Abstract
The misfolded α-synuclein protein, phosphorylated at serine 129 (pSer129 α-syn), is the hallmark of Parkinson disease (PD). Detected also in the enteric nervous system (ENS), it supports the recent theory that PD could start in the gut, rather than the brain. In a previous study, using a transgenic mouse model of human synucleinopathies expressing the A53T mutant α-synuclein (TgM83), in which a neurodegenerative process associated with α-synuclein occurs spontaneously in the brain, we have shown earlier onset of pSer129 α-syn in the ENS. Here, we used this model to study the impact of paraquat (PQ) a neurotoxic herbicide incriminated in PD in agricultural workers) on the enteric pSer129 α-syn expression in young mice. Orally delivered in the drinking water at 10 mg/kg/day for 6–8 weeks, the impact of PQ was measured in a time-dependent manner on weight, locomotor abilities, pSer129 α-syn, and glial fibrillary acidic protein (GFAP) expression levels in the ENS. Remarkably, pSer129 α-syn was detected in ENS earlier under PQ oral exposure and enteric GFAP expression was also increased. These findings bring additional support to the theory that neurotoxic agents such as PQ initiate idiopathic PD after oral delivery.
Keywords: α-Synuclein, A53T, Chronic oral exposure, Enteric nervous system, Paraquat, Parkinson disease
INTRODUCTION
Parkinson disease (PD) is the second most frequent neurodegenerative disease in the world after Alzheimer disease. In Europe, PD crude prevalence rate ranges between 65.6 per 100 000 and 12 500 per 100 000 (1). PD belongs to the so called alpha-synuclein (α-syn) aggregation diseases, also named synucleinopathies, including at least 3 diseases: PD, dementia with Lewy bodies and multiple system atrophy (2). These diseases are characterized by a neurodegeneration associated with accumulation of a pathological form of α-syn and propagated in cerebral structures, such as Lewy neurites and bodies, neuronal inclusions for PD or dementia with Lewy bodies, and glial cytoplasmic inclusions for multiple system atrophy (3, 4). Lewy bodies are composed mostly of α-syn and ubiquitin (5–7). Phosphorylation at serine 129 (pSer129 α-syn) and oligomerization of α-syn are recognized as key events in PD pathogenesis (8, 9). The distribution of Lewy inclusions in the brain has been associated with PD staging (10–12). The temporal progression of the disease revealed by these studies suggests that the pathology could initiate other than in the brain, such as the digestive tract and olfactory bulb, possibly as a result of oral or inhalation exposure to xenobiotic agents carrying neurotoxicity. This hypothesis, called Braak’s theory, was reinforced by several descriptions of pathological α-syn deposits in various peripheral nervous tissues, particularly in the enteric nervous system (ENS) of advanced as well as of preclinical PD cases (13, 14). Among the suspected xenobiotics, agricultural chemicals were identified by several epidemiological studies (15–17). Tanner et al reported a list of pesticides that present a higher correlation with the appearance of PD (18). Among them, pesticides inhibiting the mitochondrial Complex I and increasing oxidative stress appear to be more prone to induce sporadic PD upon exposure. Amid suspected products, rotenone, maneb and paraquat (PQ) have been studied experimentally and confirmed their neurotoxic effect in accordance with typical PD features (19, 20). PQ has a structure similar to that of MPP+ (deriving from 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridin [MPTP]), a well-known toxic molecule that can cause permanent Parkinsonian syndrome in humans. Also known as 1, 1′-dimethyl-4, 4′-bipyridinium, PQ is a quaternary ammonium nonselective herbicide progressively banned from the market in several countries but is still widely used in many countries all over the world. PQ was shown to be linked with early PD onset and its neurotoxicity has been assessed by several studies (16, 21).
When it comes to linking PD initiation with oral exposure to pesticides there is a paucity of published documentation. One study has shown the link between mice oral exposure to pesticide (i.e. rotenone) with pathological staging of PD, supporting Braak’s hypothesis of the peripheral initiation of the disease (22). Very few studies are using oral exposure to study the impact of pesticides on PD and, to the best of our knowledge, there have been no studies investigating the impact of oral exposure to pesticides on the α-syn pathology within the ENS, with the exception of the work published by Pan-Montojo et al (22). However, studies using other routes of administration of pesticides (e.g. intravenous or intraperitoneal routes) represent the majority of the available research. In 2007, Prasad’s team showed that PQ can accumulate in the mouse brain after oral administration in a dose-dependent manner. Systemic injection at the dose of 10 mg/kg showed that PQ persists in the ventral midbrain of mice for a prolonged time compared with other organs, with a half-life of ∼1 month, indicating that PQ crosses the blood–brain barrier and persists within the brain (23, 24).
With the above considerations, we set up the first study to investigate the potential neurotoxic effects of PQ, administrated orally, on the ENS in mutant TgHuA53T transgenic mice overexpressing human α-syn, with the A53T mutation along with murine α-syn (TgM83 lineage) (25). In this mouse model, the pathological α-syn (pSer129 α-syn) appears spontaneously in the brain and ENS several months before the symptoms appear (26). The pSer129 α-syn is detectable clearly from the age of 6 months in the brain, and as early as 4 months in the ENS (26); therefore, we used young mice (2 months-old) exposed chronically for a period of 2 months. Using immunohistochemical (IHC) techniques, we analyzed the expression of the pSer129 α-syn in ENS of PQ-exposed mice compared with controls.
MATERIALS AND METHODS
Animals and Experimental Design
We used a transgenic mouse model of human synucleinopathy, TgM83 (HuA53T α-syn transgenic mice), that overexpresses A53T mutant human α-syn under a prion promoter (B6; C3H-Tg(SNCA)83Vle/J) (25). Groups of 23 (exposed) and 11 (control) transgenic mice were composed of young (2 months-old, 19–23 g) homozygous TgM83 male and female mice (produced in Anses Lyon approved animal facilities, PFEA). A wild type control group of 12 young, female C57Bl/6 mice (8 weeks-old, #18–22 g body weight) expressing only murine α-syn (Janvier, Le Genest-Saint-Isle, France) was used for PQ exposure (n = 6) and control tap water exposure (n = 6). Mice were maintained in the PFEA with a controlled temperature and hygrometry, 12-hour day/night cycle and were fed ad libitum. All the experimental procedures were conducted in compliance with procedures agreed by ANSES/ENVA/UPEC ethic committee (N°14-004).
PQ (methyl viologen dichloride hydrate, ref. 856177, Sigma–Aldrich, St. Louis, MO) was administered to TgM83 mice by drinking water (50 µg/mL), ad libitum, for 6, 7, or 8 weeks (n = 7–8/time point; Fig. 1A) and to C57Bl/6 mice by drinking water (50 µg/mL), ad libitum, for 6 weeks (n = 6; Fig. 1B); approximate daily PQ intake is roughly equivalent to 10 mg/kg bw/day taking account an average daily water intake estimated to be ∼1.5 mL/10 g body weight/day; http://web.jhu.edu/animalcare/procedures/mouse.html). Because PQ is stable in water for more than 14 days at 54 °C (http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Evaluation04/paraquat.pdf) and sensitive to UV actions, a fresh PQ solution was prepared every 14 days and kept at 4 °C in glass bottle covered with aluminum sheet. The solution was used as drinking water that was changed twice a week. Weights were monitored weekly. After initial training, basal locomotor performances for each mouse were recorded prior to pesticide exposure by performing 2 rotarod tests weekly: endurance (3 × 5 minutes at 20 RPM) and acceleration (2 minutes at 4–40 RPM). The mean latency to fall was recorded and analyzed. At each end-point, mice were killed by 200 µL of pentobarbital (54.7 mg/mL) i.p. injection. Intestines were collected from the distal stomach to the distal colon, opened and rinsed in a bath of 0.1 M PBS, and then rolled in spiral shape directly within an inclusion cassette with the upper section at the center, using a method adapted from Moolenbeek et al (27) (Supplementary Data Fig. S1A). Once post-fixed for at least 48 hours in a buffered 10% formalin solution, samples were embedded in paraffin following a routine protocol. Five-micrometer sections of guts were collected on adhesive slides (Surgipath X-tra adhesive, Leica, Wetzlar, Germany).
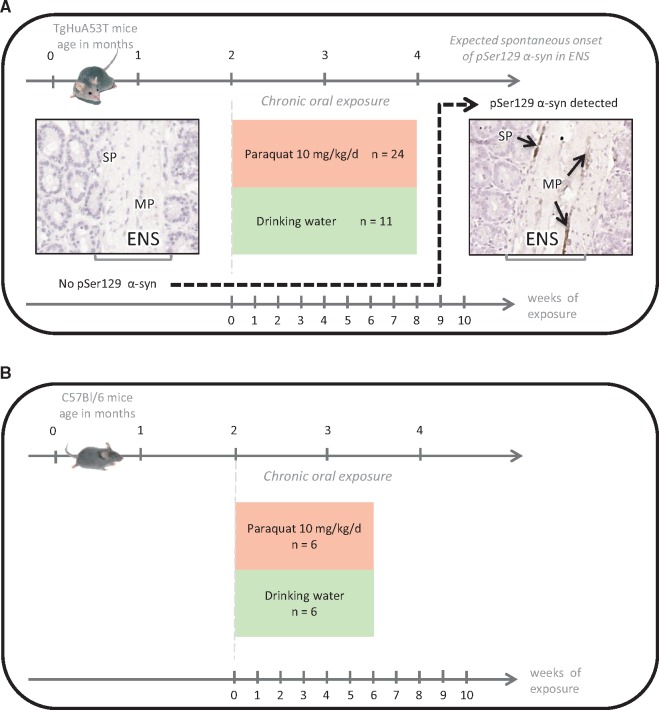
FIGURE 1.
(A) Experimental design of the chronic paraquat (PQ) oral exposure experiments in TgM83 in order to study the impact on pSer129 α-synuclein expression in the ENS. PQ was delivered in the drinking water (50 µg/mL, ad libitum) for 6, 7, and 8 weeks (n = 23) compared with control mice (n = 11) exposed to only tap water as drinking water. The endpoints were selected with the knowledge of absence pSer129 α-synuclein expression in the ENS expected in young mice of control groups. This is illustrated in the left panel by a microphotograph of an intestine section, compared with obvious expression in each animal from the age of 4 months. The right panel shows the pSer129 α-synuclein expression detected by IHC in the ENS, which appeared as dark brown deposits in neurons of the muscular plexus). (B) Experimental design of the chronic PQ oral exposure experiments in the control wild type group (C57Bl/6). PQ was delivered in the drinking water (50 µg/mL, ad libitum) for 6 weeks (n = 6) compared with control mice (n = 6) exposed to only tap water as drinking water.
IHC Detection of pSer129 α-Synuclein and GFAP in ENS
Identification of the pSer129 α-syn (1/500 ab 51253, AbCam, Cambridge, UK) as a marker of α-synucleinopathy, and the detection of glial fibrillary acidic protein (GFAP) (1/500, ab7260, AbCam, Cambridge, UK) as a marker of a reactive gliosis in the ENS was accomplished by IHC. Importantly, if several antiphosphorylated α-synuclein antibodies successfully detect phosphorylated form in the CNS of mouse ([P-syn/81 A] [ab184674], [EP1536Y], and [ab 51253]), only one allows its detection in the ENS (ab 51253) (Supplementary Data Fig. S2). The IHC procedure applied was similar to that described previously (26). Briefly, successive slides of gut sections were used to reveal each of the markers. Biotinylated secondary antibodies used at 1/200 (antiRabbit ref. 4010-05 and antimouse ref. 1010-05, Cliniscience, Nanterre, France), combined with avidin biotin complex (Vectastain ABC HRP Kit, Vector Laboratories, Burlingame, CA) and 3, 3′-diaminobenzidine peroxidase intensified with nickel (DAB Peroxidase (HRP) Substrate Kit, Vector Laboratories), revealed the specific staining that appeared as dark brown deposits (Supplementary Data Fig. S1C–E). Finally, the histological organization of each tissue sample was visualized using aqueous hematoxylin staining.
To characterize the type of enteric neurons that sustain pSer129 α-synuclein expression, a double-labeling experiment was conducted, as described previously (28), using either rabbit polyclonal anti tyrosine hydroxylase (TH) antibodies (1: 200, Abcam) or mouse monoclonal anticholine acetyltransferase (ChAT) antibodies (1: 200, Chemicon, Temecula, CA). Gut samples were first labeled with pSer129 α-synuclein antibodies following the same protocol as above. Then, after DAB-NiCl2 staining, the slides were thoroughly rinsed in 0.1 M PBS prior to a 30-minute incubation in a blocking reagent (Roche-Boehringer, Meylan, France) solution at RT. Subsequently, tissue samples were incubated with antiTH or antiChAT antibodies overnight at RT. Again, the avidin–biotin complex system was used to detect antibody binding sites, but peroxidase activity was detected using AEC (3 amino-9 ethyl-carbazol) that produces red precipitates. Finally, the histological organization of each tissue sample was visualized using aqueous hematoxylin staining.
Quantitative Study of pSer129 α-Synuclein and GFAP Expression in ENS
Observations and image captures (10× objective) were performed on an Olympus BX51 microscope (Olympus, Tokyo, Japan) coupled to an image analysis station (Explora Nova, La Rochelle, France). The sample preparation (Swiss roll method) allowed complete access of the entire gut from the duodenum to the end of the colon using only 1 slide. To cover a representative area of ENS, 10 (GFAP) to 15 (pSer129 α-syn) images were randomly collected in the largest part available, the jejunum (Supplementary Data Fig. S1B). A classical scoring system of phosphorylated α-syn and GFAP markers in each mouse and among the 10–15 images per mouse was refined to a semiquantitative approach. The surrounding background for each image to be analyzed was subtracted systematically and automatically. Densities of phosphorylated α-syn and GFAP in the ENS of the jejunum were assessed using Morpho Strider software (Explora Nova, La Rochelle, France) by measuring the staining inside the region of interest (ROI), the ENS for the gut (Supplementary Data Fig. S1F–I). The density of staining was calculated by the formula:
Results are expressed as means of density of pSer129 α-syn or GFAP staining within the nervous plexus.
Statistical Analyses
Survival estimates were computed using the Kaplan Meier method and survival distributions were compared between groups using the log rank test. Comparison of the weight between exposed and control mice was done the same way. The Welch t-test was used to compare the latency between control and PQ groups every week for acceleration and endurance. Statistical analyses for quantification of density of staining of pSer129 α-syn and GFAP were done comparing exposed to control groups using the one way ANOVA test, after having verified the homoscedasticity with the Levene test. Statistical analyses were made with R software (R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org).
RESULTS
Chronic Oral Exposure to PQ up to 8 Weeks Had No Significant Effect on Mouse Weight, Survival, and Locomotor Performances
PQ oral exposure was from 6 to 8 weeks on 3.5–4-month-old TgM83 mice. At this age, transgenic mice do not yet show PD-like pathology (paralysis) that appears rather from 8 months of age; however, as shown in our previous study, pSer129 α-syn starts to be detectable at the age of 4 months in the ENS and 6 months in the brain (26). Weight and survival of young TgM83 mice indicated no significant differences between PQ-exposed mice and controls (Supplementary Data Fig. S3A, B). The 2 locomotor tests (endurance and acceleration) performed weekly on mice using rotarod apparatus showed no significant impairment of mice locomotor skills along PQ oral exposure (Supplementary Data Fig. S3C, D). In the wild type control group, PQ oral exposure performed during 6 weeks on C57Bl6 mice did not trigger weight or survival differences with the nonexposed group. There was no significant alteration either in the locomotor performances measured by the rotarod tests (data not shown).
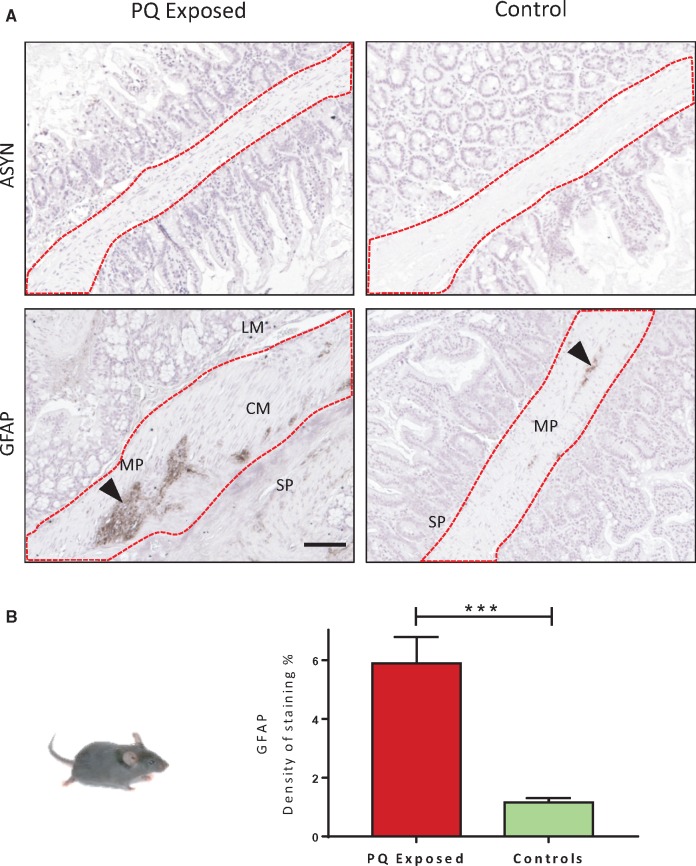
Oral Exposure to PQ Does Not Induce pSer129 α-Synuclein Expression in the ENS of C57Bl/6 Mice
The phosphorylated S129 α-syn was not observable in any of the C57BL6 mice exposed to PQ for 6 weeks (Fig. 2A). However, the response of the glial compartment of the ENS investigated through the level of GFAP expression appeared significantly (p < 0.0001) increased (Fig. 2B) compared with the controls. This suggests the presence of a reactive gliosis in the ENS compartment of PQ-exposed mice.
FIGURE 2.
pSer129 α-syn IHC analyses in the ENS of wild type control group, C57Bl/6 mice exposed to paraquat (PQ) by drinking water or not (control). (A) Absence of pSer129 α-syn in the ENS of wild type mice, whereas a significant increase of GFAP (dark brown staining, arrow) is clearly seen in exposed animals. (B) Numeric quantification of GFAP density of staining in PQ-exposed and control mice. Data were analyzed by unpaired t-test ****p < 0.0001. SP, submucosal plexus; MP, myenteric plexus; CM, circular muscle; LM, longitudinal muscle. Scale bar: 100 µm.
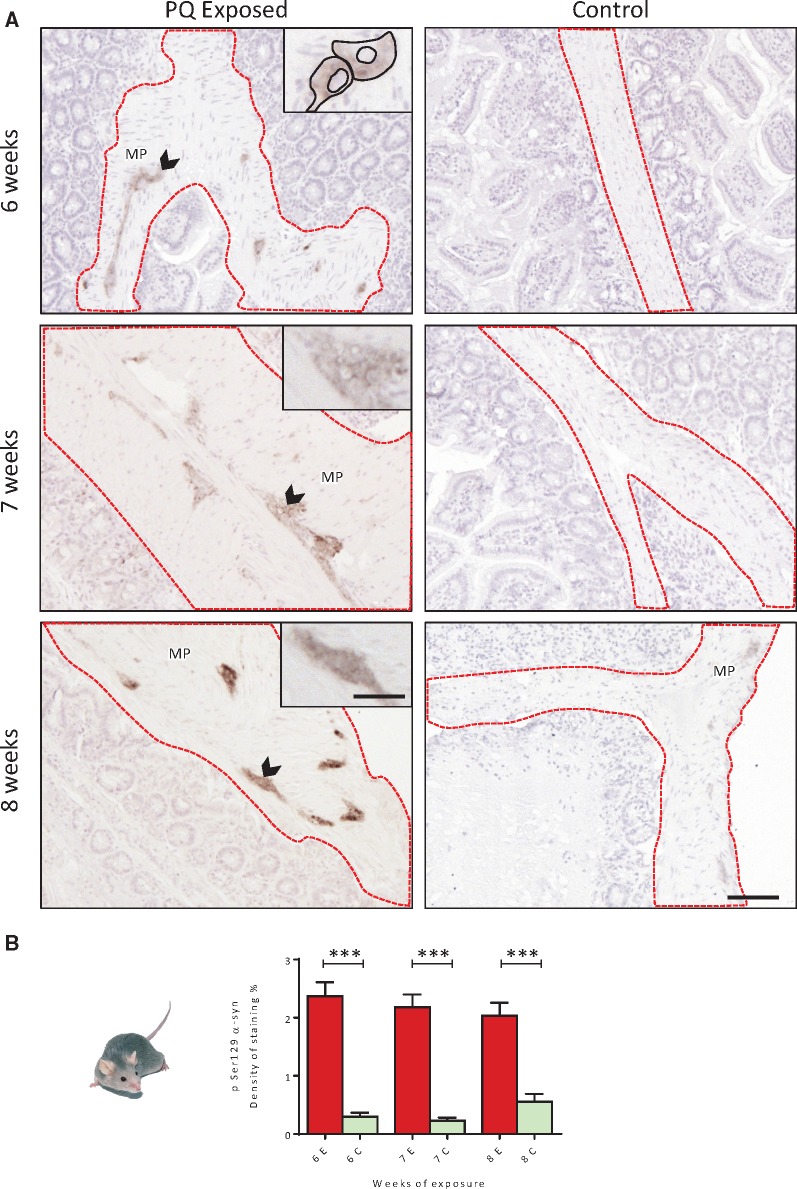
Oral Exposure to PQ Leads to Earlier pSer129 α-Synuclein Expression in the ENS of TgM83 Mice
The expression of pSer129 α-syn revealed by IHC was quantified in several portions of the jejunum both visually and numerically. In control groups (n = 4) exposed to normal drinking water, pSer129 α-syn appeared to be faintly detectable (∼4/15 images faintly stained per positive mouse) in the ENS of some mice (3/11 animals), and within a few neurons of the ENS, mostly in the myenteric plexus. At 8 weeks, the expression of pSer129 α-syn appeared more clearly in 2 out of 4 mice as revealed by the higher density detected using quantitative analysis (Fig. 3A). This could be interpreted as the first signs of local accumulation expected to appear more clearly in older mice (26). Strikingly, in the PQ-treated group, pSer129 α-syn was clearly detectable in 19/23 mice, and as early as from 6 weeks of exposure in 7/8 mice (Fig. 3A, B). Quantitative analyses showed that pSer129 α-syn immunolabeling was significantly higher (p < 0.001) in PQ-exposed mice and these differences in the levels of expression appeared similar after 7 and 8 weeks of exposure.
FIGURE 3.
(A) Immunohistochemical detection of pSer129 α-syn (dark brown deposits) in the ENS of TgM83 mice exposed to PQ by drinking water up to 8 weeks at 10 mg/kg/day. (B) Time-dependent evolution of density of staining of pSer129 α-syn expressed in the ENS. PQ orally delivered was associated with a significant pSer129 α-syn increase as early as 6 weeks after the beginning of exposure. One way ANOVA test was used to analyze this data set; ***p < 0.001. E, Exposed; C, Control. Scale bars: Images 100 µm—insets 270 µm.
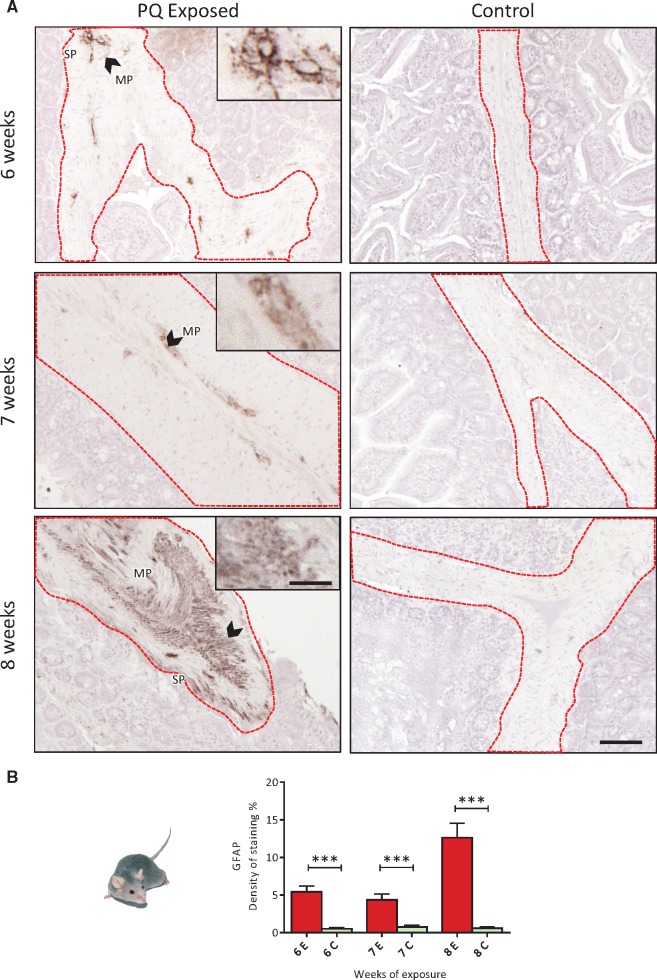
Oral Exposure to PQ Leads to GFAP Expression in the ENS of TgM83 Mice
To complete this observation, the response of the glial compartment of the ENS was studied through the level of GFAP expression. The control group revealed no obvious GFAP staining in the jejunum sections whatever the time point considered. After 6 weeks of exposure, in the PQ-exposed group, GFAP level of expression was significantly (p < 0.001) increased compared with control group, and was similarly increased after 7 weeks of exposure; after 8 weeks of PQ exposure, GFAP expression was the highest (2.5× compared with the level reached after 6 weeks of exposure) suggesting a progressive reactive gliosis (Fig. 4A, B).
FIGURE 4.
(A) Immunohistochemical detection of GFAP (dark brown deposits) in the ENS of TgM83 mice exposed to PQ by drinking water up to 8 weeks at 10 mg/kg/day. (B) Time-dependent evolution of density of staining of GFAP expressed in the ENS. PQ orally delivered was associated with a significant increase of GFAP expression in TgM83 mice ENS was observed (up to 5-fold after 8 weeks of exposure). One way ANOVA test was used to analyze this data set; ***p < 0.001. E, Exposed; C, Control. Scale bars: Images 100 µm—insets 270 µm.
Phosphorylated α-Synuclein Is Mainly Expressed by ENS Neurons
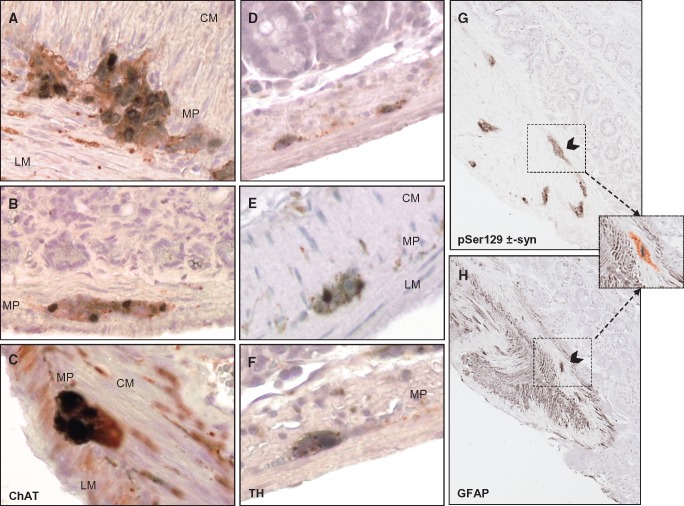
The GFAP and pSer129 α-synuclein immunostainings on successive gut sections (Fig. 5G, H) clearly showed that neuronal cells rather than glial cells express abnormal α-syn. However, a computer-assisted superposition of the labeling obtained on 2 successive slides suggested that some glial cells may accumulate a pathological form of α-syn (Fig. 5 inset G, H). In the PQ-exposed mice, cholinergic and catecholaminergic neurons most likely hosted the pSer129 α-synuclein. Indeed, in terminal stage TgM83 mice, double labeling using a specific antiChAT antibody revealed that cholinergic neurons of the myenteric plexus were accumulating pSer129 α-synuclein within their cell bodies, and apparently less so in their neuronal processes (Fig. 5A–C). Not all aggregates of pSer129 α-synuclein colocalized with ChAT-labeled cell bodies. Although myenteric neurons are not frequently reported to be positively labeled with TH antibody, the possibility that catecholaminergic neurons may also accumulate pSer129 α-synuclein in the ENS was examined. The TH staining was less intense compared with ChAT staining in terms of number of labeled cells; some myenteric as well as submucosal neurons were immunoreactive for TH (Fig. 5D–F), and in the myenteric plexus, double labeling revealed that TH-positive cell bodies hosted pSer129 α-synuclein aggregates (Fig. 5E, F).
FIGURE 5.
Cholinergic and catecholaminergic neurons of the enteric nervous system express phosphorylated α-synuclein. (A) Double immunolabeling shows that pSer129 α-synuclein (diffuse staining black-stained areas) colocalized with ChAT-immunoreactive neurons (red-stained areas) in the myenteric plexus. (B) Similarly, spheroid-(Lewy-bodies) like inclusions of pSer129 α-synuclein also colocalized with ChAT-immunoreactive neurons. (C) At higher magnification, large inclusions of pSer129 α-synuclein are detected within the soma of cholinergic neurons, but less so in their processes. (SP, submucosal plexus; MP, myenteric plexus; CM, circular muscle; LM, longitudinal muscle). (D–F) Double labeling shows that pSer129 α-synuclein expression (black-stained areas) in the submucosal plexus can be associated with TH-immunoreactive cells (red-staining) as illustrated in the myenteric plexus (E, F). Phosphorylated α-Syn (G) and GFAP (H) immunostainings driven on successive gut sections clearly show that neuronal cells rather than glial cells express abnormal α-syn. However, some enteric glial cells may be able to host phosphorylated α-Syn as shown by the computer assisted superposition of these images (inset).
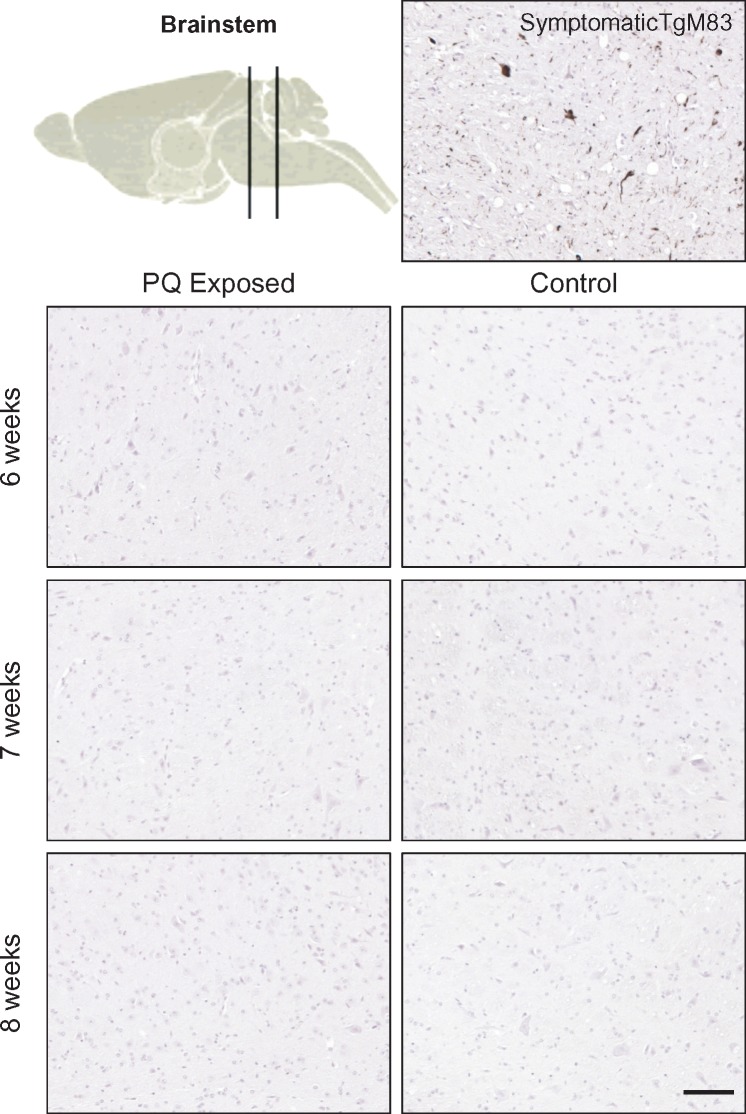
Eight-Week Oral Exposure to PQ Does Not Lead to Earlier pSer129 α-Synuclein Expression in the Brain of TgM83 Mice
To investigate the possible impact of PQ on brain expression of phosphorylated α-Syn, the brainstem and other brain regions were carefully analyzed. Compared with a positive control brain, no induction of phosphorylated α-Syn was seen, either in the dorsal nucleus of vagus nerve or elsewhere in the brain (Fig. 6), whatever the conditions studied, suggesting no effect of PQ administration on central expression of pathological α-Syn after 6 weeks of consecutive exposure through drinking water.
FIGURE 6.
Absence of pSer129 α-syn in the brainstem of TgHuA53T mice after 6–8 weeks of oral exposure to PQ. A brain of 1.5-year-old TgHuA53T mouse was used as positive control. At the final stage of the disease, many neurons were accumulating pSer129 α-syn in the brainstem. Scale bar: 25 µm.
DISCUSSION
PQ dichloride, a widely used herbicide all over the world, is one of the pesticides that has been recognized as a possible factor in the pathogenesis of PD (18). Still, its neurotoxicity, far from being completely understood, has been mainly studied experimentally in central nervous system cells and not in peripheral nervous system cells, despite the possibility of interaction of PQ with the peripheral nervous system, notably in the case of oral exposure. The present original in vivo experiment using chronic PQ exposure by oral route was designed in order to explore the latest hypothesis on early onset of PD in peripheral nervous compartments (29–31). PQ can reproduce some of the features of PD in animals, specifically a loss of dopaminergic neurons and an increase of α-syn aggregation in the brain (32, 33). Also, PQ is known to induce PD-like symptomatology in mice (34) but nothing has been reported on the effects of PQ in the ENS. In addition, the oral route is the less investigated, and as already underlined by Prasad et al in earlier studies, the manner of exposure appears to be critical (23). For the oral route, intragastric administration of PQ at 10 mg/kg/day in wild type mice was reported to be efficient but also triggered some deaths among mice (23, 35). In our experiment, we used the same daily dose, but PQ was delivered chronically using drinking water in order to mimic in a more realistic way what could happen if someone is orally exposed to that kind of environmental xenobiotic. Thus, the present study explored in a relevant way the question of how PQ administrated through drinking water may trigger early adverse effects on the ENS.
Our results show that up to 6 weeks of chronic exposure at 10 mg/kg/day through drinking water, PQ is well tolerated by the C57Bl6 mice, as indicated by absence of loss of weight and of mortality. Rotarod tests show no motor deficiencies suggesting no impact of dietary exposure to PQ on the brain. In the ENS present in the digestive tract, we report the absence of phosphorylated α-syn expression in the wild type mice exposed to PQ after 6 weeks; however, this observation cannot be considered as a proof of absence of neurotoxicity of PQ on the ENS of wild type mice since PQ exposure leads to reactive gliosis, displayed by the significant overexpression of GFAP. Still the duration of exposure appears not long enough to induce any pSer129 α-syn expression. This first observation in wild type mice could not be related to previous studies as there is no other study focusing on the effect of PQ in the ENS. In contrast, the results are different in the transgenic mice: at 8 weeks of chronic exposure at 10 mg/kg/day through drinking water, PQ is well tolerated by the transgenic mice, as indicated by absence of loss of weight and of mortality. Rotarod tests show no motor deficiencies, suggesting there is no effect of dietary exposure to PQ on the brain. In the meantime, the IHC analyses of the intestines revealed remarkable effects on pSer129 α-syn and GFAP expression. Indeed, pSer129 α-syn appeared to be detectable faintly in the ENS of control group and in few animals only. This shows the beginning of the spontaneous accumulation of the phosphorylated protein, observed in this model more clearly from 4 months of age (26). In contrast, in the PQ-exposed group, pSer129 α-syn was clearly detected in >80% of the mice, with strong expression levels, revealing a major impact seen after only 6 weeks of exposure and signifying an acceleration of the pathological process associated to this mouse model. Most probably cholinergic neurons harbor these pSer129 α-syn aggregates in accordance to the double labeling observed in the ENS of transgenic mice showing advanced disease. However, the Lewy bodies or inclusion bodies observed in old symptomatic TgM83 mice were not observed in the younger animals exposed to PQ. It does not mean that Lewy bodies could not be induced by PQ exposure at higher dose or longer time of exposure.
In contrast to these observations in the ENS, the brains of these mice were all devoid of any phosphorylated α-Syn. This indicates that the neurotoxic effect of PQ is expressed earlier in the ENS compared with the CNS and suggests that the dose of PQ and/or duration of the study were not sufficient to trigger an induction of pSer129 α-syn in the CNS compartment too. The difference between ENS and CNS could be explained by the fact that enteric neurons are more directly exposed to the ingested PQ. The intensity of the staining observed at this first experimental time point available suggests that expression of pSer129 α-syn may have possibly started even before. This first observation in TgM83 mice could not be related to previous studies as, to the best of our knowledge, there is no other study focusing on the effect of PQ in the ENS. An article published by Pan-Montojo et al studied the effect of rotenone orally administered to C57Bl/6 mice for 8 or 16 weeks in the ENS (22). Among other results, they showed that rotenone oral exposure by IG route leads to release and accumulation of pSer129 α-syn in the ENS. Therefore, using a different pesticides related to PD, our study adds experimental arguments in favor of PD initiation within this compartment and demonstrates a reason to focus on the ENS along with other toxin exposure. Altogether, our data suggest that the transgenic model could be more sensitive to PQ compared with wild type mice, but at this dose and quite short duration of exposure it is not sufficient to trigger pathological α-syn expression in the brain.
To complete this observation, we also introduced the study of the enteric glial cells. The contrast between control and exposed guts was even more obvious for the glial marker GFAP. The overexpression of GFAP, revealing a reactive gliosis when the phosphorylated α-syn is detectable, suggests a possible cooperation between enteric glial cells and neurons accumulating pathological α-syn. However, the likelihood that some glial cells may directly accumulate a pathological form of α-syn cannot be excluded, as suggested in some cases. This possibility is known and described in the brain, notably in specific forms of human alpha-synucleinopathies, such as multiple system atrophy, characterized by glial cytoplasmic inclusions. Reactive gliosis has been reported in the intestines of patients with inflammatory bowel disease (36, 37). Further data collected in cultured enteric glial cells support the link between enteric glial cells and inflammation; notably, these in vitro experiments report that pro-inflammatory cytokines such as tumor necrosis alpha increase the expression levels of GFAP (38) and that once reactive, enteric glia can secrete interleukin-6 (39). However, because there is not as much knowledge on the involvement of glial cells in the ENS compared with CNS, it is difficult to better interpret the link between the overexpression of GFAP and pathological α-syn expression observed in our model. An enteric glial reaction was suggested to occur in PD patients as shown by an upregulation of GFAP mRNA in colon biopsies (40) and was confirmed by increased GFAP protein levels in both mucosal and submucosal enteric glial cells (41). Indeed, even within the brain, the complexity of the role of astroglial cells in the initiation and progression of PD is far from being understood (42). This could, however, indicate that, to a greater extent, the inflammatory pathway may be activated with the pathological α-syn presence, supporting the hypothesis that inflammation is involved in α-syn misfolding process. Interestingly, a recent study reported that inflammation may be even more severe in A53T mutant context (43); further studies will be necessary, starting with a more precise following of the kinetic response of both markers pSer129 α-syn and GFAP in the ENS under a xenobiotic stressor. Additionally, a deeper characterization of the pathological form of α-syn, using markers of other forms of α-syn, should be done, and in these longer studies, appearance of inclusion bodies should be combined with analysis of other markers such as ubiquitin and p62.
It is also known that PQ is weakly metabolized by the brain compared with other organs, as shown by local PQ accumulation in different brain areas (23). Therefore, it would be important to assess whether any PQ accumulation would also be possible in the ENS. Indeed, this could probably play an important role in the local response to such a chronic xenobiotic exposure. Thus, an immediate goal of the next study might be the precise quantification of PQ in the ENS compared with the brain. This would allow for exploration of the link between local PQ quantity and pathological α-synuclein accumulation and also to precisely determine the daily doses that could be associated with a deleterious effect on α-synuclein expression in the ENS. In the meantime, our study suggests that new studies need to be performed after longer exposures, resulting in higher cumulated doses to identify possible effects on pSer129 α-synuclein appearance within the brain, studies that should help to better delineate the hypothetical role of the ENS in the initiation of PD after chronic xenobiotic exposure.
Conclusions
Our study demonstrates that orally delivered, PQ is able to induce a neurotoxic response in the ENS of A53T mutant human α-synuclein transgenic mice, as shown by earlier and clear expression of pathological α-syn in this compartment combined to reactive gliosis induction, signifying an acceleration of the pathological process associated to this mouse model. This could be explained by the fact that enteric neurons are more directly exposed to the environmental xenobiotics ingested. Our study parallels Braak’s theory in highlighting the ENS as a possible initiation site for α-synucleinopathy-related pathologies such as PD.
Finally, we show that the TgM83 transgenic mouse line may be a sensitive model addressing environmental stressors such as PQ, and is relevant for the study of how oral ingestion of such neurotoxic agents may initiate the accumulation of phosphorylated α-synuclein, the hallmark of PD, in the ENS. By showing an acceleration/exacerbation of the pathology within the ENS, where α-syn is the molecular target, this model should be a valuable tool for studying early events triggered by the other pesticides suspected to be linked to PD.
Supplementary Material
REFERENCES
- 1. von Campenhausen S, Bornschein B, Wick R, et al. Prevalence and incidence of Parkinson's disease in Europe. Eur Neuropsychopharmacol 2005;15:473–90 [DOI] [PubMed] [Google Scholar]
- 2. Goedert M, Spillantini MG.. Lewy body diseases and multiple system atrophy as alpha-synucleinopathies. Mol Psychiatry 1998;3:462–5 [DOI] [PubMed] [Google Scholar]
- 3. Li JY, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med 2008;14:501–3 [DOI] [PubMed] [Google Scholar]
- 4. Xia Q, Liao L, Cheng D, et al. Proteomic identification of novel proteins associated with Lewy bodies. Front Biosci 2008;13:3850–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gomez-Tortosa E, Newell K, Irizarry MC, et al. alpha-Synuclein immunoreactivity in dementia with Lewy bodies: morphological staging and comparison with ubiquitin immunostaining. Acta Neuropathol 2000;99:352–7 [DOI] [PubMed] [Google Scholar]
- 6. Shults CW. Lewy bodies. Proc Natl Acad Sci U S A 2006;103:1661–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spillantini MG, Crowther RA, Jakes R, et al. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci U S A 1998;95:6469–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walker DG, Lue LF, Adler CH, et al. Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Exp Neurol 2013;240:190–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou J, Broe M, Huang Y, et al. Changes in the solubility and phosphorylation of alpha-synuclein over the course of Parkinson's disease. Acta Neuropathol 2011;121:695–704 [DOI] [PubMed] [Google Scholar]
- 10. Braak H, Del Tredici K.. Potential pathways of abnormal tau and alpha-synuclein dissemination in sporadic Alzheimer's and Parkinson's diseases. Cold Spring Harb Perspect Biol 2016;8:a023630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braak H, Del Tredici K, Bratzke H, et al. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson's disease (preclinical and clinical stages). J Neurol 2002;249(Suppl 3):III/1–5 [DOI] [PubMed] [Google Scholar]
- 12. Hawkes CH, Del Tredici K, Braak H.. Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 2007;33:599–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruffmann C, Parkkinen L.. Gut feelings about alpha-synuclein in gastrointestinal biopsies: biomarker in the making? Mov Disord 2016;31:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hilton D, Stephens M, Kirk L, et al. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson's disease. Acta Neuropathol 2014;127:235–41 [DOI] [PubMed] [Google Scholar]
- 15. Ascherio A, Schwarzschild MA.. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol 2016;15:1257–72 [DOI] [PubMed] [Google Scholar]
- 16. Baltazar MT, Dinis-Oliveira RJ, de Lourdes Bastos M, et al. Pesticides exposure as etiological factors of Parkinson's disease and other neurodegenerative diseases–a mechanistic approach. Toxicol Lett 2014;230:85–103 [DOI] [PubMed] [Google Scholar]
- 17. van der Mark M, Brouwer M, Kromhout H, et al. Is pesticide use related to Parkinson disease? Some clues to heterogeneity in study results. Environ Health Perspect 2012;120:340–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanner CM, Kamel F, Ross GW, et al. Rotenone, paraquat, and Parkinson's disease. Environ Health Perspect 2011;119:866–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giraldez-Perez R, Antolin-Vallespin M, Munoz M, et al. Models of alpha-synuclein aggregation in Parkinson's disease. Acta Neuropathol Commun 2014;2:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murakami S, Miyazaki I, Miyoshi K, et al. Long-term systemic exposure to rotenone induces central and peripheral pathology of Parkinson's disease in mice. Neurochem Res 2015;40:1165–78 [DOI] [PubMed] [Google Scholar]
- 21. Moretto A, Colosio C.. The role of pesticide exposure in the genesis of Parkinson's disease: epidemiological studies and experimental data. Toxicology 2013;307:24–34 [DOI] [PubMed] [Google Scholar]
- 22. Pan-Montojo F, Schwarz M, Winkler C, et al. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep 2012;2:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prasad K, Tarasewicz E, Mathew J, et al. Toxicokinetics and toxicodynamics of paraquat accumulation in mouse brain. Exp Neurol 2009;215:358–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prasad K, Winnik B, Thiruchelvam MJ, et al. Prolonged toxicokinetics and toxicodynamics of paraquat in mouse brain. Environ Health Perspect 2007;115:1448–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giasson BI, Duda JE, Quinn SM, et al. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron 2002;34:521–33. [DOI] [PubMed] [Google Scholar]
- 26. Bencsik A, Muselli L, Leboidre M, et al. Early and persistent expression of phosphorylated alpha-synuclein in the enteric nervous system of A53T mutant human alpha-synuclein transgenic mice. J Neuropathol Exp Neurol 2014;73:1144–51 [DOI] [PubMed] [Google Scholar]
- 27. Moolenbeek C, Ruitenberg EJ.. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab Anim 1981;15:57–9 [DOI] [PubMed] [Google Scholar]
- 28. Bencsik A, Lezmi S, Baron T.. Autonomic nervous system innervation of lymphoid territories in spleen: a possible involvement of noradrenergic neurons for prion neuroinvasion in natural scrapie. J Neurovirol 2001;7:447–53 [DOI] [PubMed] [Google Scholar]
- 29. Braak H, de Vos RA, Bohl J, et al. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett 2006;396:67–72 [DOI] [PubMed] [Google Scholar]
- 30. Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197–211 [DOI] [PubMed] [Google Scholar]
- 31. Del Tredici K, Braak H.. Review: sporadic Parkinson's disease: development and distribution of alpha-synuclein pathology. Neuropathol Appl Neurobiol 2016;42:33–50 [DOI] [PubMed] [Google Scholar]
- 32. Fahim MA, Shehab S, Nemmar A, et al. Daily subacute paraquat exposure decreases muscle function and substantia nigra dopamine level. Physiol Res 2013;62:313–21 [DOI] [PubMed] [Google Scholar]
- 33. Manning-Bog AB, McCormack AL, Li J, et al. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem 2002;277:1641–4 [DOI] [PubMed] [Google Scholar]
- 34. Bove J, Perier C.. Neurotoxin-based models of Parkinson's disease. Neuroscience 2012;211:51–76 [DOI] [PubMed] [Google Scholar]
- 35. Mitra S, Chakrabarti N, Bhattacharyya A.. Differential regional expression patterns of alpha-synuclein, TNF-alpha, and IL-1beta; and variable status of dopaminergic neurotoxicity in mouse brain after Paraquat treatment. J Neuroinflamm 2011;8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cornet A, Savidge TC, Cabarrocas J, et al. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? Proc Natl Acad Sci U S A 2001;98:13306–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. von Boyen GB, Schulte N, Pfluger C, et al. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol 2011;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. von Boyen GB, Steinkamp M, Reinshagen M, et al. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 2004;53:222–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ruhl A, Franzke S, Collins SM, et al. Interleukin-6 expression and regulation in rat enteric glial cells. Am J Physiol Gastrointest Liver Physiol 2001;280:G1163–71 [DOI] [PubMed] [Google Scholar]
- 40. Devos D, Lebouvier T, Lardeux B, et al. Colonic inflammation in Parkinson's disease. Neurobiol Dis 2013;50:42–8 [DOI] [PubMed] [Google Scholar]
- 41. Clairembault T, Kamphuis W, Leclair-Visonneau L, et al. Enteric GFAP expression and phosphorylation in Parkinson's disease. J Neurochem 2014;130:805–15 [DOI] [PubMed] [Google Scholar]
- 42. Bruck D, Wenning GK, Stefanova N, et al. Glia and alpha-synuclein in neurodegeneration: a complex interaction. Neurobiol Dis 2016;85:262–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoenen C, Gustin A, Birck C, et al. Alpha-synuclein proteins promote pro-inflammatory cascades in microglia: stronger effects of the A53T mutant. PLoS One 2016;11:e0162717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.