Abstract
A radical-mediated strategy for β C–H amination of alcohols has been developed. This approach employs a radical relay chaperone, which serves as a traceless director that facilitates selective C–H functionalization via 1,5-hydrogen atom transfer (HAT) and enables net incorporation of ammonia at the β carbon of alcohols. The chaperones presented herein enable direct access to imidate radicals, allowing their first use for H atom abstraction. A streamlined protocol enables rapid conversion of alcohols to their β-amino analogs (via in situ conversion of alcohols to imidates, directed C–H amination, and hydrolysis to NH2). Mechanistic experiments indicate HAT is rate-limiting, whereas intramolecular amination is product- and stereo-determining.
The β-amino alcohol is a privileged motif prevalent in pharmaceuticals, agrochemicals, natural products, and catalyst architectures. Yet, its typical synthesis inelegantly relies on iterative, functional group manipulation or unselective, alkene difunctionalization.1 Conversely, directed sp3 C–H amination2 of an alcohol offers a powerful synthetic alternative applicable to complex molecules that contain this ubiquitous functional handle. To date, only the groups of Du Bois, He, and Lebel have reported methods for β C–H amination of alcohol derivatives: via Rh or Ag nitrene-mediated, vicinal amination of carbamates.3 However, metal-based approaches typically afford γ selectivity, due to requisite metallacycle geometries.4 As part of our program to develop radical relay strategies for selective C–H functionalization,5 we hypothesized that an open-shell pathway might enable valuable, synthetic complementarity.
Remote C–H functionalizations via radical intermediates are known to exhibit wide functional group tolerance, as well as robust selectivity via 1,5-hydrogen atom transfer (HAT).6 When radical precursors, or chaperones, are appended to alcohols, π-cyclizations are typical,7 whereas HAT is much rarer,8 especially compared with radicals tethered to amines.9,10 Yet, given the synthetic utility of alcohols, it is notable that their HAT-mediated C–H amination remains unsolved, despite the propensity for selective C–H amination via N-centered radicals,6 and recent progress in C–H functionalization of alcohol derivatives via metal-mediated mechanisms.11
C–H functionalization promoted by radical relay enables robust, predictable selectivity via 1,5-HAT due to a six-membered, cyclic transition state.6 To exploit this intrinsic selectivity, we rationalized that a two-atom chaperone, bearing a terminal N, appended to an alcohol should enable β C–H amination via 1,5-HAT (Figure 1a). Yet, we were also cognizant that N-centered radicals, including those of hemiaminals, are prone to β-scission and undesired, competitive mechanisms.12 Thus, we hypothesized that masking the central C of the chaperone within an imidate should obviate any undesired degradation. Next, to address the challenge of C–H functionalization, we noted that although cyclizations of imine-based sp2 N-centered radicals are known,13 their use in HAT is rare.14 Furthermore, imidate radicals have never been reported to effect HAT.15 Thus, to explore the feasibility of imidate-based chaperones to mediate radical relays, we performed ground state calculations on the SOMO energies of imidate radicals with varying substitution (Figure 1b).16 We were pleased to find that variation of the traceless R′ group in our proposed imidate chaperone scaffold affords significant tunability of the SOMO energies of the sp2 N-centered radicals (e.g., R = Ph, −0.02 eV; Me, −0.69 eV; CCl3, −1.47 eV). Notably, these open-shell intermediates span a wider range of energies than related sp3 N-centered radicals that are known to exhibit variable HAT reactivity (see SI).17 Lastly, upon selective 1,5-HAT, we envisioned in situ iodination of the ensuing C-radical and N-displacement,6 or 5-endo-trig radical cyclization and subsequent oxidation,18 should afford C–H aminated oxazoline products.
Figure 1.
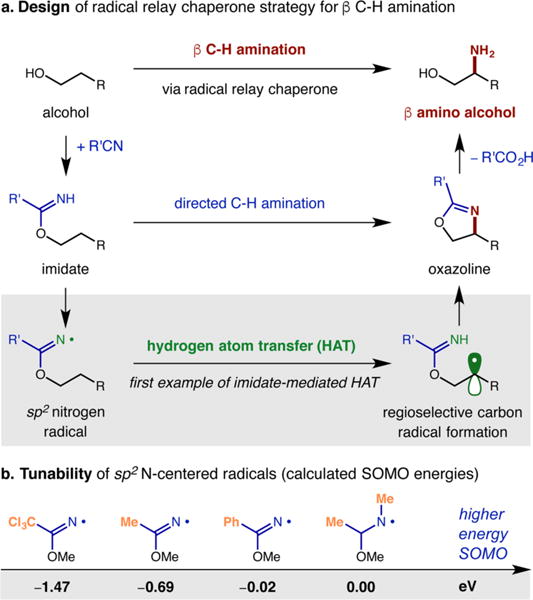
Design of a radical relay chaperone strategy for selective β C–H amination of alcohols.
To test our hypothesis, we first synthesized three imidates of 2-phenylethanol by addition into a nitrile, or via transimidation. Each imidate was then subjected to our recently developed, triiodide-based C–H amination conditions (Scheme 1).5 Gratifyingly, these mild conditions (NaI, PhI(OAc)2) convert all three imidates (1–3) to the desired oxazoline heterocycles (4–6), albeit with divergent efficiencies (e.g., trace to quantitative), reflecting a difference in stability of the imidate intermediates, as well as their respective N-centered radicals. Fortunately, trichloroacetimidate 3 affords C–H amination product 6, quantitatively. Given its synthetic convenience and utility (e.g., Overman rearrangement),19 as well as relative stability of this precursor and subsequent radical, we chose to further explore the Cl3C–CN chaperone in our β C–H amination protocol. Additionally, we expected the resultant oxazoline (bearing a −CCl3) to be primed for acidic hydrolysis, allowing direct isolation of free β-amino alcohols via an exceptionally simple process.20 In practice, we found in situ subjection of crude oxazoline 6 to HCl (aq) in MeOH facilitates hydrolysis and chromatography-free isolation (via acid/base extraction) of pure β-amino alcohol 7 in 87% yield from 3 (or 80% yield, directly from the alcohol).
Scheme 1. Discovery of β C–H Amination Chaperones.
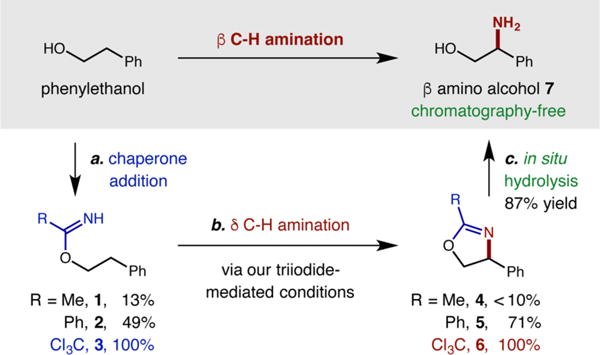
aCl3CCN, DBU or R(C=N)OCH2CF
b3NaI, PhI(OAc)2, visible light
cHCl (aq), MeOH. Isolated yields.
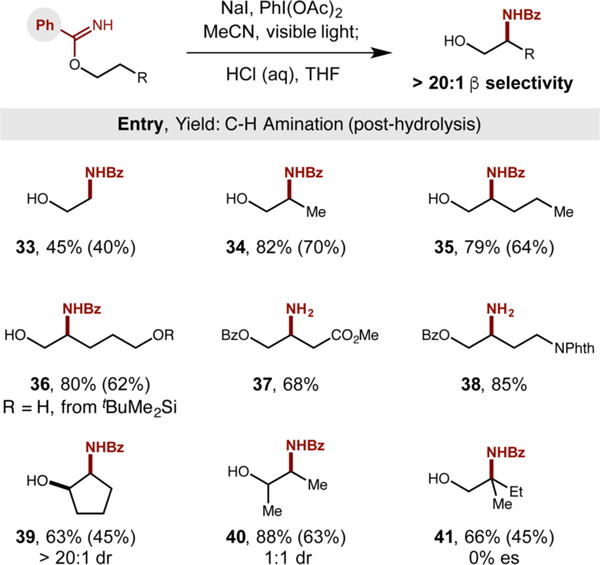
With our new protocol in hand for the net installation of −NH2 to the β position of alcohols, we then subjected this method to various alcohols bearing a range of sterically and electronically diverse functionalities (Table 1). As in the case of phenylethanol, this imidate radical-mediated C–H amination method proved remarkably efficient, affording nearly quantitative oxazoline formation in all cases, typically with >80% isolated yields after acidic hydrolysis and purification via extraction. As shown in Table 1, ortho-, meta-, and para-substitution of either electronically donating or withdrawing groups is tolerated (8–15, > 74% yield). Notably, heteroarenes such as pyridine 16 and thiophene 17 are also tolerant of the mild, triiodide-based C–H amination reaction conditions. To prevent HCl-mediated decomposition of the sensitive thio-phene product, heterobenzyl amide 17 was isolated via a milder TsOH-based hydrolysis to provide a trichloroacetamide (Ac* = COCCl3) instead of the free amine. In further probing the generality of this transformation, we observed that alcohols with tertiary β C–H bonds are aminated efficiently (18, 19). Similarly, secondary alcohols are readily β-aminated, affording remarkably high diastereoselectivity (20–22, >20:1 dr). We were pleased to find that cholesterol and reduced ibuprofen are converted to their β-amino alcohol analogs (19, 22), illustrating the benefit of this approach to drug discovery by enabling streamlined, postsynthetic modification of medicinal candidates.
Table 1.
Scope and Generality of β C–H Amination
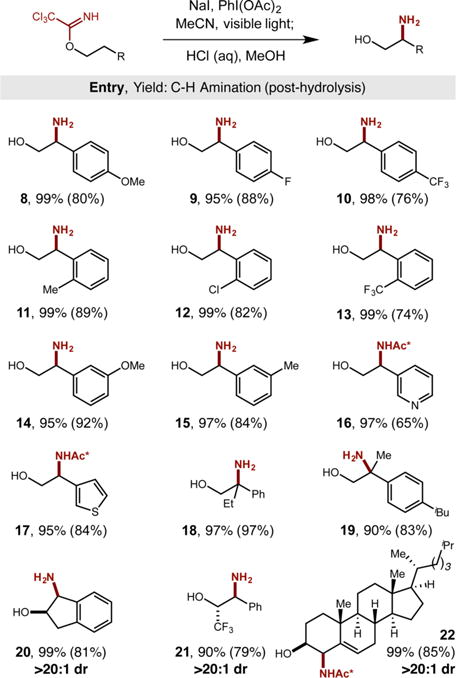
|
Having demonstrated the generality of this imidate-radical-mediated β-amination for substitution of benzylic and allylic C–H bonds, we next turned our attention to address the challenge of alcohols containing stronger C–H bonds (BDE; aliphatic, 97 vs benzylic, 90 kcal/mol).21 Three key mechanistic observations enabled us to identify a solution in this regard. First, we observed that only the trans isomer of 2-phenyl cyclohexanol is aminated via this protocol (80% yield; see SI), providing a mixture of diastereomers (2:1). The failure of the cis imidate to undergo amination suggests HAT is highly geometry-dependent, and a critical step in this transformation. Second, to probe the precise role of the HAT step, we investigated the presence of a kinetic isotope effect (KIE) in the β C–H amination (eq 1).22 A large primary KIE (8.1) was
 |
Eq 1 |
observed via intramolecular H/D competition in the imidate derived from 23, suggesting HAT is likely product- and rate-determining. Similarly, intermolecular competition between 3 and its β-d2 analog (of 24) yielded KIE values >2 via single flask (4.4) and parallel (2.1) experiments.
Third, upon exploring the feasibility of β-amination of an alcohol containing a stronger tertiary C–H bond (Figure 2, 27), we observed successful HAT from the radical of imidate 28 to afford β-iodide 30 (53% yield). This interception of intermediate 29 supports our proposed mechanistic hypothesis of I· trap, and explains the importance of subsequent nucleophilic I− displacement by the imidate for overall β-amination (since substitution is more facile for benzyl iodides than nonbenzyl iodides).
Figure 2.
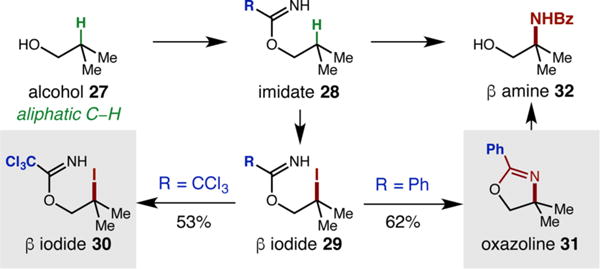
New radical chaperone enables extension to aliphatic alcohols.
This observation, coupled with an understanding of the viability, and importance, of HAT in overall amination, encouraged us to investigate benzimidate 28 (R = Ph), which simultaneously has a more reactive N-radical (Figure 1b) and more nucleophilic N. In this case, oxazoline 31 is obtained exclusively (62% yield), without any iodide 29 remaining after 24 h. Finally, in contrast to the trichloroacetimidate-derived products, benzimidates afford protected β-amido alcohols. However, free amino alcohol 32 can also be obtained upon hydrolysis with NaOH, or via acyl migration (e.g., 37, 38).
With a second-generation, benzimidate chaperone enabling β C–H amination of aliphatic alcohols, we sought to explore the scope and generality of this alternate approach (Table 2). Notably, even an alcohol with strong, primary β C–H bonds (BDE: 101 kcal/mol),21 such as ethanol, affords β-amido alcohol 33. Longer alcohols with unbiased, secondary β C–H bonds (34, 35) provide even greater efficiency–consistent with our observation that triiodide-based C–H amination enables methylene functionalization.5 Orthogonal functionality is tolerated under these mild, radical-mediated conditions, including ethers, esters, and amines (36–38). Secondary alcohols can also undergo β-amination (39, 40), complementary to metal-mediated approaches.3 Last, subjecting enantio-pure (S)-2-methyl-butanol to the C–H amination provides amide 41 with complete stereoablation (0% es), reinforcing the presence of planar radical or cationic intermediates.
Table 2.
β C–H Amination of Aliphatic Alcohols
|
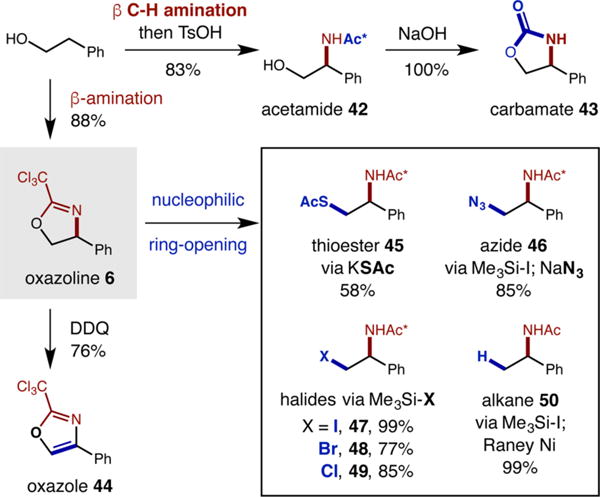
A synthetic benefit of our radical chaperone strategy is the intermediacy of an oxazoline heterocycle that can be easily derivatized to many, useful synthetic functionalities.23 To demonstrate this wide utility, the β C–H amination products were converted to several valuable motifs (Scheme 2). First, in situ hydrolysis of the oxazoline with TsOH provides acetamide 42 as a complementary product to the unprotected β amino alcohols shown in Table 1 (via HCl hydrolysis). Next, subjecting 42 to NaOH afforded carbamate 43. Importantly, oxazoline 6 could be isolated in 88% yield, enabling several further derivatizations. For example, DDQ oxidation provides a unique method of synthesizing oxazole 44 from an alcohol and nitrile. Additionally, nucleophilic ring-opening of oxazoline 6 provides access to a family of β-amines, including thioester 45 (via KSAc), azide 46 (via Me3Si–I, then NaN3), and halides 47–49 (via Me3Si-X). Lastly, deoxygenation via iodination and Raney Ni reduction affords alkane 50, wherein the alcohol is a traceless activator in a formal β C–H amination.
Scheme 2.
Synthesis of a Family of β-Amines
In summary, this radical relay chaperone strategy for rapidly converting ubiquitous alcohols into β-amino analogs, in a single afternoon, provides a powerful synthetic alternative to access these privileged motifs. We expect this broadly applicable β C–H amination scheme, and its wide product versatility, will enable streamlined syntheses of complex, amine-containing molecules from their abundant alcohol precursors. Finally, building on this first example of imidate-mediated HAT, we anticipate the use of radical relay chaperones will enable further discovery of regioselective C–H functionalizations.
Supplementary Material
Acknowledgments
We thank The Ohio State University, National Institutes of Health (NIH R35 GM119812), and American Chemical Society Petroleum Research Fund for financial support.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.7b05214.
Experimental procedures, spectroscopic data for all new compounds, including 1H and 13C NMR spectra (PDF)
ORCID
David A. Nagib: 0000-0002-2275-6381
Notes
The authors declare no competing financial interest.
References
- 1.Synthetic strategies for β-amino alcohols and their applications:; (a) Ager DJ, Prakash I, Schaad DR. Chem Rev. 1996;96:835. doi: 10.1021/cr9500038. [DOI] [PubMed] [Google Scholar]; (b) Bergmeier SC. Tetrahedron. 2000;56:2561. [Google Scholar]; (c) Karjalainen OK, Koskinen AMP. Org Biomol Chem. 2012;10:4311. doi: 10.1039/c2ob25357g. [DOI] [PubMed] [Google Scholar]
- 2.C–H amination reviews:; (a) Davies HML, Manning JR. Nature. 2008;451:417. doi: 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Roizen JL, Harvey ME, Du Bois J. Acc Chem Res. 2012;45:911. doi: 10.1021/ar200318q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jeffrey JL, Sarpong R. Chem Sci. 2013;4:4092. [Google Scholar]
- 3.β-amination of carbamates:; (a) Espino CG, Du Bois J. Angew Chem, Int Ed. 2001;40:598. doi: 10.1002/1521-3773(20010202)40:3<598::AID-ANIE598>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]; (b) Cui Y, He C. Angew Chem, Int Ed. 2004;43:4210. doi: 10.1002/anie.200454243. [DOI] [PubMed] [Google Scholar]; (c) Lebel H, Huard K, Lectard S. J Am Chem Soc. 2005;127:14198. doi: 10.1021/ja0552850. [DOI] [PubMed] [Google Scholar]; Pd-catalyzed amination of carbamate, allylic C–H bonds:; (d) Fraunhoffer KJ, White MC. J Am Chem Soc. 2007;129:7274. doi: 10.1021/ja071905g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For metal-mediated γ-amination of alcohol derivatives, see SI.
- 5.Wappes EA, Fosu SC, Chopko TC, Nagib DA. Angew Chem, Int Ed. 2016;55:9974. doi: 10.1002/anie.201604704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Intramolecular HAT:; (a) Wolff ME. Chem Rev. 1963;63:55. [Google Scholar]; (b) Barton DHR, Hesse RH, Pechet MM, Smith LC. J Chem Soc, Perkin Trans. 1979;1:1159. [Google Scholar]; (c) Breslow R. Acc Chem Res. 1980;13:170. [Google Scholar]; (d) Francisco CG, Herrera AJ, Suárez E. J Org Chem. 2003;68:1012. doi: 10.1021/jo026314h. [DOI] [PubMed] [Google Scholar]; (e) Čeković Ž. Tetrahedron. 2003;59:8073. [Google Scholar]; (f) Chiba S, Chen H. Org Biomol Chem. 2014;12:4051. doi: 10.1039/c4ob00469h. [DOI] [PubMed] [Google Scholar]
- 7.Seminal examples of alcohol-tethered, radical precursors (e.g., chaperones) without HAT:; (a) Ueno Y, Chino K, Watanabe M, Moriya O, Okawara M. J Am Chem Soc. 1982;104:5564. [Google Scholar]; (b) Stork G, Mook R, Biller SA, Rychnovsky SD. J Am Chem Soc. 1983;105:3741. [Google Scholar]; (c) Nishiyama H, Kitajima T, Matsumoto M, Itoh K. J Org Chem. 1984;49:2298. [Google Scholar]
- 8.Remote C–H functionalization via intramolecular HAT of alcohol-tethered radicals:; (a) Breslow R, Winnik MA. J Am Chem Soc. 1969;91:3083. [Google Scholar]; (b) Breslow R, Baldwin SW. J Am Chem Soc. 1970;92:732. [Google Scholar]; (c) Curran DP, Kim D, Liu HT, Shen W. J Am Chem Soc. 1988;110:5900. [Google Scholar]; (d) Chen K, Richter JM, Baran PS. J Am Chem Soc. 2008;130:7247. doi: 10.1021/ja802491q. [DOI] [PubMed] [Google Scholar]; (e) Voica AF, Mendoza A, Gutekunst WR, Fraga JO, Baran PS. Nat Chem. 2012;4:629. doi: 10.1038/nchem.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Parasram M, Chuentragool P, Sarkar D, Gevorgyan V. J Am Chem Soc. 2016;138:6340. doi: 10.1021/jacs.6b01628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.In contrast, intramolecular HAT of amine-tethered radicals afford remote C–C formation:; (a) Snieckus V, Cuevas JC, Sloan CP, Liu H, Curran DP. J Am Chem Soc. 1990;112:896. [Google Scholar]; (b) Yoshikai N, Mieczkowski A, Matsumoto A, Ilies L, Nakamura E. J Am Chem Soc. 2010;132:5568. doi: 10.1021/ja100651t. [DOI] [PubMed] [Google Scholar]
- 10.Amidine HAT affords C–O or C–N:; (a) Wang YF, Chen H, Zhu X, Chiba S. J Am Chem Soc. 2012;134:11980. doi: 10.1021/ja305833a. [DOI] [PubMed] [Google Scholar]; (b) Chen H, Sanjaya S, Wang YF, Chiba S. Org Lett. 2013;15:212. doi: 10.1021/ol303302r. [DOI] [PubMed] [Google Scholar]
- 11.Metal-mediated C–H functionalization of alcohol derivatives:; (a) Huang C, Chattopadhyay B, Gevorgyan V. J Am Chem Soc. 2011;133:12406. doi: 10.1021/ja204924j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Simmons EM, Hartwig JF. Nature. 2012;483:70. doi: 10.1038/nature10785. [DOI] [PubMed] [Google Scholar]; (c) Ren Z, Mo F, Dong G. J Am Chem Soc. 2012;134:16991. doi: 10.1021/ja3082186. For other (non-alcohol) directed C–H aminations, see SI. [DOI] [PubMed] [Google Scholar]
- 12.(a) Moriyama K, Kuramochi M, Fujii K, Morita T, Togo H. Angew Chem, Int Ed. 2016;55:14546. doi: 10.1002/anie.201607223. [DOI] [PubMed] [Google Scholar]; (b) Kim S, Joe GH, Do JY. J Am Chem Soc. 1993;115:3328. [Google Scholar]
- 13.Zard SZ. Chem Soc Rev. 2008;37:1603. doi: 10.1039/b613443m. [DOI] [PubMed] [Google Scholar]
- 14.(a) Forrester AR, Gill M, Thomson RH. J Chem Soc, Perkin Trans. 1979;1:621. doi: 10.1039/j39700001081. [DOI] [PubMed] [Google Scholar]; (b) Shu W, Nevado C. Angew Chem, Int Ed. 2017;56:1881. doi: 10.1002/anie.201609885. [DOI] [PubMed] [Google Scholar]; (c) See also ref 10.
- 15.(a) Glover SA, Beckwith ALJ. Aust J Chem. 1987;40:701. [Google Scholar]; (b) Glover SA, Hammond GP, Harman DG, Mills JG, Rowbottom CA. Aust J Chem. 1993;46:1213. [Google Scholar]
- 16.DFT calculations indicate N-H BDE’s are nearly identical for all imidates in Figure 1b (100 ± 1 kcal/mol), suggesting that HAT is more likely related to the SOMO energies. Ground state calculations on the SOMO energies of imidate radicals, as well as N–H BDE calculations, performed using the ωB97X-D/6-31G(D) hybrid functional.
- 17.Šakić D, Zipse H. Adv Synth Catal. 2016;358:3983. [Google Scholar]
- 18.Ishibashi H, Sato T, Ikeda M. Synthesis. 2002;2002:695. [Google Scholar]
- 19.Overman LE. J Am Chem Soc. 1974;96:597. [Google Scholar]; Interestingly, conversion of imidates to oxazolines was the expected product that serendipitously led to discovery of the Overman rearrangement:; Overman LE. Tetrahedron. 2009;65:6432. doi: 10.1016/j.tet.2009.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.For comparison, metal-mediated β C-H aminations (ref 3) require carbamate N-deprotection via two subsequent steps.
- 21.Blanksby SJ, Ellison GB. Acc Chem Res. 2003;36:255. doi: 10.1021/ar020230d. [DOI] [PubMed] [Google Scholar]
- 22.Simmons EM, Hartwig JF. Angew Chem, Int Ed. 2012;51:3066. doi: 10.1002/anie.201107334. [DOI] [PubMed] [Google Scholar]
- 23.(a) Gant TG, Meyers AI. Tetrahedron. 1994;50:2297. [Google Scholar]; (b) Wong GSK, Wu W. Oxazolines. In: Palmer DC, editor. Oxazoles: Synthesis, Reactions, and Spectroscopy. Wiley & Sons; Hoboken: 2004. pp. 331–528. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


