Abstract
Objective
Respiratory sinus arrhythmia (RSA) is a parasympathetic-mediated biomarker of self-regulation linked to lifespan mental and physical health outcomes. Intergenerational impacts of mothers’ exposure to prenatal stress have been demonstrated, but evidence for biological embedding of maternal preconception stress, including adverse childhood experiences (ACEs), on infant RSA is lacking. We examine the independent effects of maternal ACEs and prenatal stress on infant RSA, seeking to broaden the understanding of the earliest origins of mental and physical health risk.
Method
Mothers reported on ACEs and prenatal stress. RSA was recorded in a sample of 167 4-month-old infants (49% female and 51% male) during a dyadic stressor, the Still Face Paradigm.
Results
Independent contributions of maternal ACEs and prenatal stress to infant RSA were observed. High maternal ACEs were associated with lower RSA, whereas prenatal stress was associated with failure to recover following the stressor. Sex but not race differences were observed. Prenatal stress was associated with higher RSA among boys but lower RSA among girls.
Conclusion
Infants’ RSA is affected by mothers’ life course experiences of stress, with ACEs predicting a lower set point and prenatal stress dampening recovery from stress. For prenatal stress but not ACEs, patterns vary across sex. Findings underscore that stress-reducing interventions for pregnant women or those considering pregnancy may lead to decreased physical and mental health risk across generations.
Keywords: respiratory sinus arrhythmia, adverse childhood experiences, prenatal stress, trauma, still face paradigm
Adverse childhood experiences (ACEs) are associated with negative behavioral and physical health outcomes across the lifespan, and alterations to stress response systems (SRSs) are hypothesized underlying mechanistic pathways.1 Beyond the effect of adversity on an individual’s SRSs, there is evidence that mothers’ experiences of stress during pregnancy can affect her developing child’s SRSs, including the autonomic nervous system (ANS).2–4 ACEs also appear to have effects on the next generation, as preconception maternal early life adversity is associated with preterm birth and low birth weight, and maternal sexual abuse history independently predicts poorer birth outcomes.5,6 Furthermore, maternal history of childhood abuse predicts hypothalamic–pituitary–adrenal (HPA) axis functioning in her offspring.7 These findings are consistent with life course theory, which posits that health trajectories are influenced by recent events and reflect cumulative exposure, suggesting that consideration of both current stressors and experiences across a mother’s lifespan are needed to understand intergenerational health disparities.8,9 An infant’s developing ANS, and therefore future self-regulation and mental health risk, are likely influenced by a combination of a mother’s experience of prenatal stress and her own earlier exposures to adversity.2,10 No previous study has examined concurrently maternal ACE exposure and prenatal stress on her child’s ANS regulation. To address this gap, mothers were recruited prenatally and queried about both their ACE exposure and levels of pre-natal stress. We then tested their independent and interactive influence on 4-month-old infants’ respiratory sinus arrhythmia (RSA).
ANS Activity and Stress Regulation
Emotional and biobehavioral self-regulation is moderated by the interaction of the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS), components of the ANS that work in dynamic balance to promote homeostasis.11,12 Respiratory sinus arrhythmia (RSA) is an index of PNS activity10 and can be measured by baseline activity, indicating the potential to engage with the environment, or by reactivity, reflecting stimulus response.13 Both baseline and reactivity are associated with behavioral and physical health outcomes across the lifespan. High baseline RSA is generally considered adaptive and, in infants, associated with higher emotional reactivity and attention.10 In youth and adult populations, lower baseline RSA and divergent RSA reactivity are physiologic markers of emotion dysregulation that, consistent with the Research Domain Criteria, exhibit associations across diagnostic categories.10,14 Furthermore, RSA has been linked to stress-related physical health outcomes, including cardiovascular disease, diabetes, and obesity, making it a “unifying” risk factor across the life course and across physical and mental health outcomes.15
Among infants, RSA reactivity has been assessed with the Still Face Paradigm (SFP),16 an observational dyadic laboratory paradigm assessing infants’ self-regulatory ability and dyadic function. During the SFP, mothers first play freely with the infant. Then, during the second epoch, mothers maintain a neutral expression, the “still face,” thereby removing typical maternal responsiveness expected by the infant. In the final epoch, mothers resume interaction, providing an opportunity for infant recovery with behavioral support. Infants typically demonstrate a decrease in RSA from free play to still-face, followed by a subsequent increase in RSA from still-face to the reunion as PNS activity returns to baseline following stressor removal.17,18
Intergenerational Influences
Prenatally and throughout the first years of life, the ANS is undergoing rapid development.19–21 In utero, maternal stress has an almost immediate impact on fetal ANS activity, and maternal chronic stress and depression predict neonate ANS activity.22,23 High prenatal stress has been shown to predict blunted RSA recovery following the SFP in 6-month-old infants, with effects persisting into childhood.2,3,24 In addition to prenatal influences, genetic factors and maternal experiences prior to pregnancy may also contribute to the development of the ANS.25 Although environmental factors explain 36% to 50% of variance in RSA, heritability estimates range from 24% to 54%, pointing to the ability of the ANS to adapt to postnatal environments.26–28 Maternal history of childhood abuse has been linked to fetal ANS activity29 and to ANS markers later in childhood.30 Taken together, these data provide compelling evidence that both preconception and prenatal factors affect the ANS and other SRSs both within and across generations.
Sex Differences in Emergent SRSs
A significant body of literature reports sex differences in relation to prenatal stress, including differences in ANS outcomes, findings consistent with current National Institutes of Health directives to specifically address sex as a biological variable in all studies.19,31,32 The Adaptive Calibration Model (ACM) presupposes sex differences in stress reactivity.21 The relation between RSA and trauma exposure in older children also exhibits sex differences, with females appearing to be more affected, leading to greater psychopathology risk, although these connections are not universal and may differ based on the specific SRS examined.33–36 In addition, sex differences in mental health outcomes associated with ANS activity also exist.37 Controlling for sex rather than explicitly testing sex differences, typical in RSA research, may obscure true sex differences.36
Race Differences in Emergent SRSs
The recalcitrant nature of health disparities is driving increased efforts to define underlying mechanisms and the earliest developmental time points when racial disparities appear. Unfortunately, most existing RSA research comes from majority-white samples and fails to investigate race-specific pathways, despite evidence of differences.13,36,38 In previous studies, African American children exhibited greater resting RSA compared to white children.38 Similar to sex differences, racial differences are observed in lifespan stress-related health conditions linked to ANS function, including mental health and cardiovascular disease.15,39,40 Identifying mechanistic markers underlying intergenerational racial disparities might illuminate intervention targets or, at the very least, validate similarities across racial groups.
The Current Study
To address gaps in the literature, we tested the association between maternal self-report ACE score and prenatal stress with infant RSA, specifically exploring race and sex differences. We predicted that high maternal ACEs would be associated with lower infant RSA activity, consistent with a lower physiological starting point that may represent lower openness to environmental influence found in literature exploring other SRSs.7,21,41 We expected that prenatal stress would be associated with dampened recovery following a stressor, consistent with previous RSA literature.2
METHOD
Study Participants
The current report includes 167 infants (49% female) of mothers (61% African American, 39% white) enrolled in a longitudinal study. Recruitment of pregnant women (18–41 years) took place in prenatal and Women, Infant, and Children (WIC) clinics and from other studies. Mothers were >18 years of age (mean = 28.17, SD = 5.82), and only English-speaking mothers were recruited. Gestational age ranged from 32 to 43 weeks (mean = 39.04, SD = 1.63). Twelve infants were born preterm (<37 weeks; 10 late preterm [34–36 weeks] and 2 preterm [<34 weeks]). Premature infants were not tested at corrected age. Of the mothers, 17% had less than a high school degree, whereas 37% had a college degree or higher.
Procedure
During pregnancy, mothers reported on ACEs, indicators of prenatal stress, and demographics. Mothers were recruited at any stage of pregnancy (mean = 28.3 ± 8.4 weeks); the majority of mothers were in their third trimester (54%; n = 90; n = 68 second trimester; n = 9 first trimester). Gestational age and pregnancy complications were abstracted from medical records.
When the infant was 4 months old, the mother and child completed the Still Face Paradigm (SFP), a well-established dyadic stressor validated across sociodemographically diverse samples designed to assess behavioral and physiological regulatory capacities of infants, at the laboratory.16 The procedure contained three 2.5-minute epochs: Play Period 1 (PP1): mothers instructed to play freely with infants; Still Face (SF): mothers maintained a neutral face and avoided interacting with their infant; Play Period 2 (PP2): mothers resumed play. Mothers were instructed not to touch infants, who were seated in a car seat facing mothers at eye level approximately 3 feet away. A research assistant was present but hidden throughout the procedure. If infants expressed distress, operationalized as 20 seconds of continuous crying, epochs were discontinued.
A total of 235 infants completed the SFP. Fifteen mothers completed the prenatal interview after parturition and were not included, given the focus on prenatal stress. Twenty-four identified as a race other than African American or white and were excluded to facilitate race analyses. Twenty-nine infants did not have valid RSA data due to movement artifact, hardware malfunction, human error, or insufficient duration of SFP epochs. The resultant analytical sample (N = 167) did not differ from infants who did not complete the SFP on any study variables.
Mothers were compensated for study visits. The study was approved by the university institutional review board.
Measures
Prenatal Stress Index
Consistent with a cumulative risk perspective, whereby multiple risk factors in concert typically explain more variance than individual factors,42 we created a prenatal stress index consisting of 5 indicators: pregnancy-related anxiety, measured by the 10-item Pregnancy Related Anxiety Scale,43 assessing mothers’ worry about health, labor, delivery, and infant care; chronic strain, measured with the Chronic Strain Questionnaire,44 on which mothers rated frequency of 10 types of chronic stressors (e.g., having trouble paying bills); stressful prenatal life events, assessed with the Prenatal Life Events Scale–Revised,45 assessing the presence or absence of 18 stressful life events during the prenatal period (e.g., experiencing a loss); perceived stress, measured by the 4-item version of the Perceived Stress Scale,46 probing mothers’ appraisal of their lives as stressful; and prenatal depression, assessed by the 10-item Edinburgh Depression Scale,47 shown to effectively detect prenatal depression.48
For the indicator with a dichotomous clinical cut-off (depression), a score over the clinical threshold was coded “1.” For other indicators, participants with scores in the top quartile were coded “1.” The following cut-off thresholds were derived: pregnancy-related anxiety: ≥20; chronic strain: ≥27; prenatal life events: ≥4; and perceived stress: ≥7. A factor analysis of these 5 indicators was consistent with a unitary construct by scree plot examination and eigenvalue assessment (one factor with eigenvalue ≥1); all items loaded above 0.45, the threshold for “fair” (range, 0.49–0.73).49 The 5 indicators were summed, yielding a prenatal stress variable with a range of 0 to 5 (mean = 1.31, SD = 1.40). “High” was designated as ≥3 indicators (21% of sample).
Adverse Childhood Experiences
Mothers reported on adverse experiences during their first 18 years using the Adverse Childhood Experiences survey,50 indicating “present” or “absent” for 10 types of early life stress (e.g., parental mental illness or divorce). Consistent with prior research, items were summed and “high” classified as ≥4 (18% of sample).50
Respiratory Sinus Arrhythmia
Data on heart period, the time in milliseconds from one QRS wave to the next, were collected from continuous electrocardiography (ECG) recording during the SFP with James Long Company equipment (Caroga Lake, NY). ECG signal processing and analysis were conducted using the Inter-beat Interval (IBI) Analysis System. Rising edges of R waves were automatically identified with a multiple-pass, self-scaling algorithm, represented graphically for artifact identification, and manually corrected.51 IBIs were prorated to equal time intervals (125 milliseconds) and detrended using a moving polynomial filter to remove slow trends from data prior to spectral analysis. RSA estimated from power spectral analysis was conducted using a discrete Fourier transform with a 32-second Hanning window and 50% overlap between consecutive windows. Spectral power was quantified in the validated high-frequency band range for 4-month-old infants (0.30–0.75 Hz).52 Mean RSA values were computed for each SFP epoch. RSA values were winsorized to 3 standard deviations from the mean and natural log transformed.
Data Analysis
For all analyses, a stepwise approach was taken; gestational age and maternal education were covaried. Trimester of prenatal data collection, infant age (in weeks) at visit, and pregnancy complications (preeclampsia, fetal growth restriction, gestational diabetes, gestational hypertension) were considered as covariates and found not to contribute significantly. We present parsimonious models with gestational age, infant sex, and maternal education and race.
To investigate whether prenatal stress was associated with RSA during dyadic play, a generalized linear model was run with infant RSA during PP1 as the outcome and sex, race, prenatal stress, and ACEs as predictors. First, main effects of ACEs and prenatal stress were examined; then, 2-way interactions examining sex and race differences were entered. For RSA reactivity analyses, mixed models nested within subject were tested using the PROC MIXED statement in SAS 9.4. RSA was modeled as within subject over time (PP1, SF, PP2), and prenatal stress, ACEs, and sex and race as between-subjects factors. Time was modeled with a linear and a quadratic term, with interactions placed on the quadratic term. For reactivity analyses, we modeled main effects of ACEs and prenatal stress, then 2-way interaction terms examining differences in RSA reactivity over time (time2) by ACEs and prenatal stress, as well as 2-way interaction terms examining sex and race differences (sex/race×prenatal stress/ACEs, sex/race×time2).
RESULTS
Correlations and descriptive statistics are presented in Table 1; a total of 113 infants (67%) were low on both ACEs and prenatal stress, 44 (26%) high on one indicator, and 10 (6%) high on both. Boys and girls were equally likely to be classified as high prenatal stress, χ2(2, N = 167) = 0.34, p = .56 (boys n = 18, girls n = 16), and high ACEs, χ2(2, N = 167) = 0.03, p = .86 (boys n = 15, girls n = 15). Prenatal stress and ACEs did not differ between African American and white mothers (prenatal: χ2[2, N = 167] = 0.78, p = .38; ACEs: χ2[2, N = 167] = 1.23, p = .27). High ACEs were associated with high prenatal stress, χ2(2, N = 167) = 3.80, p = .05. ACEs but not prenatal stress were associated with infant RSA in bivariate tests, with higher ACEs associated with lower RSA during PP1 and SF epochs.
TABLE 1.
Correlations and Descriptive Statistics for Study Variables
| Child Sex | Prenatal Stress | ACEs | PP1 RSA | SF RSA | PP2 RSA | Gestational Age | Maternal Race | |
|---|---|---|---|---|---|---|---|---|
| Prenatal stress | 0.06 | |||||||
| ACEs | 0.05 | 0.15* | ||||||
| PP1 RSA | −0.00 | 0.06 | −0.18** | |||||
| SF RSA | −0.05 | −0.03 | −0.16* | 0.66*** | ||||
| PP2 RSA | 0.10 | −0.09 | −0.12 | 0.56*** | 0.57*** | |||
| Gestational age | 0.04 | −0.01 | 0.03 | −0.00 | 0.05 | −0.04 | ||
| Maternal race | −0.06 | −0.07 | −0.09 | 0.08 | −0.00 | 0.12 | 0.11 | |
| Maternal education | −0.06 | −0.18* | −0.05 | −0.01 | −0.02 | 0.10 | 0.19* | 0.41*** |
| Mean (SD) or % | 51% Male | 21% High | 18% High | 2.70 (0.47) | 2.53 (0.55) | 2.56 (0.61) | 39.03 (1.62) | 61% AA |
Note: Prenatal stress and adverse childhood experiences (ACEs) coded as 0 = low, 1 = high; sex coded as 1 = male, 2 = female; race coded as 0 = African American, 1 = white; gestational age reported in weeks. N = 167. AA = African American; PP1 = play period 1; PP2 = play period 2; RSA = respiratory sinus arrhythmia; SF = still face.
p < .05;
p < .01,
p < .001.
All race interactions were nonsignificant (p > .41), suggesting that RSA did not vary by race. Additionally, ACE×sex and ACE×time2 interactions were nonsignificant; thus, we present parsimonious models with prenatal stress×sex 2-way interactions and main effects of ACEs only.
Dyadic Play RSA
Prenatal Stress
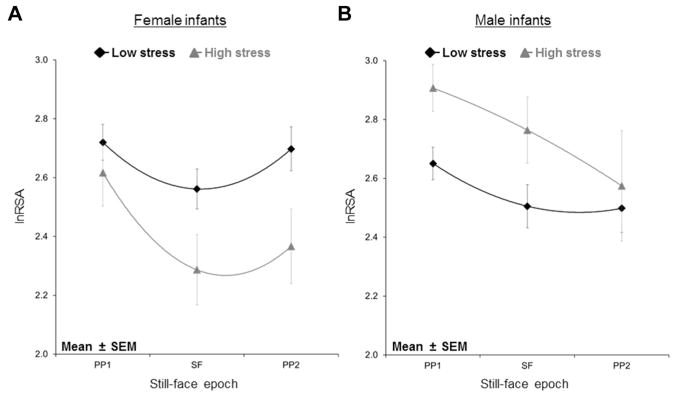
No main effect of prenatal stress on RSA was observed during PP1, but a significant prenatal stress×sex interaction was present, β = −0.35, p = .04. Post hoc analyses indicated that boys but not girls with high prenatal stress had significantly higher RSA during PP1 compared to infants with low prenatal stress, t84 = −2.10, p = .04 (Figure 1, PP1 epoch). No race differences were observed.
FIGURE 1.
Infants with high prenatal stress do not show parasympathetic recovery during still-face reunion; high-stress girls show lower respiratory sinus arrhythmia than low-stress girls, whereas high-stress boys show higher respiratory sinus arrhythmia than low-stress boys. Note: lnRSA = respiratory sinus arrhythmia; PP1 = play period 1; PP2 = play period 2; SEM = standard error of the mean; SF = still face.
Maternal ACEs
There was a main effect of mothers’ ACEs. Higher ACEs predicted lower infant RSA during dyadic play, β = −0.21, p = .02. No sex or race differences were observed.
RSA Reactivity
A majority of variance was accounted for within subject (intraclass correlation = 56%). In the final model including ACEs, prenatal stress, and interactions, both linear and quadratic time terms were significant, indicating significant RSA response (Table 2). Girls showed greater reactivity than boys (time2×sex; Figure 1).
TABLE 2.
Mixed Model Showing Main Effects of Maternal Adverse Childhood Experiences (ACEs) on Infant Respiratory Sinus Arrhythmia (RSA), Effects of Prenatal Stress on RSA Reactivity, and Sex Differences
| Infant RSA | B (SE) | p Value |
|---|---|---|
| Intercept | 3.22 (0.89) | <.001 |
| Time | −0.48 (0.13) | <.001 |
| Time2 | 0.07 (0.03) | .05 |
| Sex | 0.03 (0.09) | .75 |
| Race | 0.09 (0.02) | .26 |
| Prenatal stress | 0.75 (0.04) | .05 |
| ACEs | −0.20 (0.09) | .04 |
| Gestational age | −0.00 (0.02) | .84 |
| Maternal education | −0.02 (0.03) | .55 |
| Prenatal stress×time2 | −0.02 (0.01) | .05 |
| Prenatal stress×sex | −0.43 (0.18) | .02 |
| Time2×sex | 0.02 (0.01) | .02 |
Note: Data in boldface indicate significant effects.
Prenatal Stress
Prenatal stress was associated with lower RSA across the SFP. However, 2 significant 2-way interactions, prenatal stress×time2 and prenatal stress×sex, were identified (Table 2). Post hoc analyses indicated that whereas low prenatal stress infants demonstrated expected RSA return to baseline following the SF, high prenatal stress infants did not. High prenatal stress was associated with higher RSA among boys, whereas high prenatal stress was associated with lower overall RSA in girls. Although the 3-way interaction term (prenatal×time2×sex) was not significant, visual inspection indicated that differences were driven by high-stress boys.
Maternal ACEs
ACEs demonstrated an independent, significant main effect on infant RSA across the SFP, with high ACEs associated with lower RSA. No sex or race differences were observed.
DISCUSSION
This study describes how a mother’s exposures across her lifespan influence her offspring’s SRS, likely altering future risk for psychopathology and health. Although both maternal ACEs and prenatal stress predicted infant RSA at 4 months of age, their effects differed. Prenatal stress influenced RSA reactivity over time with infants whose mothers reported high prenatal stress, demonstrating persistent RSA suppression following removal of the stressor, consistent with previous literature.2,4 Clear sex effects were demonstrated in relation to prenatal stress, consistent with the frequently observed relation between prenatal anxiety and infant outcomes, including RSA.2 We extend this work and demonstrate a unique effect of maternal ACEs on infant RSA. Consistent with emerging data and theoretical models such as ACM and life course theory that suggest that alterations to the SRSs may contribute mechanistically to effects across generations, infants of mothers with high ACEs demonstrated lower overall RSA, indicating a lower physiologic start point.21,41
These data replicate previous findings of prolonged RSA suppression, suggestive of “spillover” PNS deactivation, among infants with high maternal prenatal stress.2 Infants’ failure to return to baseline RSA during the reunion episode is associated with lower infant regulatory behavior.2,17,53 Although PNS deactivation during the SF epoch (a stressor) permits infant vigilance to environmental cues, persistent PNS deactivation when the mother re-engages with the infant (PP2) may impede the infant’s ability to use the mother for biobehavioral regulation.54 The development of self-regulatory abilities during infancy, intricately tied to early caregiving and attachment, has direct implications for future psychopathology.55
Consistent with the prenatal programming literature19 and our prediction, sex differences were observed for RSA and prenatal stress.34 These results are also consistent with the larger body of literature reporting sex-specific effects in the link between prenatal stress and SRSs, including the ANS and HPA axis.34,56 For boys, high prenatal stress was associated with higher RSA during PP1 and over the course of the SFP. In girls, higher prenatal stress was associated with lower RSA. As higher RSA is associated with heightened responsiveness to environmental stimuli among infants,10 it may be that an evolutionary sex-differentiated advantage is conferred to boys to be more responsive in stressful environments. Surprisingly, sex differences were not observed in the relation between maternal ACEs and infant RSA, providing additional evidence that pathways by which prenatal and preconception events influence offspring development differ. As longitudinal studies of RSA are limited, the implications of these patterns for sex differences in developmental psychopathology remain unknown. Given theoretical justifications21 and downstream sex differences in outcomes linked to RSA such as depression and cardiovascular disease,15,37 continued exploration of sex-differentiated patterns is needed.
This is the first study to report on the concurrent impact of maternal ACEs and prenatal stress on infant RSA. Maternal ACEs exerted unique effects on infant RSA, suggesting that the intergenerational tuning of ANS activity begins prior to conception. Indeed, bivariate correlations between infant RSA and maternal ACEs were stronger than between infant RSA and prenatal stress. Consistent with life course theory and ACM,21,57 our results suggest that inter-generational stress influences the physiologic start point,41 dampening PNS activity and setting a lower potential responsiveness, perhaps in anticipation that reactivity to expected repeated or chronic stressors would be too biologically costly. This pattern, while isolating the infant from negative environmental influences, is putatively adaptive at a cost: initially protective from the negative environment, but later leading to elevated psychopathology risk. Prenatal exposures, in contrast, appear to affect the reactivity of the emerging PNS, maintaining periods of alertness even when the stressor is removed.
Surprisingly, we did not detect race effects despite evidence of race differences in RSA and racial disparities in life course stress-related health conditions, including mental health and cardiovascular disease.8,14,36,38 Differing from previous studies, our sample did not exhibit race differences in either ACEs or prenatal stress, although our rates of ACE exposure were significantly higher than previous work with more representative samples.50 Our findings indicate that if race differences in the impact of ACEs and prenatal stress on RSA exist, then they may appear later in development or be influenced by differences not captured by our measures. Notwithstanding our findings, continuing to search for stress-related mechanistic markers that underlie intergenerational health disparities is crucial.
The current study has limitations. First, ACEs and prenatal stress are based only on maternal report. As with other retrospective report measures, ACE report is subject to bias and may reflect a wider range of negative exposures. However, a substantial literature supports its utility; for instance, in a large prospective cohort, the impact of perceived rather than validated ACEs was most predictive of mental illness.58,59 A second limitation is the focus on a single SRS, which may oversimplify patterns, as SRSs are likely interactive.2,60,61 Third, the sample size is only moderate. Fourth, mothers were enrolled at any time point during pregnancy; prenatal stress measurement does not capture exposure across the entire pregnancy. However, controlling for trimester of enrollment did not influence findings. Fifth, we did not assess mothers’ lifetime psychopathology or RSA, which have both demonstrated heritability.26–28 Accounting for maternal psychopathology or RSA may help to disambiguate heritability from impact of stress exposure. Finally, although infant RSA was predictive of child behavior in other studies,2 the current study does not include behavioral measures.
Despite these limitations, there are several strengths. Most notable is the large number of African American participants comparable to white participants in levels of ACEs and prenatal stress. Other strengths include direct examination of sex and race differences and the use of RSA reactivity across a stressor, as individual differences are more often detected during reactivity.26
Early experiences are known to contribute to mental illness risk across the life span. The life course perspective extends this in 2 important ways. First, life course theory suggests that when exposures happen is relevant, with exposures occurring during time periods of rapid development resulting in greater impacts on the individual or physiologic system. Second, life course theory posits that how an individual reacts, both behaviorally and physiologically, to stressors in the current generation is associated with both the exposures and subsequent calibration of SRSs in previous generations.52 Our data are consistent with both aspects of this theory. As the ANS is one of the earliest developing SRSs, it is not surprising that the impact of maternal stress is detected in RSA. However, this impact may change over the course of development; longitudinal studies that integrate maternal physiologic patterns and are sufficiently powered to examine race and sex differences are needed. Early changes to the ANS likely also influence other biological systems, potentially shifting subsequent development of later-developing SRSs, leading to a cascade effect. These impacts may increase over time, compounded by both continued exposure to environmental stressors and potential impairments in sensitive caregiving as a consequence of maternal stress or stress-related psychopathology.2,60,61
The life course perspective also provides a theoretical backdrop for the unique impact of a mother’s own preconception exposure to adversity driving baseline stress responsiveness in the next generation, a potentially adaptive but costly tactic. RSA can also reflect an infant’s environmental receptiveness42; lower RSA in high-ACE infants may suggest decreased openness to the environment, potentially buffering these infants from expected continued adversity but limiting their ability to engage and to be regulated by their caregiver. Beyond the impact of maternal exposures on the infant’s emerging SRSs, dyadic interactions and early caregiving environments can either accentuate or mitigate these impacts.53,62 Dyadic coordination between mother–child physiology, affect, and behavior has meaningful links to infants’ physiological organization, behavior, and development.54 Thus, infant RSA, although affected by prenatal and preconception influences, is calibrated postnatally by potentially modifiable environmental influences such as sensitive parenting. Existing evidence-based interventions targeting dyadic functioning therefore represent under-used opportunities for mitigating the intergenerational effects of early life adversity on mental and physical well-being.63 Clinically, these data call for considering children’s emergent regulatory abilities in dyadic and intergenerational contexts and suggest that early interventions, including prior to and during pregnancy, may mitigate risk for divergent SRS patterns linked to developmental psychopathology across generations.
Acknowledgments
This research was supported by the National Institutes of Health (NIH) R01MH101533 (S.S.D.), K12HD043451 (S.A.O.G.; PI: Krousel-Wood), and L30HD085275 (S.A.O.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors would like to thank all of the families that participated in the Infant Development Study for sharing their time and enthusiasm with the researchers.
Footnotes
Disclosure: Dr. Gray has received research funding from the National Institutes of Health, the National Alliance for Research on Schizophrenia and Depression, and the Louisiana Board of Regents. Dr. Theall has received research funding from the National Institutes of Health, the Kellogg Foundation, and the Health Resources and Services Administration. Dr. Drury has received research funding from the National Institutes of Health, the Bill and Melinda Gates Foundation, Tulane University, the Patient-Centered Outcomes Research Institute, the National Science Foundation, and the Substance Abuse and Mental Health Services Administration. Mr. Jones and Ms. Glackin report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Dr. Sarah A.O. Gray, Tulane University, New Orleans, LA.
Mr. Christopher W. Jones, Program in Neuroscience and The Brain Institute, Tulane University.
Dr. Katherine P. Theall, Tulane University, New Orleans, LA.
Ms. Erin Glackin, Tulane University, New Orleans, LA.
Dr. Stacy S. Drury, The Brain Institute, Tulane University.
References
- 1.Poggi-Davis E, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2011;52:119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suurland J, van der Heijden KB, Smaling HJ, Huijbregts SC, van Goozen SH, Swaab H. Infant autonomic nervous system response and recovery: associations with maternal risk status and infant emotion regulation. Dev Psychopathol. 2016;29:1–15. doi: 10.1017/S0954579416000456. [DOI] [PubMed] [Google Scholar]
- 3.Field T, Diego M, Hernandez-Reif M, et al. Pregnancy anxiety and co-morbid depression and anger: effects on the fetus and neonate. Depress Anxiety. 2003;17:140–151. doi: 10.1002/da.10071. [DOI] [PubMed] [Google Scholar]
- 4.Peltola MJ, Makela T, Paavonen EJ, et al. Respiratory sinus arrhythmia moderates the impact of maternal prenatal anxiety on infant negative affectivity. Dev Psychobiol. 2017;59:209–216. doi: 10.1002/dev.21483. [DOI] [PubMed] [Google Scholar]
- 5.Cheng ER, Park H, Wisk LE, et al. Examining the link between women’s exposure to stressful life events prior to conception and infant and toddler health: the role of birth weight. J Epidemiol Community Health. 2016;70:245–252. doi: 10.1136/jech-2015-205848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wosu AC, Gelaye B, Williams MA. Maternal history of childhood sexual abuse and preterm birth: an epidemiologic review. BMC Pregnancy Childbirth. 2015;15:174–182. doi: 10.1186/s12884-015-0606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand SR, Brennan PA, Newport DJ, Smith AK, Weiss T, Stowe ZN. The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period. Psychoneuroendocrinology. 2010;35:686–693. doi: 10.1016/j.psyneuen.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. 2003;7:13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- 9.Buss C, Entringer S, Moog NK, et al. Intergenerational transmission of maternal childhood maltreatment exposure: implications for fetal brain development. J Am Acad Child Adolesc Psychiatry. 2017;56:373–382. doi: 10.1016/j.jaac.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beauchaine T. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- 11.Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol Rev. 1991;98:459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- 12.El-Sheikh M, Kouros CD, Erath S, Cummings EM, Keller P, Staton L. Marital conflict and children’s externalizing behavior: pathways involving interactions between parasympathetic and sympathetic nervous system activity. Monogr Soc Res Child Dev. 2009;74:vii–79. doi: 10.1111/j.1540-5834.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graziano P, Derefinko K. Cardiac vagal control and children’s adaptive functioning: a meta-analysis. Biol Psychol. 2013;94:22–37. doi: 10.1016/j.biopsycho.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Trolnick E, Heidelise A, Adamson L, Wise S, Brazelton B. The infant’s response to entrapment between contradictory messages in face-to-face interaction. J Am Acad Child Psychiatry. 1978;17:1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- 17.Bazhenova OV, Plonskaia O, Porges SW. Vagal reactivity and affective adjustment in infants during interaction challenges. Child Dev. 2001;72:1314–1326. doi: 10.1111/1467-8624.00350. [DOI] [PubMed] [Google Scholar]
- 18.Moore GA, Calkins SD. Infants’ vagal regulation in the still-face paradigm is related to dyadic coordination of mother-infant interaction. Dev Psychol. 2004;40:1068–1080. doi: 10.1037/0012-1649.40.6.1068. [DOI] [PubMed] [Google Scholar]
- 19.Phillips DI, Jones A. Fetal programming of autonomic and HPA function: do people who were small babies have enhanced stress responses? J Physiol. 2006;572:45–50. doi: 10.1113/jphysiol.2005.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips DI. Programming of the stress response: a fundamental mechanism underlying the long-term effects of the fetal environment? J Intern Med. 2007;261:453–460. doi: 10.1111/j.1365-2796.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- 21.Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci Biobehav Rev. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiPietro JA, Costigan KA, Gurewitsch ED. Fetal response to induced maternal stress. Early Hum Dev. 2003;74:125–138. doi: 10.1016/j.earlhumdev.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Jacob S, Byrne M, Keenan K. Neonatal physiological regulation is associated with perinatal factors: a study of neonates born to healthy African American women living in poverty. Infant Ment Health J. 2009;30:82–94. doi: 10.1002/imhj.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Dijk AE, Van Eijsden M, Stronks K, Gemke RJ, Vrijkotte TG. Prenatal stress and balance of the child’s cardiac autonomic nervous system at age 5–6 years. PLoS One. 2012;7:e30413. doi: 10.1371/journal.pone.0030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaze J, Roth TL. Evidence from clinical and animal model studies of the long-term and transgenerational impact of stress on DNA methylation. Semin Cell Dev Biol. 2015;43:76–84. doi: 10.1016/j.semcdb.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Geus E, Kupper N, Boomsma DI, Snieder H. Bivariate genetic modeling of cardiovascular stress reactivity: does stress uncover genetic variance? Psychosom Med. 2007;69:356–364. doi: 10.1097/PSY.0b013e318049cc2d. [DOI] [PubMed] [Google Scholar]
- 27.Snieder H, Boomsma DI, Van Doornen LJ, De Geus EJ. Heritability of respiratory sinus arrhythmia: dependency on task and respiration rate. Psychophysiology. 1997;34:317–328. doi: 10.1111/j.1469-8986.1997.tb02402.x. [DOI] [PubMed] [Google Scholar]
- 28.Boomsma DI, van Baal GC, Orlebeke JF. Genetic influences on respiratory sinus arrhythmia across different task conditions. Acta Genet Med Gemellol (Roma) 1990;39:181–191. doi: 10.1017/s0001566000005419. [DOI] [PubMed] [Google Scholar]
- 29.Gustafsson H, Doyle C, Gilchrist M, Werner E, Monk C. Maternal abuse history and reduced fetal heart rate variability: abuse-related sleep disturbance is a mediator. Dev Psychopathol. 2016;29:1023–1034. doi: 10.1017/S0954579416000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jovanovic T, Smith A, Kamkwalala A, et al. Physiological markers of anxiety are increased in children of abused mothers. J Child Psychol Psychiatry. 2011;52:844–852. doi: 10.1111/j.1469-7610.2011.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyle C, Werner E, Feng T, et al. Pregnancy distress gets under fetal skin: maternal ambulatory assessment and sex differences in prenatal development. Dev Psychobiol. 2015;57:607–625. doi: 10.1002/dev.21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institutes of Health. [Accessed June 28, 2017];Consideration of sex as a biological variable in NIH-funded research. Available at: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-102.html. Published June 2015.
- 33.Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res. 2007;32:1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- 34.Tibu F, Hill J, Sharp H, Marshall K, Glover V, Pickles A. Evidence for sex differences in fetal programming of physiological stress reactivity in infancy. Dev Psychopathol. 2014;26:879–888. doi: 10.1017/S0954579414000194. [DOI] [PubMed] [Google Scholar]
- 35.Maxwell SD, Fineberg AM, Drabick DA, Murphy SK, Ellman LM. Maternal prenatal stress and other developmental risk factors for adolescent depression: spotlight on sex differences [epub ahead of print] J Abnorm Child Psychol. 2017 doi: 10.1007/s10802-017-0299-0. http://dx.doi.org/10.1007/s10802-017-0299-0. [DOI] [PMC free article] [PubMed]
- 36.Gray SAO, Theall K, Lipschutz R, Drury S. Sex differences in the contribution of respiratory sinus arrhythmia and trauma to children’s psychopathology. J Psychopathol Behav Assess. 2017;39:67–78. doi: 10.1007/s10862-016-9568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nolen-Hoeksema S. Gender differences in depression. Curr Dir Psychol Sci. 2001;10:173–176. [Google Scholar]
- 38.Hinnant JB, Elmore-Staton L, El-Sheikh M. Developmental trajectories of respiratory sinus arrhythmia and preejection period in middle childhood. Dev Psychobiol. 2011;53:59–68. doi: 10.1002/dev.20487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Hare T, Shen C, Sherrer M. Racial differences in response to trauma: comparing African-American, white, and Hispanic people with severe mental illness. J Ethn Cult Divers Soc Work. 2017:1–14. [Google Scholar]
- 40.Alexander A, Ali J, McDevitt-Muprhy M, Forde D, Stockton M, Read M. Racial differences in posttraumatic stress disorder vulnerability following Hurricane Katrina among a sample of adult cigarette smokers from New Orleans. J Racial Ethn Health Disparities. 2017;4:94–103. doi: 10.1007/s40615-015-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 42.Sheinkopf SJ, Lagasse LL, Lester BM, et al. Vagal tone as a resilience factor in children with prenatal cocaine exposure. Dev Psychopathol. 2007;19:649–673. doi: 10.1017/S0954579407000338. [DOI] [PubMed] [Google Scholar]
- 43.Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol. 1999;18:333–345. doi: 10.1037//0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- 44.Dunkel-Schetter C, Schafer P, Lanzi RG, Clark-Kauffman E, Raju TN, Hillemeier MM. Shedding light on the mechanisms underlying health disparities through community participatory methods: the stress pathway. Perspect Psychol Sci. 2013;8:613–633. doi: 10.1177/1745691613506016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27:604–615. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- 46.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 47.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 48.Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EDS) J Reprod Infant Psychol. 1990;8:99–107. [Google Scholar]
- 49.Tabachnick BG, Fidell LS. Using Multivariate Statistics. Boston, MA: Pearson Education; 2007. [Google Scholar]
- 50.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 51.Byrne EA, Porges SW. Data-dependent filter characteristics of peak-valley respiratory sinus arrhythmia estimation: a cautionary note. Psychophysiology. 1993;30:397–404. doi: 10.1111/j.1469-8986.1993.tb02061.x. [DOI] [PubMed] [Google Scholar]
- 52.Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Dev Psychobiol. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 53.Conradt E, Ablow J. Infant physiological response to the still-face paradigm: contributions of maternal sensitivity and infants’ early regulatory behavior. Infant Behav Dev. 2010;33:251–265. doi: 10.1016/j.infbeh.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Feldman R, Magori-Cohen R, Galili G, Singer M, Louzoun Y. Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behav Dev. 2011;34:569–577. doi: 10.1016/j.infbeh.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Feldman R, Greenbaum CW, Yirmiya N. Mother–infant affect synchrony as an antecedent of the emergence of self-control. Dev Psychol. 1999;35:223–231. doi: 10.1037//0012-1649.35.1.223. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein JM, Handa RJ, Tobet SA. Disruption of fetal hormonal programming (prenatal stress) implicates shared risk for sex differences in depression and cardiovascular disease. Front Neuroendocrinol. 2014;35:140–158. doi: 10.1016/j.yfrne.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ellis B, Essex M. Family environments, adrenarche, and sexual maturation: a longitudinal test of a life history model. Child Dev. 2007;78:1799–1817. doi: 10.1111/j.1467-8624.2007.01092.x. [DOI] [PubMed] [Google Scholar]
- 58.Reuben A, Moffitt TE, Caspi A, et al. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. J Child Psychol Psychiatry. 2016;57:1103–1112. doi: 10.1111/jcpp.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naicker SN, Norris SA, Mabaso M, Richter LM. An analysis of retrospective and repeat prospective reports of adverse childhood experiences from the South African Birth to Twenty Plus cohort. PLoS One. 2017;12:e0181522. doi: 10.1371/journal.pone.0181522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Sheikh M, Arsiwalla DD, Hinnant JB, Erath SA. Children’s internalizing symptoms: the role of interactions between cortisol and respiratory sinus arrhythmia. Physiol Behav. 2011;103:225–232. doi: 10.1016/j.physbeh.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rash JA, Thomas JC, Campbell TS, et al. Developmental origins of infant stress reactivity profiles: a multi-system approach. Dev Psychobiol. 2016;58:578–599. doi: 10.1002/dev.21403. [DOI] [PubMed] [Google Scholar]
- 62.Enlow MB, King L, Schreier HM, et al. Maternal sensitivity and infant autonomic and endocrine stress responses. Early Hum Dev. 2014;90:377–385. doi: 10.1016/j.earlhumdev.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bick J, Dozier M. The effectiveness of an attachment-based intervention in promoting foster mothers’ sensitivity toward foster infants. Infant Ment Health J. 2013;34:95–103. doi: 10.1002/imhj.21373. [DOI] [PMC free article] [PubMed] [Google Scholar]