Abstract
The red cell distribution width (RDW) is a component of the automated complete blood count (CBC) that quantifies heterogeneity in the size of circulating erythrocytes. Higher RDW values reflect greater variation in red blood cell (RBC) volumes and are associated with increased risk for cardiovascular disease (CVD) events. The mechanisms underlying this association are unclear, but RBC deformability might play a role. CBCs were assessed in 293 adults who were clinically examined. RBC deformability (expressed as the elongation index) was measured using a micro fluidic slit-flow ektacytometer. Multivariate regression analysis identified a clear threshold effect whereby RDW values above 14.0% were significantly associated with decreased RBC deformability (β = −0.24; p = 0.003). This association was stronger after excluding anemic participants (β = −0.40; p = 0.008). Greater variation in RBC volumes (increased RDW) is associated with decreased RBC deformability, which can impair blood flow through the microcirculation. The resultant hypoxia may help to explain the previously reported increased risk for CVD events associated with elevated RDW.
Keywords: Ektacytometer, Erythrocyte count, Erythrocyte deformability, Erythrocyte indices
1 Introduction
The red cell distribution width (RDW) is a component of the complete blood count (CBC) that quantifies heterogeneity in the size of circulating erythrocytes. Nearly all modern automated blood cell counters report the RDW as the coefficient of variation of red blood cell (RBC) volume, which is computed by dividing the standard deviation of RBC volume by the mean corpuscular volume (MCV) and multiplying this quantity by 100. Therefore, higher values of RDW reflect greater variation in the population distribution of RBC volumes.
Several epidemiologic studies have shown that higher RDW is strongly associated with increased risk for cardiovascular disease (CVD) events (e.g., myocardial infarction, stroke) and mortality in middle-aged and older adults, independent of hemoglobin concentration and nutritional status [1–7]. These associations have been observed in a variety of settings, including in the general community-dwelling population [3, 4], in patients with clinically significant CVD [1, 2, 5, 7], and among hospitalized patients [5–7]. The mechanisms underlying these associations are unclear; however, it is conceivable that RBC deformability might play a role as elevated RDW is associated with increased inflammation and decreased levels of antioxidants [8], which can reduce RBC deformability and survival. Accordingly, we sought to determine whether higher RDW values are associated with decreased RBC deformability.
2 Methods
2.1 Study Population
Blood was collected in 293 community-dwelling adults participating in the Baltimore Longitudinal Study of Aging (BLSA), an ongoing prospective cohort study. Participants in the current study were 32–98 years of age, were examined between May 2009 and March 2010, and provided written informed consent. The protocol of the BLSA was reviewed and approved by the Institutional Review Board of the National Institute on Aging, Intramural Research Program.
2.2 RBC Measures
Fasting blood draws were completed in the morning. A Sysmex XE-2100 automated blood cell counter was used to obtain the CBC, which included the following Erythrocyte indices: hemoglobin concentration, MCV, mean corpuscular hemoglobin concentration (MCHC), and RDW. In each whole blood sample, RBC deformability was measured using a micro fluidic RheoScan-D slit-flow ektacytometer (Rheo Meditech, Seoul, South Korea). The methodological details of this instrument have previously been published [9]. Briefly, blood (~6 µl) was suspended (~1/100 dilution) by slowly mixing it in 600 µl of the highly viscous PVP360 solution (viscosity ~30 cP) provided in the test kits from Rheo Meditech, and then 500 µl of this solution were loaded onto the sample reservoir of the microfluidic chip. During operation of the ektacytometer, the vacuum generating mechanism allowed RBCs to flow through the micro-channel at a range of shear stresses, while the elliptical diffraction patterns of the flowing cells were generated by a laser beam (wavelength 635 nm from a 1.5 mW laser diode). The elliptical diffraction patterns of the flowing RBCs, at different shear stress levels, are projected on a screen and captured by a CCD-video camera. The image data are then analyzed by an ellipse-fitting software. Deformability of RBCs was expressed as the elongation index (EI), which is defined as (L − W)/(L + W), where L and W are the major and minor axes of the ellipse, respectively, at various shear stress values (0–20 Pa). However, for comparative analysis of the deformability values of multiple blood samples, EI values (mean and standard deviation of three measurements) at 3 Pa were used, which is approximately at the halfway point on the EI-shear stress curve [10].
2.3 Statistical Analysis
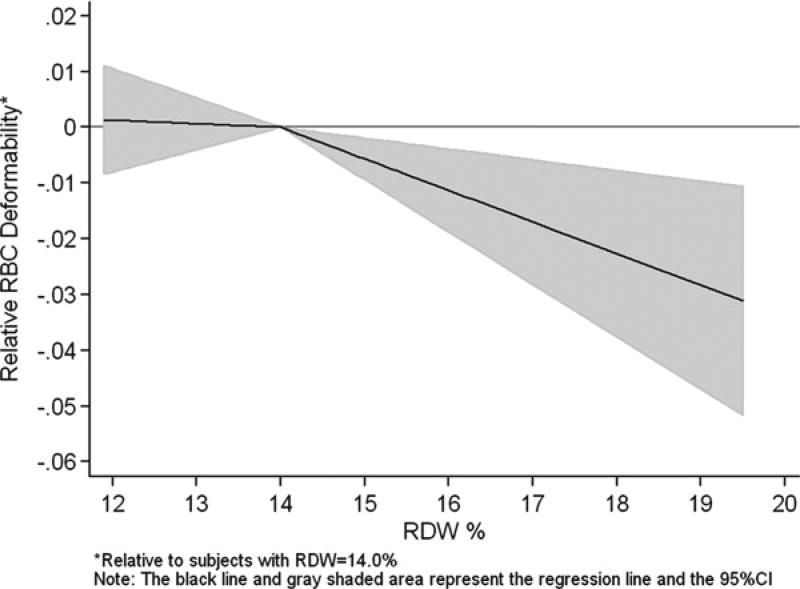
Means and standard deviations (SD) were used to describe the distribution of participants’ age and RBC measurements (Table 29.1). Multivariable linear regression analysis was used to test the association of RDW with RBC deformability (Table 29.2). The elongation index values (RBC deformability) were first standardized into Z-scores and then were regressed on age, sex, hemoglobin concentration, MCV, MCHC, and RDW. An initial exploratory graphical analysis showed a clear nonlinear association between RBC deformability and RDW with an inflection point near an RDW value of 14.0%; a threshold above which mortality risk is substantially increased [3, 4]. Spline functions are often used in epidemiology to fit a nonlinear dose–response curve. Therefore, linear splines were added to the regression analysis with a knot arbitrarily set at RDW = 14.0% (Fig. 29.1 and Table 29.2). All analyses were completed using Stata/SE statistical software (version 10.1; StataCorp LP, College Station, Texas).
Table 29.1.
Characteristics of the study sample (N = 293)
| Characteristic | Mean (SD) or n (%) |
|---|---|
| Age in years, mean (SD) | 71.1 (13.3) |
| Women, n (%) | 136 (46.4) |
| Hemoglobin in g/dL, mean (SD) | 13.7 (1.3) |
| MCV in fL, mean (SD) | 90.3 (4.7) |
| MCHC in g/dL, mean (SD) | 33.5 (1.1) |
| RDW %, mean (SD) | 13.6 (1.0) |
| RBC deformability (elongation index), mean SD | 0.32 (0.02) |
Table 29.2.
Association of RDW with RBC deformability (elongation index)a, adjusting for age, sex, and other Erythrocyte indices (N = 293)
| Regression coefficient | 95% Confidence interval | p-Value | |
|---|---|---|---|
| Age in years | 0.006 | −0.002, 0.013 | 0.152 |
| Men (vs. women) | −0.327 | −0.522, −0.131 | 0.001 |
| Hemoglobin in g/dL | 0.081 | −0.003, 0.165 | 0.060 |
| MCV in fL | 0.104 | 0.083, 0.125 | <0.001 |
| MCHC in g/dL | −0.228 | −0.329, −0.127 | <0.001 |
| RDW % | |||
| Spline below 14.0% | −0.027 | −0.224, 0.171 | 0.791 |
| Spline above 14.0% | −0.242 | −0.401, −0.082 | 0.003 |
Note. RBC deformability is expressed as the elongation index and it was standardized into a Z-score (mean = 0, SD = 1) for this regression analysis. The model adjusted R2 = 0.37
Fig. 29.1.
Association of RDW with RBC deformability (elongation index), adjusted for age, sex, hemoglobin concentration, MCV, and MCHC (N = 293)
3 Results
The characteristics of the study sample are shown in Table 29.1. Participants had a mean age of 71.1 years and 46.4% were women. The distributions of the Erythrocyte indices are consistent with normative data from population studies of adults.
Figure 29.1 illustrates the relationship between RBC deformability and RDW. Compared to participants with an RDW value of 14.0%, there was no difference in RBC deformability among those with RDW <14.0%; however, participants with RDW above 14.0% had significantly decreased RBC deformability. The detailed results of this regression model are shown in Table 29.2. While there was no association of the participants’ age with RBC deformability, men had significantly decreased RBC deformability compared with women. Higher hemoglobin concentration was associated with increased RBC deformability, although the p-value for this association was 0.06. As expected, lower MCV and higher MCHC were associated with decreased RBC deformability, reflecting the effects that reduced cell membrane and increased intracellular hemoglobin concentration can have on deformability. After excluding 51 participants with anemia, the effects of age, sex, and the RBC indices on deformability were essentially the same as those shown in Table 29.2, including for RDW whereby values above 14.0% were significantly associated with decreased RBC deformability (regression coefficient = −0.40; p = 0.008).
4 Conclusions
In this study of 293 community-dwelling adults, greater variation in RBC volumes (increased RDW) was associated with decreased RBC deformability, which can impair blood flow through the microcirculation [11]. The resultant hypoxia may help to explain the previously reported increased risk for CVD events associated with elevated RDW, particularly among those with RDW values above 14.0%. To our knowledge, this is first study to examine RDW with erythrocyte deformability. Future studies are needed to replicate the current findings as well as to investigate other aspects of hemorheology with RDW and identify the mechanisms through which RDW is associated with adverse health outcomes.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Contributor Information
Kushang V. Patel, Laboratory of Epidemiology, Demography, and Biometry, National Institute on Aging/NIH, 7201 Wisconsin Ave, Suite 3C309, Gateway Building, Bethesda, MD 20814, USA
Joy G. Mohanty, Molecular Dynamics Section, National Institute on Aging/NIH, Bethesda, MD, USA
Bindu Kanapuru, Clinical Research Branch, National Institute on Aging/NIH, Bethesda, MD, USA.
Charles Hesdorffer, Clinical Research Branch, National Institute on Aging/NIH, Bethesda, MD, USA.
William B. Ershler, Institute for Advanced Studies in Aging, Gaithersburg, MD, USA
Joseph M. Rifkind, Molecular Dynamics Section, National Institute on Aging/NIH, Bethesda, MD, USA
References
- 1.Felker GM, Allen LA, Pocock SJ, et al. Red cell distribution width as a novel prognostic marker in heart failure. J Am Coll Cardiol. 2007;50:40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Sacks F, Arnold M, et al. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 3.Patel KV, Ferrucci L, Ershler W, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169:515–523. doi: 10.1001/archinternmed.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel KV, Semba RD, Ferrucci L, et al. Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2010;65A:258–265. doi: 10.1093/gerona/glp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lippi G, Filippozzi L, Montagnana M, et al. Clinical usefulness of measuring red cell distribution width on admission in patients with acute coronary syndromes. Clin Chem Lab Med. 2009;47:353–357. doi: 10.1515/cclm.2009.066. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Pan W, Pan S, et al. Red cell distribution width as a novel predictor of mortality in ICU patients. Ann Med. 2011;43:40–46. doi: 10.3109/07853890.2010.521766. [DOI] [PubMed] [Google Scholar]
- 7.Zalawadiya SK, Zmily H, Farah J, et al. Red cell distribution width and mortality in predominantly African American population with decompensated heart failure. J Card Fail. 2011;17:292–298. doi: 10.1016/j.cardfail.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Semba RD, Patel KV, Ferrucci L, et al. Serum antioxidants and inflammation predict red cell distribution width in older women. Clin Nutr. 2010;29:600–604. doi: 10.1016/j.clnu.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin S, Hou JX, Suh JS, Singh M. Validation and application of a microfluidic ektacytometer (RheoScan-D) in measuring erythrocyte deformability. Clin Hemorheol Microcirc. 2007;37:319–328. [PubMed] [Google Scholar]
- 10.Baskurt OK, Boynard M, Cokelet GC, et al. New guidelines for hemorheological laboratory techniques. Clin Hemorheol Microcirc. 2009;42:75–97. doi: 10.3233/CH-2009-1202. [DOI] [PubMed] [Google Scholar]
- 11.Simchon S, Jan KM, Chien S. Influence of reduced red cell deformability on regional blood flow. Am J Physiol. 1987;253:H898–H903. doi: 10.1152/ajpheart.1987.253.4.H898. [DOI] [PubMed] [Google Scholar]