Abstract
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide. Pathways responsible for the activation of IL1 family cytokines are key in the development of NAFLD but underlying mechanisms are not fully understood. Many studies have focused on the inflammasome/caspase-1 pathway and have shown that this pathway is an important inducer of inflammation in NAFLD. However, this pathway is not solely responsible for the activation of pro-inflammatory cytokines. Also neutrophil serine proteases (NSPs) are capable of activating cytokines and recent studies reported that these proteases also contribute to NAFLD. These studies provided, for the first time, evidence that this inflammasome-independent pathway is involved in NAFLD. In our opinion, these new insights open up new approaches for therapeutic intervention.
Keywords: Non-alcoholic fatty liver disease, obesity, inflammation, inflammasome, neutrophil serine proteases, interleukin-1
Non-alcoholic fatty liver disease: an increasing public health issue
Non-alcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease worldwide. The prevalence of NAFLD in the general population of Western countries is 17-46% and is rapidly increasing in parallel with the increasing prevalence of obesity and metabolic syndrome (1). NAFLD can range from simple steatosis (non-alcoholic fatty liver or NAFL) to non-alcoholic steatohepatitis (NASH) characterized by liver inflammation and hepatocyte ballooning -with or without fibrosis. Due to this wide spectrum of conditions, NAFLD and NASH are associated with increased morbidity and mortality (2). Given the high prevalence and the fact that no treatment is available, a better understanding of its pathogenesis is essential to be able to identify targets for drug development in order to overcome the epidemic.
Although the mechanisms underlying obesity-induced NAFLD are not fully understood, it is known that the induction of inflammation and production of inflammatory cytokines such as TNF and members of the IL-1 cytokine family play a crucial role in disease process (3, 4). Many pathogens or danger signals derived from the host are able to trigger innate immune responses that contribute to liver diseases. Innate immune cells in the liver are able to sense molecules derived from pathogens, commonly known as pathogen-associated molecular patterns (PAMPs) (see Glossary) and also endogenous alarm signals known as damage associated molecular patterns (DAMPs) through specific receptors called pattern recognition receptors (PRRs). After binding to PRRs, both PAMPs and DAMPs trigger the immune system to produce and activate pro-inflammatory cytokines. It is now established that the assembly of a cytosolic protein complex called the NLRP3 inflammasome is important for the activation of pro-inflammatory cytokines. This protein complex activates the protease caspase-1 which cleaves pro-IL-1β and pro-IL-18 converting them to their active forms (5). Several studies investigating both NAFLD mouse models and patients with metabolic syndrome shown that someinflammasome and its downstream effects are important for the induction and prolongation of obesity-induced NAFLD (5, 6). However, this inflammasome-dependent pathway is not solely responsible for the activation of pro-inflammatory cytokines. The neutrophil serine proteases (NSPs) neutrophil elastase (NE), proteinase 3 (PR3) and cathepsin G (CG) are also capable of processing pro-inflammatory cytokines into their bio-active form. NSPs are responsible for the activation of cytokines such as membrane-bound TNF, IL-1α, IL-1β, IL-18 and IL-33 (7, 8). Recent studies investigating mouse models of obesity-induced NAFLD showed for the first time that these inflammasome-independent mechanisms are also important for the induction of liver inflammation and progression into liver fibrosis (9–11). In our opinion, these recent findings provide new insights into how dysregulation of inflammatory pathways contribute to the development of NAFLD and NASH. In addition, these results open up new perspectives for therapeutic intervention.
Glossary.
Alpha-1 antitrypsin (AAT): is a serine peptidase inhibitor produced by the liver. Among its targets are neutrophil serine proteases and caspase-1.
Anakinra: is a recombinant form of IL-1Ra, the natural inhibitor of IL-1. Anakinra inhibits the binding of IL-1α and IL-1β to their receptor.
Canakinumab: is a human monoclonal antibody that inhibits IL-1β effects by binding it and preventing ligation to its receptor.
Caspase-1: it is a molecule associated to the NLRP3 complex. Upon inflammasome activation, the inactive form called pro-caspase-1 it is cleaved and will further processes IL-1β and IL-18 to their active forms.
Damage associated molecular patterns (DAMPs): are self-molecules capable of initiating an inflammatory response. They can be represented by nuclear or mitochondrial DNA, heat shock proteins, metabolites such as ATP, uric acid, cholesterol crystals.
Dipeptidyl peptidase I (DPPI): is an enzyme that processes neutrophil serine proteases to their active forms.
IL-1 cytokine family: are molecules that mediate innate immune responses. They display both pro-inflammatory and anti-inflammatory functions. Among these molecules, both IL-1 isoformes (IL-1α and IL-1β), IL-18 and IL-33 exhibit pro-inflammatory properties.
Neutrophil serine proteases (NSPs): are antimicrobial peptides stores in the azurophilic granules of neutrophiles.
NLPR3 (NOD-like receptor P3) inflammasome: is an intracellular protein complex formed by the NACHT, LRR and PYD domains, an adaptor molecule called ASC (apoptosis associated peck like protein containing a CARD) and pro-caspase-1. NLRP3 components assemble upon PRR stimulation and initiate activation of IL-1β and IL-18 through the effector protein caspase-1.
Pathogen-associated molecular patterns (PAMPs): are constitutive components of pathogens (sugar or lipid molecules from the cell wall, nucleic acids) that are able to trigger an immune response.
Pattern recognition receptors (PRRs): are special receptors of the innate immune system capable of detecting PAMPs and DAMPs.
Here, we will first provide an overview of the recent literature regarding the role of pro-inflammatory cytokines in the development of NAFLD and NASH. We will focus on both the underlying inflammasome-dependent and inflammasome-independent mechanisms responsible for the processing and activation of these cytokines during the development of NAFLD and NASH. Although we will provide a full overview of these mechanisms, we will emphasize more on recent findings regarding the involvement of the less-established inflammasome-independent pathways in NAFLD and NASH. Additionally, we discuss the potential of targeting these pathways as novel therapeutic approaches in obesity-induced NAFLD.
IL-1 family cytokines in obesity-induced NAFLD
IL-1 family cytokines are one of the main drivers of inflammation in NAFLD. When activated, these pro-inflammatory cytokines are able to disrupt insulin and lipid signaling pathways thereby influencing insulin sensitivity and lipid metabolism (12). The cytokines IL-1β, IL-1α, IL-18 and IL-33, all members of the IL-1 family, are most intensively studied for their role in NAFLD.
IL-1β has an important role in liver disease being involved in all the stages of the disease. It promotes liver steatosis, inflammation and fibrosis by signaling through the IL-1 receptor widely expressed on the different liver cell subpopulations (13). IL-1β promotes hepatic steatosis by stimulating triglycerides and cholesterol accumulation in primary liver hepatocytes and lipid droplets formation (4). Acting on liver sinusoidal endothelial cells, IL-1β promotes liver inflammation by up-regulating ICAM-1 (intercellular adhesion molecule 1) expression which attracts neutrophils in the liver. This effect was elegantly demonstrated in an in vivo model where wild-type (WT) C57Bl/6 mice and Icam1 deficient mice were exposed to Il-1β expression by injecting a nonreplicating adenoviral vector that contained the Il-1β gene. After 4 days there was a significant influx of neutrophils in the livers of WT mice but not in the livers of Icam1 deficient mice. Blocking other adhesion molecules in the WT mice did not affect neutrophil recruitment suggesting that IL-1β stimulates neutrophil recruitment in the liver through ICAM-1 (14). In addition, IL-1β stimulates local inflammation by inducing production of IL-6, another pro-inflammatory cytokine (15). Several mouse models of sterile inflammation in the liver have shown that IL-1β together with IL-6 and TNF activate local immune cells and attract other leucocytes to the liver, leading to a chronic inflammatory state (16, 17). Finally, IL-1β contributes to the progression from liver inflammation to liver fibrosis as shown in different rodent models of liver fibrosis. For example, in a mouse model of thioacetamine (TAA)-induced liver fibrosis, Il-1r (Il-1 receptor) knock out mice were protected from liver fibrosis as assessed by HE staining. When compared to WT mice, these mice showed lower alanine transaminase (ALT) concentrations in plasma and lower mRNA espression of α-smooth muscle actin (αSMA), a marker for hepatic stellate cells (HSC) activation (18). In another study, Il-1r knock out mice were fed a CDAA (choline deficient amino acid defined) diet, a well-established diet for the induction of inflammation and liver fibrosis. Il-1r knock out mice showed, when compared to WT controls, lower ALT plasma levels and lower mRNA expression of the fibrosis markers collagen type I alpha 1 (Col1a1)and collagen type IV alpha 1 (Col4a1). Also lower mRNA expression levels were observed for Timp-1 (tissue inhibitor of metalloproteinases), a molecule that prevents degradation of the extracellular matrix and inhibits HSC apoptosis, thereby favoring the process of fibrosis. Furthermore, HSC were isolated from both WT and Il-1r knock out mice and stimulated with IL-1β. HSC from WT mice had an increased production of TIMP-1 protein, elevated mRNA levels of Col1a1 and Col4a1 and suppressed mRNA expression of Bambi (BMP and activin membrane-bound inhibitor), an inhibitor of TGF-β, the main promoter of liver fibrosis (19). In conclusion, IL-1β promotes liver fibrosis by stimulating hepatic stellate cell activation, proliferation and survival and by inducing the production of pro-fibrogenic factors. Altogether, these studies indicate that IL-1β has a major role in development of non-alcoholic fatty liver disease by contributing to steps of the disease ranging from simple steatosis to steatohepatitis and liver fibrosis.
Next to IL-1β, IL-1α has been investigated regarding its role in metabolic disturbances. The induction of steatohepatitis has been assessed in Il-1α knock out (k.o.) mice fed an atherogenic diet for 18 weeks (16). The study indicated that these mice were protected from developing steatohepatitis when compared to wild type mice (WT) that were fed the same diet. Il-1α k.o mice showed improved liver histology, and less injury and inflammation suggested by lower ALT (serum alanine aminotransferase) and SAA (serum amyloid alpha) concentrations. In addition, Il-1α k.o. mice had decreased expression of inflammatory genes mRNA such as Il-1β, Il-6, Tnf, P-selectin, Cxcl1 and Tgf-β when compared to WT mice (20).
IL-18 is also implicated in NAFLD and other metabolic conditions but results between human and animal studies are contradictory. Studies using mice deficient for Il-18 reported that these mice develop features of the metabolic syndrome like dyslipidemia, obesity, atherosclerosis and insulin resistance, thereby suggesting a protective role for this cytokine in glucose homeostasis and lipid metabolism (21, 22). In contrast, patients with metabolic syndrome showed a positive correlation between plasma concentration of IL-18 and the development of atherosclerosis, insulin resistance and type 2 diabetes suggesting that IL-18 might promote the development of the metabolic syndrome (23–25). These intriguing but contradictive results urge the need for further studies in order to establish whether high plasma concentrations of IL-18 are indeed involved in promoting inflammation and metabolic disturbances or whether the increased concentrations reflect a compensatory mechanism.
Recently, a newly described member of the IL-1 family cytokines, IL-33, was also found to be involved in the later stages of NAFLD whereby fibrosis is prominent. Mouse models of experimentally-induced liver fibrosis showed that Il-33 mRNA expression was higher in fibrotic mice than wild-type controls (26). Mouse models of diet-induced liver steatosis and inflammation showed that administration of IL-33 intraperitoneally worsened the liver fibrosis content by inducing a shift of invading macrophages and T cells towards M2 macrophages, respectively T helper 2 cells which were abundant in IL-33 treated mice and produced pro-fibrotic cytokines (IL-4, IL-5, IL-13) (26). In line with these findings studies on patients with liver fibrosis versus healthy controls showed increased serum concentrations of IL-33 in those with liver fibrosis, increased mRNA expression of IL-33 and increased IL-33 protein content in the fibrotic livers suggesting a role for this cytokine in the mechanisms of liver fibrosis (26–28).
These studies clearly show that pro-iflammatory cytokines are key in the onset and prolonging of NAFLD. Imparing the action of these cytokines could be a potentially successful approach for treating the disease. We are convinced that preventing their activation is a promising strategy, as these activation pathways are responsible for the activation of multiple pro-inflammatory cytokines. By targeting these underlying activation pathways, multiple cytokines are inhibited by a single drug. It is therefore of utmost importance to have a clear an comprehensive overview of all the molecular mechanisms responsible for cytokine activation in NAFLD.
The NLRP3 inflammasome and NAFLD
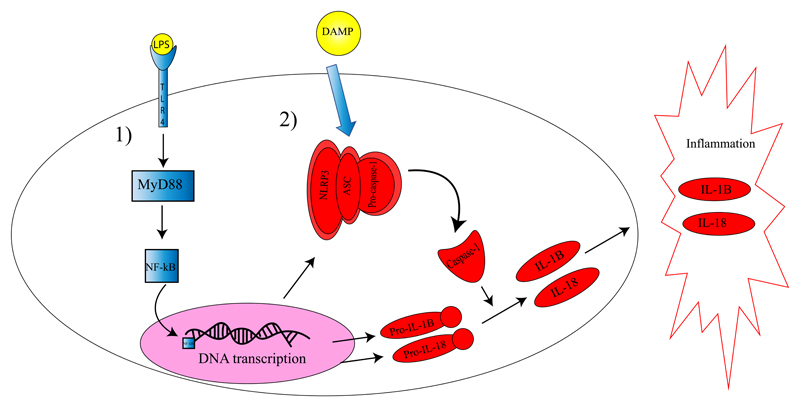
The discovery of the NLRP3 inflammasome provided an important molecular mechanism for the activation of cytokines and induction of inflammation. The NLRP3 protein complex activates the protease caspase-1 which cleaves pro-IL-1β and pro-IL-18 thereby converting them to their active forms (29). In the liver, the NLRP3–caspase-1 complex is predominantly expressed by Kupffer cells, resident macrophages of the liver, but other inflammatory cells and parenchimal cells are also known to be able to express the complex (5). During recent years, many studies have focused on caspase-1 and the NLRP3 inflammasome and their role in liver diseases. Several mouse models have shown that in liver steatosis the NLRP3 inflammasome is activated (Figure 1) promoting progression to steatohepatits and liver fibrosis (30). For example, mice deficient for the Nlrp3 gene did not develop liver steatohepatitis when fed a CDAA when compared with WT controls on the same diet (31). In contrast, mice overexpressing Nlrp3 and fed a normal diet, developed severe liver inflammation and fibrosis when compared to WT controls (31). These results revealed that long signaling via the NLRP3 inflammasome leads to liver inflammatory changes and development of liver steatohepatitis and liver fibrosis (31). In support of these findings, studies investigating patients with NASH showed that these patients have increased mRNA expression of the NLRP3 inflammasome gene and of the genes PYCARD (PYD and CARD domain containing; members of the inflammasome protein complex), CASP1 (caspase-1), IL-1β and IL-18 when compared to patients having simple steatosis (31, 32).
Figure 1. The role of the NLRP3 inflammasome in IL-1 family cytokine activation.
Activation of the NLRP3 inflammasome and IL-1 family cytokines is a two-step process. In the first step (1) a PAMP, such as LPS, binds to its receptor (TLR4 for LPS) and activates via the adaptor protein MyD88 the transcription factor NF-kB. Next, NF-kB will translocate to the nucleus and up regulates the transcription of genes encoding for the NLRP3 protein complex and the pro-inflammatory cytokines IL-1β and IL-18. In the second step (2), a DAMP signal leads to association of the NLRP3 complex and activation of the effector protein caspase-1. In turn, caspase-1 cleaves pro-IL-1β and pro-IL-18 thereby releasing their active forms. These cytokines will be excreted from the cells and promote local (and systemic) inflammation. LPS: lypopolysacharide; TLR4: toll-like receptor; MyD88: myeloid differentiation primary response 88; NF-kB: nuclear factor kB; DAMP: damage associated molecular pattern; ASC: apotosis like associated protein containing a CARD domain (component of the inflammasome); NLRP3: NOD like receptor associated protein 3; (pro)-IL-1β: (pro) interleukin-1β; (pro)- IL-18: (pro)- interleukin 18.
Caspase-1 itself is also involved in mediating inflammation in NAFLD and promoting the progression to steatohepatitis and fibrosis. Knockout of caspase-1 in mice fed a NAFLD/NASH inducing diet, showed a protective effect regarding the development of liver steatosis, steatohepatitis and early fibrogenesis when compared with WT age-matched controls (33, 34).
Taken together, these studies clearly indicate that inflammasome-mediated activation of pro-inflammatory cytokines is an important mechanism in the development of inflammation-induced NAFLD and NASH.
The role of neutrophil serine proteases in liver steatosis and steatohepatitis
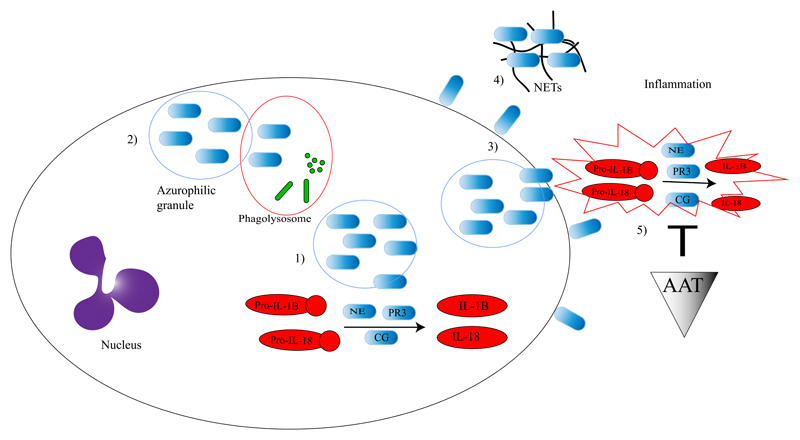
Although important and well-established, the NLRP3 inflammasome and its effector protein caspase-1 are not the only mechanisms responsible for activating pro-inflammatory cytokines. Neutrophil serine proteases (NSPs) proteinase-3 (PR3), neutrophil elastase (NE) and cathepsin G (CG) are also able to process/activate cytokines thereby regulating inflammatory responses (Figure 2) (35). Next to neutrophils, also macrophages are able to produce PR3 (35).
Figure 2. The role of neutrophil serine proteases in IL-1 family cytokine activation.
NSPs are stored in the azurophilic granules of neutrophils. Upon neutrophil activation, neutrophil serine proteases will be released into the cytosol (1) where they can activate pro-inflammatory cytokines. They can also be released into the phagolysosome where they have antimicrobial effects (2) or outside the cell where they can bind to the membrane (3) and activate extracellular cytokines, form NETs (4) and migrate into the extracellular space and activate pro-inflammatory cytokines (5). In the extracellular matrix AAT can inhibit NSPs which leads to the inhibition of cytokine activation (5).
Although it is known that NSPs are involved in the onset and prolongation of several “classical” inflammatory and infectious diseases (36), their role in inflammation-associated metabolic diseases like obesity-induced type 2 diabetes, NAFLD and NASH is less well investigated. Given that IL-1 family cytokines are drivers of inflammation contributing to the disease process, it is very well possible that NSPs, as underlying mechanisms for cytokine activation, are also involved in these metabolic diseases. So far, studies investigating the role of these enzymes show interesting results. PR3 has been shown to play an important role in insulin resistance by degrading IGF-1 (insulin-like growth factor 1) and IGFBP3 (insulin-like growth factor binding protein-3) (two molecules involved in insulin signaling) in an in-vitro system using L6 skeletal muscle cells that espress IGF-1 (31). In vivo experiments from the same study showed that administration of PR3 in WT Balb/c mice alters the glucose tolerance compared to mice that were administered vehicle. In addition, 8 out of 36 type 2 diabetes patients had high protein concentrations of PR3 in their urine while no PR3 was detected in the urine from 36 healthy volunteers. Altogether, the authors of the study concluded that PR3 may contribute to the development of type 2 diabetes in at least a subset of patients (37).
As well as PR3, neutrophil elastase (NE) has been shown to play a role in metabolic conditions by promoting insulin resistance, liver steatosis and obesity driven inflammation (11, 38). For example, neutrophil elastase activity was shown to be increased in the serum of both obese (ob/ob) mice and WT mice fed a high fat diet (HFD) (11). Additionally, serum concentrations of the neutrophil serine protease inhibitor alpha-1 antitrypsin (AAT) were severely reduced in these mice, suggesting that an imbalance between neutrophil elastase activity and its regulator might lead to obesity-driven metabolic syndrome. This theory was also confirmed by the fact that NE deficient mice and mice overexpressing human AAT fed a HFD failed to develop liver steatosis, insulin resistance and adipose tissue inflammation when compared to WT age-matched controls on the same diet (11). Moreover, a similar imbalance between neutrophil elastase activity and AAT protein concentrations was observed in the serum of obese subjects when compared to lean subjects (11). In line with this data, another study showed an increased ratio between plasma levels of NE and AAT (hence, an increasing disbalance between NE and AAT concentrations) in patients with NAFLD that correlated with disease severity ranging from simple steatosis to NASH or liver fibrosis (10). These results lead to the idea that a disequilibrium between protein levels of neutrophil elastase and AAT might (at least partly) be responsible for the development of obesity-related liver steatosis, insulin resistance and adipose tissue inflammation. Supporting this data, low AAT levels were associated with development of obesity, cardiovascular disease and insulin resistance (39, 40).
The studies reviewed above reported results that are in line with our own observations. We recently investigated the development of NAFLD and insulin resistance in double knockout mice for neutrophil elastase/proteinase 3 and neutrophil elastase/cathepsin G and in WT control mice. We showed that these double k.o. mice were, after a HFD, protected from developing insulin resistance, liver steatosis and obesity-induced inflammation suggesting and important role for NSPs in NAFLD development (9).
Although we believe that the proteases NE and PR3 are at least partly responsible for the onset and prologation of NAFLD and NASH, also other factors may be involved. For instance, it might be interesting to investigate the role of neutrophil extracellular traps (NETs) in NAFLD and NASH. NETs have an important role during infection by helping neutrophils to capture and kill pathogens. They contain proteins from azurophilic granules such as NE, CG and myeloperoxidase (MPO) and uses these proteases for disarming pathogens. Evidence suggest that uncontrolled or excessive production of NETs is related to the exacerbation of inflammation thereby contributing to the development of autoimmunity, cancer and inappropriate thrombosis (41). Also granzyme B, a serine protease expressed by natural killer cells and cytotoxic T cells might be an interesting candidate to further investigate its role in NAFLD and NASH (42). However, at this moment, studies providing evidence that these factors are involved in NAFLD or NASH are lacking.
Collectively, these recent findings provide new insights pointing towards alternative mechanisms that are, next to inflammasomes, very important in the process of inflammatory-driven induction of metabolic conditions.
Therapeutic approaches in NAFLD
Given the prominent role of inflammatory cytokines in NAFLD and NASH, it is a conceivable approach to target these cytokines in order to treat the disease. IL-1 signaling can be inhibited in humans using canakinumab (43) or by anakinra (Table1). Although not studied in NAFLD, administration of anakinra in patients with type 1 or type 2 diabetes showed an improvement of insulin sensitivity and reduced systemic inflammation (44, 45) which could be also beneficial in NAFLD where insulin resistance and systemic inflammation contribute to progression from simple steatosis to steatohepatits. However, at this point it is unclear whether targeting a single pro-inflammatory cytokine is sufficient to attenuate inflammation, as multiple inflammatory signals are involved in the pathogenesis of these conditions. In this context, new insights that both inflammasomes and NSPs are involved in the development of NAFLD and NASH are of particular interests and offer new targets for therapeutic intervention. Targeting inflammasomes and/or NSPs prevents the activation of multiple pro-inflammatory cytokines instead of only one. This strategy is appealing, especially in the view that no treatments are available. Currently, several compounds targeting inflammasomes or NSPs pathways are being investigated as potential therapy for NAFLD and NASH (Table l).
Table 1. Potential pharmacological agents for NAFLD.
| Targeted pathway | Pharmacological agent | Targeted molecule | Targeted diseases | Species | Methods and Results | Conclusion regarding efficacy | References |
|---|---|---|---|---|---|---|---|
| IL-1β signaling | Canakinumab | IL-1β | Cardiovascular disease | Human | 10061 patients with previous myocardial infarction and hsCRP<2mg/L were admistered placebo and three different doses of canakinumab at a ratio 1,5:1:1:1. Patients with canakinumab had significant reduction of hs CRP at 48 months and IL-6 at 12 months. Patients that received 150 mg canakinumab had a 15% reduced risk of a secondary cardiovascular event than patients on placebo. | Good | Ridker PM et al [43] |
| Anakinra | IL-1 receptor1 | Type 1 and type 2 diabetes | Human | 1) After receiving anakinra for 8 days, 14 type 1 diabetes patients had lower counts in neutrophils and leucocytes, improved insulin sensitivity assessed by euglycemic hyperinsulinimic clamp with lower glucose levels, lower dose of insulin used that lasted for 4 weeks and decrease of HbA1c at 4 weeks. 2) 70 patients with type 2 diabetes were divided in two groups and administered either anakinra or placebo subcutaneously for 13 weeks. Patients that received anakinra had lower HbA1c, lower fasting plasma glucose levels, lower neutrophil counts and improved beta-cell secretory function as assessed by the ratio pro-insulin to insulin when compared to patients that received placebo. |
Good | 1)van Asseldonk EJ et al [44]; 2)Larsen CM et al [45] | |
| NLRP3 and caspase-1 | Sulforaphane | NLRP3 inflammasome | NAFLD | Mice | Sulphorafane prevented development of hepatic steatosis in a mouse model of obesity-induced NAFLD as assessed by histological examination and hepatic lipid content. Mice that received sulphorafane had lower ALT plasma levels, lower IL-1β protein content in the liver and lower mRNA expression of ASC and caspase-1 (components of the NLRP3 inflammasome) as well as reduced caspase-1 activity in the liver when compared to mice that received placebo. All these results are indicating that targeting NLRP3 inflammasome in the liver could prevent development of liver steatosis. | Mild | Yang G et al [46] |
| GW501516 | NLRP3 inflammasome/PPAR delta | NAFLD | Mice, human cells | Administration of GW501516 in a mouse model of HFD-induced obesity with LPS challenge significantly reduced serum ALT and AST levels and prevented development of hepatic steatosis when compared to controls. However, there was no difference in mRNA levels of caspase-1 and mRNA and protein levels of IL-1 β in the liver, so the observed effect was independent of NLRP3 inflammasome pathway. In human HepG2 cells challenged with pro-inflammatory stimuli, administration of GW501516 significantly reduced NLRP3 inflammasome mRNA expression. | Mild | Lee HJ et al [47] | |
| Isoliquiritigenin | NLRP3 inflammasome | HFD-induced obesity | Mice, human cells | Isoliquiritigenin prevented development of hepatic steatosis in a mouse model of HFD-induced obesity by reducing hepatic lipid content. Mice that received isoliquiritigenin had improved insulin sensitivity, reduced ALT levels and reduced mRNA expression of pro-inflammatory cytokines and NLRP3 inflammasome components in the adipose tissue when compared to controls. In mouse bone marrow derived macrophages and in human THP-1 macrophages challenged with LPS and ATP, administration of isoliquiritigenin significantly decreased active protein levels of caspase-1 and IL-1β, suggestive for an inhibition of NLRP3 inflammasome activity. |
Mild | Honda H et al [48] | |
| B-hydroxybutirate | NLRP3 inflammasome | Inflammation | Mouse cells | B-hydroxybutirate inhibited activation of caspase-1 and IL-1β, assessed by western blot analysis by preventing ASC oligomerization in a dose-dependent manner, in mouse bone marrow derived macrophages challenged with several pro-inflammatory stimuli that triggered NLRP3 inflammasome. | Good | Youm YH et al [49] | |
| MCC950 | NLRP3 inflammasome | NASH | Mice | Administration of MCC950 in two mouse models of diet-induced NASH significantly reduced NLRP3 components and IL-1β mRNA expression and IL-1β, IL-6, MCP-1 plasma levels, as well as NASH score, liver inflammation and liver fibrosis assessed by histological analysis. No effect of MCC950 was observed on liver steatosis. | Good | Mridha AR et al [50] | |
| Glyburide | NLRP3 inflammasome | Type 2 diabetes | Cells | Glyburide administration on bone marrow derived macrophages challenged with inflammasome triggeres significantly reduced pro-caspase-1 and pro-IL-1β NLRP3 dependent activation. | Gooid | Lamkanfi M et al [51] | |
| Parthenolide | NLRP3 inflammasome | Inflammation | Cells | Bone marrow derived macrophages were challenged with LPS and pre-treated with parthenolide and bay 11-7082 before ATP challenge for the two-step NLRP3 activation. Administrating these two molecules significantly reduced pro-caspase-1 and IL-1β NLRP3-dependent activation as assessed by Western Blot analysis. | Good | Juliana C et al [52] | |
| Bay 11-7082 | Good | ||||||
| Emricasan | Caspase family | NAFLD/NASH | Mice | In a mouse model of diet-induced NASH, mice treated with Emricasan had significant reduction of scores for liver apoptosis, inflammation and fibrosis, assessed by histopatholgical analysis, when compared to non-treated controls on the same diet. | Mild | Barreyro FJ et al [53] | |
| Vx-166 | Caspase family | NAFLD/NASH | Mice | Administrating Vx-166 in two mouse models of HFD-induced NAFLD and MCD (methyl choline deficient)-induced NASH had shown that this compound can reduce significantly liver apoptosis and inflammation as scored by histopathological analysis when compared with mice administered a placebo. However, no effect was observed on the overall NASH score and no effect was observed on hepatic steatosis. | Mild | Wittek RP et al [54]; Anstee QM et al [55] | |
| Nivocasan | Caspase family | NAFLD/NASH | Rats | Nivocasan prevented development of liver fibrosis as assessed by histopatological analysis and reduced mRNA expression of fibrosis markers αSMA, collagen 1α1 and TGF-β in a rat model of thioacetamine-induced liver fibrosis. | Good | Kim DY et al [56] | |
| Ac-YVAD | Caspase-1 | NAFLD/NASH | Mice | Ac-YVAD administration significantly reduced liver steatosis and fibrosis development, as assessed by histological analysis, in LDLR-/- mice fed a HFD when compared to LDLR-/- mice on HFD that did not receive treatment. | Mild | Morrison MC et al [57] | |
| Neutrophil serine proteases | Alpha-1 antitrypsin | NE, PR3, CG | COPD, cystic fibrosis | Human | Administration of AAT in patients with COPD or cystic fibrosis has proven efficient in inhibiting neutrophil elastase activity in the lungs and has shown an improvement in lung function. AAT is used as treatment in some cases of lung diseases due to AAT deficiency. | Good | Siekmeier R [60] |
| Dipetide vinyl sulfones | DPPI | Not tested | Not tested | Unknown | Unknown | Korver GE et al [64] | |
| Semicarbazide-derived inhibitors | DPPI | Not tested | Not tested | Unknown | Unknown | Bondebjerg J et al[65] | |
| E-64c-Hydrazide-derived inhibitors | DPPI | Not tested | Not tested | Unknown | Unknown | Radzey H et al [66] |
Regarding inflammasomes, several reagents inhibiting this pathway have been developed and evaluated in animal models of liver diseases (46–52). The efficacy of sulforaphane (SFN), a NLRP3 inhibitor, has been investigated in a mouse model for obesity-induced NAFLD (46). Mice treated with SFN showed decreased caspase-1 activity, reduced IL-1β production in the liver and reduced steatosis. Although steatohepatitis was not assessed in this study, these results are encouraging since sulphorafane appears to show efficacy at least in the early stages of NAFLD (46). These results clearly indicate that NLRP3 inhibition might be promising for treating metabolic disorders. However, no NLRP3 inhibitor has been tested in a clinical setting yet.
For caspase-1 inhibition, most compounds described target all the caspases in the cell (pan-caspase inhibitors) instead of caspase-1 alone (53–56). Studies investigating these compounds are more focused on caspases involved in apoptosis, another mechanism for liver inflammation, as opposed to caspase-1 which promotes inflammation by activating pro-inflammatory cytokines. An inhibitor that is specific for caspase-1, namely Ac-YVAD, has been investigated using low density lipoprotein receptor (LDLr-/-), Leiden mice, that display features of the metabolic syndrome when fed a HFD (57). Mice treated with this caspase-1 inhibitor showed reduced liver steatosis and reduced liver fibrosis, as assessed by Sirius red staining. In addition, these mice had less neutrophils infiltrates, lower ALT plasma concentration and reduced Tnf gene expression in the liver (57). Taken together, these results show that inhibiting the inflammasomes and/or caspase-1 has potential for the treatment of NAFLD.
As discussed above, NSPs are also able to regulate inflammation thereby contributing to liver diseases. Therefore, these pathways might offer promising targets for therapeutic intervention as well. An interesting candidate for inhibiting this pathway is alpha-1 antitrypsin (AAT), the natural inhibitor of NSPs (Figure 2). Next to NSPs, AAT was described to inhibit caspase-1 as well in a mouse model of acute myocardial infarction (58). Therefore, using a compound that is able to inhibit both NSPs as caspase-1, might be very effective for treating sterile inflammation. Several groups have reported promising findings regarding the use of AAT as an effective and safe treatment for inflammatory diseases. The potential role of AAT as a drug has been investigated in a mouse model for rheumatoid arthritis. Human AAT protein therapy delayed onset, ameliorated disease manifestations and reduced autoimmunity in arthritic mice when compared to control mice that were administered a saline solution (59).
Regarding the use of AAT in NAFLD there are a few results indicating its importance as potential treatment. As described above, correcting an imbalance between protein levels of AAT and NSPs might be a good therapeutic approach (11). We also have investigated alpha-1 antitrypsin as a potential therapy for NAFLD. In our study, WT mice were treated with human AAT during the last 10 days of a 16-weeks HFD intervention. Treatment reduced hepatic lipid content and decreased fasting glucose levels when comared to non-treatment (9). Similar observations were made in patient studies. High protein levels of neutrophil elastase were negatively correlated with the protein levels of AAT in patients suffering from liver steatosis and NASH (10). In conclusion, a deficit of AAT is responsible for insufficient inhibition of NSPs and therefore also for inflammation-induced liver diseases. Although we do not know yet the efficacy of AAT and the possible adverse event in NAFLD patients, it is a promising candidate also because it is a natural occurring inhibitor of both inflammasome-dependent and independent cytokines activation pathways (59). In patients, AAT already has demonstrated to be a successful therapy in NSPs-mediated inflammatory diseases such as chronic obstructive pulmonary disease (COPD) and cystic fibrosis (60). Besides inhibiting NSPs and caspase-1, AAT is also a potent inducer of IL-1Ra, the natural antagonist of IL-1β (61); another reason to consider this protein as a good candidate for treating IL-1 mediated diseases.
Another interesting approach for inhibiting NSPs pathways might be targeting of their activator dipeptidyl peptidase I (DPPI). Studies investigating arthritis and NSPs-mediated autoimmune diseases have shown that DPPI knockout mice do not develop full-blown inflammation when compared to WT mice. DPPI k.o. mice had significantly decreased enzyme activity for NE, PR3 and CG and decreased protein levels of pro-inflammatory cytokines such as TNF-α and IL-1β when compared to WT controls (36, 62, 63). These results strengthen the hypothesis that targeting DPPI might also be an interesting therapeutic approach for NSPs-mediated metabolic diseases. To date, only a few DPPI inhibitors have been described but they were not tested yet in an in-vivo model for NSPs mediated inflammatory diseases (Table 1) (64–66).
Although future research is needed to provide insights into the balance between the beneficial and harmful effects of inhibiting these pathways, we believe that these initial studies show encouraging results regarding the inhibition of both inflammasome-dependent and independent pathways in treating inflammatory-driven liver diseases.
Concluding remarks
It is known that IL-1 family cytokines play a major role in the development of liver inflammation. Since these cytokines need enzymatic processing in order to become active, their activators are also important in the process of liver inflammation. In this Opinion article we provide new insights that next to the classical NLRP3 inflammasome–caspase-1 cytokine activation pathway, NSPs are also potent cytokine activators that contribute to liver disease progression. This might be an explanation why pharmacological agents against the NLRP3-caspase-1 pathway have a rather mild effect on cytokine inhibition and liver inflammatory events. The realization that NSPs are also involved in cytokine activation, opens new perspectives for NAFLD treatment. Additional studies are needed to further investigate whether the NSPs pathway can be targeted. Next to that, we believe it is worthwhile to investigate suppression of both NLRP3-caspase-1 and NSPs pathways to systematically inhibit cytokine activation thereby reducing inflammation. Comprehensive studies are needed to investigate not only efficacy but also safety aspects of blocking multiple cytokines in inflammatory-induced metabolic conditions (see also Oustanding questions box). A knockout mouse for both caspase-1 and NSPs would provide a valuable tool for future studies investigating simultaneous suppression of these pathways. Additionally, experiments in transgenic mice overexpressing NSPs could provide useful information regarding the systemic consequences of overexpressing these enzymes. Next to animal models, investigating genetic variants in candidate genes using well-characterized NAFLD patient cohorts would provide a better understanding of NSPs regulation in humans and may highlight new targets that have therapeutic potential.
Outstanding questions.
What are the interactions between neutrophil serine proteases (NSPs) and NLRP3 inflammasome - caspase-1 pathways? Treating the disease has shown to be difficult and downregulating one pathway might directly or indirectly upregulate the other via interactions not yet revealed.
What are the consequences of systemic inhibition of multiple pro-inflammatory cytokines as they are important for the normal immune response to infections.
Which (experimental) drugs are able to inhibit both caspase-1 and NSPs? Is alpha-1 antitrypisn (AAT) a good candidate to target both pathways? What are both the short- and long-term effects of these (experimental) drug regarding efficacy and adverse events?
During which disease stages would treatment have beneficial effects? Should these pathways be targeted during the early stages of disease of or would it also have effects during later stages? Can it be administered as preventive therapy for patients at risk to develop NAFLD?
Which biomarkers are suitable for monitoring disease progression and/or therapy response in inflammatory-induced NAFLD?
A limitation in reviewing current literature is that most studies investigated animal models of NAFLD. At this point it is unclear how much of the results obtained in these animal studies could be translated to the human situation. In addition, human studies are hampered by the fact that it is difficult to obtain relevant samples as a liver biopsy is an invasive method that has potential complications. Therefore, it is challenging to investigate inflammatory mechanisms in human patients with NAFLD and most data in human studies is obtained by measuring markers in plasma/serum. However, it is often unclear whether these plasma/serum markers reflect the local inflammatory status in the liver.
In conclusion, new insights now reveal that NSPs, next to the NLRP3-caspase-1 pathway also contribute to the development of NAFLD and NASH. The realization that NSPs contribute to the disease process in an inflammasome-independent manner should move the field forward and opens up new perspectives for therapeutic interventions for the treatment of NAFLD and NASH.
Clinician’s corner.
Non-alcoholic fatty liver disease (NAFLD) can range from simple steatosis to liver inflammation (non-alcoholic steatohepatitis-NASH) and fibrosis which affect the survival rate of patients. Currently, there is no treatment available to stop disease progression.
Pro-inflammatory cytokines play an important role in the disease progression from liver steatosis to liver inflammation and fibrosis. IL-1 family cytokines are one of the major promoters of liver inflammatory events. Blocking the action of these cytokines could potentially stop NAFLD progression. Cytokines need enzymatic processing in order to become active. Therefore, blocking the pathways responsible for cytokine processing/activation might also have therapeutic potential.
The NLRP3 inflammasome complex and its effector protein caspase-1 is one of the most studied pathways for activation of IL-1 family cytokines. Many studies have reported that this pathway is involved in the development of NAFLD and other metabolic disturbances. However, this pathway is not the only mechanism available for cytokine activation. Neutrophil serine proteases also play an important role in cytokine activation during NAFLD.
Targeting both the NLRP3 - caspase-1 and neutrophil serine protease pathways might have potential as a new therapy for the treatment of inflammatory-induced NAFLD.
Highlights.
Cytokines are key in the development of NAFLD and NASH but mechanisms responsible for the activation of these cytokines are not fully understood.
The NLRP3-inflamasome pathway is capable of activating cytokines and is a known inducer of inflammation in NAFLD and NASH.
Also neutrophil serine proteases (NSPs) can activate cytokines. Studies have now shown that NSPs also contribute to NAFLD and NASH.
The realization that NSPs are involved in NAFLD and NASH in an inflammasome-independent manner provides new insights into how inflammatory pathways contribute to these conditions.
NSPs are inhibited by alpha-1 antitrypsin (AAT) and mice overexpressing AAT are protected against the development of NAFLD. Mice treated with AAT showed reduced hepatic lipid content in NAFLD mice models.
The recent findings that NSPs contribute to NAFLD and NASH opens up perspectives for new therapeutics.
Acknowledgements
This work was supported by a grant of the Else- Kröner-Fresenius-Stiftung (to ET, LJ and TC). TC was also supported by the European research Council (DEMETINL).
Footnotes
Competing interests
The authors declare to have no competing interests
References
- 1.European Association for the Study of the L, European Association for the Study of D, European Association for the Study of O. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Journal of hepatology. 2016;64(6):1388–402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. International journal of molecular sciences. 2016;17(5) doi: 10.3390/ijms17050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kakino S, Ohki T, Nakayama H, Yuan X, Otabe S, Hashinaga T, et al. Pivotal Role of TNF-alpha in the Development and Progression of Nonalcoholic Fatty Liver Disease in a Murine Model. Horm Metab Res. 2017 doi: 10.1055/s-0043-118666. [DOI] [PubMed] [Google Scholar]
- 4.Negrin KA, Roth Flach RJ, DiStefano MT, Matevossian A, Friedline RH, Jung D, et al. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PloS one. 2014;9(9):e107265. doi: 10.1371/journal.pone.0107265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nature reviews Gastroenterology & hepatology. 2015;12(7):387–400. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
- 6.Mehal WZ. The inflammasome in liver injury and non-alcoholic fatty liver disease. Digestive diseases. 2014;32(5):507–15. doi: 10.1159/000360495. [DOI] [PubMed] [Google Scholar]
- 7.Afonina IS, Muller C, Martin SJ, Beyaert R. Proteolytic Processing of Interleukin-1 Family Cytokines: Variations on a Common Theme. Immunity. 2015;42(6):991–1004. doi: 10.1016/j.immuni.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong L, Godinho SI, Uppington KM, Whittington HA, Millar AB. Tumour necrosis factor-alpha processing in interstitial lung disease: a potential role for exogenous proteinase-3. Clinical and experimental immunology. 2009;156(2):336–43. doi: 10.1111/j.1365-2249.2009.03906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toonen EJ, Mirea AM, Tack CJ, Stienstra R, Ballak DB, van Diepen JA, et al. Activation of proteinase 3 contributes to Non-alcoholic Fatty Liver Disease (NAFLD) and insulin resistance. Molecular medicine. 2016;22 doi: 10.2119/molmed.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zang S, Ma X, Zhuang Z, Liu J, Bian D, Xun Y, et al. Increased ratio of neutrophil elastase to alpha1-antitrypsin is closely associated with liver inflammation in patients with nonalcoholic steatohepatitis. Clinical and experimental pharmacology & physiology. 2016;43(1):13–21. doi: 10.1111/1440-1681.12499. [DOI] [PubMed] [Google Scholar]
- 11.Mansuy-Aubert V, Zhou QL, Xie X, Gong Z, Huang JY, Khan AR, et al. Imbalance between neutrophil elastase and its inhibitor alpha1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell metabolism. 2013;17(4):534–48. doi: 10.1016/j.cmet.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annual review of immunology. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 13.Tan Q, Hu J, Yu X, Guan W, Lu H, Yu Y, et al. The Role of IL-1 Family Members and Kupffer Cells in Liver Regeneration. Biomed Res Int. 2016;2016 doi: 10.1155/2016/6495793. 6495793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patrick AL, Rullo J, Beaudin S, Liaw P, Fox-Robichaud AE. Hepatic leukocyte recruitment in response to time-limited expression of TNF-alpha and IL-1beta. American journal of physiology Gastrointestinal and liver physiology. 2007;293(4):G663–72. doi: 10.1152/ajpgi.00070.2007. [DOI] [PubMed] [Google Scholar]
- 15.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39(6):1003–18. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143(5):1158–72. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Digestive diseases. 2010;28(1):179–85. doi: 10.1159/000282083. [DOI] [PubMed] [Google Scholar]
- 18.Gieling RG, Wallace K, Han YP. Interleukin-1 participates in the progression from liver injury to fibrosis. American journal of physiology Gastrointestinal and liver physiology. 2009;296(6):G1324–31. doi: 10.1152/ajpgi.90564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139(1):323–34 e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamari Y, Shaish A, Vax E, Shemesh S, Kandel-Kfir M, Arbel Y, et al. Lack of interleukin-1alpha or interleukin-1beta inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. Journal of hepatology. 2011;55(5):1086–94. doi: 10.1016/j.jhep.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nature medicine. 2006;12(6):650–6. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 22.Yamanishi K, Maeda S, Kuwahara-Otani S, Watanabe Y, Yoshida M, Ikubo K, et al. Interleukin-18-deficient mice develop dyslipidemia resulting in nonalcoholic fatty liver disease and steatohepatitis. Translational research : the journal of laboratory and clinical medicine. 2016;173:101–14 e7. doi: 10.1016/j.trsl.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Hung J, McQuillan BM, Chapman CM, Thompson PL, Beilby JP. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(6):1268–73. doi: 10.1161/01.ATV.0000163843.70369.12. [DOI] [PubMed] [Google Scholar]
- 24.Troseid M, Seljeflot I, Arnesen H. The role of interleukin-18 in the metabolic syndrome. Cardiovascular diabetology. 2010;9:11. doi: 10.1186/1475-2840-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troseid M, Seljeflot I, Weiss TW, Klemsdal TO, Hjerkinn EM, Arnesen H. Arterial stiffness is independently associated with interleukin-18 and components of the metabolic syndrome. Atherosclerosis. 2010;209(2):337–9. doi: 10.1016/j.atherosclerosis.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Liu Y, Yang M, Guo X, Zhang M, Li H, et al. IL-33 treatment attenuated diet-induced hepatic steatosis but aggravated hepatic fibrosis. Oncotarget. 2016;7(23):33649–61. doi: 10.18632/oncotarget.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marvie P, Lisbonne M, L'Helgoualc'h A, Rauch M, Turlin B, Preisser L, et al. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J Cell Mol Med. 2010;14(6B):1726–39. doi: 10.1111/j.1582-4934.2009.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiskirchen R, Tacke F. Interleukin-33 in the pathogenesis of liver fibrosis: alarming ILC2 and hepatic stellate cells. Cellular & molecular immunology. 2017;14(2):143–5. doi: 10.1038/cmi.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular cell. 2002;10(2):417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 30.Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59(3):898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wree A, McGeough MD, Pena CA, Schlattjan M, Li H, Inzaugarat ME, et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. Journal of molecular medicine. 2014;92(10):1069–82. doi: 10.1007/s00109-014-1170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54(1):133–44. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon LJ, Flask CA, Papouchado BG, Feldstein AE, Nagy LE. Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PloS one. 2013;8(2):e56100. doi: 10.1371/journal.pone.0056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dixon LJ, Berk M, Thapaliya S, Papouchado BG, Feldstein AE. Caspase-1-mediated regulation of fibrogenesis in diet-induced steatohepatitis. Laboratory investigation; a journal of technical methods and pathology. 2012;92(5):713–23. doi: 10.1038/labinvest.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA. Inflammasome-independent regulation of IL-1-family cytokines. Annual review of immunology. 2015;33:49–77. doi: 10.1146/annurev-immunol-032414-112306. [DOI] [PubMed] [Google Scholar]
- 36.Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis and rheumatism. 2009;60(12):3651–62. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bae S, Choi J, Hong J, Jhun H, Hong K, Kang T, et al. Neutrophil proteinase 3 induces diabetes in a mouse model of glucose tolerance. Endocrine research. 2012;37(1):35–45. doi: 10.3109/07435800.2011.620579. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, Su S, Wang X, Barnes V, De Miguel C, Ownby D, et al. Obesity is associated with more activated neutrophils in African American male youth. International journal of obesity. 2015;39(1):26–32. doi: 10.1038/ijo.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engstrom G, Stavenow L, Hedblad B, Lind P, Eriksson KF, Janzon L, et al. Inflammation-sensitive plasma proteins, diabetes, and mortality and incidence of myocardial infarction and stroke: a population-based study. Diabetes. 2003;52(2):442–7. doi: 10.2337/diabetes.52.2.442. [DOI] [PubMed] [Google Scholar]
- 40.Engstrom G, Hedblad B, Stavenow L, Lind P, Janzon L, Lindgarde F. Inflammation-sensitive plasma proteins are associated with future weight gain. Diabetes. 2003;52(8):2097–101. doi: 10.2337/diabetes.52.8.2097. [DOI] [PubMed] [Google Scholar]
- 41.Honda M, Kubes P. Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal system. Nature reviews Gastroenterology & hepatology. 2018 doi: 10.1038/nrgastro.2017.183. [DOI] [PubMed] [Google Scholar]
- 42.Omoto Y, Yamanaka K, Tokime K, Kitano S, Kakeda M, Akeda T, et al. Granzyme B is a novel interleukin-18 converting enzyme. J Dermatol Sci. 2010;59(2):129–35. doi: 10.1016/j.jdermsci.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. The New England journal of medicine. 2017;377(12):1119–31. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 44.van Asseldonk EJ, van Poppel PC, Ballak DB, Stienstra R, Netea MG, Tack CJ. One week treatment with the IL-1 receptor antagonist anakinra leads to a sustained improvement in insulin sensitivity in insulin resistant patients with type 1 diabetes mellitus. Clin Immunol. 2015;160(2):155–62. doi: 10.1016/j.clim.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, et al. Interleukin-1-eceptor antagonist in type 2 diabetes mellitus. The New England journal of medicine. 2007;356(15):1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 46.Yang G, Lee HE, Lee JY. Erratum: A pharmacological inhibitor of NLRP3 inflammasome prevents non-alcoholic fatty liver disease in a mouse model induced by high fat diet. Scientific reports. 2016;6:26218. doi: 10.1038/srep24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee HJ, Yeon JE, Ko EJ, Yoon EL, Suh SJ, Kang K, et al. Peroxisome proliferator-activated receptor-delta agonist ameliorated inflammasome activation in nonalcoholic fatty liver disease. World journal of gastroenterology. 2015;21(45):12787–99. doi: 10.3748/wjg.v21.i45.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Honda H, Nagai Y, Matsunaga T, Okamoto N, Watanabe Y, Tsuneyama K, et al. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. Journal of leukocyte biology. 2014;96(6):1087–100. doi: 10.1189/jlb.3A0114-005RR. [DOI] [PubMed] [Google Scholar]
- 49.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nature medicine. 2015;21(3):263–9. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. Journal of hepatology. 2017;66(5):1037–46. doi: 10.1016/j.jhep.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. The Journal of cell biology. 2009;187(1):61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. The Journal of biological chemistry. 2010;285(13):9792–802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barreyro FJ, Holod S, Finocchietto PV, Camino AM, Aquino JB, Avagnina A, et al. The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver international: official journal of the International Association for the Study of the Liver. 2015;35(3):953–66. doi: 10.1111/liv.12570. [DOI] [PubMed] [Google Scholar]
- 54.Witek RP, Stone WC, Karaca FG, Syn WK, Pereira TA, Agboola KM, et al. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50(5):1421–30. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 55.Anstee QM, Concas D, Kudo H, Levene A, Pollard J, Charlton P, et al. Impact of pan-caspase inhibition in animal models of established steatosis and non-alcoholic steatohepatitis. Journal of hepatology. 2010;53(3):542–50. doi: 10.1016/j.jhep.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 56.Kim DY, Chung SI, Ro SW, Paik YH, Lee JI, Jung MK, et al. Combined effects of an antioxidant and caspase inhibitor on the reversal of hepatic fibrosis in rats. Apoptosis: an international journal on programmed cell death. 2013;18(12):1481–91. doi: 10.1007/s10495-013-0896-5. [DOI] [PubMed] [Google Scholar]
- 57.Morrison MC, Mulder P, Salic K, Verheij J, Liang W, van Duyvenvoorde W, et al. Intervention with a caspase-1 inhibitor reduces obesity-associated hyperinsulinemia, non-alcoholic steatohepatitis and hepatic fibrosis in LDLR-/-.Leiden mice. International journal of obesity. 2016;40(9):1416–23. doi: 10.1038/ijo.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toldo S, Seropian IM, Mezzaroma E, Van Tassell BW, Salloum FN, Lewis EC, et al. Alpha-1 antitrypsin inhibits caspase-1 and protects from acute myocardial ischemia-reperfusion injury. Journal of molecular and cellular cardiology. 2011;51(2):244–51. doi: 10.1016/j.yjmcc.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Grimstein C, Choi YK, Wasserfall CH, Satoh M, Atkinson MA, Brantly ML, et al. Alpha-1 antitrypsin protein and gene therapies decrease autoimmunity and delay arthritis development in mouse model. Journal of translational medicine. 2011;9:21. doi: 10.1186/1479-5876-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siekmeier R. Lung deposition of inhaled alpha-1-proteinase inhibitor (alpha 1-PI) - problems and experience of alpha1-PI inhalation therapy in patients with hereditary alpha1-PI deficiency and cystic fibrosis. Eur J Med Res. 2010;15 Suppl 2:164–74. doi: 10.1186/2047-783X-15-S2-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joosten LA, Crisan TO, Azam T, Cleophas MC, Koenders MI, van de Veerdonk FL, et al. Alpha-1-anti-trypsin-Fc fusion protein ameliorates gouty arthritis by reducing release and extracellular processing of IL-1beta and by the induction of endogenous IL-1Ra. Annals of the rheumatic diseases. 2016;75(6):1219–27. doi: 10.1136/annrheumdis-2014-206966. [DOI] [PubMed] [Google Scholar]
- 62.Adkison AM, Raptis SZ, Kelley DG, Pham CT. Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. The Journal of clinical investigation. 2002;109(3):363–71. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schreiber A, Pham CT, Hu Y, Schneider W, Luft FC, Kettritz R. Neutrophil serine proteases promote IL-1beta generation and injury in necrotizing crescentic glomerulonephritis. Journal of the American Society of Nephrology : JASN. 2012;23(3):470–82. doi: 10.1681/ASN.2010080892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korver GE, Kam CM, Powers JC, Hudig D. Dipeptide vinyl sulfones suitable for intracellular inhibition of dipeptidyl peptidase I. International immunopharmacology. 2001;1(1):21–32. doi: 10.1016/s0162-3109(00)00267-8. [DOI] [PubMed] [Google Scholar]
- 65.Bondebjerg J, Fuglsang H, Valeur KR, Kaznelson DW, Hansen JA, Pedersen RO, et al. Novel semicarbazide-derived inhibitors of human dipeptidyl peptidase I (hDPPI) Bioorganic & medicinal chemistry. 2005;13(14):4408–24. doi: 10.1016/j.bmc.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 66.Radzey H, Rethmeier M, Klimpel D, Grundhuber M, Sommerhoff CP, Schaschke N. E-64c-hydrazide: a lead structure for the development of irreversible cathepsin C inhibitors. ChemMedChem. 2013;8(8):1314–21. doi: 10.1002/cmdc.201300093. [DOI] [PubMed] [Google Scholar]