Abstract
Taste and smell receptor expression has been traditionally limited to the tongue and nose. We have identified bitter taste receptors (TAS2Rs) and olfactory receptors (ORs) on human airway smooth muscle (HASM) cells. TAS2Rs signal to PLCβ evoking an increase in [Ca2+]i causing membrane hyperpolarization and marked HASM relaxation ascertained by single cell, ex vivo, and in vivo methods. The presence of TAS2Rs in the lung was unexpected, as was the bronchodilatory function which has been shown to be due to signaling within specific microdomains of the cell. Unlike β2-adrenergic receptor-mediated bronchodilation, TAS2R function is not impaired in asthma and shows little tachyphylaxis. HASM ORs do not bronchodilate, but rather modulate cytoskeletal remodeling and hyperplasia, two cardinal features of asthma. We have shown that short chain fatty acids, byproducts of fermentation of polysaccharides by the gut microbiome, activate HASM ORs. This establishes a non-immune gut-lung mechanism that ties observations on gut microbial communities to asthma phenotypes. Subsequent studies by multiple investigators have revealed expression and specialized functions of TAS2Rs and ORs in multiple cell-types and organs throughout the body. Collectively, the data point towards a previously unrecognized chemosensory system which recognizes endogenous and exogenous agonists. These receptors and their ligands play roles in normal homeostatic functions, predisposition or adaptation to disease, and represent drug targets for novel therapeutics.
Keywords: Receptor, G-protein, smooth muscle, asthma, bronchodilator, airway
1. Introduction
G-protein coupled receptors (GPCRs) are well known to be expressed throughout the body, and they represent the largest superfamily of signaling proteins in the genome. We have recently found “sensory GPCRs” of the bitter taste receptor (TAS2R) family [1], and the olfactory receptor (OR) family [2] expressed deep in the lung, on human airway smooth muscle (HASM) cells. These findings of receptors “in the wrong place” was initially met with skepticism because of the bias that such specialized receptors were only expressed on taste cells of the tongue and sensory neurons in the nose, responding to external ligands involved in taste and smell perceptions, respectively.
We have now extensively characterized these receptors on HASM [1–7], and it is now clear that TAS2Rs and ORs are expressed on other cell-types in different organs as well. They represent a previously unrecognized chemosensory system that is activated by endogenous and exogenous agonists, representing potential homeostatic/disease loci as well as novel targets for therapeutic intervention. At the molecular level, these receptors also appear to signal differently than was expected from the pharmacology in taste and smell perception, indicating a plasticity of receptor-activated events with these receptors that is cell-type dependent. Herein we review TAS2R and OR expression in HASM, the biochemistry of their cellular signaling, and their physiologic function, including development of enabling single-cell technologies for ascertaining mechanical effects of receptor activation and performing screening for new therapeutics.
2. TAS2Rs on HASM
2.1. Expression of TAS2Rs on HASM
There are 25 TAS2R subtypes in the human genome [8, 9]. Quantitative RT-PCR was performed using 25 subtype-specific primers to determine mRNA levels in cultured HASM cells derived from subjects without lung disease. Six subtypes (TAS2R10, 14, 31, 5, 4, 19) were found at levels greater than the β2-adrenergic receptor (β2AR) (Fig 1A) [1]. In taste cells, TAS2Rs couple to the G-protein gustducin, and the βy subunits released from the heterotrimer activate phospholipase Cβ (PLCβ), resulting in an increase in inositol 1, 4, 5-triphosphate (IP3). IP3 activates the IP3 receptor on the endoplasmic reticulum resulting in release of Ca2+ from this intracellular depot. Released [Ca2+]i is thus readily assayed as a second messenger for activated TAS2Rs. In taste cells the increase in [Ca2+]i activates a transient receptor potential (TRP) channel, depolarizing the membrane, with release of neurotransmitter and subsequent activation of the Type III cell which signals to the brain. A search of available expression databases and our own studies indicated expression of Gαgust, PLCβ, IP3 receptor, but not the classic TRP channel (TRPM5), in HASM. This suggested a deviation of signaling, if these receptors were functional, in HASM compared to taste cells. Because of their higher expression, most of our studies have been targeted to TAS2R10, 14, and 31.
Figure 1.
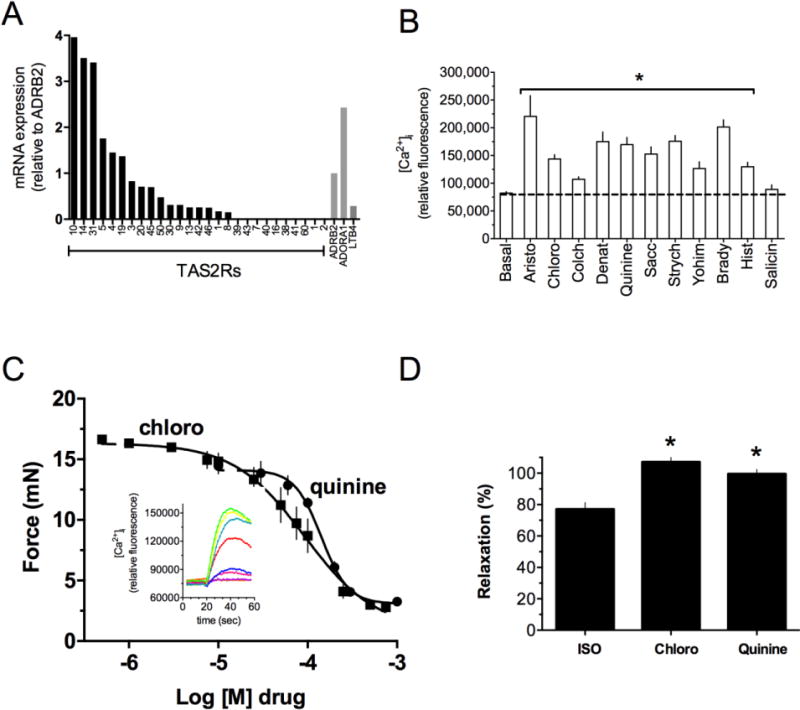
TAS2R expression and function in human airway smooth muscle (HASM). (A) Relative expression of the 25 TAS2R subtypes in HASM as determined by quantitative RT-PCR. A high expressing (ADORA1) and low expressing (LTB4R) GPCR known to be expressed on HASM were controls. (B) [Ca2+]i response to TAS2R agonists. Bradykinin and histamine, acting at Gq-coupled receptors, acted as positive controls. The bitter substance salicin is an agonist for TAS2R16 which is not expressed (see A), and showed no [Ca2+]i response. Results are from 4–6 experiments. *p < 0.05 vs basal. (C) Relaxation of intact mouse airway by the TAS2R agonists quinine and chloroquine. Airways were precontracted with serotonin (n = 4 experiments). The inset shows [Ca2+]i traces in HASM in response to increasing doses of chloroquine. (D) Maximal relaxation of intact human airways to the β-agonist isoproterenol and the TAS2R agonists quinine and chloroquine. *, p < 0.05 vs isoproterenol (n = 5).
2.2. TAS2Rs increase [Ca2+]i in HASM
Using a panel of known TAS2R agonists we observed increases in [Ca2+]i in HASM cells that were consistent with the subtype expression profiles (Fig 1B) [1]. Agonists such as saccharin, strychnine, denatonium, chloroquine, aristolochic acid, yohimbine, and quinine, which activate the higher expressing subtypes, stimulated [Ca2+]i the greatest, while an agonist to a lower expressing subtype (colchicine, TAS2R4) showed a lower response. Furthermore, the bitter tastant salicin, an agonist for TAS2R16 which is not expressed, showed no increase in [Ca2+]i. The stimulation of [Ca2+]i by TAS2R agonists was fully blocked by the βγ sequestering agent gallein and the PLC inhibitor U73122, and was inhibited >50% by the IP3 receptor antagonist 2APB. TAS2R agonists had no effect on HASM intracellular cAMP levels as determined by two sensitive methods. Unlike what is observed in taste cells, though, TAS2R agonists caused hyperpolarization of the cell membrane [1]. Such hyperpolarization is observed using cells in the native state, or, when first depolarized by a constrictive GPCR agonist such as histamine.
2.3. Methods for determining physiologic function of sensory GPCRs in HASM
To address the functional consequences of HASM TAS2R activation, we have utilized multiple techniques including myography of mouse and human bronchi [1], measurement of airway resistance in the ventilated intact mouse [1], quantitative airway area measurements in precision-cut lung slices (PCLS) [10], and three methods for studying HASM cell mechanical and biophysical properties in culture. These methods enable the study of hundreds of cells under multiple conditions and, thereby, provide a robust dataset for single-cell analyses of cellular mechanics.
In magnetic twisting cytometry (MTC), a ferrimagnetic microbead (~5 μm in diameter) is functionalized to the cytoskeleton of living cells (both stress-bearing cytoskeletal structures and the contractile apparatus) through cell surface integrin receptors using a Arg-Gly-Asp linker [11]. By imposing a uniform magnetic field upon the magnetized bead, a small torque is applied and resulting bead motions deform structures deep in the cell interior [12, 13]. Such forced bead motions are impeded by mechanical stresses developed within the cell body, and the ratio of specific torque to lateral bead displacements is measured optically and is taken as a measure of cell stiffness [12]. Using this technique, it has been previously demonstrated that HASM cells in culture exhibit pharmacomechanical coupling to a wide panel of contracting and relaxing agonists [14]. For example, HASM cell stiffness increases in response to known contractile agonists reported to stimulate [Ca2+]i or IP3 formation and decreases in response to known relaxant agonists that generate cAMP. In addition, changes in the stiffness responses in single cells require, as in the intact tissue, actin polymerization as well as myosin activation [15]. As such, dynamic changes in cell stiffness measured with MTC are robust indices of single-cell contraction and/or relaxation of isolated HASM [16, 17]. Indeed, changes in cell stiffness as measured by MTC closely track active stresses within individual HASM cells, as measured by the other methods [18].
In Fourier transformed traction microscopy (FTTM), fluorescent beads (~0.2 μm in diameter) are embedded in a flexible polyacrylamide gel and used as fiduciary markers for deformation fields exerted by adherent cells [19]. The polyacrylamide gels can be coated with any extracellular matrix protein of interest (i.e. collagen) and can be “tuned” to precise rigidities that span a physiologic range of matrix stiffness [20]. Using FTTM, high resolution traction stress, delineated in space and time, can be imaged [21]. By knowing the elastic properties of the gels, traction stress can be quantitated from the deformation field arising at the interface between each adherent cell and the elastic matrix [19]. Importantly, mechanical responsiveness of airway smooth muscle measured at the level of the single cell in vitro using MTC and FTTM is consistent with physiological responses measured at tissue and organ levels [15, 16, 22].
Spontaneous nanoscale tracer motion (SNTM) involves measurement of unforced trajectories of ferrimagnetic beads attached to the cell. SNTM quantitates, in real-time, the rate of spontaneous cytoskeletal rearrangement (termed “cytoskeletal remodeling”) of adherent cells [23–25]. The mean square displacements (MSDs) of spontaneous bead motions increase with time (t) as a power law with an exponent α greater than unity, indicating non-thermal, ongoing molecular-level fluctuations of the underlying cytoskeleton [2]. Although the precise nature of cytoskeletal remodeling is unknown, it is clear that in HASM, the internal networks of cytoskeleton (both actin and myosin) are evanescent structures that are in a continuous state of remodeling [26, 27], and the rate at which these events occurs may contribute to a pathologic feature of chronic asthma [28].
2.4. TAS2Rs relax HASM and dilate airways
In the isolated intact mouse airway, we found that TAS2Rs markedly dilated the airway from a passive stretch baseline, or, from an actively contracted state (Fig 1C) [1]. Similar findings were observed using intact human airways [1] and PCLS obtained from surgical specimens [7]. The efficacy of TAS2R agonists is equal to or greater than that of β-agonists such as isoproterenol and formoterol (Fig 1D) [4, 7]. However, the potencies of most TAS2R agonists are relatively low, with EC50 values in the μM range. Studies using FTTM revealed that TAS2R agonists decrease the contractile stress (traction) both spatially and temporally in individual HASM cells (Fig 2A) [29]. Since these studies were performed on isolated HASM cells, the results confirm that the action of TAS2R agonists on intact bronchi is due to receptors being activated on HASM, rather than indirectly via signaling from the airway epithelium or other airway cells. Additional experiments using MTC revealed a decrease in cell stiffness (i.e. single-cell relaxation) of HASM in response to TAS2R agonists such as saccharine and chloroquine (Fig 2B) [1]. This relaxation response was blocked by PLC inhibition but not PKA inhibition (Fig 2C), thus linking the biochemical data to the physiologic response. Additional studies also showed partial inhibition of saccharin-mediated relaxation by charybdotoxin and iberiotoxin (Fig 2C), suggesting a role for the large capacitance Ca2+-gated K+ channel (BKCa) [1, 29]. Studies performed with depletion of [Ca2+]i using thapsigargin showed a total loss of TAS2R-mediated relaxation, again linking the [Ca2+]i stimulatory pathway to relaxation [1].
Figure 2.
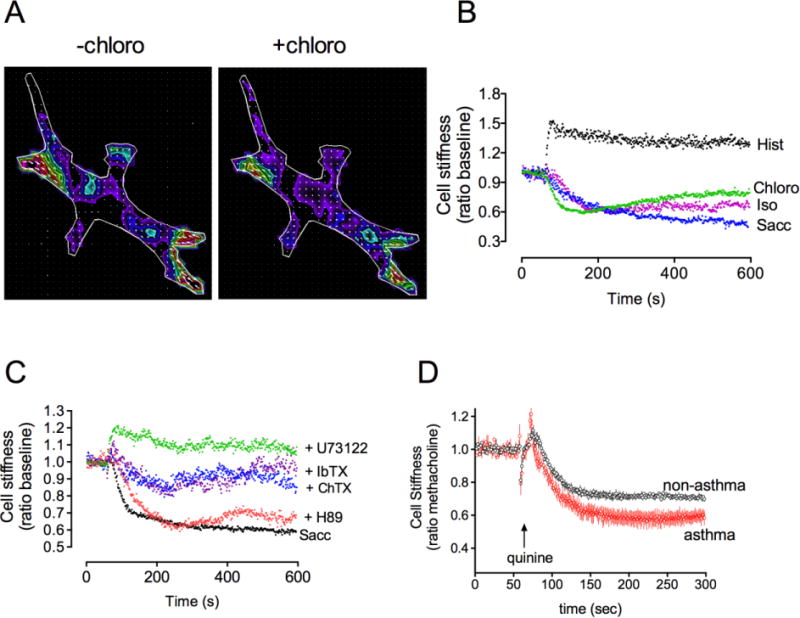
Single cell responses to TAS2R agonists in HASM. (A) Fourier transformed traction microscopy of a single HASM before and 30 sec after exposure to the TAS2R agonist chloroquine. The colors show the magnitudes of the various traction stresses throughout the cell in Pa. The wavelength of the color is proportional to the stress (red > blue; black = 0). (B) Magnetic twisting cytometry (MTC) studies of HASM show a relaxation response to TAS2R agonists chloroquine and saccharin. The expected relaxation to the β-agonist isoproterenol (ISO) and the contraction response to histamine (Hist) are also indicated. (C) MTC reveals physiologic responses to various pathway blockers in HASM. The relaxation response to saccharin was not affected by the PKA inhibitor H89, was partially inhibited by Ca2+-dependent K+ channel antagonists charybdotoxin (ChTx) and iberiotoxin (IbTx), and fully blocked by the PLCβ inhibitor U73122. (D) The relaxation response to quinine is preserved in asthmatic HASM. The MTC data in (B–D) are from > 500 measurements per condition.
2.5. TAS2Rs signal to specialized [Ca2+]i pools
Virtually all contractile signals mediated by GPCRs in HASM act through Gq-coupled receptors. This contraction is mediated by an increase in [Ca2+]i which would be expected from Gq-coupled receptors acting at PLCβ. Thus there has been this general assumption that any agent that increases [Ca2+]i in smooth muscle would lead to contraction. Of note, the magnitude of these increases in [Ca2+]i with Gq-coupled contractile GPCRs and that of TAS2R-mediated [Ca2+]i increases is similar (Fig 1B), and yet TAS2Rs relax. This suggests that the contractile [Ca2+]i signals and the relaxation [Ca2+]i signals are different in some way, which may not be readily discernable in plate-based whole cell [Ca2+]i assays. To address this possibility, we utilized high-resolution real-time confocal imaging. We found that activation of the Gq-coupled contractile H1-histamine receptor caused a relatively global increase in [Ca2+]i that is first seen about 10 sec after addition of histamine. In contrast, the [Ca2+]i signal from saccharin was observed within 2.5 sec, and was most intense at the slender ends and sarcolemmal regions of the cell [1]. When examined in line-scan mode parallel to the cell membrane, distinct Ca2+ puff-like events were observed. Taken together with the partial loss of the relaxation effect when BKCa channels are blocked and the hyperpolarization of the membrane, this localized [Ca2+]i from TAS2Rs appears to be in a microenvironment conducive to activation of BKCa and the expected relaxation. We have not explored this aspect of TAS2R signaling in HASM extensively, and note that others have suggested alternative mechanisms [30]. Regardless, it appears that TAS2R-mediated increases in [Ca2+]i have different effects on membrane potential and physiologic function compared to Gq-mediated receptor activation. In fact, recent data have revealed that IP3 itself, the initiator of [Ca2+]i release from the sarcoplasmic reticulum, is much more localized than previously thought [31]. And, there is precedence for distinct [Ca2+]i microdomains promoted by different GPCRs [32].
2.6. TAS2Rs are functional in models of inflammation and asthma
Asthma is an inflammatory disease of the airways where airway obstruction occurs due to airway smooth muscle contraction as well as increased smooth muscle mass and mucous plugging. The contraction is due to release of local factors that act on bronchoconstrictive GPCRs such as the M3-muscarinic, H1-histamine, and leukotriene receptors. While antagonists to these receptors could act to block these actions, these “indirect bronchodilators” are not particularly effective in asthma as monotherapy for bronchospasm, in part because of their specificity for one pathway. In contrast, β-agonists act as “direct bronchodilators” because activation of airway smooth muscle β2AR evokes relaxation regardless of the constrictive signal. β-agonists are the only available class of direct bronchodilators, but their use is associated with interindividual variability due to genetic polymorphisms, increased airway hyperresponsiveness, tachyphylaxis, asthma exacerbations, and mortality [33–39]. Furthermore, β2AR function on airway smooth muscle is depressed in many models of airway inflammation and asthma [1, 7, 40, 41]. Agonists at TAS2Rs would provide a new class of direct bronchodilators which relax airway smooth muscle by a different mechanism than β-agonists. We have found that cultured HASM cells derived from asthmatic donors retain several asthmatic phenotypes [42]. To ascertain if the asthmatic diathesis alters TAS2R function in HASM, we have compared the [Ca2+]I responses and relaxation responses (by MTC) of HASM from asthmatic and nonasthmatic donors. We found no differences in TAS2R signaling or physiologic function between the two groups [6]. Additional studies, using PCLS, where airways were treated with or without IL-13 showed a loss of β-agonist-mediated relaxation, but TAS2R-mediated relaxation was unaffected [7]. In terms of agonist-promoted desensitization, we have observed a small degree (~30%) loss of TAS2R function due to pre-exposure of HASM to high-dose TAS2R agonist [6]. Additional studies are underway to delineate the mechanisms of this desensitization. Thus taken together, a number of limitations that are associated with β-agonists appear to be absent or minimal with TAS2R agonists. This points the way towards development of TAS2R agonists as primary or adjunct therapy for chronic prevention or acute treatment of bronchospasm.
3. Olfactory receptors on HASM
3.1. Expression of ORs on HASM
The identification of TAS2Rs on HASM prompted a search for other sensory receptors on this cell type, with a goal of understanding the basis of asthmatic phenotypes and/or finding new targets for treatment. Using RNA-Seq we proceeded to identify ORs in HASM using a gene model from the UCSC database, which contained 375 ORs (pseudogenes were excluded). Several ORs were identified and subsequently verified by quantitative RT-PCR [2]. Of particular initial interests were OR51E2, OR152, OR10Q1, OR2A1, OR2W3, OR1J4, OR2A7, OR1Q1, OR6A2, and OR1J1. In nasal olfactory epithelium, ORs couple to adenylyl cyclase type 3 (AC3) via the G-protein Golf, generating cAMP. Both Gαolf and AC3 were detected in HASM, so we felt that determining function of ORs in HASM would be relatively straightforward. Like TAS2Rs, we found different functions compared to traditional dogma. We have so far concentrated on OR51E2 since it was readily expressed on the cell surface of transfected HEK-293 cells, and, OR51E2 and its murine ortholog Olfr78 have been reported to respond to short chain fatty acids, which are metabolic byproducts of anaerobic bacteria. These “endogenous” substances are readily absorbed into the bloodstream and could thus act as OR agonists. We considered that they might form part of a gut-lung interface that has been implicated as a mechanism in asthma pathogenesis [43–45]. Transfected HEK-293 cells showed an increase in intracellular cAMP in response to acetate and propionate [2], but not formate or butyrate, consistent with other studies of OR51E2/Olfr78.
3.2. OR51E2 signaling in HASM
Based on the transfection studies, we fully expected that activation of OR51E2 in HASM would lead to relaxation, given that it generated cAMP in the transfected cells. However, using two sensitive methods of detection we were unable to detect an increase in cAMP in HASM cells with short- or long-term exposure to acetate or propionate, even in the presence of phosphodiesterase inhibitors. In functional studies employing MTC, we also did not observe acute relaxation of isolated HASM cells in response to these compounds, consistent with the lack of an acute cAMP response. We did note a small decrease in baseline cell stiffness with prolonged exposure, prompting investigation using functionalized ferrimagnetic beads attached to HASM in the unforced condition as motion tracers (SNTM) to investigate spontaneous cytoskeletal remodeling (see above). We found that HASM cells exposed to the two inactive compounds formate and butyrate did not modify the cytoskeletal remodeling dynamics and were no different than vehicle control. However, cytoskeletal remodeling dynamics were significantly reduced by the known agonists for OR51E2, acetate and propionate (Fig 3A). Airway smooth muscle cells from C57BL/6 mice also displayed this response to acetate and propionate, which was absent in CRISPR/Cas9 promoted Olfr78 gene knockout cells [2].
Figure 3.
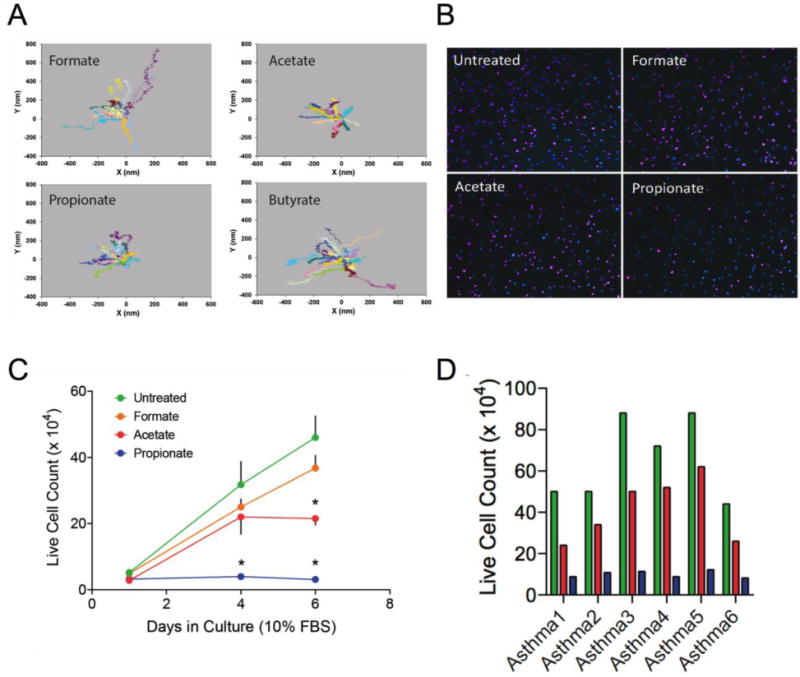
Properties of OR51E2 expressed on human airway smooth muscle. (A) Cytoskeletal remodeling in HASM is inhibited by OR51E2 agonists. Shown are representative trajectory maps of unforced ferrimagnetic beads attached to HASM using SNTM methodology (see text). Acetate and propionate, but not formate or butyrate, inhibited spontaneous cytoskeletal motions of HASM. (B) EdU incorporation reveals that propionate decreases HASM proliferation. EdU-positive nuclei are labeled with Alexa Fluor 647 (purple color) while all nuclei are labeled with DAPI (blue color). Shown is a representative study. (C) HASM proliferation is inhibited by OR51E2 agonists acetate and proprionate (n = 4 experiments). *, p < 0.05 vs untreated. (D) Inhibition of HASM cell proliferation by OR51E2 agonists is preserved in cells derived from asthmatic lungs (color code same as in C).
Another feature of increased smooth muscle mass in asthma is smooth muscle cell hyperplasia. We reasoned that decreases in cytoskeletal remodeling might slow the rate of cellular proliferation. Of note, asthmatic HASM cells are known to proliferate at higher rates than nonasthmatic HASM cells, and they also exhibit enhanced cytoskeletal remodeling dynamics (unpublished data). We measured cell proliferation in response to the active and inactive compounds over a six day period with sparsely seeded cells in culture in serum-containing media. Typically a ~4 fold increase in cells is expected over this time period. As shown in Fig 3B and 3C, propionate and to a lesser extent acetate, reduced cell proliferation. The effects of these two compounds were also observed in six cell lines derived from the lungs of asthmatic donors, indicating effectiveness in cells from individuals with the disease of interest (Fig 3D). Of note, another report shows expression of two ORs in HASM that we find at very low expression levels (OR2AG1 and OR1D2), with the former apparently mediating relaxation or contraction depending on the agonist, and the latter promoting cytokine release [46].
4. Conclusions: A previously unrecognized widespread chemosensory system
As illustrated by our finding functional TAS2Rs and ORs on HASM, there is growing evidence that these receptors, previously relegated to isolated regions of the body for perception of external stimuli such as taste and smell, are expressed on many cell-types. And, it is apparent that their signaling in these other cell-types is not readily apparent from the usual paradigms. Besides airway smooth muscle, TAS2Rs have also been identified on central cortical neurons, vascular smooth muscle, upper and lower airway epithelium, cardiac myocytes, several cell types within the gastrointestinal tract, and thyroid follicular cells [47, 48]. ORs have also been detected in multiple cell types throughout the body and have been shown to regulate sperm chemotaxis, respiratory rate, myogenesis, proliferation of prostate cancer, blood pressure regulation, and as reviewed here, airway smooth muscle cytoskeletal remodeling and proliferation [49–54].
We propose that there is a relatively unrecognized chemosensory system in the body that is activated or deactivated by ligands of TAS2Rs and ORs. Clearly one source of bitter tasting substances and odors (the agonists for TAS2Rs and ORs) is the external environment. Bitter substances from plants are often toxic, so TAS2Rs on the tongue may have evolved for avoidance of such substances. This evolutionary pathway does not readily explain TAS2Rs expressed outside the oral cavity. We have hypothesized [1] that TAS2Rs on HASM, which are known to be receptors for acyl-homoserine lactones generated by bacteria, may act to open airways and, along with TAS2Rs of cilia, maintain patency and promote clearance of pathogenic bacteria and debris in the lung during infection. Digestion of certain bitter substances would be expected to directly act on TAS2Rs in the digestive tract, thereby promoting multiple responses. It is unclear, though, whether circulating bitter tastants from food reach concentrations in the blood that are relevant for activation of TAS2Rs on thyroid follicular cells [55], leading to modulation of thyroid stimulating hormone secretion. In the case of OR51E2 expression on HASM, as we summarized earlier, this receptor acts on two processes that lead to increased airway smooth muscle mass, a hallmark of asthma. Since agonists for this receptor include short chain fatty acids which are generated by fermentation of polysaccharides by the gut microbial community, we have postulated a non-immune gut-lung axis that may affect asthma susceptibility. Given the known link between obesity and asthma, this potential interaction requires further exploration to understand the basic mechanisms involved in this complex disease. Agonists directed to OR51E2 might serve to mitigate against increases in airway smooth muscle in asthma. Alternatively, dietary modification to promote microbial communities (and the appropriate substrates) to generate the short-chain fatty acids that are agonists for OR51E2, might represent a nonpharmacological approach for treatment.
We propose further research in this area using a multipronged approach. First, identification of expression patterns of these sensory receptors throughout human and mouse in multiple organs and cell types should be undertaken. This will require some innovative approaches, given that the human OR family has > 500 receptors. Secondly, characterization of their function should be carried out using the cell type of interest, because of the differences in signaling that may arise between transfected cells or a single “model” cell, and the cell of interest. Physiologic function should be ascertained at the cellular and organ levels, and a broad net should be cast do delineate physiologic relevance. Biophysical measurements using innovative technologies will be necessary, such as FTTM, MTC, and SNTM, to tease-out physiologic function at the cell level. Such function should ultimately be considered in a manner consistent with a cohesive chemosensory system in the body, similar to how adrenergic receptor structure and physiology has been considered in terms of the sympathetic nervous system. There also needs to be effort towards understanding the physiology of a given receptor-cell-organ combination in terms of its evolutionary basis and its relationship to disease states. Finally, these ectopically expressed chemosensory receptors need to be considered potential drug targets for novel therapeutics. There has long been a need for a new class of direct bronchodilators for treating asthma (and chronic obstructive lung disease), and TAS2R agonists are even more effective in bronchodilating human airways than the full β-agonists. Based on the inhibitory effects of OR51E2 on asthmatic smooth muscle, receptor agonists may prevent the long-term consequences of the disease. Fortunately for lung targets, the inhalation route of administration allows for higher concentrations of drug delivery, and thus lower affinity drugs (such as many of the known TAS2R and OR agonists) can be considered.
Taken together, the results from multiple studies in diverse and widespread cells and organs indicate a complex, physiologically relevant, chemosensory network in the body which responds to endogenous and exogenous ligands. These sensory receptors appear to carry out adaptive functions, serve to predispose to disease, and represent drug targets for novel therapeutics.
Highlights.
Bitter taste receptors and olfactory receptors are expressed throughout the body.
In airway smooth muscle, bitter taste receptors evoke relaxation and bronchodilation.
Olfactory receptors on airway smooth muscle decrease remodeling and proliferation.
This localization suggests a widespread chemosensory system in the body.
Acknowledgments
This work is supported by National Institutes of Health HL45967, HL114471, HL107361, and HL107261. Steven An was also funded by Johns Hopkins University Discovery Award. The authors thank Tara Rosin for manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16(11):1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aisenberg WH, Huang J, Zhu W, Rajkumar P, Cruz R, Santhanam L, Natarajan N, Yong HM, De Santiago B, Oh JJ, Yoon AR, Panettieri RA, Homann O, Sullivan JK, Liggett SB, Pluznick JL, An SS. Defining an olfactory receptor function in airway smooth muscle cells. Sci Rep. 2016;6:38231. doi: 10.1038/srep38231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camoretti-Mercado B, Pauer SH, Yong HM, Smith DC, Deshpande DA, An SS, Liggett SB. Pleiotropic Effects of Bitter Taste Receptors on [Ca2+]i Mobilization, Hyperpolarization, and Relaxation of Human Airway Smooth Muscle Cells. PLoS One. 2015;10(6):e0131582. doi: 10.1371/journal.pone.0131582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deshpande DA, Robinett KS, Wang WC, Sham JS, An SS, Liggett SB. Bronchodilator activity of bitter tastants in human tissue. Nat Med. 2011;17(7):776–778. doi: 10.1038/nm0711-776b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim D, Pauer SH, Yong HM, An SS, Liggett SB. beta2-Adrenergic Receptors Chaperone Trapped Bitter Taste Receptor 14 to the Cell Surface as a Heterodimer and Exert Unidirectional Desensitization of Taste Receptor Function. J Biol Chem. 2016;291(34):17616–28. doi: 10.1074/jbc.M116.722736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinett KS, Deshpande DA, Malone MM, Liggett SB. Agonist-promoted homologous desensitization of human airway smooth muscle bitter taste receptors. Am J Respir Cell Mol Biol. 2011;45(5):1069–1074. doi: 10.1165/rcmb.2011-0061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinett KS, Koziol-White CJ, Akoluk A, An SS, Panettieri RA, Jr, Liggett SB. Bitter taste receptor function in asthmatic and nonasthmatic human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2014;50(4):678–83. doi: 10.1165/rcmb.2013-0439RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100(6):693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 9.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100(6):703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 10.An SS, Wang WC, Koziol-White CJ, Ahn K, Lee DY, Kurten RC, Panettieri RA, Jr, Liggett SB. TAS2R activation promotes airway smooth muscle relaxation despite beta(2)-adrenergic receptor tachyphylaxis. Am J Physiol Lung Cell Mol Physiol. 2012;303(4):L304–11. doi: 10.1152/ajplung.00126.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260(5111):1124–7. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 12.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. Scaling the microrheology of living cells. Phys Rev Lett. 2001;87(14):148102. doi: 10.1103/PhysRevLett.87.148102. [DOI] [PubMed] [Google Scholar]
- 13.Hu S, Chen J, Fabry B, Numaguchi Y, Gouldstone A, Ingber DE, Fredberg JJ, Butler JP, Wang N. Intracellular stress tomography reveals stress focusing and structural anisotropy in cytoskeleton of living cells. Am J Physiol Cell Physiol. 2003;285(5):C1082–90. doi: 10.1152/ajpcell.00159.2003. [DOI] [PubMed] [Google Scholar]
- 14.Hubmayr RD, Shore SA, Fredberg JJ, Planus E, Panettieri RA, Jr, Moller W, Heyder J, Wang N. Pharmacological activation changes stiffness of cultured human airway smooth muscle cells. Am J Physiol. 1996;271(5 Pt 1):C1660–8. doi: 10.1152/ajpcell.1996.271.5.C1660. [DOI] [PubMed] [Google Scholar]
- 15.An SS, Laudadio RE, Lai J, Rogers RA, Fredberg JJ. Stiffness changes in cultured airway smooth muscle cells. Am J Physiol Cell Physiol. 2002;283(3):C792–801. doi: 10.1152/ajpcell.00425.2001. [DOI] [PubMed] [Google Scholar]
- 16.An SS, Fabry B, Trepat X, Wang N, Fredberg JJ. Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness? Am J Respir Cell Mol Biol. 2006;35(1):55–64. doi: 10.1165/rcmb.2005-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.An SS, Mitzner W, Tang WY, Ahn K, Yoon AR, Huang J, Kilic O, Yong HM, Fahey JW, Kumar S, Biswal S, Holgate ST, Panettieri RA, Jr, Solway J, Liggett SB. An inflammation-independent contraction mechanophenotype of airway smooth muscle in asthma. J Allergy Clin Immunol. 2016;138(1):294–297 e4. doi: 10.1016/j.jaci.2015.12.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang N, Tolic-Norrelykke IM, Chen J, Mijailovich SM, Butler JP, Fredberg JJ, Stamenovic D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol. 2002;282(3):C606–16. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- 19.Butler JP, Tolic-Norrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Physiol Cell Physiol. 2002;282(3):C595–605. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 20.An SS, Kim J, Ahn K, Trepat X, Drake KJ, Kumar S, Ling G, Purington C, Rangasamy T, Kensler TW, Mitzner W, Fredberg JJ, Biswal S. Cell stiffness, contractile stress and the role of extracellular matrix. Biochem Biophys Res Commun. 2009;382(4):697–703. doi: 10.1016/j.bbrc.2009.03.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tolic-Norrelykke IM, Butler JP, Chen J, Wang N. Spatial and temporal traction response in human airway smooth muscle cells. Am J Physiol Cell Physiol. 2002;283(4):C1254–66. doi: 10.1152/ajpcell.00169.2002. [DOI] [PubMed] [Google Scholar]
- 22.Maksym GN, Fabry B, Butler JP, Navajas D, Tschumperlin DJ, Laporte JD, Fredberg JJ. Mechanical properties of cultured human airway smooth muscle cells from 0.05 to 0.4 Hz. J Appl Physiol (1985) 2000;89(4):1619–32. doi: 10.1152/jappl.2000.89.4.1619. [DOI] [PubMed] [Google Scholar]
- 23.Bursac P, Fabry B, Trepat X, Lenormand G, Butler JP, Wang N, Fredberg JJ, An SS. Cytoskeleton dynamics: fluctuations within the network. Biochem Biophys Res Commun. 2007;355(2):324–30. doi: 10.1016/j.bbrc.2007.01.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An SS, Fabry B, Mellema M, Bursac P, Gerthoffer WT, Kayyali US, Gaestel M, Shore SA, Fredberg JJ. Role of heat shock protein 27 in cytoskeletal remodeling of the airway smooth muscle cell. J Appl Physiol (1985) 2004;96(5):1701–13. doi: 10.1152/japplphysiol.01129.2003. [DOI] [PubMed] [Google Scholar]
- 25.Bursac P, Lenormand G, Fabry B, Oliver M, Weitz DA, Viasnoff V, Butler JP, Fredberg JJ. Cytoskeletal remodelling and slow dynamics in the living cell. Nat Mater. 2005;4(7):557–61. doi: 10.1038/nmat1404. [DOI] [PubMed] [Google Scholar]
- 26.Mehta D, Gunst SJ. Actin polymerization stimulated by contractile activation regulates force development in canine tracheal smooth muscle. J Physiol. 1999;519(Pt 3):829–40. doi: 10.1111/j.1469-7793.1999.0829n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seow CY, Pratusevich VR, Ford LE. Series-to-parallel transition in the filament lattice of airway smooth muscle. J Appl Physiol (1985) 2000;89(3):869–76. doi: 10.1152/jappl.2000.89.3.869. [DOI] [PubMed] [Google Scholar]
- 28.An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chitano P, Deng L, Dowell M, Eidelman DH, Fabry B, Fairbank NJ, Ford LE, Fredberg JJ, Gerthoffer WT, Gilbert SH, Gosens R, Gunst SJ, Halayko AJ, Ingram RH, Irvin CG, James AL, Janssen LJ, King GG, Knight DA, Lauzon AM, Lakser OJ, Ludwig MS, Lutchen KR, Maksym GN, Martin JG, Mauad T, McParland BE, Mijailovich SM, Mitchell HW, Mitchell RW, Mitzner W, Murphy TM, Pare PD, Pellegrino R, Sanderson MJ, Schellenberg RR, Seow CY, Silveira PS, Smith PG, Solway J, Stephens NL, Sterk PJ, Stewart AG, Tang DD, Tepper RS, Tran T, Wang L. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J. 2007;29(5):834–60. doi: 10.1183/09031936.00112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.An SS, Robinett KS, Deshpande DA, Wang WC, Liggett SB. Reply to: Activation of BK channels may not be required for bitter tastant-induced bronchodilation. Nat Med. 2012;18(5):650–1. doi: 10.1038/nm.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang CH, Lifshitz LM, Uy KF, Ikebe M, Fogarty KE, ZhuGe R. The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol. 2013;11(3):e1001501. doi: 10.1371/journal.pbio.1001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickinson GD, Ellefsen KL, Dawson SP, Pearson JE, Parker I. Hindered cytoplasmic diffusion of inositol trisphosphate restricts its cellular range of action. Science signaling. 2016;9(453):ra108. doi: 10.1126/scisignal.aag1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lechleiter J, Girard S, Clapham D, Peralta E. Subcellular patterns of calcium release determined by G protein-specific residues of muscarinic receptors. Nature. 1991;350(6318):505–8. doi: 10.1038/350505a0. [DOI] [PubMed] [Google Scholar]
- 33.Sears MR, Taylor DR. The b2-agonist controversy: Observations, explanations and relationship to asthma epidemiology. Drug Saf. 1994;11(4):259–283. doi: 10.2165/00002018-199411040-00005. [DOI] [PubMed] [Google Scholar]
- 34.Beasley R, Pearce N, Crane J, Burgess C. Beta-agonists: what is the evidence that their use increases the risk of asthma morbidity and mortality? J Allergy Clin Immunol. 1999;103:S18–S30. doi: 10.1016/s0091-6749(99)70270-8. [DOI] [PubMed] [Google Scholar]
- 35.Lipworth BJ. Airway subsensitivity with long-acting beta 2-agonists. Is there cause for concern? Drug Saf. 1997;16:295–308. doi: 10.2165/00002018-199716050-00002. [DOI] [PubMed] [Google Scholar]
- 36.Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull. 2000;56(4):1054–1070. doi: 10.1258/0007142001903535. [DOI] [PubMed] [Google Scholar]
- 37.Salpeter SR, Wall AJ, Buckley NS. Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med. 2010;123(4):322–328. doi: 10.1016/j.amjmed.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 38.Cheung D, Timmers MC, Zwinderman AH, Bel EH, Dijkman JH, Sterk PJ. Long-Term Effects of a Long-Acting Beta-2-Adrenoceptor Agonist, Salmeterol, on Airway Hyperresponsiveness in Patients with Mild Asthma. N Engl J Med. 1992;327(17):1198–1203. doi: 10.1056/NEJM199210223271703. [DOI] [PubMed] [Google Scholar]
- 39.Kraan J, Koeter GH, Vandermark TW, Sluiter HJ, Devries K. Changes in Bronchial Hyperreactivity Induced by 4 Weeks of Treatment with Antiasthmatic Drugs in Patients with Allergic-Asthma - a Comparison between Budesonide and Terbutaline. J Allergy Clin Immunol. 1985;76(4):628–636. doi: 10.1016/0091-6749(85)90786-9. [DOI] [PubMed] [Google Scholar]
- 40.McGraw DW, Elwing JM, Fogel KM, Wang WC, Glinka CB, Mihlbachler KA, Rothenberg ME, Liggett SB. Crosstalk between Gi and Gq/Gs pathways in airway smooth muscle regulates bronchial contractility and relaxation. J Clin Invest. 2007;117(5):1391–8. doi: 10.1172/JCI30489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trian T, Burgess JK, Niimi K, Moir LM, Ge Q, Berger P, Liggett SB, Black JL, Oliver BG. b2-agonist induced cAMP is decreased in asthmatic airway smooth muscle due to increased PDE4D. PLoS One. 2011;6(5):e20000. doi: 10.1371/journal.pone.0020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An SS, Mitzner W, Tang WY, Ahn K, Yoon AR, Huang J, Kilic O, Yong HM, Fahey JW, Kumar S, Biswal S, Holgate ST, Panettieri RA, Jr, Solway J, Liggett SB. An inflammation-independent contraction mechanophenotype of airway smooth muscle in asthma. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2015.12.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–66. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 44.Kumari M, Kozyrskyj AL. Gut microbial metabolism defines host metabolism: an emerging perspective in obesity and allergic inflammation. Obes Rev. 2017;18(1):18–31. doi: 10.1111/obr.12484. [DOI] [PubMed] [Google Scholar]
- 45.Xanthopoulos M, Tapia IE. Obesity and common respiratory diseases in children. Paediatr Respir Rev. 2016 doi: 10.1016/j.prrv.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Kalbe B, Knobloch J, Schulz VM, Wecker C, Schlimm M, Scholz P, Jansen F, Stoelben E, Philippou S, Hecker E, Lubbert H, Koch A, Hatt H, Osterloh S. Olfactory Receptors Modulate Physiological Processes in Human Airway Smooth Muscle Cells. Front Physiol. 2016;7:339. doi: 10.3389/fphys.2016.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark AA, Liggett SB, Munger SD. Extraoral bitter taste receptors as mediators of off-target drug effects. FASEB J. 2012;26(12):4827–31. doi: 10.1096/fj.12-215087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu P, Zhang CH, Lifshitz LM, ZhuGe R. Extraoral bitter taste receptors in health and disease. J Gen Physiol. 2017 doi: 10.1085/jgp.201611637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, Schnermann J, Caplan MJ. Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci U S A. 2009;106(6):2059–64. doi: 10.1073/pnas.0812859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299(5615):2054–8. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- 51.Griffin CA, Kafadar KA, Pavlath GK. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev Cell. 2009;17(5):649–61. doi: 10.1016/j.devcel.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pluznick JL. Renal and cardiovascular sensory receptors and blood pressure regulation. Am J Physiol Renal Physiol. 2013;305(4):F439–44. doi: 10.1152/ajprenal.00252.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110(11):4410–5. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang AJ, Ortega FE, Riegler J, Madison DV, Krasnow MA. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature. 2015;527(7577):240–4. doi: 10.1038/nature15721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark AA, Dotson CD, Elson AE, Voigt A, Boehm U, Meyerhof W, Steinle NI, Munger SD. TAS2R bitter taste receptors regulate thyroid function. FASEB J. 2015;29(1):164–72. doi: 10.1096/fj.14-262246. [DOI] [PMC free article] [PubMed] [Google Scholar]


