six affected members), suggesting that these mutations are moderate-risk alleles, which is similar to the risk conferred by the MITF(E318K) (14) variant and consistent with EBF3’s calculated odds ratio of approximately 5.
EBF3 belongs to a family of transcription factors (EBF1-4) known to be involved in B cell differentiation and the pathogenesis of several tumor types (36,37). Although speculative, it is conceivable that the role of EBF proteins in immune cell specification (36,38) could explain, in part, the observed association between EBF3 and the “high immune” melanoma subclass. The EBF3 gene is located on the chromosome 10q26.3 and encodes a 596 amino acid protein with a conserved N-terminal DNA binding region, an IPT/TIG domain, an unusual helix-loop-helix-loop-helix (HLHLH) motif and a C-terminal PST domain (39). Functionally, the EBF transcription factors bind to DNA with a consensus sequence of 5′-CCCNNGGG-3′ as homo- or heterodimers and can interact with p300 (36). Our mutations do not appear to cluster in any single domain though genotype/functional experiments are currently underway.
For melanoma, there is a single report of EBF1 SNPs being correlated with survival among stage III and IV patients (40). In our study, EBF3 showed a strong association in all tests except the loss of freedom (LoF) burden test. The gene is highly intolerant of LoF variation (pLI = 1.0, ExAC) thereby suggesting that full loss of EBF3 function may undergo strong negative selection. The precise mechanism of EBF3 action in melanoma remains to be elucidated though one recent report suggests that EBF3 might play a role in cell migration, and possibly proliferation, in a subset of melanoma lines (41). There is precedence for transcription factors in cancer predisposition. Perhaps the most relevant is a low-prevalence SNP in the MITF transcription factor (p.E318K) that alters a sumoylation site and confers risk for both cutaneous melanoma and renal cell carcinoma (14,42). Germline mutations in other transcription factors such as TP53 and RUNX1 also produce strong cancer phenotypes in Li-Fraumeni syndrome (OMIM No. 151623) and familial AML (OMIM No. 601399), respectively.
The design of our study incorporates several classic RVAS strategies (24) though there are also several limitations. Full-exome sequencing is still more expensive compared with genotyping, but the costs are converging. Although we enriched for genetic causation by focusing on rarer familial and multiple tumor cases, statistical power is still lower than those reported for common-variant GWAS. Recognizing this risk, we subjected all available cases to our analytical pipeline including the CDKN2A and BAP1 families and were reassured that statistically significant association signals were readily detected for these loci.
Despite limitations, the analyses in this report represent a major first step toward understanding the landscape of rare mutations in hereditary melanoma. The statistical methodologies that were blindly deployed in the case/control design unequivocally recovered several anticipated signals (ie, CDKN2A and BAP1). Moreover, several subthreshold loci have been nominated for future studies, including a proof-of-concept validation of one such gene, EBF3.
Funding
These studies were supported in part by grants from the National Institutes of Health (K24-CA149202, P01- CA163222, U54 HG003067) and the Melanoma Research Alliance and by generous donors to the MGH Millennium Fund for Melanoma and the MGH Innovations in Melanoma Care Fund (all to HT). AS was funded by the European Union (European Social Fund [ESF]) and Greek national funds through the Operational Program ‘‘Education and Lifelong Learning’’ of the National Strategic Reference Framework (NSRF), Research Funding Program Aristeia I–1094. GJ was funded by The Swedish Cancer Society and the Swedish Research Council.
Notes
The funders had no role in design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The authors disclose no potential conflict of interest. We also want to thank the patients for their participation in these studies.
Authors: Mykyta Artomov, Alexander J. Stratigos, Ivana Kim, Raj Kumar, Martin Lauss, Bobby Y. Reddy, Benchun Miao, Carla Daniela Robles-Espinoza, Aravind Sankar, Ching-Ni Njauw, Kristen Shannon, Evangelos S. Gragoudas, Anne Marie Lane, Vivek Iyer, Julia A. Newton-Bishop, D. Timothy Bishop, Elizabeth A. Holland, Graham J. Mann, Tarjinder Singh, Jeffrey Barrett, David J. Adams, Göran Jönsson, Mark J. Daly, Hensin Tsao
Affiliations of authors: MGH Analytic and Translational Genetics Unit, MGH and Broad Institute, Boston, MA (MA, MJD); Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA (MA); 1st Department of Dermatology, University of Athens School of Medicine, Andreas Sygros Hospital, Athens, Greece (AJS); Retina Service, Massachusetts Eye and Ear Infirmary, Boston, MA (IK, ESG, AML); Department of Dermatology, Wellman Center for Photomedicine, MGH, Boston, MA (RK, BYR, BM, CNN, HT); Department of Oncology, Clinical Sciences, Lund University, Lund, Sweden (ML, GJ); Experimental Cancer Genetics, Wellcome Trust Sanger Institute, Cambridge, UK (CDRE, AS, VI, DJA); Laboratorio Internacional de Investigación sobre el Genoma Humano, Universidad Nacional Autónoma de México, Santiago de Querétaro, Mexico (CDRE); Melanoma Genetics Program, MGH Cancer Center, MGH, Boston, MA (KS, HT); Section of Epidemiology and Biostatistics, Leeds Institute of Cancer and Pathology, University of Leeds, Leeds, UK (JANB, DTB); Centre for Cancer Research, Westmead Institute for Medical Research, University of Sydney, Westmead, Australia (EAH, GJM); Melanoma Institute Australia, University of Sydney, North Sydney, NSW, Australia (GJM); Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, UK (TS, JB)
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2016. CA Cancer J Clin. 2016;661:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Jovanovic P, Mihajlovic M, Djordjevic-Jocic J, et al. Ocular melanoma: An overview of the current status. Int J Clin Exp Pathol. 2013;67:1230–1244. [PMC free article] [PubMed] [Google Scholar]
- 3. Greene MH, Clark WH Jr., Tucker MA, et al. High risk of malignant melanoma in melanoma-prone families with dysplastic nevi. Ann Intern Med. 1985;1024:458–465. [DOI] [PubMed] [Google Scholar]
- 4. Kraemer KH, Greene MH, Tarone R, et al. Dysplastic naevi and cutaneous melanoma risk. Lancet. 1983;2:1076–1077. [DOI] [PubMed] [Google Scholar]
- 5. Ford D, Bliss JM, Swerdlow AJ, et al. Risk of cutaneous melanoma associated with a family history of the disease. The International Melanoma Analysis Group (IMAGE). Int J Cancer. 1995;624:377–381. [DOI] [PubMed] [Google Scholar]
- 6. Mucci LA, Hjelmborg JB, Harris JR, et al. Familial risk and heritability of cancer among twins in nordic countries. JAMA. 2016;3151:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bale SJ, Dracopoli NC, Tucker MA, et al. Mapping the gene for hereditary cutaneous malignant melanoma-dysplastic nevus syndrome to chromosome 1p. N Engl J Med. 1989;320:1367–1372. [DOI] [PubMed] [Google Scholar]
- 8. Cannon-Albright LA, Goldgar DE, Meyer LJ, et al. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13-p22. Science. 1992;258:1148–1152. [DOI] [PubMed] [Google Scholar]
- 9. Jonsson G, Bendahl PO, Sandberg T, et al. Mapping of a novel ocular and cutaneous malignant melanoma susceptibility locus to chromosome 9q21.32. J Natl Cancer Inst. 2005;9718:1377–1382. [DOI] [PubMed] [Google Scholar]
- 10. Falchi M, Spector TD, Perks U, et al. Genome-wide search for nevus density shows linkage to two melanoma loci on chromosome 9 and identifies a new QTL on 5q31 in an adult twin cohort. Hum Mol Genet. 2006;1520:2975–2979. [DOI] [PubMed] [Google Scholar]
- 11. Gillanders E, Hank Juo SH, Holland EA, et al. Localization of a novel melanoma susceptibility locus to 1p22. Am J Hum Genet. 2003;732:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soura E, Eliades PJ, Shannon K, et al. Hereditary melanoma: Update on syndromes and management: Genetics of familial atypical multiple mole melanoma syndrome. J Am Acad Dermatol. 2016;743:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soura E, Eliades PJ, Shannon K, et al. Hereditary melanoma: Update on syndromes and management: Emerging melanoma cancer complexes and genetic counseling. J Am Acad Dermatol. 2016;743:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yokoyama S, Woods SL, Boyle GM, et al. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature. 2011;4807375:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Law MH, Bishop DT, Lee JE, et al. Genome-wide meta-analysis identifies five new susceptibility loci for cutaneous malignant melanoma. Nat Genet. 2015;479:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elwood JM, Gallagher RP, Hill GB, et al. Pigmentation and skin reaction to sun as risk factors for cutaneous melanoma: Western Canada Melanoma Study. Br Med J (Clin Res Ed). 1984;2886411:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holman CD, Armstrong BK.. Pigmentary traits, ethnic origin, benign nevi, and family history as risk factors for cutaneous malignant melanoma. J Natl Cancer Inst. 1984;722:257–266. [PubMed] [Google Scholar]
- 18. MacKie RM, Freudenberger T, Aitchison TC.. Personal risk-factor chart for cutaneous melanoma. Lancet. 1989;28661:487–490. [DOI] [PubMed] [Google Scholar]
- 19. Gallagher RP, McLean DI, Yang CP, et al. Suntan, sunburn, and pigmentation factors and the frequency of acquired melanocytic nevi in children. Similarities to melanoma: The Vancouver Mole Study. Arch Dermatol. 1990;1266:770–776. [PubMed] [Google Scholar]
- 20. Bliss JM, Ford D, Swerdlow AJ, et al. Risk of cutaneous melanoma associated with pigmentation characteristics and freckling: Systematic overview of 10 case-control studies. The International Melanoma Analysis Group (IMAGE). Int J Cancer. 1995;624:367–376. [DOI] [PubMed] [Google Scholar]
- 21. Holly EA, Aston DA, Cress RD, et al. Cutaneous melanoma in women. II. Phenotypic characteristics and other host-related factors. Am J Epidemiol. 1995;14110:934–942. [DOI] [PubMed] [Google Scholar]
- 22. Naldi L, Altieri A, Imberti GL, et al. Sun exposure, phenotypic characteristics, and cutaneous malignant melanoma. An analysis according to different clinico-pathological variants and anatomic locations (Italy). Cancer Causes Control. 2005;168:893–899. [DOI] [PubMed] [Google Scholar]
- 23. Iles MM, Bishop DT, Taylor JC, et al. The effect on melanoma risk of genes previously associated with telomere length. J Natl Cancer Inst. 2014;10610: dju267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zuk O, Schaffner SF, Samocha K, et al. Searching for missing heritability: Designing rare variant association studies. Proc Natl Acad Sci U S A. 2014;1114:E455–E464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Samocha KE, Robinson EB, Sanders SJ, et al. A framework for the interpretation of de novo mutation in human disease. Nat Genet. 2014;469:944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li H, Durbin R.. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;2514:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van der Auwera GA, Carneiro MO, Hartl C, et al. From FastQ data to high confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;111110:11.10.1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DePristo MA, Banks E, Poplin R, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;435:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;209:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;5367616:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Price AL, Patterson NJ, Plenge RM, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;388:904–909. [DOI] [PubMed] [Google Scholar]
- 32. Purcell S, Neale B, Todd-Brown K, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;813:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purcell S. http://zzz.bwh.harvard.edu/plink/. Accessed August 3, 2016.
- 34. McLaren W, Pritchard B, Rios D, et al. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics. 2010;2616:2069–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee S, Abecasis GR, Boehnke M, et al. Rare-variant association analysis: Study designs and statistical tests. Am J Hum Genet. 2014;951:5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liao D. Emerging roles of the EBF family of transcription factors in tumor suppression. Mol Cancer Res. 2009;712:1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tao YF, Xu LX, Lu J, et al. Early B-cell factor 3 (EBF3) is a novel tumor suppressor gene with promoter hypermethylation in pediatric acute myeloid leukemia. J Exp Clin Cancer Res. 2015;34:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Medina KL, Pongubala JM, Reddy KL, et al. Assembling a gene regulatory network for specification of the B cell fate. Dev Cell. 2004;74:607–617. [DOI] [PubMed] [Google Scholar]
- 39. Siponen MI, Wisniewska M, Lehtio L, et al. Structural determination of functional domains in early B-cell factor (EBF) family of transcription factors reveals similarities to Rel DNA-binding proteins and a novel dimerization motif. J Biol Chem. 2010;28534:25875–25879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fang S, Wang Y, Chun YS, et al. Association of common genetic polymorphisms with melanoma patient IL-12p40 blood levels, risk, and outcomes. J Invest Dermatol. 2015;1359:2266–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chatterjee A, Stockwell PA, Ahn A, et al. Genome-wide methylation sequencing of paired primary and metastatic cell lines identifies common DNA methylation changes and a role for EBF3 as a candidate epigenetic driver of melanoma metastasis. Oncotarget. 2016;84:6085–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bertolotto C, Lesueur F, Giuliano S, et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature. 2011;4807375:94–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.