Abstract
An increase in production of commercial products containing graphene-family nanomaterials (GFNs) has led to concern over their release into the environment. The fate and potential ecotoxicological effects of GFNs in the environment are currently unclear, partially due to the limited analytical methods for GFN measurements. In this review, the unique properties of GFNs that are useful for their detection and quantification are discussed. The capacity of several classes of techniques to identify and/or quantify GFNs in different environmental matrices (water, soil, sediment, and organisms), after environmental transformations, and after release from a polymer matrix of a product is evaluated. Extraction and strategies to combine methods for more accurate discrimination of GFNs from environmental interferences as well as from other carbonaceous nanomaterials are recommended. Overall, a comprehensive review of the techniques available to detect and quantify GFNs are systematically presented to inform the state of the science, guide researchers in their selection of the best technique for the system under investigation, and enable further development of GFN metrology in environmental matrices. Two case studies are described to provide practical examples of choosing which techniques to utilize for detection or quantification of GFNs in specific scenarios. Since the available quantitative techniques are somewhat limited, more research is required to distinguish GFNs from other carbonaceous materials and improve the accuracy and detection limits of GFNs at more environmentally relevant concentrations.
TOC Artwork

INTRODUCTION
Graphene family nanomaterials (GFNs) are a class of carbonaceous nanomaterials, similar in chemical structure to graphite, but with a thickness on the order of nanometers and lateral dimensions typically in the micron range. GFNs contain an sp2-hybridized network of fused benzene rings existing as a single sheet or a few layers of sheets. There are many categories of GFNs; definitions provided by Bianco et al. will be used throughout this paper.1 Graphene, the most widely known type of GFN, is a fully graphenic, single-layer sheet of sp2 hybridized carbon. Graphene is typically prepared by chemical vapor deposition, micromechanical cleavage of graphite, or reduction of graphene oxide.2 Graphene oxide (GO) is similarly composed of a single sheet of graphenic carbon that contains areas of disrupted aromaticity where carbon atoms are oxidized. Oxygen functional groups can include epoxide, hydroxyl, carbonyl, and carboxyl groups, which can reside along the basal plane or the edges of the graphenic structure.3, 4 Generally, GO has high carbon/oxygen (C/O) ratios around 2.0 and sometimes as high as 3.0. GO is typically prepared by the oxidation of graphite in strong acids and other oxidants followed by sonication.5 Reduced graphene oxide (rGO) is GO in a form that contains fewer oxygen functional groups and a greater proportion of graphenic carbon; rGO can be prepared by exposure of GO to thermal,6, 7 ultraviolet (UV),8, 9 biodegradation,10 and chemical processes.4 Few-layer graphene (FLG) are composed of several graphene layers, typically 2 to 5. Graphene quantum dots (GQDs) are similar to graphene, but have lateral dimensions on the nanometer-scale, rather than the micron-scale. They are often produced for biomedical imaging, photonic devices, electronic devices, and catalysis applications and are tuned for their fluorescence properties.11, 12 Unlike fullerenes but similar to carbon nanotubes, GFNs typically exist as a distribution of particles with varying defects, sizes, thicknesses, and oxidation levels.4
Graphene family nanomaterials (GFNs) have novel properties that include high electrical and thermal conductivity, and tensile strength as high as 130,000 MPa compared to 300 MPa to 440 MPa for low carbon steel.13, 14 As a result, GFNs have the potential for use in a broad range of fields and commercial applications.15, 16 Globally, over 26,000 graphene-related patents have been filed since graphene was first isolated in 2003.17 Overall, the total annual sales of graphene was $12 million in 2013 and was projected to reach $20 million in sales by 2016.17, 18 In 2027, the production volume of graphene is expected to reach 3800 metric tons with total annual sales of $300 million.19 GFNs are being developed for use in functional coatings, anti-corrosion applications, antifouling and antibacterial applications, membranes, conductive inks, supercapacitors, optoelectronics, and touch screens.20–22 Bendable phones containing graphene are also in development.23 On the market, a range of products are readily available from pre-mixed graphene/epoxy resins and graphene-modified polymer masterbatches (pre-mixed granular pellets) to graphene scratch-resistant and heat-cooling coatings, graphene conductivity agents, inks, bike helmets, tennis and badminton rackets, and batteries.24–29
With the increased production and use of GFNs in consumer products and their potential for release into the environment and exposure to humans, it is critical to understand their environmental fate and potential health and ecological risks.30, 31 In terms of GFN fate, the ranges of critical coagulation concentrations (CCC) reported for GO in aqueous media are 38 mmol/L to 200 mmol/L of NaCl and 0.9 mmol/L to 2.6 mmol/L of CaCl2, depending on lateral size, number of layers, initial GO concentration, and solution pH.32–36 Graphene and rGO have lesser or no functionalization (compared to GO), and are thus less stable than GO in aqueous media. In a recent study, the CCC in NaCl decreased from 200 mmol/L for pristine GO to 35 mmol/L and 30 mmol/L upon Solvothermal reduction of pristine GO for 1 h and 2 h, respectively.36 The CCC of NaCl for graphene is about 1.6 mmol/L to 10 mmol/L, depending on initial concentration and lateral size.37 Based on these colloidal stability behaviors, GFNs may be unstable in some surface waters and groundwater,32, 36–38 and may result in exposure of organisms in the pelagic zone initially, and then organisms in the benthic zones as the nanomaterials agglomerate and settle out. Organisms in terrestrial environments will also be exposed, for instance, if biosolids containing GFNs are applied to farmlands.38 Most of the studies on the environmental persistence and fate of GFNs have been conducted in simple environmental media (e.g., water with natural organic matter (NOM) but not soil or sediment media).39–44 While the concentration of GFNs in natural waters has not yet been modeled or measured, useful estimates for the expected range can be based on the average concentrations for CNTs and fullerenes, which have been modeled to be in the low ng/L range or less, concentrations orders of magnitude lower than those for current GFN detection/quantification methods.45 In addition, studies on the ecotoxicity and fate of GFNs have almost exclusively focused on the GFNs as produced by the manufacturer and not on the particles released from consumer products containing GFNs such as polymer nanocomposites, due in part, to a lack of methods for quantifying GFNs in the presence of other carbonaceous materials. Therefore, methods for quantification of GFNs at low concentrations and in complex environmental media and consumer product-relevant matrices are urgently needed.
Organisms are likely to come into contact with GFNs that have been released into the environment, and it is important to understand the implications of these exposures. Numerous studies on the potential environmental impacts of GFNs have focused on trophic transfer of GFNs,46, 47 bioaccumulation of GFNs, or toxicological effects to bacteria,48–53 pelagic (e.g., fish, zooplankton, etc.),37, 41, 42, 54–58 soil (e.g., earthworms,57 plants59, 60), and benthic organisms (e.g., organisms that burrow in sediments).57 Similar to carbon nanotubes (CNTs), GFNs show varying degrees of toxicity that depend on oxidation level, dispersion quality, size, surface area, orientation or alignment, and organism type,52, 53, 61–65 and have shown the capacity to impact the toxicity of organic and inorganic co-contaminants.66, 67 Concentrations as low as 0.01 mg/L GO have caused elevated β-galactosidase biosynthesis in zebrafish embryos.68 Conversely, Artemia larvae showed no effects with GO levels as high as 100 mg/L.69, 70 Bacterial effects generally occurred at GFN concentrations ranging from 5 to 100 mg/L.52, 71, 72 A bacterial community from a wastewater treatment plant (WWTP) showed effects at GO concentrations less than 10 mg/L.73 Graphene and GO inhibited algal growth at ≥ 0.675 mg/L and ≥ 1.25 mg/L, respectively.74 In-vitro cell exposure shows effects of GO and rGO between 2 mg/L and 25 mg/L for blue mussel hemocytes,75 zebrafish gill cells,76 and mouse fibroblasts.77 This four orders of magnitude difference is not surprising based on the variety of organisms, endpoints, types of material tested, and different exposure durations and conditions. Although the concentration of GFNs in the environment are expected to be lower than the toxicity thresholds reported in most current studies, different endpoints may be required to determine molecular level effects (such as DNA damage, metabolism interference), effects on sensitive populations, and long-term effects of low (ug/L to ng/L) concentrations.78 Furthermore, most of these studies did not provide measurements of the GFN body burden, a measurement which may be more predictive of the toxic effects observed as compared to the exposure concentration, and typically the exposure concentration was not measured after the exposure period. More robust measurements of the GFN in the exposure media and in the organisms tested can reduce uncertainties in assessing the potential ecotoxicological risks of GFNs.
While insights can be drawn from quantitative procedures used for other carbon nanomaterials (CNMs), the unique properties of GFNs indicate that new or modified procedures may be needed for detection and quantification of these materials. For example, many chromatographic techniques (e.g., liquid chromatography-mass spectrometry) have been utilized to accurately quantify fullerene particles, but this approach will likely not work for GFNs since these materials possess higher polydispersity than individually-dispersed fullerene particles that have controlled stoichiometry.79–81 In addition, GFNs do not have the same near-infrared fluorescence patterns that have been used for quantification of individually dispersed single-wall CNTs (SWCNTs);82–84 the reason that GFNs cannot be quantified using near-infrared fluorescence spectroscopy is that they are not composed of varying conformations or chiralities as are SWCNTs. Carbon nanotubes also sometimes contain residual metal catalysts from the manufacturing process,85 which can be used as a proxy to measure CNT concentration with single particle-inductively coupled mass spectrometry (spICP-MS)86, 87 and total inorganic elemental analysis using, for example, ICP-atomic emission spectroscopy (ICP-AES).88 Unlike some methods of CNT synthesis, graphene is not typically manufactured with metal nanoparticles that can be used for detection. However, other methods used for the detection and quantification of carbon nanotubes and fullerenes may be similarly applied to GFNs. While reviews have been conducted on quantitative methods for the analysis of CNTs and fullerenes,80, 89–91 the applicability of many of these methods for GFNs is still unclear.
This paper provides a comprehensive review of analytical methods for detection and quantification of GFNs in various environmental media, such as water, soil, sediments, and organisms. Measurements of GFNs in these media are critical for studies assessing the environmental fate and potential ecotoxicological effects of GFNs. Given that GFNs will likely be released into the environment after use in consumer products, quantification techniques for the assessment of GFN release from polymer nanocomposites will also be evaluated. The unique properties of GFNs that can be useful for quantification and identification in environmental media and consumer-relevant matrices will be discussed. Potential biases and detection limits, when available, will be provided for relevant techniques in each type of environmental medium, as well as the current ability to differentiate GFNs from other carbonaceous nanomaterials. Key topics for future work will also be described which include the importance of GFN extraction, a process necessary in many cases to separate GFNs from interfering compounds and concentrate GFNs to reach detection limit requirements. Extraction will be considered in the context of current studies and future research needs. Furthermore, case studies will be provided to apply the techniques described to two different environmentally important scenarios.
UNIQUE PROPERTIES OF GFNS THAT ALLOW FOR DETECTION/QUANTIFICATION
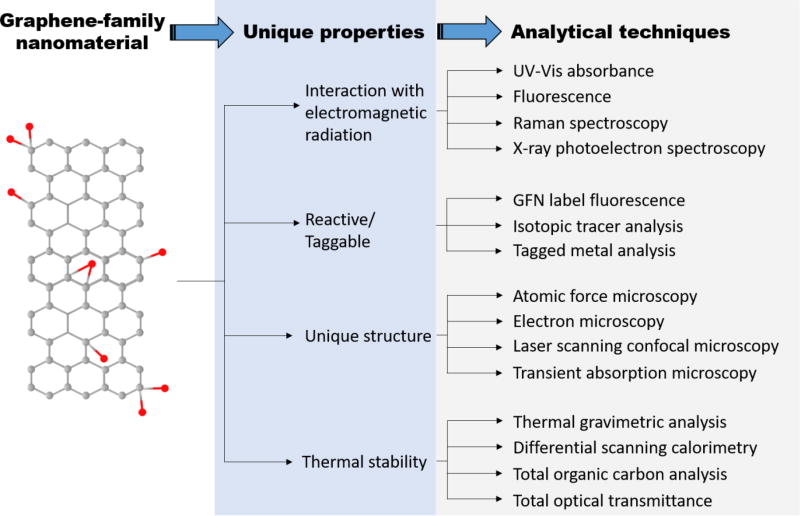
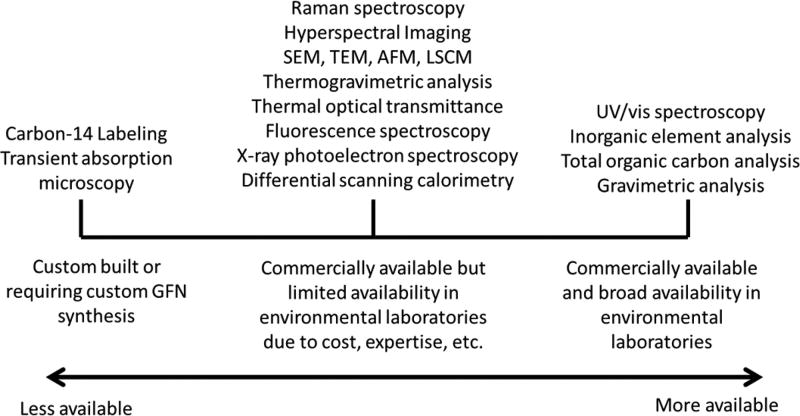
The detection and quantification of GFNs in simple and complex systems requires measurements that are specific to the unique properties of GFNs. These properties can include the interaction of GFNs with light, the graphitic and electronic structure, and the two-dimensional shape and size of GFNs.3, 4, 16, 92 Figure 1 provides an overview of selected techniques grouped by spectroscopic, spectrometric, microscopic, thermal, and labeling categories for GFN measurements and Table 1 provides technique descriptions with strengths and limitations. Table S1 summarizes the detection limits of GFNs for the few techniques for which this information has been provided in the literature.
Figure 1.
A selection of unique graphene-family nanomaterial (GFN) properties and the analytical techniques that can be used to measure these properties.
Table 1.
Selected techniques for GFN characterization and quantification
| Method | Overview | Strengths | Limitations |
|---|---|---|---|
| Spectroscopic | |||
| Absorbance 8, 32, 40, 44, 92, 96, 113, 114, 116, 230 | Measures absorbance of aqueous sample; can include ultraviolet, visible, or near infrared wavelengths; absorbance can be related to mass concentration using the Beer-Lambert law; with analytical ultracentrifugation (AUC), different fragment sizes of material can be measured with absorbance | Except for AUC, absorbance spectrophotometers are readily available in many environmental laboratories | Interference from other sample components; relatively high detection limit; only applicable for aqueous samples; controlled GFN dispersion quality required |
| Raman 113, 136, 137, 145, 159, 160, 231–233 | Measures G, D and G’ vibrational bands in dry powder, polymer nanocomposites, and tissues | Minimal sample preparation; enables GFN characterization; compatible with in vitro and in vivo samples; can be used with a microscope; low detection limits achieved using resonance Raman conditions | Some matrices may produce interferences; sensitive to laser power; requires calibration or a reference peak for quantitative analysis; background fluorescence can interfere; samples dispersed in an organic solvent are less common but this is possible |
| Fluorescence 103 | Measures fluorescence emission intensity after excitation of GFN at a known adsorption band | Available in many environmental laboratories; fluorimetry is highly sensitive; rapid technique | Interference of other fluorescent materials (e.g. polymer or environmental matrix); non-specificity of GFN signal; only applicable for aqueous samples; controlled GFN dispersion quality required; may work better for graphene oxide (GO) versus graphene because GO is more highly fluorescent |
| X-ray Photoelectron Spectroscopy (XPS)234–236 | Measures the atomic surface concentration of carbon (top 10 nm) and can provide some information on oxidation state; relative concentration of GFN can be determined in a dry matrix if matrix has a very different conductivity relative to the GFN | Provides atomic information and oxidation state of GFN | Requires dry down and a high vacuum environment; doesn’t distinguish nanomaterial carbon from background carbon unless charging occurs and background material identity is known |
| Spectrometric | |||
| Inorganic Elemental Analysis of Metal Coordination to GFN Functional Groups 141, 155 | Measures divalent metal cations coordinated to GO functional groups | Multi-elemental capability and extreme sensitivity of ICP-MS allow for an accurate and selective determination of metal content coordinated to GFN in a wide range of matrices at ngL−1 or sub ngL−1 levels, the rapid sample throughput of this method is attractive for routine screening; potential for covalent attachment of metals rather than coordination to minimize desorption of the metal tags during measurements | Carbon is generally not detectable with standard ICP-MS methods; metal release from carboxyl groups using strong acid is required prior to analysis; other carboxyl groups in environmental samples can interfere; carboxyl group content can vary between different GFNs; divalent metal cations can dissociate from carboxyl groups since they are not covalently attached; divalent metal cations can increase agglomeration state in water samples; will not work for pristine graphene since it does not contain functional groups |
| Microscopic | |||
| Atomic Force Microscopy (AFM) 32, 113, 115, 237–241 | Measures the surface features of a sample by dragging or tapping a cantilever over the sample; the dimensions of identifiable GFN particles can be determined by the movements of the cantilever | A reliable technique for determining sheet thickness and lateral dimensions | Deposition bias, measurement bias, and detection errors are all possible in most samples |
| Hyperspectral Imaging 156, 242–247 | Measures reflectance (or absorbance and transmittance) spectra of GFNs in a darkfield (visual near infrared /short-wave infrared spectral range) mode using a high-power halogen light source, resulting in 2D-optical images with full spectral information (400 nm to 1000 nm or 900 nm to 1700 nm, respectively) in each pixel (a pixel can be as small as 128 nm) 248 | Easy sample preparation, provides optical and spectral information, allows spatial localization of particles without the need for labelling, can provide semi-quantitative information | Spectral mixing in complex samples/composites, long analysis times, spatial resolution may not be sufficient to differentiate individual small-sized GFNs from their aggregates (especially when stacked), which might impact quantification. Relatively expensive instrumentation. |
| Scanning Electron Microscopy (SEM) 137, 158, 159, 237, 247, 249 | Measures the interaction of a finely collimated electron beam with the GFNs; secondary electrons emitted by atoms excited by the electron beam can be used for image formation | Provides 3-D morphological properties of GFNs; GFNs may be identifiable in complex matrices based on morphological criteria | Labor-intensive, often only qualitative information |
| Transmission Electron Microscopy (TEM) and Scanning Transmission Electron Microscopy (STEM) 112, 113, 157, 159, 160, 178, 237, 244, 250 | A TEM passes a parallel beam of electrons through a selected sample area and detects the transmitted electrons that pass through the samples. The main difference with the STEM mode is that it scans very finely focused beam of electrons over the sample selected area in a raster pattern. | Provides morphological properties of GFNs; GFNs can be identified in energy filtered TEM images | Challenging sample preparation for tissues; it may be very hard to detect GFNs in complex samples at low concentrations |
| Laser Scanning Confocal Microscopy 110, 159, 178, 179 | Uses a laser to excite fluorophores from a fluorescent marker tagged to GFNs or optically detects reflected light. The technique generates a series of focused image planes in the z direction by scanning with point illumination suppressing out-of-focus signal using a pinhole in front of the detector; three dimensional images are generated by combining the series of focused image planes. | Relatively easy technique for tracking translocation of GFNs in biological tissues | Only qualitative, or at best, semi-quantitative. Fluorescence probes may photo-bleach, and may be cytotoxic or interfere with normal biological processes. Reflection mode may be unable to distinguish GFN from other materials in the matrix that scatter light similarly. |
| Transient Absorption Microscopy 133, 251–254 | A typical pump–probe technique whereby a modulated pump field (typically a pulsed laser) excites the electrons in the sample. A probe (another light source) then interacts with the photoexcited sample to obtain an absorption spectrum | Relatively fast, highly sensitive, and label-free technique that can be used to visualize GFNs in living cells and live animals. May provide quantitative data in well-dispersed GFNs | Light-absorbing matrices may introduce strong background signals. GFNs may have to be functionalized to improve their dispersability for quantitative analyses |
| Thermal | |||
| Thermal Gravimetric Analysis (TGA) 173–176 | Quantification of mass percentage of phases with distinct thermal stabilities under a variety of reactive gases (usually inert or air) and relatively rapid temperature programs (e.g., heating rates of 5 °C/min to 20 °C/min; room temperature to ca. 950 °C); each sample takes 1 h to 2 h total; a systematic shift in the TGA profile as a function of GFN loading can potentially be measured since GFNs can enhance the thermal stability of materials | A rapid technique that allows for the quantification of multiple phases in a single sample; good for complex matrices; no special sample preparation needed | Effect of thermal ramp rate and reactive atmospheres on apparent phase distribution is not well understood (and is largely ignored), detection limits are relatively high for solid matrices since only small masses can be analyzed, potential for interferences between sample matrix (e.g., polymer, other carbon nanomaterials, soot, or black carbon) and GFN decomposition temperatures; good GFN dispersion quality required for systematic TGA profile shift; drying required |
| Differential Scanning Calorimetry (DSC) 173 | Measures the thermal transitions of materials relative to a reference pan. The relative energy required or released is measured as a material is heated or cooled through a thermal transition; this technique has been used to measure the shift in the glass transition temperature (Tg) as a function of GO loading | A rapid technique and good for complex matrices; no special sample preparation needed | Thermal ramp rate can affect the transition temperatures; detection limits are relatively high for solid matrices; good GFN dispersion quality required for systematic DSC profile shift; dry-down required; might only be useful for samples containing polymer |
| Total Organic Carbon (TOC) Analysis 255–257 | TOC analysis can be conducted on water or soil samples by oxidizing (chemical, heated catalyst, UV) carbon to carbon monoxide or dioxide which is detected by infrared or other types of detectors | TOC analysis has been used successfully with CNTs and fullerenes and once with few layer graphene (FLG) to investigate binding of NOM to FLG | Very little optimization of temperature or catalytic conditions have been examined; its application to CNT stock solutions have been consistent with prepared masses; any organics, such as natural organic matter, in solution or soils will interfere; this is a non-specific method and thus matrices that contain sufficiently high concentrations of other carbon nanomaterials (e.g., graphene), soot, or black carbons would impact the technique; with the more common instrument setups (680 °C maximum temperature), the temperature used is not sufficiently high to combust the FLG but would most likely be high enough for GO to combust |
| Programmed Thermal Analysis (PTA) 96, 170, 211, 258 | While the temperature is ramped, there are two phases: inert followed by oxidizing for measuring organic and elemental carbon, respectively. Detects carbon by having evolved organic carbon be converted to CO2, then converted back to methane, and analyzed using a flame ionization detector. If organic carbon is converted to elemental carbon during the inert phase, there is a correction that can be performed. | Very reliable technique for detecting elemental carbon in environmental matrices, this technique could differentiate between types of GFNs based on their thermal stability; there is an ability to quantify mass | Too much organic carbon in a sample causes peak overlapping between elemental and organic carbon which affects the accuracy; similar carbonaceous materials such as CNTs and fullerenes will be counted in the GFN peak if they exist in the sample; unless the peak from GFN is far enough from the peaks for other carbonaceous material, it is difficult to exclude the other carbonaceous materials, however, adjusting the temperature program might help to some extent; GO does not separate from matrix unless a strong reducing agent is used followed by extraction prior to sample analysis |
| Isotopic Labeling | |||
| Carbon-14 Labeling 37, 42, 57, 106, 154 | Can be used to quantify carbon-14 labeled GFNs following combustion in a biological oxidizer or direct addition to a scintillation cocktail; measures beta emissions using liquid scintillation counting (LSC); autoradiography can provide spatial distribution of radioactivity | Provides definitive quantification of GFNs in complex matrices; can be used as an orthogonal technique to develop other analytical techniques; can be used to identify degradation products and GFN quantities in tissues or released from polymer nanocomposites | High cost to synthesize radioactively labeled GFN; safety concerns; limited availability of radioactively labeled GFN; C-14 not inherently part of GFN that would be released into the environment |
| Additional Techniques | |||
| Gravimetric 259, 260 | GFN mass concentration in air is estimated by determining total particle number (e.g. during GFN production) while accounting for background particle concentration. In suspensions, GFN concentration is estimated by drying a fraction of the suspension and weighing it, or by determining the fraction of GFNs not suspended by weighing the mass of GFN particles settled at the bottom of the container | Uses readily available equipment except in airborne measurements which require special instrumentation | Limited to high GFN concentrations, except in airborne measurements where the sensitivity of equipment may be reasonably high. The technique is nonspecific, and thus only applicable in relatively simple systems/matrices |
The GFN size and oxidation level can significantly alter the measurement obtained from a given technique. GFNs tend to be composed of a heterogeneous distribution of sizes, amorphous impurities, and levels of exfoliation which adds complexity to their quantification. Currently, there is information about the impact of lateral size and GFN agglomeration state on quantification methods, but information about the impact of GFN thickness on quantification is not yet readily available.32, 37, 40 In terms of lateral size, the ratio of edge defects to graphitic regions decreases with GFN lateral size, changing the electronic properties. Oxidation generally leads to a change in the chemical and electronic structure of the GFN. Oxidation leads to an increasing number of defect sites containing oxygen functional groups (e.g., epoxides, carboxylic acids, alcohols, carbonyls), which disrupt the aromaticity of the graphitic structure and, generally, decrease the electrical conductivity.4 These oxygen functional groups often serve as anchor points for derivatization or metal ion tagging, which can enable GFN detection and quantification.40, 41 In comparison to graphene, GO has the advantage of being readily dispersible in water.4 This facilitates detection and quantification of GO in aqueous systems, since only minor agglomeration occurs except in waters with high ionic strength.32 Graphene, on the other hand, agglomerates readily and requires extensive exfoliation processes and addition of surfactants to be suspended in water. This presents a challenge for detection, since graphene can exist in many different agglomeration states from system to system. However, this is not as substantial of an issue with thermal, isotopic, or radioactive labeling methods. Environmentally relevant processes such as ultraviolet (UV), chemical, and biological degradation have shown the capacity to transform GFNs through oxidation to CO2, reduction of GO, and GFN fragmentation.40, 41, 72, 93–95 The large variations in GFN structure observed as a function of oxidation level and material size as a result of these environmental processes presents challenges for quantification. Nevertheless, a combination of techniques can usually be employed to identify the presence of GFNs and sometimes quantify them.40
The measurement limitations presented must also be considered in the context of the media and systems in which GFNs will be detected and quantified. These can include aqueous and complex environmental media such as soils and sediments, polymer fragments containing GFNs released from products, and biological systems such as cells and tissues (Figure S1). The main challenge with all of these systems is that detection of CNMs must often take place in a matrix containing high amounts of carbon.96 As a result, there are several potential ways that the media, matrix, or system can cause interferences such as absorbance overlap in the same region of the UV-Visible (UV-Vis) spectrum, thermal profile overlap with NOM, and obscuration of the two-dimensional GFN shape in the presence of other materials using microscopy (Figure S1). Table S2 describes the methods presented in Table 1 as applied to different matrices with information on what has been previously studied in these systems, when extraction is or might be required, and the potential biases associated with these matrices. In the following sections, these matrices are described and considered in the context of the classes of techniques used for measurement of GFNs that are subsequently described.
RELEVANT MATRICES
One key factor related to GFN detection and/or quantification is that various environmentally and biologically relevant matrices may impact the type of techniques used. In the following sections, general details will be provided about the potential impact of matrix on GFN quantification. Then, in the Classes of Techniques Used for Detection and/or Quantification of GFNs section, different classes of techniques and their use with different matrices will be discussed in depth.
Measurement of pristine GFNs in aqueous systems
Over the course of their life cycle, GFNs are likely to end up in aqueous systems such as freshwater, wastewater and marine water bodies including bottom sediments.30, 38 A large majority of GFN measurements that have been made in a laboratory setting involve suspensions of GFNs prepared in purified (i.e., deionized (DI) water) aqueous systems or synthetic media (e.g., EPA hard water) rather than in natural water.32, 36, 97–99 For example, GFNs have been measured in purified water using UV-Vis spectroscopy,16, 100 Raman spectroscopy, 101, 102 and fluorimetry.103 More complex natural waters typically contain NOM, microorganisms, inorganic species, suspended particles, and pollutants, all of which have the potential to interfere with GFN detection and/or quantification (Table 1).
Measurement of GFNs in soils/sediments
Soils and sediments are extremely complex, and they constitute some of the largest sinks for engineered nanomaterials.104, 105 There is currently no study measuring GFNs in soils and sediments without carbon-14 labeling, and the complexity of these matrices will most likely require extraction of GFNs prior to detection and quantification.57
Measurement of GFNs in cells/organism tissues
Detection and quantification of GFNs in biological matrices is important for understanding the fate, bioavailability, bioaccumulation, and potential adverse effects of the GFNs on organisms. Analytical techniques for detection and/ or quantification of GFNs in carbon based biological matrices present similar challenges to detection and/or quantification of GFNs in soils and sediments. These techniques also will require that the GFNs be extracted from biological systems prior to measurements, while only a few techniques (e.g. using labeled GFNs) can be used to analyze GFNs in situ.42, 57, 58, 106
Measurement of released GFNs from consumer products such as polymer nanocomposites
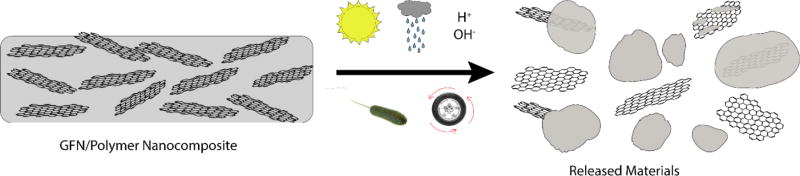
A large fraction of GFNs will be used as additives in consumer products. Many of these consumer products will use GFNs embedded in polymer matrices to enhance material properties. For example, GFNs can enhance mechanical strength, electrical properties, and barrier properties of a polymer.107, 108 As these GFN/polymer nanocomposites go through their life cycle, GFNs can potentially be released from the consumer product into the environment via mechanical wear, thermal, UV, and other weathering conditions.109, 110 GFN release from polymer matrices is not a simple process and can generate different types of released particles that include freely released GFNs, GFN(s) partly encapsulated in polymer fragments, and GFN(s) fully encapsulated in polymer fragments (Figure 2). Therefore, the polymer matrix can interfere with GFN detection and quantification. This has previously been shown with abraded CNT/polymer nanocomposites during simulated wear experiments.111, 112 Methods to detect the heterogenous mixture of particles released from polymer nanocomposites as well as methods to remove polymer interferences are needed. Furthermore, the detection of GFNs becomes even more challenging when a polymer matrix and environmental matrix, such as natural water and soils/sediments, are combined.
Figure 2.
Degradation of GFN/polymer nanocomposites by environmental processes such as UV-weathering, rain, acid rain, alkaline conditions, microbial activity, and mechanical wear can lead to the release of a heterogeneous mixture of polymer fragments, polymer fragments containing GFNs, GFNs coated in polymer, and free GFNs.
CLASSES OF TECHNIQUES USED FOR DETECTION AND/OR QUANTIFICATION OF GFNS
Spectroscopic Techniques
Spectroscopically, the interaction of GFNs with light can enable GFN-specific measurements. In this case, oxidation level, lateral size, and agglomeration state must be considered since they change the interaction of GFNs with light. The spectroscopic techniques considered in this review include UV-Vis spectroscopy, fluorescence spectroscopy, Raman spectroscopy, X-ray photoelectron spectroscopy, and a few other specialized techniques.
UV-Vis Spectroscopy
UV-Vis spectroscopy (absorbance mode) is the most commonly used method for quantifying GFNs in purified, synthetic, and natural waters due to the ease of use, low cost, and availability of spectrophotometers in environmental laboratories.32, 40, 44, 92, 113–116 The absorbance of a GFN can be related to its mass concentration in suspension using the Beer-Lambert law, but the particles must be well-dispersed.117, 118 The absorbance of GFNs is typically measured at wavelengths around 220 nm to 300 nm. The absorption peak of graphene around 265 nm is due to π → π* transitions, which shifts to shorter wavelengths (around 230 nm) when graphene is oxidized to GO.92, 119, 120 For graphene, surfactants are often required for colloidal stability in water so that consistent UV-Vis measurements can be obtained. It is often challenging to prevent the surfactant from absorbing in the same region of the spectrum as graphene.121 Alternatively, organic solvents can sometimes be used to suspend graphene.122 UV-Vis measurements of GO around 300 nm targets the peak (shoulder) originating from the n → π* transitions of the oxygen functional groups.120, 123, 124 In natural and synthetic waters, it is typically challenging to detect and/or quantify GFNs via UV-Vis spectroscopy because of the complexity of the medium, non-specificity of the technique, and potential for agglomeration of the GFNs.32, 36, 38, 96, 114 For instance, several constituents of natural or synthetic waters such as salts, nutrients, NOM, and suspended solids absorb light in the UV region, making it impossible to use UV-Vis spectroscopy to quantify GFNs in these media without extracting the nanomaterials. For these reasons, it is useful to have a reference spectrum of the GFN material in purified water whenever possible. It is also important to have measurements of the natural/synthetic water without GFNs and of the natural/synthetic water after adding a known amount of GFN to determine if measurements can be made without significant interference using a specified technique. This same approach has been taken in biological systems where CNTs were quantified in cells by lysing the cells and determining the absorbance of the lysate spiked with known amounts of CNTs to develop a calibration curve.125 Another approach used for CNT suspensions has been to measure absorbance increases at longer wavelengths from light-scattering by the suspended particles, which is proportional to CNT mass concentration, but this approach has not yet been shown to be effective with GFNs.126 In addition, the low expected average environmental concentrations of GFNs (i.e., average in the low ng/L range if the concentrations are similar to those modeled for CNTs) makes UV-Vis spectroscopy, with detection limits estimated to be in the tens of µg/L to mg/L range for GFNs (Table S1), likely unsuitable for quantifying GFNs in natural surface waters.96 In laboratory studies where challenges arising from matrix effects and high detection limits are overcome, biases may still arise from GFN size distribution, method of dispersion, and agglomeration state, all of which may influence the absorption coefficients.
UV-Vis measurements of GFNs in other matrices (e.g., polymer fragments, soil/sediment, cells or tissue components) can prove to be even more complex. These measurements must be performed in a liquid medium, usually water, that is part of or surrounding the matrix (e.g., released particles from a GFN polymer nanocomposite suspended in water). Spectroscopically, the interaction of the matrix (e.g. polymer fragment, soil/sediment, cells or tissue components) with electromagnetic radiation must be sufficiently different from that of the GFN to avoid overlap in the GFN spectrum. This is challenging because many polymers, inorganic particles from soils/sediments, and biological materials absorb light around the wavelength of GFN absorption (200 nm to 300 nm range).117, 127, 128 Another approach is to make use of analytical ultracentrifugation with UV detection, as has been performed with CNTs to separate various CNT structures by size prior to detection.127–129 Overall, the UV-Vis approach is likely to work for quantifying GFNs in matrices when they are well-defined and do not have significant interferences at the wavelength used for GFN quantification.
Fluorescence
The ability of GFNs to fluoresce is sometimes useful for GFN characterization in purified and synthetic waters.103, 113, 123 Both GO and rGO are detectable by instruments capable of measuring near-infrared (NIR), visible (vis) and ultraviolet (UV) fluorescence.103, 130 Also, graphene quantum dots, or graphene fragments with a lateral dimension on the nanoscale (rather than micron scale), are designed specifically for their unique fluorescence ‘tunability’ but are still challenging to prepare synthetically in terms of size, surface chemistry, and photoluminescence properties.11 Pristine graphene, on the other hand, is not readily fluorescent because it has a zero band gap.3, 131 In general, the fluorescence properties of GFNs will vary as the result of changes to the electronic structure caused by alterations in size and oxidation level, which can happen via transformation processes in the environment.103 Thus, fluorimetry is not used as widely as UV-Vis spectroscopy to quantify GFNs in laboratory studies conducted in aqueous media. This may also be due, in part, to the non-linear relationship between fluorescence intensity and the concentration of GFNs in aqueous media—making the technique mostly useful for semi-quantitative analysis.113
Similar to UV-Vis spectroscopy, the applicability of fluorescence in detecting GFNs in natural waters, soils/sediment, polymer fragments, and cells/tissues is limited. Fluorimetry requires well-dispersed particles and is non-specific, making it impossible to use the technique for in situ quantification of GFNs in natural waters containing other fluorescent materials. In addition, the interactions of salts and NOM with GFNs can interfere with GFN fluorescence. In biological matrices, the intrinsic photoluminescence of GO can ideally be used to trace GO. However, the emission efficiency of GO is low,132, 133 and may be affected by interference from cellular components. For GFN/polymer nanocomposites, degraded or highly oxidized polymer fragments generated during polymer degradation processes often fluoresce strongly and will likely interfere with GFN detection.134 Therefore, it is highly unlikely that fluorescence will be utilized to detect GFNs in most environmental matrices since 1) GFN structures are not homogenous and may change in the environment, which leads to a changing fluorescence spectra and 2) many components in environmental matrices and polymer fragments will interfere since they are also fluorescent.
Raman Spectroscopy
Raman spectroscopy offers better specificity than UV-Vis spectroscopy and fluorimetry for identifying graphitic forms of carbon such as GFNs. In Raman spectroscopy, GFNs can be detected using the signature defective (D, ~1350 cm−1) and graphitic (G, ~1580 cm−1) bands representative of the sp2 hybridized network of carbon disrupted by edges and defects along the basal plane.101, 102 With Raman spectroscopy, higher oxygen functional group levels increase the D band intensity and decrease the G band intensity, leading to higher D/G band ratios for GO than for graphene. A decrease in lateral size also increases the number of defect sites relative to the graphitic carbon regions, increasing the D/G ratio.40, 135 The intensity of the D and G bands can be used to quantify the GFN concentration in a consistent Raman configuration or by measuring the intensity of the D or G band relative to a reference peak.136, 137 The G’ band (~2650 cm−1) can also be used with pristine graphene, but decreases in intensity occur much more readily than in the G band with an increasing number of defects in the graphitic structure, which are likely to form in the environment.101
Raman instruments are configured differently depending on their use for dry or liquid samples and the choice of which configuration to use can depend on the GFN form and matrix under investigation. For example, it is more appropriate to measure GFNs in powder form with a Raman microscope while it is more appropriate to measure GFNs in an aqueous suspension with a Raman system built to hold cuvettes. Raman instruments are also widely availability at universities but less available in environmental testing laboratories.
Raman spectroscopy is commonly used for detection of GFNs in purified aqueous systems and some synthetic media, but not in natural waters, which contain other types of graphitic carbons (such as humic acid, clays, black carbon, and other graphitic carbon) that can have overlapping D and G bands.138, 139 When GFNs are analyzed in water with Raman spectroscopy, the G band of GFNs overlaps with the H-O-H bending transition of water band (1640 cm−1), which has previously been shown to limit the quantification of CNTs, at least with respect to the G band.140
The characteristic nature of the D and G bands of GFNs allows for the use of Raman spectroscopy in detecting and quantifying GFNs in biological matrices and polymer fragments provided there is a reference peak to use for normalization.137, 141 Raman spectroscopy has low throughput, however, which makes it challenging to use for probing large sample areas in dried-down polymer fragments and determining the detection limit in tissue matrices. Another challenge is that degraded or highly oxidized polymer fragments generated during environmental weathering processes, often fluoresce strongly and can interfere with the D and G bands in the Raman spectrum, either through a rising background or development of overlapping bands from fluorescent byproducts. Nevertheless, the D and G bands of GFNs in Raman spectroscopy are fairly unique and can be used to distinguish the GFNs from the polymer matrix if polymer byproduct peaks do not overlap and the fluorescent background is adequately corrected.141 Thus, it is likely that Raman spectroscopy will be used and continually developed for GFN detection in biological matrices and polymer fragments. In contrast, soils and sediments contain numerous forms of carbonaceous substances (including graphitic forms), making it impossible to utilize Raman spectroscopy except after an extraction procedure is performed.142, 143
X-ray Photoelectron Spectroscopy (XPS)
X-ray photoelectron spectroscopy (XPS) analyses are widely used in studies for characterizing and, at times, detecting GFNs present in purified and synthetic waters.72, 93, 113, 115, 144–148 It is important to note that for XPS, samples must be deposited on a substrate and any water present must be evaporated prior to measurement. XPS is subject to interferences from the abundant, naturally-occurring carbon constituents in natural waters, sediments and biological matrices, and is therefore not very useful for quantifying GFNs in these matrices. However, this may change if sophisticated extraction techniques are developed. Nevertheless, the carbon content of GFNs can be quantified relative to another element in the absence of other carbonaceous species or the presence of a less conductive carbonaceous material (e.g., a polymer).63, 149 When one carbonaceous material such as a polymer is less conductive than the GFN, the charge neutralizer of the XPS system can be turned off, and the polymer component of the C(1s) peak can differentially charge or shift away from the GFN component, thus allowing (hypothetically) for GFN component deconvolution, integration, and semi-quantification. This has been demonstrated with CNTs and is yet to be demonstrated with GFNs.63, 149 It is only likely to be successful with graphene or rGO, since they are conductive; in contrast, GO is not conductive with a graphenic structure disrupted by oxygen functional groups. Another important point is that XPS is a surface sensitive technique that can probe only the top ~10 nm of a material so it cannot be used for reliable bulk measurements of larger polymer fragments.150 Furthermore, GFNs must be homogeneously distributed within the sample since the spot size covers an area on the order of microns and in terms of sample amount, a few milligrams of material are needed for analysis. Other disadvantages include the high cost of XPS and the fact that samples are prone to contamination through adventitious carbon adsorbed onto sample surfaces.151 Overall, it is unlikely that XPS will be used to detect GFNs in environmental matrices, since all matrices contain carbonaceous species. However, XPS may be useful to detect GFNs in small polymer fragments released from polymeric nanocomposites into pure aqueous systems.
Other Spectroscopic Approaches
Transient absorption spectroscopy, a specialized technique, has also been shown to provide fast visualization and quantitation of GFNs within living cells but the accuracy of quantitation is dependent on the dispersion state of the nanomaterials.133 This technique is only likely to be applied in specialized laboratories due to its high cost and complexity.
GFNs also have unique X-ray diffraction patterns, which may make their detection possible with X-ray diffraction (XRD) techniques. However, a large amount of material 10 mg to 100 mg is required.152, 153 Furthermore, a GFN reference is necessary to distinguish a particular GFN from the matrix it resides in. Consequently, XRD may be most useful for evaluating released GFN/polymer nanocomposite fragments in mg quantities.152 Furthermore, XRD instrumentation is generally expensive and not always available to environmental testing laboratories.
Microscopic Techniques
The two-dimensional shape and lateral size of GFNs (tens of nanometers to several micrometers) enables their detection with a variety of microscopic techniques such as atomic force microscopy (AFM), scanning electron microscopy (SEM), transmission electron microscopy (TEM), laser scanning confocal microscopy (LSCM), and hyperspectral imaging.16, 154 When compared to CNTs which have cylindrical structures, the 2D morphology of GFNs is less distinct, which may make GFN identification in natural matrices via microscopy very challenging. GFN quantification is possible with microscopic techniques but it may be overly time-consuming and as a result, is often impractical or infeasible in complex matrices.155 For electron microscopy, limitations may include the choice of dilution factor when drying down GFNs so that particles do not overlap, assessing consistency in and determination of thicknesses and agglomeration state, and evaluating the degree to which the GFNs wrinkle, which could make GFN counting a challenging task. There are no reports, to our knowledge, where the researchers counted the number of graphene layers and the number of graphene particles present, especially in an environmental sample. In addition, these instruments are often fairly expensive, and accessibility is limited to universities and other user facilities rather than environmental testing laboratories.
For microscopic techniques, any water present must be completely evaporated, except when using techniques such as environmental SEM (ESEM), cryo SEM (CSEM), AFM, or low vacuum SEM (LVSEM).113, 115, 137, 147, 156–158 Drying of samples for microscopy may introduce artifacts, but this can often be avoided with careful sample preparation. For instance, salts left after evaporating synthetic media or natural water can deposit onto or even mask GFNs (depending on the salinity of the synthetic media or natural water), but an ultrafiltration step prior to drying can substantially reduce the salt concentration present. In general, the amount of GFNs expected to be present in natural waters is very low compared to the amount of other particle types (e.g., clay), which may make the detection of GFNs in natural waters challenging via microscopy. With proper dilution, microscopic techniques such as energy-filtered TEM (EFTEM) and hyperspectral imaging may be used to identify GFNs in some environmental matrices based on unique GFN interactions with electrons and the electromagnetic spectrum.156 With proper dilution, semi-quantitative analysis of GFNs may be possible with microscopic techniques such as TEM (e.g. by using software programs such as ImageJ),159–161 laser scanning confocal microscopy (LSCM),159 and hyperspectral imaging.156 Due to the complexity of soils and sediments, GFNs would have to be extracted from these matrices prior to identifying them using microscopy.
In polymer nanocomposite fragments, GFNs can be observed with techniques such as SEM and TEM if GFNs are close to the polymer surface (within 10 nm to 100 nm).162 Some light microscopy techniques, such as laser scanning confocal microscopy (LSCM), may also be employed depending on the size of the particles with respect to the diffraction limit of light used in the microscope. However, detection and quantification of different fragment types and freely released GFNs is time-consuming and often impractical since a high number of images are required for robust statistical inferences to be made. In general, microscopy will continue to be a useful tool for GFN detection, and sometimes quantification. However, efforts are needed to decrease the time it takes to prepare and image samples, and the improvements that can be made will likely only be incremental. Nevertheless, microscopy will continue to be useful as a supplementary characterization technique.
Thermal Techniques
The graphitic structure of graphene also leads to high thermal stabilities which decrease with increasing GFN oxidation level.96 The high thermal stability of graphene permits its detection at much higher temperatures than GO.96, 163, 164 Figure 3 reports the temperature range at which GFNs show the most change during thermal decomposition under inert conditions. Areas of overlap with the different media, matrices, and systems presented in this text are shown (Figure 3) and illustrations of thermal gravimetric analysis (TGA) profiles for different GFNs and polymer matrices are shown in Figure S2a and S2b, respectively. Unlike for graphene, the decreased thermal stability of GO causes its thermal profile to overlap with many carbonaceous species, thus hindering its quantification using thermal methods.
Figure 3.
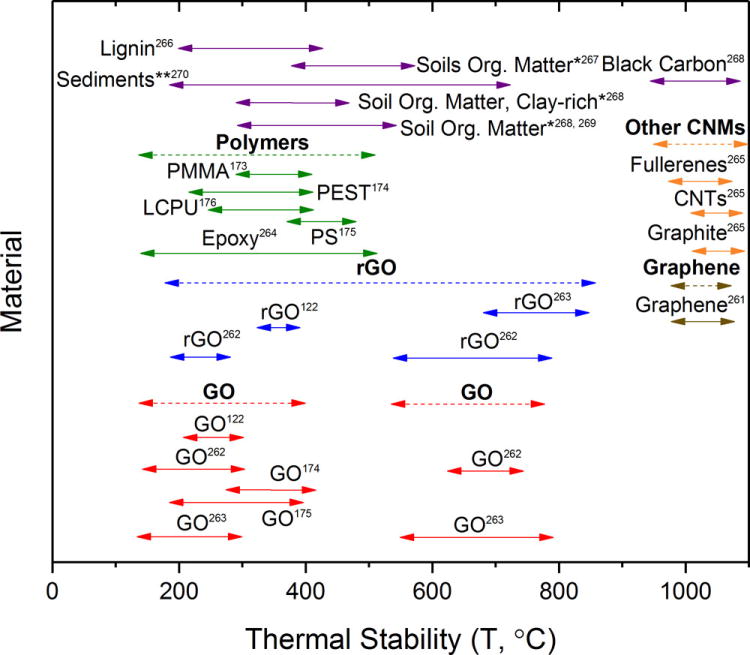
The thermal stability as a function of mass loss for graphene,261 graphene oxide122, 174, 175, 262, 263 and reduced graphene oxide122, 262, 263 relative to polymer matrices (LCPU = liquid crystalline polyurethane, PS = polystyrene, PMMA = poly(methyl methacrylate), PEST = polyester, and epoxy),173–176, 264 other carbonaceous nanomaterials,265 and plant material (lignin),266 soils or soil materials,267–269 and sediments.270 An asterisk (*) indicates that clay was not included as part of the thermal gravimetric analysis (TGA) profile while a double asterisk (**) indicates that clay was included as part of the TGA profile. The plot shows where overlap can occur between the thermal profile of the CNM and the thermal profile of the matrix. Ranges provided are the most dramatic change(s) observed with TGA under inert conditions (N2 or Ar) with ramp rates ranging from 5 °C/min to 20 °C/min.
Analytical techniques that leverage the unique thermal properties of GFNs such as thermal gravimetric analysis (TGA), total organic carbon (TOC) analysis,145 and programmed thermal analysis (PTA)96 are useful both for characterizing and quantifying CNMs, by drying down an aliquot from aqueous media. Since graphene is thermally stable, there is not much interference when using thermal techniques in purified, synthetic, and natural waters which contain mostly labile forms of carbon. However, interference is typically higher in natural waters that contain large amounts of suspended particles or in complex media such as untreated wastewater.96 In such systems, the thermal profiles of GFNs, the matrix (e.g., natural water) without GFN if available, and GFNs mixed into the matrix should be characterized to assess any matrix interferences and determine if the matrix impacts the thermal stability of the GFNs. Overlaps between the thermal profiles of GFNs and the matrix can be easily accounted for when they are not substantial.
Unlike graphene, GO and sometimes rGO have a very high oxygen content and number of defect sites, which make them less thermally stable.165 The application of thermal techniques, such as PTA, to quantify GO (without further modifications) may be restricted to purified or synthetic waters without added NOM if there is an overlap between the GO and NOM thermal profiles. For aqueous media containing NOM or natural waters, it may be necessary to chemically reduce (using hydrazine, sodium borohydride, ascorbic acid, etc.92, 96, 165–167) GO in order to increase its thermal stability relative to that of the organic carbon in the matrix.96 In addition, other carbonaceous (nano)materials such as soot, CNTs, and fullerenes may be present with GFNs in natural waters (e.g., wastewater), which may make the detection and quantification of GFNs via thermal techniques more complicated. One possible solution is to selectively extract GFNs from the matrix while excluding other carbonaceous materials like soot and CNTs, but there are currently very few methods for achieving such a selective extraction and all extraction procedures result in some loss of the analyte.168 Alternatively, it may be possible to add known quantities of the GFN material of interest (or a GFN with a similar thermal profile) to the water matrix to quantify the amount of background carbonaceous materials interfering with GFN quantification.96 Another probable challenge with using thermal techniques for quantifying GFNs in natural waters is the potentially high detection limit of some thermal instruments relative to the amount of GFNs expected in natural waters. A detection limit of 1.7 µg was reported for GO in pure water using PTA while other thermal techniques such as TOC analysis and TGA may have even higher detection limits.96 More so, the detection limit of these instruments may increase substantially when GFNs are mixed into complex natural waters depending upon the overlap in the thermal profile of the matrix and GFN. Overall, none of the techniques described so far can conclusively detect GFNs without measurement of a reference GFN, the matrix without added GFN, and/or extraction.
Analytical methods relying on the thermal properties of GFNs may be applicable to quantify the nanomaterials in biological matrices without extraction if there is not substantial interference between the thermal profiles of the nanomaterials and matrices. Given the small amount of GFNs expected to be internalized in cells and tissues, even slight interferences from the matrix can overshadow the GFN signals. However, high concentrations may be present in the gut tract of organisms such as Daphnia magna; therefore, if voiding of the gut tract is not performed, measurements of total body burden will be dominated by the GFN mass in the gut tract, yet, unlike bioaccumulation measurements for dissolved chemicals, these values will not reflect the GFN mass adsorbed across epithelial surfaces. 41, 58 Similar to other matrices, the thermal instability of GO compared to graphene, may make it difficult to detect GO in biological matrices via thermal techniques due to substantial interference. Also, changes in the chemical and thermal properties of intracellular/internalized GFNs (relative to their pristine forms) are currently unknown, and may interfere with analyses. Quantification of GFNs in biological matrices via thermal techniques may be less challenging if the nanomaterials are extracted by digestion or lysis of the cells/tissues either chemically or mechanically.96 For example, PTA has been used to quantify CNTs in rat lungs169 and GFNs in wastewater biomass after extraction.96, 118 Care has to be taken to ensure that the chemicals used for digestion do not degrade or oxidize GFNs if the analysis technique can be impacted by the GFN oxidation state.
Studies have not yet been conducted to investigate the ability to detect and quantify GFNs in soils and sediments using thermal techniques, but studies have shown that CNTs can be measured in these matrices using thermal techniques albeit with varying levels of success.170–172 The major challenge of using thermal techniques for characterizing GFNs in soils and sediments is that these matrices contain thermally stable elemental carbon (e.g., soot), which can substantially interfere with GFNs, as was the case with CNTs.170, 171 However, the ion ratios of gases evolved upon thermal degradation of GFNs may be substantially different from the ion ratios of gases originating from soil and sediments, which provides an opportunity to quantify the nanomaterials in these matrices.172 The required instrumentation for this type of analysis is relatively expensive and uncommon in environmental science laboratories, as the thermal instrument has to be coupled with a mass spectrometer to analyze the gases evolved. Thus, there is a need for methods that are more readily available for practical detection and quantification of GFNs in soils and sediments.
Graphene can be easily distinguished from a polymer matrix using thermal analytical techniques since the thermal stability of polymers tends to be below 400 °C, well below the thermal stability of graphene (Figure S2a). In contrast, the thermal profile of GO can overlap with the thermal profile of many polymers (Figure S2b). Furthermore, the mass loss of GO and rGO is gradual over a large temperature range (Figure 3) in TGA, making it challenging to differentiate the polymer from the GFN. Experimentation with conditions such as a switchover from inert gas to air flow at different temperatures may be useful, in some cases, for differentiation of polymer from GFN. Small differences in thermograms, such as first derivative shifts in TGA or slight shifts in glass transition temperatures (Tg) with differential scanning calorimetry (DSC), can be employed to measure the mass fraction of GFNs in polymer fragments or polymer matrices.173–176 These approaches, however, require a reference polymer material and a calibration curve of similarly dispersed GFNs in the polymer at varied concentrations. If lower mass fractions (< 1 %) of GFNs are incorporated into polymers, their signal must be discernible from the polymer background and from polymer charring. This approach is not practical in every application.
Labeling Techniques
Metal ion labeling, isotopic labeling, and fluorescence labeling of GFNs provides a unique opportunity for the detection and quantification of GFNs that avoids some of the interferences observed with other methods. However, these approaches are typically only applicable for laboratory studies given that GFNs in the environment will not be labeled. Labeling of GFNs with materials that are (or have properties that are) not intrinsically found in environmental matrices provides an opportunity for detecting and quantifying the nanomaterials within these complex matrices.
In aqueous systems, especially natural water, there are very few interferences (with well-designed labels) compared to most other methods. Similar to CNTs, metal ions can potentially be coordinated to GO or incorporated into a GFN structure for use as a GFN proxy.141, 177 Inorganic elemental analysis using techniques such as ICP-MS can then be employed to detect and quantify the GFN concentration.141, 155 The metal ion used must be properly chosen so that the metal is not present in the natural water at sufficiently high concentrations to bias the measurements. Furthermore, the coordination of the metal to the GO oxygen functional groups must remain unchanged throughout the experiment or proper controls must be run to measure the percentage of coordinated metal ion loss during any environmental transformation.155
Isotopic labeling of graphitic carbon can also be used as a means for detection and quantification.42 14C-isotopes are stable, and techniques based on their detection and quantification have been used to study the fate and transformations of GFNs (mostly graphene) in aqueous systems.37, 42 Quantification of isotopically labeled GFNs allows for laboratory studies to be carried out at very low GFN concentrations (ng/L to µg/L range)—much lower than would be possible with most other analytical techniques.37, 42 The radioactivity of isotopically labeled GFNs is quantified using liquid scintillation counting (LSC) with or without combustion of the graphene; combustion transforms the GFN to 14CO2 prior to the LSC analysis. In one study, direct addition of a FLG suspension to scintillation cocktail followed by scintillation counting was hypothesized to underestimate FLG radioactivity due to interferences with beta emissions, likely from self-quenching of graphene agglomerates or within the layers of the FLG.58 Higher radioactivity recovery rates have been achieved by combustion of FLG stock suspensions in a biological oxidizer with capture of the released 14CO2 in scintillation cocktail followed by quantitation using LSC; biological oxidation eliminated the potential for self-quenching, but led to a lower precision.58
In biological systems, labeling GFNs with metals, fluorescent dyes, and 14C isotopes may enhance the ability to detect and quantify intracellular GFNs in situ. Labeling of GFNs with materials such as fluorescene isothiocyanate (FITC) and 14C-isotopes allows for their detection and quantification using techniques such as LSCM and radioactivity measurements, respectively.37, 42, 159 Label-based GFN detection techniques are also capable of providing information on the bioaccumulation and translocation of GFNs within biological matrices.58, 154, 159, 178 Real time investigation of uptake and localization within small organisms (such as zebrafish embryos) is also possible with FITC-labeled GFNs.159 In addition, GFNs can also be labeled with metals (preferably metals that are not inherently found in cells and tissues) which can be used as a tracer for the GFNs. One important consideration is that metallic and organic labels may interfere with normal cellular processes and/or may be toxic to organisms.141, 179 The potential occurrence of these label-induced adverse effects to cells or organisms should be tested before using labeled GFNs in laboratory studies.
In soils and sediments, some techniques using labeled GFNs may be less prone to interferences compared to other types of techniques. Specifically, 14C-labeled GFNs can typically be detected and quantified at low concentrations in soils and sediments.57 In contrast, metal ions in these soils/sediments are often present at high concentrations and can interfere with metal ion labels. For fluorescence, the potential for fluorescence quenching by components in soils and sediments may limit the applicability of this type of labeling approach. Overall, labeling is very useful for laboratory studies that model outdoor conditions, but is unlikely to be used for detection and quantification of GFNs found in the environment.
DIFFERENTIATION OF OTHER CARBONACEOUS NANOMATERIALS FROM GFNS
All of the techniques applied to GFNs in Table 1 have been previously used for other graphenic nanomaterials such as CNTs and fullerenes; the differences in CNM structure are shown in Figure S3.89 Therefore, the ability to differentiate GFNs from other carbonaceous nanomaterials must be considered as techniques are developed for the detection and quantification of GFNs present in environmental matrices. With the exception of microscopy, almost all of the techniques presented cannot completely differentiate GFNs from other graphenic nanomaterials.16 For example, CNTs and fullerenes absorb/optically scatter light, have D and G bands at similar wavenumbers in Raman spectroscopy, and can have similar thermal profiles.89 However, subtle differences between spectra and the use of nanomaterial controls can sometimes be used to differentiate CNMs. For example, the wavelength of absorption in UV-Vis spectroscopy is strongly dependent on the nanomaterial structure and dispersability.92 In Raman spectroscopy, GFNs tend to have larger D bands relative to CNTs since there are more edge defects per total area in a flat structure than the number of defects present at the ends of a CNT cylinder (where the majority of defects reside). Thus, the D/G ratio can be much larger for two-dimensional versus three-dimensional graphitic carbon structures.135, 180 Due to their strained curvature, CNTs and fullerenes tend to be less thermally stable than graphene. Thus, graphene may be differentiated from CNMs using thermal techniques with proper controls.181 Microscopic analysis allows for differentiation of fullerenes, carbon nanotubes, and GFNs based on their unique physical structures and through the use of EFTEM utilizing differences in their electron energy loss spectra. However, GFNs can be difficult to detect with microscopy as a result of their two dimensional structure.16 Microwave-induced heating methods have been successfully used to quantify MWCNTs in biological samples by measuring the rapid thermal response of MWCNTs relative to the surrounding matrix, but a slower microwave-induced heating response was found for graphene powder. Thus, microwave-induced heating methods have not yet been shown to be useful for GFNs.182 Other techniques such as metal ion labeling combined with inorganic elemental analysis and isotopic labeling can be used to differentiate GFNs from other types of graphitic nanomaterials but are limited to laboratory studies. Overall, the techniques previously developed for CNTs and fullerenes can be similarly applied to GFNs, with only some small differences that permit GFN differentiation in a CNM mixture.
EFFECT OF GFN INTERACTIONS AND TRANSFORMATION ON GFN QUANTIFICATION
In general, GFNs possess extremely large surface areas and highly negative charge densities when oxidized (i.e., GO),3, 92 which enable them to adsorb various organic and inorganic compounds in the aqueous phase—including nutrients, NOM, and metal ions.183–189 These interactions, mediated by π-bonding and hydrophobic interactions, electrostatic interactions, and hydrogen bonding, influence the surface charge of GFNs and thus their homo-agglomeration, and heteroagglomeration with other particles in aquatic systems such as clays, metallic colloids and organic particles.30, 97, 188, 190, 191 Adsorption of inorganic ions (e.g., metal ions) neutralizes the surface charge of GFNs, which typically leads to decreased colloidal stability;34, 37, 99, 114 while adsorption of organic materials (such as NOM) increases the colloidal stability of GFNs via increased electrostatic repulsion and/or steric hindrance.37, 114 The interactions of organic and inorganic compounds (including other colloids) with GFNs also lead to formation of agglomerates with different morphological conformations,34, 97, 192 which (like changes to colloidal stability) can interfere with techniques such as UV-Vis spectroscopy and fluorimetry. More readily water-dispersible GO will also decrease in colloidal stability if the salt concentration is sufficiently high due to suppression of GO’s electric double-layer by the cations in salts.30, 32–34, 99 Importantly, the adsorption of organic compounds (such as NOM) and nutrients onto GFNs may contribute to the signals obtained from non-specific analytical methods such as UV-Vis and TOC analysis.
Transformations of GFNs have been shown in natural conditions, and these transformations can potentially interfere with GFN detection and quantification. For instance, exposure of graphene to water changes its morphology, and results in greater disorder of the structure (increased D/G band ratio), and expansion of the d-spacing (the distance between adjacent planes in the crystal structure).193 These physicochemical changes are further enhanced when graphene is exposed simultaneously to water and visible light.193 In the case of GO, sunlight reduces the primary particle size and colloidal stability, which may interfere with techniques such as UV-Vis spectroscopy and fluorimetry.40, 43, 72, 95 These transformations should be considered when quantifying sunlight-exposed GO with spectroscopic and thermal techniques.8, 40 GFNs in the natural environment are also subject to chemical transformation by strong, naturally occurring oxidizing agents such as hydrogen peroxide (H2O2), found in rain and natural waters. The degradation of graphene (and most likely, other GFNs) can occur at concentrations of H2O2 that naturally occur in surface waters (51 mg/L to 231 mg/L or 1 × 10−3 mol/L to 7 × 10−3 mol/L),147, 194 leaving defects on the surface of the GFN. Similarly, iron/H2O2-driven Fenton chemistry (with or without UV irradiation), a treatment technique commonly applied in wastewater treatment plants, can generate reactive oxygen species (ROS; such as hydroxyl radical, •OH) which can cause defects in the GFN structure, and even lead to complete degradation to CO2 at sufficiently high concentrations of the reactants.42, 93, 195 Structural defects can make the detection and quantification of GFNs more complicated when using analytical techniques that rely on structural properties. Furthermore, when using non-degraded GFNs for calibration, the use of UV-Vis spectroscopy and fluorimetry to quantify degraded GFNs may lead to inaccurate estimation of GFN concentration.8, 40, 93, 195
Transformation of GFNs arising from interactions with cells and organisms has not been widely investigated but a few studies have shown that GO can be reduced by microorganisms via direct contact and electron shuttling.196–199 Reduction of GO by bacterial genera (including Shigella, Shewanella, and Escherichia) occurs as the nanomaterial acts as an electron acceptor for the electrons generated during respiration. Similar reduction of GO has been shown by other biological molecules, including plant extracts.200, 201 As mentioned earlier, reducing the oxygen-containing functional groups on GO will influence its spectroscopic and thermal response and thus may interfere with measurements. The size and thickness of graphene were shown to decrease in a chemical reaction catalyzed by horseradish peroxidase, showing that the enzyme can change the morphology of GFNs.202 The effects of horseradish peroxidase (in the presence of H2O2) on the structure of GO was even stronger (than that of graphene)—resulting in the formation of holes (up to 27 nm wide after 10 days) in the graphitic lattice of the basal plane, and complete oxidation to CO2 after 20 days.203 The study however found no effects of horseradish peroxidase on rGO possibly due to tighter binding between the rGO and enzymes, which retarded the dynamic motion of the enzymes.203 Overall, these enzyme-catalyzed changes should be considered when analyzing enzyme-exposed GFNs with spectroscopic and microscopic techniques.
Changes in the physicochemical state of GFNs in soils and sediments can further complicate their measurements in these matrices. For example Shewanella, a microorganism that has the ability to transfer electrons extracellularly (i.e., an exoelectrogen), which has been shown to reduce GO in laboratory studies, is present in freshwater and marine sediments and may also use GO as a terminal electron acceptor in these matrices.204 In fact, in a study testing five strains of Shewanella obtained from different natural environments, the strain obtained from marine sediments (Pacific Ocean) achieved the highest GO reduction—more than 95% of the carbon left in the GO was in a reduced state after 24 h (compared to 83% obtained by using hydrazine, a commonly used GO reducing agent).196 Additionally, E. coli, a bacterium found in almost all environmental media, including soils and sediments, has also shown the ability to reduce GO.10, 199 Chemical degradation of GFNs can occur via Fenton reactions in soils and sediments but has not been studied. In addition, all GFNs are likely to strongly bind to dissolved organic carbon and colloids in marine sediments due to the high ionic strength of marine waters. Further, GFNs with low surface charges (i.e., graphene and rGO) will adsorb to soil/clay particles due to weak repulsive forces.98, 205, 206 It is also likely that GFNs will behave similarly to CNTs and strongly interact with organic matter in soils and sediments.207–209 These interactions will affect the bioavailability of the nanomaterials as well as their extractability (and thus, measurements in soils and sediments).
EXTRACTION
Isolation of GFNs from other materials present in an environmental matrix is an important component of GFN detection since GFNs can have similarities to other matrix materials, which can also be carbonaceous and graphenic, and can hinder identification of GFN via microscopy. The process of isolating GFNs from an environmental matrix by transfer of the GFNs from the matrix phase to another phase is termed extraction.89 Extraction methods typically involve transfer of the GFNs out of the initial matrix phase into a phase where the interfering compounds are less soluble. Conversely, removal of the interfering compounds to another phase can also be applied.
Methods to extract GFNs from environmental matrices can be considered in the context of CNT and fullerene extraction methods that have already been successfully employed. For examples, CNTs have been extracted from environmental and biological matrices with asymmetric field flow fractionation (AF4),210 matrix digestion,211 and sonication with surfactants.82 Techniques used for CNT purification (i.e., separating a distribution of CNTs into homogeneous fractions) such as gel permeation chromatography, capillary electrophoresis, density ultracentrifugation, and two-phase polymer extraction may also be considered with respect to the extraction of GFNs from environmental matrices.212–214 Fullerene extraction has been even more thoroughly studied than CNT extraction, most likely due to having a less heterogeneous distribution of particles, at least in terms of size, and their affinity for many organic solvents such as toluene. Fullerenes have been extracted from complex matrices using solid phase extraction techniques (i.e., chromatography) and liquid-liquid phase extraction, mostly with toluene as the non-polar phase, and sometimes, the addition of salt to destabilize the nC60 particles.80, 91, 215 Importantly, extraction approaches have been successfully used to enable quantification of fullerene concentrations in complex matrices such as sediments,216, 217 soils,218, 219 and organisms.220, 221 An approach for detection of oxidized fullerenes (i.e., fullerols) has been the addition of salt and toluene for liquid or solid phase extraction, and, occasionally, solid phase extraction of oxidized fullerenes in an aqueous phase after less oxidized fullerenes are separated out using toluene.80, 215 Since GFNs have a different shape than CNTs and fullerenes, a distribution and range of physical dimensions, surface chemistries that can range from hydrophobic to hydrophilic, and are affected by transformation processes in the environment, testing of CNT and fullerene extraction methods with GFNs needs to be attempted and modified as needed. Although it is unlikely that a “one-size-fits-all” approach will work considering the range of physicochemical properties that GFNs can have, development of efficient and simple extraction techniques for GO, rGO, FLG, and single-layer graphene along with extraction techniques for GFNs with various lateral sizes and thicknesses would be very useful. Furthermore, extraction of all types of carbonaceous nanomaterials requires development of more general combined strategy approaches of filtration, differential extraction and functionalization/defunctionalization.91
Only a few studies have used extraction methods to isolate GFNs from environmental matrices. In Doudrick et al,96 both FLG and GO were extracted from biomass grown from return activated sludge. Solvable, a tissue solubilizer consisting of sodium hydroxide, C10-16-alkyldimethyl, N-oxide, and C11-15-secondary, ethoxylated alcohol, was used for matrix digestion (at 60 °C for 24 h). For GO, a sodium borohydride (NaBH4) reduction step was required to remove surface-bound oxygen from GO and thus increase its hydrophobicity for pellet formation during centrifugation. The samples were centrifuged, washed twice with water, and the formed pellet was analyzed with PTA. Extraction of 20 µg GFNs from 200 mg dried biomass/L wastewater followed by PTA analysis yielded recoveries of 52 ± 8 % and 80 ± 6 % for FLG and GO, respectively.96 The authors also tried phase-separation of reduced GO by heating for longer times in the NaBH4 step and allowing the surfactant phase of Solvable, containing rGO, to separate from the aqueous phase; this increased the reduced GO recovery, but was not successful at phase-transferring the FLG.96 Overall, this study demonstrates the challenge in recovering GFNs with a range of surface functionalities, especially without reduction of GO to a more thermally stable form. In another study, graphene was extracted from water using oil, toluene, and hexanes, but this study did not consider the effect of these solvents on environmental media where other hydrophobic components would transfer to the hydrophobic phase.222 Finally, a separate study made use of GO and rGO as a microbarrier between water and dispersed organic droplets, where spontaneous assembly of GO and rGO sheets was observed at the droplet interfaces with ‘tunability’ of the process possible using multivalent cations.223 Applications of approaches such as this to environmental matrices may be worthwhile to investigate further as there are currently no studies involving GFN extraction methods for cells, tissues, soils, sediments, and complex waters other than wastewater. The approaches may, in some cases, be matrix specific such as lysing cells first in biological systems or filtration techniques in the case of soils and sediments.
Ideally, extraction techniques should be able to completely separate interfering materials or compounds in the matrix from the GFN of interest or completely eliminate the interfering materials while preserving the GFN. However, methods that can sufficiently reduce the amount of interfering material such these substances no longer overwhelm the signal from the GFN of interest may also be successful. For instance, thermally stable or graphenic materials (other than GFNs) from complex media such as soil and sediment must not phase-transfer when using techniques such as PTA and Raman spectroscopy, but if they do, microscopy (which is time-consuming) may become a more viable option since the matrix materials will be less abundant. For released GFNs from polymer nanocomposites, many GFNs will be encased partially or fully in polymer fragments, which will alter their ability to phase-transfer, and extraction techniques that phase-transfer particular polymer types might be more suitable or need to be used in addition to GFN extraction techniques.
Currently, the ability to distinguish other carbonaceous nanomaterials (of high thermal stability) from GFNs with the centrifugal separation method using Solvable or the other methods described for CNTs in this section have not been thoroughly explored, and further study would be useful. In one study, a quantitative method based on isolating CNTs with specific DNA oligomers successfully separated CNTs from GO.168 However, it is unknown if the method will be applicable to isolating GFNs from other natural and engineered CNMs.168 Future application of this approach to a range of GFNs is worthwhile since this method was useful for extraction from an environmental matrix.168 In addition, the orientation of engineered CNMs (such as CNTs, graphene, and GO) in suspension can be ordered upon the application of external stimuli such as induced flow, or magnetic or electric field.64, 224, 225 The differences in the degree and potential for ordering of different CNMs under different scenarios may be useful for separating GFNs from other types of engineered CNMs, and this possibility is worth exploring.
GFN DETECTION/QUANTIFICATION CASE STUDY
For GFN quantification, one of the first considerations is which instruments are available in the testing laboratory. In Figure 4, the availability of different techniques for GFN quantification is compared based on their availability for purchase and their availability in environmental testing laboratories. It is interesting to note that most of the techniques discussed in this paper are commercially available, but most of the techniques are not typically available in environmental testing laboratories. Another important consideration relates to the method used to stabilize the GFN in water. If a dispersant is needed, that could influence the technique selected, while the potential for the GFNs to agglomerate in the water could also impact the feasibility of using some techniques. One helpful approach to understand the appropriate choice and use of GFN quantification techniques is to consider two case studies where there are clear advantages to using different sets of techniques. Two realistic scenarios for graphene quantification involve 1) regulatory toxicity testing of a GFN using an acute immobilization method for Daphnia magna (OECD method 202) and 2) monitoring the concentration of a GFN released from a manufacturing plant in industrial effluent discharged into a receiving river to insure GFN concentrations in the river are below a specific hazard level. To describe how to quantify the GFNs in the case studies described above, we will assume that all commercially available techniques are available to discuss how quantification could be addressed in a best-case scenario without instrumental limitations. Compared to the effluent scenario, it will be more straightforward to analyze the suspended GFN in the toxicity testing stock suspensions for the D. magna assay since there will be fewer interfering compounds present. In contrast, when the effluent is mixed with the natural water present in the river, there will likely be NOM and other suspended organic and inorganic particles. For the D. magna assay, the technique to use depends mainly on the detection limit needed, and if the stock suspension or the suspension in the test media before or after the test is being analyzed. As described in Table S1, a relatively low detection limit can be obtained with PTA analysis for the stock suspension and this technique should work in this stock suspension regardless of the GFN thermal properties because there are no other compounds present that would interfere with GFN detection (unless a dispersant is used to help suspend the GFN). Other techniques may also be applicable but would depend on their limits of detection, which would need to be tested given the lack of data on this topic in the published literature, and if interferences in the test media could impact the measurement. For example, one complication for UV/Vis spectroscopy analysis is that the GFNs may agglomerate during the exposure and this could impact the absorption coefficient which may in turn bias the concentrations measured.226 The situation is more complex for the river water scenario as described above. A first step is to collect water prior to the point where the manufacturing plant contacts the river (i.e., the influent), and to assess to what degree the properties of the river water could impact the test results for various techniques (e.g., the impact of NOM on TOC or Raman measurements). If the GFN to be tested is available, it could then be dispersed and spiked into the river water to evaluate potential matrix effects and recovery for different techniques which include Raman spectroscopy, PTA, and extraction followed by UV-Vis. Based on these results and the detection limits needed, the best technique can be selected to evaluate the test sample. As a last resort due its time-consuming nature, SEM or TEM microscopy could be applied with dried-down river water samples for counting, assuming the matrix does not overwhelm identification of the GFNs.
Figure 4.
The availability of different techniques for GFN quantification. Techniques are compared based on their availability for purchase and their availability in environmental testing laboratories
OUTLOOK AND FUTURE RESEARCH TOPICS
The concentration of GFNs released into the environment needs to be better understood. The lack of data on environmental GFN concentrations is a result of scattered information on the number of products on the market that hinders predictive modeling, the difficulty of detecting the very low GFN concentrations (e.g., ng/L) expected in the environment, environmental transformations of GFNs or interactions with other particles or NOM in the environment, and the lack of techniques for GFN detection in complex environmental media and biological systems. The quantity of GFNs released from polymer nanocomposites and other consumer-relevant matrices is also largely unknown, especially relative to CNTs where the high aspect ratio, molecular structure, and entanglement have shown low CNT release (undetectable to µg level).90, 111, 112, 149, 227
Currently, the ability to quantify GFNs in purified, natural and synthetic aqueous media is in its infancy and requires further development. For example, it may be possible to further improve PTA by combining that analysis with Raman given that the D to G band ratios may reveal insights into the thermal stability of the GFN, as has been previously demonstrated for CNTs,170 and therefore inform the thermal program to use. Additional work on hybrid Raman-PTA instruments could be valuable as could investigations into different carrier gases or the addition of new detectors to PTA instruments. Detection and quantification are even more challenging in complex matrices where creative approaches would be most valuable for environmentally relevant studies. Complex matrices often possess unique interferences that affect GFN detection and quantification. The potential biases and limitations of each method can potentially be overcome by using multiple analytical techniques for a given GFN/matrix. Some methods such as thermal analysis and UV-Vis spectroscopy were found to provide similar detection events for GFNs and other CNMs, such as CNTs, while other methods such as Raman spectroscopy and microscopy, have subtle or substantial differences allowing for a more unique level of detection of GFNs in the presence of other CNMs. Techniques such as Raman spectroscopy require further development to permit quantification in addition to detection, while labeling (e.g., with 14C or FITC) has been successfully used in multiple laboratory studies. Other techniques that can distinguish between GFNs and other carbonaceous (nano)materials, such as microscopy, may be combined with thermal techniques for a more reliable quantification of GFNs in complex aqueous matrices.
Extraction is often necessary for reliable quantification of GFNs, especially when GFNs are present in natural aquatic and terrestrial media, and biological matrices. However, methods for extracting GFNs from natural and synthetic matrices are currently scarce.96 Furthermore, the mass concentration of GFNs in the environment is expected to be in the ng/L range, and without isolation and concentration of GFNs, the use of the techniques described in this text are limited for GFN detection in environmental samples. Developing robust extraction methods can also enable more cost-effective options for environmental testing laboratories to detect and quantify GFNs present in environmental matrices. Extraction techniques must also be designed to consider the range of physicochemical properties that GFNs can have in order to specifically target GO, rGO, FLG, or single-layer graphene along with GFNs having a range of lateral sizes and thicknesses. Currently, it is not clear if the extraction methods previously developed for carbon nanotubes (CNTs) will be as effective for GFNs due to differences in physicochemical properties.82, 96, 168, 228, 229 Also, existing extraction methods are not likely to effectively distinguish between the different types of CNMs (i.e., CNTs, fullerenes, GFNs, etc.), should they co-exist in a matrix. In addition to selectivity between the different engineered CNMs, such techniques should also be able to separate GFNs from incidental and naturally-occurring carbonaceous particles such as soot and black carbon. Once methods for extracting and analyzing GFNs in natural and synthetic matrices are readily available, the ability to comprehensively quantify GFN exposure will be achieved. Consequently, research geared towards the development of extraction techniques that are specific to GFNs and work across relevant matrices is needed.
As reported in this review, several studies have shown that transformations of GFNs (including graphene and GO) can occur in aquatic ecosystems via irradiation, and chemical reactions involving enzymes, ROS, and reducing agents.40, 42, 202, 203 These transformations are important because they influence not only the fate and ecotoxicological effects of the nanomaterials,43, 50 but also how their extraction, detection and quantification is approached (especially when using spectroscopic, thermal, and microscopic techniques).203 While several studies have investigated the transformation of GFNs in aqueous media, information on the transformations of the nanomaterials in other relevant natural and anthropogenic matrices is rare. Therefore, studies investigating the transformation (both physical and chemical) of GFNs in cells and tissues, nanocomposites, soils and sediments are urgently needed to reduce biases that can result from not accounting for different GFN forms. Developing methods to extract, detect and quantify GFNs, including transformed GFNs, in complex natural systems is critical to understanding the effects of these nanoparticles on human health and ecological systems.
Increased implementation of GFNs in consumer products requires a reduction in the uncertainty surrounding their environmental impact. This reduction in uncertainty can be accomplished by the continued progress of analytical method development to detect and quantify GFNs in environmentally relevant matrices. The capacity to detect GFNs in these matrices at increasingly lower concentrations with greater precision and selectivity is expected to yield new insights into the toxicity mechanisms of GFNs in cells and organisms. It will also help accurately model the environmental fate and transformations of these materials in the natural environment. This information will ultimately enable the optimal design of GFN-enabled products that utilize their superior properties while minimizing potential adverse effects.
Supplementary Material
Footnotes
Tables describing lowest reported detection limits and potential matrix interferences for GFN quantification, and figures showing the structures of different carbonaceous nanomaterials, thermogravimetric profiles of GFNs and matrices, and examples of biases in different matrices. This information is available free of charge via the Internet at http://pubs.acs.org.
Disclaimers
Certain commercial products or equipment are described in this paper in order to specify adequately the experimental procedure. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that it is necessarily the best available for the purpose. This is NHEERL Contribution ORD-022716. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. Any mention of trade names, products, or services does not imply an endorsement by the U.S. Government or the U.S. Environmental Protection Agency. The EPA does not endorse any commercial products, services, or enterprises.
References
- 1.Bianco A, Cheng H-M, Enoki T, Gogotsi Y, Hurt RH, Koratkar N, Kyotani T, Monthioux M, Park CR, Tascon JM. All in the graphene family–A recommended nomenclature for two-dimensional carbon materials. Carbon. 2013;65:1–6. [Google Scholar]
- 2.Rao C, Subrahmanyam K, Matte HR, Abdulhakeem B, Govindaraj A, Das B, Kumar P, Ghosh A, Late DJ. A study of the synthetic methods and properties of graphenes. Sci. Technol. Adv. Mater. 2010;11(5):054502. doi: 10.1088/1468-6996/11/5/054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv.Mater. 2010;22(35):3906–3924. doi: 10.1002/adma.201001068. [DOI] [PubMed] [Google Scholar]
- 4.Dreyer DR, Park S, Bielawski CW, Ruoff RS. The chemistry of graphene oxide. Chem. Soc. Rev. 2010;39(1):228–240. doi: 10.1039/b917103g. [DOI] [PubMed] [Google Scholar]
- 5.Park S, Ruoff RS. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009;4(4):217–224. doi: 10.1038/nnano.2009.58. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Bao Q, Tang LAL, Zhong Y, Loh KP. Hydrothermal Dehydration for the “Green” Reduction of Exfoliated Graphene Oxide to Graphene and Demonstration of Tunable Optical Limiting Properties. Chem. Mater. 2009;21(13):2950–2956. [Google Scholar]
- 7.Wang H, Robinson JT, Li X, Dai H. Solvothermal Reduction of Chemically Exfoliated Graphene Sheets. J. Am. Chem. Soc. 2009;131(29):9910–9911. doi: 10.1021/ja904251p. [DOI] [PubMed] [Google Scholar]
- 8.Gengler RYN, Badali DS, Zhang D, Dimos K, Spyrou K, Gournis D, Miller RJD. Revealing the ultrafast process behind the photoreduction of graphene oxide. Nat. Commun. 2013;4 doi: 10.1038/ncomms3560. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto Y, Koinuma M, Kim SY, Watanabe Y, Taniguchi T, Hatakeyama K, Tateishi H, Ida S. Simple Photoreduction of Graphene Oxide Nanosheet under Mild Conditions. ACS Appl. Mater. Interfaces. 2010;2(12):3461–3466. doi: 10.1021/am100900q. [DOI] [PubMed] [Google Scholar]
- 10.Gurunathan S, Han JW, Eppakayala V, Kim J-H. Microbial reduction of graphene oxide by Escherichia coli: A green chemistry approach. Colloids Surf. B: Biointerfaces. 2013;102:772–777. doi: 10.1016/j.colsurfb.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Zhu S, Zhang J, Liu X, Li B, Wang X, Tang S, Meng Q, Li Y, Shi C, Hu R. Graphene quantum dots with controllable surface oxidation, tunable fluorescence and up-conversion emission. RSC Adv. 2012;2(7):2717–2720. [Google Scholar]
- 12.Choi S-H. Unique properties of graphene quantum dots and their applications in photonic/electronic devices. J. Phys. D: Appl. Phys. 2017;50(10):103002. [Google Scholar]
- 13.Lee C, Wei X, Kysar JW, Hone J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science. 2008;321(5887):385–388. doi: 10.1126/science.1157996. [DOI] [PubMed] [Google Scholar]
- 14.Harvey PD. Engineering properties of steel. ASM Intl; 1982. [Google Scholar]
- 15.Allen MJ, Tung VC, Kaner RB. Honeycomb carbon: a review of graphene. Chem. Rev. 2009;110(1):132–145. doi: 10.1021/cr900070d. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Kim F, Huang J. Seeing graphene-based sheets. Mater. Today. 2010;13(3):28–38. [Google Scholar]
- 17.Scott A. Graphene’s Global Race to Market (citing an IDTechEx market report) Chemical & Engineering News. 94:28–33. [Google Scholar]
- 18.Ciriminna R, Zhang N, Yang M-Q, Meneguzzo F, Xu Y-J, Pagliaro M. Commercialization of graphene-based technologies: a critical insight. Chem. Commun. 2015;51(33):7090–7095. doi: 10.1039/c5cc01411e. [DOI] [PubMed] [Google Scholar]
- 19.Press Release: Graphene, 2D Materials and Carbon Nanotubes: Markets, Technologies and Opportunities 2017–2027. IDTechEx [Google Scholar]
- 20.Ren W, Cheng H-M. The global growth of graphene. Nat. Nanotechnol. 2014;9(10):726–730. doi: 10.1038/nnano.2014.229. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamoorthy K, Jeyasubramanian K, Premanathan M, Subbiah G, Shin HS, Kim SJ. Graphene oxide nanopaint. Carbon. 2014;72:328–337. [Google Scholar]
- 22.Zhao J, Deng B, Lv M, Li J, Zhang Y, Jiang H, Peng C, Li J, Shi J, Huang Q, Fan C. Graphene Oxide-Based Antibacterial Cotton Fabrics. Adv. Healthc. Mater. 2013;2(9):1259–1266. doi: 10.1002/adhm.201200437. [DOI] [PubMed] [Google Scholar]
- 23.Bae S, Kim H, Lee Y, Xu X, Park J-S, Zheng Y, Balakrishnan J, Lei T, Kim HR, Song YI. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010;5(8):574–578. doi: 10.1038/nnano.2010.132. [DOI] [PubMed] [Google Scholar]
- 24.G6-Epoxy™. https://g6-epoxy.com/shop?olsPage=products&olsFocus=false (3/23/17)
- 25.Box TG. http://www.thegraphenebox.com/ (3/23/17)
- 26.Catlike Catlike Mixino Helmet. https://www.amazon.com/gp/product/B00K7LVMYK/ref=as_li_tl?ie=UTF8&camp=1789&creative=390957&creativeASIN=B00K7LVMYK&linkCode=as2&tag=grapheneinfo-20&linkId=DEL3NY5LLNJDRVXI (3/23/17)
- 27.Head GrapheNext. http://www.head.com/us/sports/tennis/technology/graphene-xt/ (2/23/17)
- 28.Nanotechnologies TPOE. Consumer Products Inventory: Graphene. http://www.nanotechproject.org/cpi/browse/nanomaterials/graphene/ (3/23/17)
- 29.Amazon Turnigy Graphene 1500mAh 4S 65C LiPo Pack w/ XT60. https://www.amazon.com/gp/product/B01HEMH2KM/ref=s9_dcacsd_bhz_bw_c_x_1_w (3/23/17)
- 30.Zhao J, Wang Z, White JC, Xing B. Graphene in the Aquatic Environment: Adsorption, Dispersion, Toxicity and Transformation. Environ. Sci. Technol. 2014;48(17):9995–10009. doi: 10.1021/es5022679. [DOI] [PubMed] [Google Scholar]
- 31.Ou L, Song B, Liang H, Liu J, Feng X, Deng B, Sun T, Shao L. Toxicity of graphene-family nanoparticles: a general review of the origins and mechanisms. Part. Fibre Toxicol. 2016;13(1):57. doi: 10.1186/s12989-016-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chowdhury I, Duch MC, Mansukhani ND, Hersam MC, Bouchard D. Colloidal Properties and Stability of Graphene Oxide Nanomaterials in the Aquatic Environment. Environ. Sci. Technol. 2013;47(12):6288–6296. doi: 10.1021/es400483k. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, Raliya R, Fortner JD, Biswas P. Graphene Oxides in Water: Correlating Morphology and Surface Chemistry with Aggregation Behavior. Environ. Sci. Technol. 2016;50(13):6964–6973. doi: 10.1021/acs.est.6b00810. [DOI] [PubMed] [Google Scholar]
- 34.Yang KJ, Chen BL, Zhu XY, Xing BS. Aggregation, Adsorption, and Morphological Transformation of Graphene Oxide in Aqueous Solutions Containing Different Metal Cations. Environ. Sci. Technol. 2016;50(20):11066–11075. doi: 10.1021/acs.est.6b04235. [DOI] [PubMed] [Google Scholar]
- 35.Wu L, Liu L, Gao B, Muñoz-Carpena R, Zhang M, Chen H, Zhou Z, Wang H. Aggregation Kinetics of Graphene Oxides in Aqueous Solutions: Experiments, Mechanisms, and Modeling. Langmuir. 2013;29(49):15174–15181. doi: 10.1021/la404134x. [DOI] [PubMed] [Google Scholar]
- 36.Chowdhury I, Mansukhani ND, Guiney LM, Hersam MC, Bouchard D. Aggregation and Stability of Reduced Graphene Oxide: Complex Roles of Divalent Cations, pH, and Natural Organic Matter. Environ. Sci. Technol. 2015;49(18):10886–10893. doi: 10.1021/acs.est.5b01866. [DOI] [PubMed] [Google Scholar]
- 37.Su Y, Yang G, Lu K, Petersen EJ, Mao L. Colloidal properties and stability of aqueous suspensions of few-layer graphene: Importance of graphene concentration. Environ. Pollut. 2017;220(Part A):469–477. doi: 10.1016/j.envpol.2016.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suárez-Iglesias O, Collado S, Oulego P, Díaz M. Graphene-family nanomaterials in wastewater treatment plants. Chem. Eng. J. 2017;313:121–135. [Google Scholar]
- 39.Wang F, Wang F, Gao G, Chen W. Transformation of graphene oxide by ferrous iron: environmental implications. Environ. Toxicol. Chem. 2015;34(9):1975–1982. doi: 10.1002/etc.3055. [DOI] [PubMed] [Google Scholar]
- 40.Hou W-C, Chowdhury I, Goodwin DG, Jr, Henderson WM, Fairbrother DH, Bouchard D, Zepp RG. Photochemical transformation of graphene oxide in sunlight. Environ. Sci. Technol. 2015;49(6):3435–3443. doi: 10.1021/es5047155. [DOI] [PubMed] [Google Scholar]
- 41.Hou W-C, Henderson WM, Chowdhury I, Goodwin DG, Chang X, Martin S, Fairbrother DH, Bouchard D, Zepp RG. The contribution of indirect photolysis to the degradation of graphene oxide in sunlight. Carbon. 2016;110:426–437. [Google Scholar]
- 42.Feng Y, Lu K, Mao L, Guo X, Gao S, Petersen EJ. Degradation of 14 C-labeled few layer graphene via Fenton reaction: Reaction rates, characterization of reaction products, and potential ecological effects. Water Res. 2015;84:49–57. doi: 10.1016/j.watres.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Chowdhury I, Hou W-C, Goodwin D, Henderson M, Zepp RG, Bouchard D. Sunlight affects aggregation and deposition of graphene oxide in the aquatic environment. Water Res. 2015;78:37–46. doi: 10.1016/j.watres.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Zhao J, Liu FF, Wang ZY, Cao XS, Xing BS. Heteroaggregation of Graphene Oxide with Minerals in Aqueous Phase. Environ. Sci. Technol. 2015;49(5):2849–2857. doi: 10.1021/es505605w. [DOI] [PubMed] [Google Scholar]
- 45.Gottschalk F, Sun T, Nowack B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013;181:287–300. doi: 10.1016/j.envpol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Dong S, Xia T, Yang Y, Lin S, Mao L. Bioaccumulation of 14C-Labeled Graphene in an Aquatic Food Chain through Direct Uptake or Trophic Transfer. Environ. Sci. Technol. 2017 doi: 10.1021/acs.est.7b04339. [DOI] [PubMed] [Google Scholar]
- 47.Su Y, Tong X, Huang C, Chen J, Liu S, Gao S, Mao L, Xing B. Green Algae as Carriers Enhance the Bioavailability of 14C-Labeled Few-Layer Graphene to Freshwater Snails. Environ. Sci. Technol. 2017 doi: 10.1021/acs.est.7b05796. [DOI] [PubMed] [Google Scholar]
- 48.Ge Y, Priester JH, Mortimer M, Chang CH, Ji Z, Schimel JP, Holden PA. Long-term effects of multiwalled carbon nanotubes and graphene on microbial communities in dry soil. Environ. Sci. Technol. 2016;50(7):3965–3974. doi: 10.1021/acs.est.5b05620. [DOI] [PubMed] [Google Scholar]
- 49.Combarros R, Collado S, Díaz M. Toxicity of graphene oxide on growth and metabolism of Pseudomonas putida. J. Hazard. Mater. 2016;310:246–252. doi: 10.1016/j.jhazmat.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 50.Hou W-C, Lee P-L, Chou Y-C, Wang Y-S. Antibacterial Property of Graphene Oxide: the Role of Phototransformation. Environ. Sci.: Nano. 2017;4(3):647–657. [Google Scholar]
- 51.Nguyen HN, Castro-Wallace SL, Rodrigues DF. Acute toxicity of graphene nanoplatelets on biological wastewater treatment process. Environ. Sci.: Nano. 2017;4(1):160–169. [Google Scholar]
- 52.Liu S-B, Zeng T-YH, Hofmann M, Burcombe E, Wei J, Jiang R-R, Kong J, Chen Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano. 2011;5(9):6971–6980. doi: 10.1021/nn202451x. [DOI] [PubMed] [Google Scholar]
- 53.Perreault F, de Faria AF, Nejati S, Elimelech M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano. 2015;9(7):7226–7236. doi: 10.1021/acsnano.5b02067. [DOI] [PubMed] [Google Scholar]
- 54.Chen M, Yin J, Liang Y, Yuan S, Wang F, Song M, Wang H. Oxidative stress and immunotoxicity induced by graphene oxide in zebrafish. Aquat. Toxicol. 2016;174:54–60. doi: 10.1016/j.aquatox.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Fan W, Liu Y, Xu Z, Wang X, Li X, Luo S. The mechanism of chronic toxicity to Daphnia magna induced by graphene suspended in a water column. Environ. Sci.: Nano. 2016;3(6):1405–1415. [Google Scholar]
- 56.Li S, Pan X, Wallis LK, Fan Z, Chen Z, Diamond SA. Comparison of TiO 2 nanoparticle and graphene–TiO 2 nanoparticle composite phototoxicity to Daphnia magna and Oryzias latipes. Chemosphere. 2014;112:62–69. doi: 10.1016/j.chemosphere.2014.03.058. [DOI] [PubMed] [Google Scholar]
- 57.Mao L, Liu C, Lu K, Su Y, Gu C, Huang Q, Petersen EJ. Exposure of few layer graphene to Limnodrilus hoffmeisteri modifies the graphene and changes its bioaccumulation by other organisms. Carbon. 2016;109:566–574. doi: 10.1016/j.carbon.2016.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo X, Dong S, Petersen EJ, Gao S, Huang Q, Mao L. Biological uptake and depuration of radio-labeled graphene by daphnia magna. Environ. Sci. Technol. 2013;47(21):12524–12531. doi: 10.1021/es403230u. [DOI] [PubMed] [Google Scholar]
- 59.Mukherjee A, Majumdar S, Servin AD, Pagano L, Dhankher OP, White JC. Carbon nanomaterials in agriculture: a critical review. Front. Plant Sci. 2016;7 doi: 10.3389/fpls.2016.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang M, Gao B, Chen J, Li Y. Effects of graphene on seed germination and seedling growth. J. Nanopart. Res. 2015;17(2):78. [Google Scholar]
- 61.Kang S, Herzberg M, Rodrigues DF, Elimelech M. Antibacterial effects of carbon nanotubes: size does matter! Langmuir. 2008;24(13):6409–6413. doi: 10.1021/la800951v. [DOI] [PubMed] [Google Scholar]
- 62.Nadres ET, Fan J, Rodrigues DF. Graphene-based Materials in Health and Environment. Springer; 2016. Toxicity and Environmental Applications of Graphene-Based Nanomaterials; pp. 323–356. [Google Scholar]
- 63.Goodwin DG, Jr, Marsh K, Sosa I, Payne J, Gorham J, Bouwer E, Fairbrother D. Interactions of microorganisms with polymer nanocomposite surfaces containing oxidized carbon nanotubes. Environ. Sci. Technol. 2015;49(9):5484–5492. doi: 10.1021/acs.est.5b00084. [DOI] [PubMed] [Google Scholar]
- 64.Lu X, Feng X, Werber JR, Chu C, Zucker I, Kim J-H, Osuji CO, Elimelech M. Enhanced antibacterial activity through the controlled alignment of graphene oxide nanosheets. Proc. Natl. Acad. Sci. U.S.A. 2017 doi: 10.1073/pnas.1710996114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Yuan H, von dem Bussche A, Creighton M, Hurt RH, Kane AB, Gao H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Natl. Acad. Sci. U.S.A. 2013;110(30):12295–12300. doi: 10.1073/pnas.1222276110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanchís J, Olmos M, Vincent P, Farré M, Barceló D. New insights on the influence of organic co-contaminants on the aquatic toxicology of carbon nanomaterials. Environ. Sci. Technol. 2015;50(2):961–969. doi: 10.1021/acs.est.5b03966. [DOI] [PubMed] [Google Scholar]
- 67.Hu X, Kang J, Lu K, Zhou R, Mu L, Zhou Q. Graphene oxide amplifies the phytotoxicity of arsenic in wheat. Sci. Rep. 2014;4:6122. doi: 10.1038/srep06122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mu L, Gao Y, Hu X. Characterization of Biological Secretions Binding to Graphene Oxide in Water and the Specific Toxicological Mechanisms. Environ. Sci. Technol. 2016;50(16):8530–8537. doi: 10.1021/acs.est.6b02494. [DOI] [PubMed] [Google Scholar]
- 69.Zhu S, Luo F, Chen W, Zhu B, Wang G. Toxicity evaluation of graphene oxide on cysts and three larval stages of Artemia salina. Sci. Total Environ. 2017;595:101–109. doi: 10.1016/j.scitotenv.2017.03.224. [DOI] [PubMed] [Google Scholar]
- 70.Mesarič T, Gambardella C, Milivojevič T, Faimali M, Drobne D, Falugi C, Makovec D, Jemec A, Sepčić K. High surface adsorption properties of carbon-based nanomaterials are responsible for mortality, swimming inhibition, and biochemical responses in Artemia salina larvae. Aquat. Toxicol. 2015;163(Supplement C):121–129. doi: 10.1016/j.aquatox.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 71.Gurunathan S, Han JW, Dayem AA, Eppakayala V, Kim J-H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomedicine. 2012;7:5901–5914. doi: 10.2147/IJN.S37397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Du T, Adeleye AS, Keller AA, Wu Z, Han W, Wang Y, Zhang C, Li Y. Photochlorination-induced transformation of graphene oxide: Mechanism and environmental fate. Water Res. 2017;124:372–380. doi: 10.1016/j.watres.2017.07.054. [DOI] [PubMed] [Google Scholar]
- 73.Ahmed F, Rodrigues DF. Investigation of acute effects of graphene oxide on wastewater microbial community: A case study. J. Hazard. Mater. 2013;256–257(Supplement C):33–39. doi: 10.1016/j.jhazmat.2013.03.064. [DOI] [PubMed] [Google Scholar]
- 74.Pretti C, Oliva M, Pietro RD, Monni G, Cevasco G, Chiellini F, Pomelli C, Chiappe C. Ecotoxicity of pristine graphene to marine organisms. Ecotoxicol. Environ. Saf. 2014;101:138–145. doi: 10.1016/j.ecoenv.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 75.Katsumiti A, Tomovska R, Cajaraville MP. Intracellular localization and toxicity of graphene oxide and reduced graphene oxide nanoplatelets to mussel hemocytes in vitro. Aquat. Toxicol. 2017;188(Supplement C):138–147. doi: 10.1016/j.aquatox.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 76.Souza JP, Baretta JF, Santos F, Paino IMM, Zucolotto V. Toxicological effects of graphene oxide on adult zebrafish (Danio rerio) Aquat. Toxicol. 2017;186(Supplement C):11–18. doi: 10.1016/j.aquatox.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 77.Wojtoniszak M, Chen X, Kalenczuk RJ, Wajda A, Łapczuk J, Kurzewski M, Drozdzik M, Chu PK, Borowiak-Palen E. Synthesis, dispersion, and cytocompatibility of graphene oxide and reduced graphene oxide. Colloids Surf. B: Biointerfaces. 2012;89(Supplement C):79–85. doi: 10.1016/j.colsurfb.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 78.Hu X, Wei Z, Mu L. Graphene oxide nanosheets at trace concentrations elicit neurotoxicity in the offspring of zebrafish. Carbon. 2017;117:182–191. [Google Scholar]
- 79.Chen Z, Westerhoff P, Herckes P. Quantification of C60 fullerene concentrations in water. Environ. Toxicol. Chem. 2008;27(9):1852–1859. doi: 10.1897/07-560.1. [DOI] [PubMed] [Google Scholar]
- 80.Isaacson CW, Kleber M, Field JA. Quantitative analysis of fullerene nanomaterials in environmental systems: a critical review. Environ. Sci. Technol. 2009;43(17):6463–6474. doi: 10.1021/es900692e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Isaacson CW, Usenko CY, Tanguay RL, Field JA. Quantification of fullerenes by LC/ESI-MS and its application to in vivo toxicity assays. Anal. Chem. 2007;79(23):9091–9097. doi: 10.1021/ac0712289. [DOI] [PubMed] [Google Scholar]
- 82.Schierz A, Parks AN, Washburn KM, Chandler GT, Ferguson PL. Characterization and quantitative analysis of single-walled carbon nanotubes in the aquatic environment using near-infrared fluorescence spectroscopy. Environ. Sci. Technol. 2012;46(22):12262–12271. doi: 10.1021/es301856a. [DOI] [PubMed] [Google Scholar]
- 83.Bisesi JH, Jr, Merten J, Liu K, Parks AN, Afrooz AN, Glenn JB, Klaine SJ, Kane AS, Saleh NB, Ferguson PL. Tracking and quantification of single-walled carbon nanotubes in fish using near infrared fluorescence. Environ. Sci. Technol. 2014;48(3):1973–1983. doi: 10.1021/es4046023. [DOI] [PubMed] [Google Scholar]
- 84.Bisesi JH, Ngo T, Ponnavolu S, Liu K, Lavelle CM, Afrooz A, Saleh NB, Ferguson PL, Denslow ND, Sabo-Attwood T. Examination of single-walled carbon nanotubes uptake and toxicity from dietary exposure: tracking movement and impacts in the gastrointestinal system. Nanomater. 2015;5(2):1066–1086. doi: 10.3390/nano5021066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adeleye AS, Keller AA. Long-term colloidal stability and metal leaching of single wall carbon nanotubes: effect of temperature and extracellular polymeric substances. Water Res. 2014;49(0):236–50. doi: 10.1016/j.watres.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 86.Reed RB, Goodwin DG, Marsh KL, Capracotta SS, Higgins CP, Fairbrother DH, Ranville JF. Detection of single walled carbon nanotubes by monitoring embedded metals. Environ. Sci.: Process. Impacts. 2013;15(1):204–213. doi: 10.1039/c2em30717k. [DOI] [PubMed] [Google Scholar]
- 87.Wang J, Lankone RS, Reed RB, Fairbrother DH, Ranville JF. Analysis of single-walled carbon nanotubes using spICP-MS with microsecond dwell time. NanoImpact. 2016;1:65–72. [Google Scholar]
- 88.Hanna SK, Miller RJ, Lenihan HS. Deposition of carbon nanotubes by a marine suspension feeder revealed by chemical and isotopic tracers. J. Hazard. Mater. 2014;279:32–37. doi: 10.1016/j.jhazmat.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 89.Petersen EJ, Flores-Cervantes DX, Bucheli TD, Elliott LC, Fagan JA, Gogos A, Hanna S, Kägi R, Mansfield E, Bustos ARM. Quantification of Carbon Nanotubes in Environmental Matrices: Current Capabilities, Case Studies, and Future Prospects. Environ. Sci. Technol. 2016;50(9):4587–4605. doi: 10.1021/acs.est.5b05647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Petersen EJ, Zhang L, Mattison NT, O’Carroll DM, Whelton AJ, Uddin N, Nguyen T, Huang Q, Henry TB, Holbrook RD. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ. Sci. Technol. 2011;45(23):9837–9856. doi: 10.1021/es201579y. [DOI] [PubMed] [Google Scholar]
- 91.Pycke BF, Chao T-C, Herckes P, Westerhoff P, Halden RU. Beyond nC60: strategies for identification of transformation products of fullerene oxidation in aquatic and biological samples. Anal. Bioanal. Chem. 2012;404(9):2583–2595. doi: 10.1007/s00216-012-6090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li D, Muller MB, Gilje S, Kaner RB, Wallace GG. Processable aqueous dispersions of graphene nanosheets. Nat. Nano. 2008;3(2):101–105. doi: 10.1038/nnano.2007.451. [DOI] [PubMed] [Google Scholar]
- 93.Bai H, Jiang W, Kotchey GP, Saidi WA, Bythell BJ, Jarvis JM, Marshall AG, Robinson RA, Star A. Insight into the mechanism of graphene oxide degradation via the photo-Fenton reaction. J. Phys. Chem. C. 2014;118(19):10519–10529. doi: 10.1021/jp503413s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kurapati R, Russier J, Squillaci MA, Treossi E, Ménard-Moyon C, Rio-Castillo D, Esaú A, Vazquez E, Samorì P, Palermo V. Dispersibility-Dependent Biodegradation of Graphene Oxide by Myeloperoxidase. Small. 2015;11(32):3985–3994. doi: 10.1002/smll.201500038. [DOI] [PubMed] [Google Scholar]
- 95.Adeleye AS, Wang X, Wang F, Hao R, Song W, Li Y. Photoreactivity of graphene oxide in aqueous system: Reactive oxygen species formation and bisphenol A degradation. Chemosphere. 2018;195:344–350. doi: 10.1016/j.chemosphere.2017.12.095. [DOI] [PubMed] [Google Scholar]
- 96.Doudrick K, Nosaka T, Herckes P, Westerhoff P. Quantification of graphene and graphene oxide in complex organic matrices. Environ. Sci.: Nano. 2015;2(1):60–67. [Google Scholar]
- 97.Huang G, Guo H, Zhao J, Liu Y, Xing B. Effect of co-existing kaolinite and goethite on the aggregation of graphene oxide in the aquatic environment. Water Res. 2016;102:313–320. doi: 10.1016/j.watres.2016.06.050. [DOI] [PubMed] [Google Scholar]
- 98.Lanphere JD, Rogers B, Luth C, Bolster CH, Walker SL. Stability and Transport of Graphene Oxide Nanoparticles in Groundwater and Surface Water. Environ. Eng. Sci. 2014;31(7):350–359. doi: 10.1089/ees.2013.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao Y, Ren X, Tan X, Hayat T, Alsaedi A, Chen C. Insights into key factors controlling GO stability in natural surface waters. J. Hazard. Mater. 2017;335:56–65. doi: 10.1016/j.jhazmat.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 100.Valentini L, Bon SB, Kenny JM. Emerging methods for producing graphene oxide composites in coatings with multifunctional properties. J. Mater. Chem. 2012;22(40):21355–21361. [Google Scholar]
- 101.Ferreira EM, Moutinho MV, Stavale F, Lucchese M, Capaz RB, Achete C, Jorio A. Evolution of the Raman spectra from single-, few-, and many-layer graphene with increasing disorder. Phys. Rev. B. 2010;82(12):125429. [Google Scholar]
- 102.Cançado LG, Jorio A, Ferreira EM, Stavale F, Achete C, Capaz R, Moutinho M, Lombardo A, Kulmala T, Ferrari A. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett. 2011;11(8):3190–3196. doi: 10.1021/nl201432g. [DOI] [PubMed] [Google Scholar]
- 103.Shang J, Ma L, Li J, Ai W, Yu T, Gurzadyan GG. The origin of fluorescence from graphene oxide. Sci. Rep. 2012;2:792. doi: 10.1038/srep00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keller AA, Lazareva A. Predicted Releases of Engineered Nanomaterials: From Global to Regional to Local. Environ. Sci. Technol. Lett. 2014;1(1):65–70. [Google Scholar]
- 105.Keller AA, McFerran S, Lazareva A, Suh S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013;15(6):1–17. [Google Scholar]
- 106.Mao L, Hu M, Pan B, Xie Y, Petersen EJ. Biodistribution and toxicity of radio-labeled few layer graphene in mice after intratracheal instillation. Part. Fibre Toxicol. 2016;13(1):1. doi: 10.1186/s12989-016-0120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim H, Abdala AA, Macosko CW. Graphene/polymer nanocomposites. Macromol. 2010;43(16):6515–6530. [Google Scholar]
- 108.Chee WK, Lim HN, Huang NM, Harrison I. Nanocomposites of graphene/polymers: a review. RSC Adv. 2015;5(83):68014–68051. [Google Scholar]
- 109.Kingston C, Zepp R, Andrady A, Boverhof D, Fehir R, Hawkins D, Roberts J, Sayre P, Shelton B, Sultan Y. Release characteristics of selected carbon nanotube polymer composites. Carbon. 2014;68:33–57. [Google Scholar]
- 110.Bernard C, Nguyen T, Pellegrin B, Holbrook RD, Zhao M, Chin J. Fate of graphene in polymer nanocomposite exposed to UV radiation. J. Phys.: Conf. Ser. 2011;304:012063/1–012063/8. [Google Scholar]
- 111.Harper S, Wohlleben W, Doa M, Nowack B, Clancy S, Canady R, Maynard A. Measuring nanomaterial release from carbon nanotube composites: review of the state of the science. J. Phys.: Conf. Ser. 2015;617:1–19. (4th International Conference on Safe Production and Use of Nanomaterials, 2014. [Google Scholar]
- 112.Wohlleben W, Kingston C, Carter J, Sahle-Demessie E, Vázquez-Campos S, Acrey B, Chen C-Y, Walton E, Egenolf H, Müller P, Zepp R. NanoRelease: Pilot interlaboratory comparison of a weathering protocol applied to resilient and labile polymers with and without embedded carbon nanotubes. Carbon. 2017;113:346–360. doi: 10.1016/j.carbon.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dhifaf AJ, Neus L, Kostas K. Synthesis of few-layered, high-purity graphene oxide sheets from different graphite sources for biology. 2D Mater. 2016;3(1):014006. [Google Scholar]
- 114.Duan L, Hao R, Xu Z, He X, Adeleye AS, Li Y. Removal of graphene oxide nanomaterials from aqueous media via coagulation: Effects of water chemistry and natural organic matter. Chemosphere. 2017;168:1051–1057. doi: 10.1016/j.chemosphere.2016.10.104. [DOI] [PubMed] [Google Scholar]
- 115.Nogueira PFM, Nakabayashi D, Zucolotto V. The effects of graphene oxide on green algae Raphidocelis subcapitata. Aquat. Toxicol. 2015;166:29–35. doi: 10.1016/j.aquatox.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 116.Ren X, Li J, Tan X, Shi W, Chen C, Shao D, Wen T, Wang L, Zhao G, Sheng G. Impact of Al2O3 on the aggregation and deposition of graphene oxide. Environ. Sci. Technol. 2014;48(10):5493–5500. doi: 10.1021/es404996b. [DOI] [PubMed] [Google Scholar]
- 117.Yousefi N, Gudarzi MM, Zheng Q, Aboutalebi SH, Sharif F, Kim J-K. Self-alignment and high electrical conductivity of ultralarge graphene oxide–polyurethane nanocomposites. J. Mater. Chem. 2012;22(25):12709–12717. [Google Scholar]
- 118.Yang Y, Yu Z, Nosaka T, Doudrick K, Hristovski K, Herckes P, Westerhoff P. Interaction of carbonaceous nanomaterials with wastewater biomass. Front. Environ. Sci. Eng. 2015;9(5):823–831. [Google Scholar]
- 119.Johra FT, Lee J-W, Jung W-G. Facile and safe graphene preparation on solution based platform. Ind. Eng. Chem. Res. 2014;20(5):2883–2887. [Google Scholar]
- 120.Rattana, Chaiyakun S, Witit-anun N, Nuntawong N, Chindaudom P, Oaew S, Kedkeaw C, Limsuwan P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012;32:759–764. [Google Scholar]
- 121.Fernández-Merino MJ, Paredes JI, Villar-Rodil S, Guardia L, Solís-Fernández P, Salinas-Torres D, Cazorla-Amorós D, Morallón E, Martínez-Alonso A, Tascón JMD. Investigating the influence of surfactants on the stabilization of aqueous reduced graphene oxide dispersions and the characteristics of their composite films. Carbon. 2012;50(9):3184–3194. [Google Scholar]
- 122.Konios D, Stylianakis MM, Stratakis E, Kymakis E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014;430:108–112. doi: 10.1016/j.jcis.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 123.Sun X, Liu Z, Welsher K, Robinson JT, Goodwin A, Zaric S, Dai H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008;1(3):203–212. doi: 10.1007/s12274-008-8021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bruice PY. Organic Chemistry. Prentice Hall; 2006. 2006. [Google Scholar]
- 125.Li R, Wang X, Ji Z, Sun B, Zhang H, Chang CH, Lin S, Meng H, Liao Y-P, Wang M, Li Z, Hwang AA, Song T-B, Xu R, Yang Y, Zink JI, Nel AE, Xia T. Surface Charge and Cellular Processing of Covalently Functionalized Multiwall Carbon Nanotubes Determine Pulmonary Toxicity. ACS Nano. 2013;7(3):2352–2368. doi: 10.1021/nn305567s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hyung H, Fortner JD, Hughes JB, Kim J-H. Natural organic matter stabilizes carbon nanotubes in the aqueous phase. Environ. Sci. Technol. 2007;41(1):179–184. doi: 10.1021/es061817g. [DOI] [PubMed] [Google Scholar]
- 127.Neubauer N, Wohlleben W, Tomović Ž. Conductive plastics: comparing alternative nanotechnologies by performance and life cycle release probability. J. Nanopart. Res. 2017;19(3):112. [Google Scholar]
- 128.Wohlleben W, Meyer J, Muller J, Muller P, Vilsmeier K, Stahlmecke B, Kuhlbusch TAJ. Release from nanomaterials during their use phase: combined mechanical and chemical stresses applied to simple and multi-filler nanocomposites mimicking wear of nano-reinforced tires. Environ. Sci.: Nano. 2016;3(5):1036–1051. [Google Scholar]
- 129.Wohlleben W, Meier MW, Vogel S, Landsiedel R, Cox G, Hirth S, Tomovic Z. Elastic CNT-polyurethane nanocomposite: synthesis, performance and assessment of fragments released during use. Nanoscale. 2013;5(1):369–380. doi: 10.1039/c2nr32711b. [DOI] [PubMed] [Google Scholar]
- 130.Vempati S, Uyar T. Fluorescence from graphene oxide and the influence of ionic, π–π interactions and heterointerfaces: electron or energy transfer dynamics. Phys. Chem. Chem. Phys. 2014;16(39):21183–21203. doi: 10.1039/c4cp03317e. [DOI] [PubMed] [Google Scholar]
- 131.Geim AK, Novoselov KS. The rise of graphene. Nat. Mater. 2007;6(3):183–191. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 132.Mei Q, Zhang K, Guan G, Liu B, Wang S, Zhang Z. Highly efficient photoluminescent graphene oxide with tunable surface properties. Chem. Commun. 2010;46(39):7319–7321. doi: 10.1039/c0cc02374d. [DOI] [PubMed] [Google Scholar]
- 133.Li J, Zhang W, Chung T-F, Slipchenko MN, Chen YP, Cheng J-X, Yang C. Highly sensitive transient absorption imaging of graphene and graphene oxide in living cells and circulating blood. Sci. Rep. 2015;5:12394. doi: 10.1038/srep12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rabek JF. Photodegradation of polymers: physical characteristics and applications. Springer Science & Business Media; 2012. [Google Scholar]
- 135.Dresselhaus MS, Jorio A, Hofmann M, Dresselhaus G, Saito R. Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy. Nano Lett. 2010;10(3):751–758. doi: 10.1021/nl904286r. [DOI] [PubMed] [Google Scholar]
- 136.Ouyang S, Hu X, Zhou Q. Envelopment–Internalization Synergistic Effects and Metabolic Mechanisms of Graphene Oxide on Single-Cell Chlorella vulgaris Are Dependent on the Nanomaterial Particle Size. ACS Appl. Mater. Interfaces. 2015;7(32):18104–18112. doi: 10.1021/acsami.5b05328. [DOI] [PubMed] [Google Scholar]
- 137.Hu XG, Gao Y, Fang Z. Integrating metabolic analysis with biological endpoints provides insight into nanotoxicological mechanisms of graphene oxide: From effect onset to cessation. Carbon. 2016;109:65–73. [Google Scholar]
- 138.Yang Y, Chase HA. Applications of Raman and Surface-Enhanced Raman Scattering Techniques to Humic Substances. Spectrosc. Lett. 1998;31(4):821–848. [Google Scholar]
- 139.Villanueva U, Raposo JC, Castro K, de Diego A, Arana G, Madariaga JM. Raman spectroscopy speciation of natural and anthropogenic solid phases in river and estuarine sediments with appreciable amount of clay and organic matter. J. Raman Spectrosc. 2008;39(9):1195–1203. [Google Scholar]
- 140.Salzmann CG, Chu BT, Tobias G, Llewellyn SA, Green ML. Quantitative assessment of carbon nanotube dispersions by Raman spectroscopy. Carbon. 2007;45(5):907–912. [Google Scholar]
- 141.Hu X, Kang W, Mu L. Aqueously released graphene oxide embedded in epoxy resin exhibits different characteristics and phytotoxicity of Chlorella vulgaris from the pristine form. Environ. Sci. Technol. 2017 doi: 10.1021/acs.est.7b00361. [DOI] [PubMed] [Google Scholar]
- 142.Jorio A, Ribeiro-Soares J, Cançado LG, Falcão NPS, Dos Santos HF, Baptista DL, Martins Ferreira EH, Archanjo BS, Achete CA. Microscopy and spectroscopy analysis of carbon nanostructures in highly fertile Amazonian anthrosoils. Soil Tillage Res. 2012;122:61–66. [Google Scholar]
- 143.Jorio A. Raman spectroscopy in graphene-based systems: Prototypes for nanoscience and nanometrology. ISRN Nanotechnol. 2012;2012 [Google Scholar]
- 144.Du T, Wang Y, Yang X, Wang W, Guo H, Xiong X, Gao R, Wuli X, Adeleye AS, Li Y. Mechanisms and kinetics study on the trihalomethanes formation with carbon nanoparticle precursors. Chemosphere. 2016;154:391–397. doi: 10.1016/j.chemosphere.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 145.Li Y, Yang N, Du T, Xia T, Zhang C, Chen W. Chloramination of graphene oxide significantly affects its transport properties in saturated porous media. NanoImpact. 2016;3–4:90–95. [Google Scholar]
- 146.Ferrari AC, Meyer JC, Scardaci V, Casiraghi C, Lazzeri M, Mauri F, Piscanec S, Jiang D, Novoselov KS, Roth S, Geim AK. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006;97(18):187401. doi: 10.1103/PhysRevLett.97.187401. [DOI] [PubMed] [Google Scholar]
- 147.Xing W, Lalwani G, Rusakova I, Sitharaman B. Degradation of Graphene by Hydrogen Peroxide. Part. Part. Syst. Charact. 2014;31(7):745–750. [Google Scholar]
- 148.Li Y, Yang N, Du T, Wang X, Chen W. Transformation of graphene oxide by chlorination and chloramination: Implications for environmental transport and fate. Water Res. 2016;103:416–423. doi: 10.1016/j.watres.2016.07.051. [DOI] [PubMed] [Google Scholar]
- 149.Petersen EJ, Lam T, Gorham JM, Scott KC, Long CJ, Stanley D, Sharma R, Alexander Liddle J, Pellegrin B, Nguyen T. Methods to assess the impact of UV irradiation on the surface chemistry and structure of multiwall carbon nanotube epoxy nanocomposites. Carbon. 2014;69:194–205. [Google Scholar]
- 150.Briggs D. Handbook of X-ray Photoelectron Spectroscopy CD Wanger, WM Riggs, LE Davis, JF Moulder and GE Muilenberg Perkin-Elmer Corp., Physical Electronics Division, Eden Prairie, Minnesota, USA. 1979. 190 pp. Surf. Interface Anal. 1981;3(4) [Google Scholar]
- 151.Thomas HR, Day SP, Woodruff WE, Vallés C, Young RJ, Kinloch IA, Morley GW, Hanna JV, Wilson NR, Rourke JP. Deoxygenation of Graphene Oxide: Reduction or Cleaning? Chem. Mater. 2013;25(18):3580–3588. [Google Scholar]
- 152.Oku T. Structure analysis of advanced nanomaterials: nanoworld by high-resolution electron microscopy. Walter de Gruyter GmbH & Co KG; 2014. [Google Scholar]
- 153.Stobinski L, Lesiak B, Malolepszy A, Mazurkiewicz M, Mierzwa B, Zemek J, Jiricek P, Bieloshapka I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron. Spectrosc. Relat. Phenom. 2014;195:145–154. [Google Scholar]
- 154.Lu K, Dong S, Petersen EJ, Niu J, Chang X, Wang P, Lin S, Gao S, Mao L. Biological Uptake, Distribution, and Depuration of Radio-Labeled Graphene in Adult Zebrafish: Effects of Graphene Size and Natural Organic Matter. ACS Nano. 2017;11(3):2872–2885. doi: 10.1021/acsnano.6b07982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schlagenhauf L, Buerki-Thurnherr T, Kuo Y-Y, Wichser A, Nuesch F, Wick P, Wang J. Carbon Nanotubes Released from an Epoxy-Based Nanocomposite: Quantification and Particle Toxicity. Environ. Sci. Technol. 2015;49(17):10616–10623. doi: 10.1021/acs.est.5b02750. [DOI] [PubMed] [Google Scholar]
- 156.Roth GA, Tahiliani S, Neu-Baker NM, Brenner SA. Hyperspectral microscopy as an analytical tool for nanomaterials. WIREs Nanomed. Nanobiotechnol. 2015;7(4):565–579. doi: 10.1002/wnan.1330. [DOI] [PubMed] [Google Scholar]
- 157.Chen YM, Ren CX, Ouyang SH, Hu XG, Zhou QX. Mitigation in Multiple Effects of Graphene Oxide Toxicity in Zebrafish Embryogenesis Driven by Humic Acid. Environ. Sci. Technol. 2015;49(16):10147–10154. doi: 10.1021/acs.est.5b02220. [DOI] [PubMed] [Google Scholar]
- 158.Du S, Zhang P, Zhang R, Lu Q, Liu L, Bao X, Liu H. Reduced graphene oxide induces cytotoxicity and inhibits photosynthetic performance of the green alga Scenedesmus obliquus. Chemosphere. 2016;164:499–507. doi: 10.1016/j.chemosphere.2016.08.138. [DOI] [PubMed] [Google Scholar]
- 159.Chen Y, Hu X, Sun J, Zhou Q. Specific nanotoxicity of graphene oxide during zebrafish embryogenesis. Nanotoxicol. 2016;10(1):42–52. doi: 10.3109/17435390.2015.1005032. [DOI] [PubMed] [Google Scholar]
- 160.Ren C, Hu X, Li X, Zhou Q. Ultra-trace graphene oxide in a water environment triggers Parkinson's disease-like symptoms and metabolic disturbance in zebrafish larvae. Biomater. 2016;93:83–94. doi: 10.1016/j.biomaterials.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 161.Talukdar Y, Rashkow J, Lalwani G, Kanakia S, Sitharaman B. The Effects of Graphene Nanostructures on Mesenchymal Stem Cells. Biomater. 2014;35(18):4863–4877. doi: 10.1016/j.biomaterials.2014.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vickerman JC, Gilmore I. Surface analysis: the principal techniques. John Wiley & Sons; 2011. [Google Scholar]
- 163.Eigler S, Grimm S, Hirsch A. Investigation of the thermal stability of the carbon framework of graphene oxide. Chem. Eur. J. 2014;20(4):984–989. doi: 10.1002/chem.201304048. [DOI] [PubMed] [Google Scholar]
- 164.Dreyer DR, Todd AD, Bielawski CW. Harnessing the chemistry of graphene oxide. Chem. Soc. Rev. 2014;43(15):5288–5301. doi: 10.1039/c4cs00060a. [DOI] [PubMed] [Google Scholar]
- 165.Rajagopalan B, Chung JS. Reduced chemically modified graphene oxide for supercapacitor electrode. Nanoscale Res. Lett. 2014;9(1):535. doi: 10.1186/1556-276X-9-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang J, Yang H, Shen G, Cheng P, Zhang J, Guo S. Reduction of graphene oxide vial-ascorbic acid. Chem. Commun. 2010;46(7):1112–1114. doi: 10.1039/b917705a. [DOI] [PubMed] [Google Scholar]
- 167.Bourlinos AB, Gournis D, Petridis D, Szabo T, Szeri A, Dekany I. Graphite oxide: Chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids. Langmuir. 2003;19(15):6050–6055. [Google Scholar]
- 168.Jeong J, Lee Y-j, Hwang Ys, Hong IS. Selective detection and quantification of carbon nanotubes in soil. Environ. Toxicol. Chem. 2015;34(9):1969–1974. doi: 10.1002/etc.3035. [DOI] [PubMed] [Google Scholar]
- 169.Silva RM, Doudrick K, Franzi LM, TeeSy C, Anderson DS, Wu Z, Mitra S, Vu V, Dutrow G, Evans JE, Westerhoff P, Van Winkle LS, Raabe OG, Pinkerton KE. Instillation versus Inhalation of Multiwalled Carbon Nanotubes: Exposure-Related Health Effects, Clearance, and the Role of Particle Characteristics. ACS Nano. 2014;8(9):8911–8931. doi: 10.1021/nn503887r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Doudrick K, Herckes P, Westerhoff P. Detection of Carbon Nanotubes in Environmental Matrices Using Programmed Thermal Analysis. Environ. Sci. Technol. 2012;46(22):12246–12253. doi: 10.1021/es300804f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sobek A, Bucheli TD. Testing the resistance of single- and multi-walled carbon nanotubes to chemothermal oxidation used to isolate soots from environmental samples. Environ. Pollut. 2009;157(4):1065–1071. doi: 10.1016/j.envpol.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 172.Plata DeL, Reddy CM, Gschwend PM. Thermogravimetry–mass spectrometry for carbon nanotube detection in complex mixtures. Environ. Sci. Technol. 2012;46(22):12254–12261. doi: 10.1021/es203198x. [DOI] [PubMed] [Google Scholar]
- 173.Vallés C, Kinloch IA, Young RJ, Wilson NR, Rourke JP. Graphene oxide and base-washed graphene oxide as reinforcements in PMMA nanocomposites. Compos. Sci. Technol. 2013;88:158–164. [Google Scholar]
- 174.Bora C, Gogoi P, Baglari S, Dolui SK. Preparation of polyester resin/graphene oxide nanocomposite with improved mechanical strength. J. Appl. Polym. Sci. 2013;129(6):3432–8. [Google Scholar]
- 175.Kattimuttathu SI, Chidambaram K, Vinod V, Rajender N, Venkateswara RM, Miroslav e. Synthesis, characterization and optical properties of graphene oxide-polystyrene nanocomposites. Polym. Adv. Technol. 2015;26(3):214–222. [Google Scholar]
- 176.Li Y, Lian H, Hu Y, Chang W, Cui X, Liu Y. Enhancement in Mechanical and Shape Memory Properties for Liquid Crystalline Polyurethane Strengthened by Graphene Oxide. Polymers. 2016;8(7):236. doi: 10.3390/polym8070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yu Y, Li Y, Pan Y, Liu C-j. Fabrication of palladium/graphene oxide composite by plasma reduction at room temperature. Nanoscale Res. Lett. 2012;7(1):234. doi: 10.1186/1556-276X-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hu XG, Lu KC, Mu L, Kang J, Zhou QX. Interactions between graphene oxide and plant cells: Regulation of cell morphology, uptake, organelle damage, oxidative effects and metabolic disorders. Carbon. 2014;80:665–676. [Google Scholar]
- 179.Alford R, Simpson HM, Duberman J, Hill GC, Ogawa M, Regino C, Kobayashi H, Choyke PL. Toxicity of organic fluorophores used in molecular imaging: literature review. Mol. Imaging. 2009;8(6) 7290.2009. 00031. [PubMed] [Google Scholar]
- 180.Bokobza L, Bruneel J-L, Couzi M. Raman spectra of carbon-based materials (from graphite to carbon black) and of some silicone composites. C. 2015;1(1):77–94. [Google Scholar]
- 181.Mauter MS, Elimelech M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 2008;42(16):5843–5859. doi: 10.1021/es8006904. [DOI] [PubMed] [Google Scholar]
- 182.Irin F, Shrestha B, Cañas JE, Saed MA, Green MJ. Detection of carbon nanotubes in biological samples through microwave-induced heating. Carbon. 2012;50(12):4441–4449. [Google Scholar]
- 183.Adeleye AS, Conway JR, Garner K, Huang Y, Su Y, Keller AA. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016;286:640–662. [Google Scholar]
- 184.Smith SC, Rodrigues DF. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: A review of mechanisms and applications. Carbon. 2015;91:122–143. [Google Scholar]
- 185.Ren XM, Wu QY, Xu H, Shao DD, Tan XL, Shi WQ, Chen CL, Li JX, Chai ZF, Hayat T, Wang XK. New Insight into GO, Cadmium(II), Phosphate Interaction and Its Role in GO Colloidal Behavior. Environ. Sci. Technol. 2016;50(17):9361–9369. doi: 10.1021/acs.est.6b02934. [DOI] [PubMed] [Google Scholar]
- 186.Vasudevan S, Lakshmi J. The adsorption of phosphate by graphene from aqueous solution. RSC Adv. 2012;2(12):5234–5242. [Google Scholar]
- 187.Lee BM, Hur J. Adsorption Behavior of Extracellular Polymeric Substances on Graphene Materials Explored by Fluorescence Spectroscopy and Two-Dimensional Fourier Transform Infrared Correlation Spectroscopy. Environ. Sci. Technol. 2016;50(14):7364–7372. doi: 10.1021/acs.est.6b01286. [DOI] [PubMed] [Google Scholar]
- 188.Chen XX, Chen BL. Macroscopic and Spectroscopic Investigations of the Adsorption of Nitroaromatic Compounds on Graphene Oxide, Reduced Graphene Oxide, and Graphene Nanosheets. Environ. Sci. Technol. 2015;49(10):6181–6189. doi: 10.1021/es5054946. [DOI] [PubMed] [Google Scholar]
- 189.Aboubaraka AE, Aboelfetoh EF, Ebeid E-ZM. Coagulation effectiveness of graphene oxide for the removal of turbidity from raw surface water. Chemosphere. 2017;181:738–746. doi: 10.1016/j.chemosphere.2017.04.137. [DOI] [PubMed] [Google Scholar]
- 190.Apul OG, Wang Q, Zhou Y, Karanfil T. Adsorption of aromatic organic contaminants by graphene nanosheets: Comparison with carbon nanotubes and activated carbon. Water Res. 2013;47(4):1648–1654. doi: 10.1016/j.watres.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 191.Wang H, Adeleye AS, Huang Y, Li F, Keller AA. Heteroaggregation of nanoparticles with biocolloids and geocolloids. Adv. Colloid Interface Sci. 2015;226(Pt A):24–36. doi: 10.1016/j.cis.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 192.Feng Y, Liu X, Huynh KA, McCaffery JM, Mao L, Gao S, Chen KL. Heteroaggregation of Graphene Oxide with Nanometer-and Micrometer-Sized Hematite Colloids: Influence on Nanohybrid Aggregation and Microparticle Sedimentation. Environ. Sci. Technol. 2017 doi: 10.1021/acs.est.7b00132. [DOI] [PubMed] [Google Scholar]
- 193.Hu XG, Zhou M, Zhou QX. Ambient Water and Visible-Light Irradiation Drive Changes in Graphene Morphology, Structure, Surface Chemistry, Aggregation, and Toxicity. Environ. Sci. Technol. 2015;49(6):3410–3418. doi: 10.1021/es503003y. [DOI] [PubMed] [Google Scholar]
- 194.IARC Monographs on the Evaluation of the Carconogenic Risks of Chemicals to Humans. International Agency for Research on Cancer; France. 1985. [Google Scholar]
- 195.Zhou X, Zhang Y, Wang C, Wu X, Yang Y, Zheng B, Wu H, Guo S, Zhang J. Photo-Fenton reaction of graphene oxide: a new strategy to prepare graphene quantum dots for DNA cleavage. ACS Nano. 2012;6(8):6592–6599. doi: 10.1021/nn301629v. [DOI] [PubMed] [Google Scholar]
- 196.Salas EC, Sun ZZ, Luttge A, Tour JM. Reduction of Graphene Oxide via Bacterial Respiration. ACS Nano. 2010;4(8):4852–4856. doi: 10.1021/nn101081t. [DOI] [PubMed] [Google Scholar]
- 197.Bansal P, Doshi S, Panwar AS, Bahadur D. Exoelectrogens Leading to Precise Reduction of Graphene Oxide by Flexibly Switching Their Environment during Respiration. ACS Appl. Mater. Interfaces. 2015;7(37):20576–20584. doi: 10.1021/acsami.5b04390. [DOI] [PubMed] [Google Scholar]
- 198.Wang G, Qian F, Saltikov CW, Jiao Y, Li Y. Microbial reduction of graphene oxide by Shewanella. Nano Res. 2011;4(6):563–570. [Google Scholar]
- 199.Akhavan O, Ghaderi E. Escherichia coli bacteria reduce graphene oxide to bactericidal graphene in a self-limiting manner. Carbon. 2012;50(5):1853–1860. [Google Scholar]
- 200.Thakur S, Karak N. Green reduction of graphene oxide by aqueous phytoextracts. Carbon. 2012;50(14):5331–5339. [Google Scholar]
- 201.Akhavan O. Bacteriorhodopsin as a superior substitute for hydrazine in chemical reduction of single-layer graphene oxide sheets. Carbon. 2015;81:158–166. [Google Scholar]
- 202.Lu K, Huang Q, Wang P, Mao L. Physicochemical Changes of Few-Layer Graphene in Peroxidase-Catalyzed Reactions: Characterization and Potential Ecological Effects. Environ. Sci. Technol. 2015;49(14):8558–8565. doi: 10.1021/acs.est.5b02261. [DOI] [PubMed] [Google Scholar]
- 203.Kotchey GP, Allen BL, Vedala H, Yanamala N, Kapralov AA, Tyurina YY, Klein-Seetharaman J, Kagan VE, Star A. The Enzymatic Oxidation of Graphene Oxide. ACS Nano. 2011;5(3):2098–2108. doi: 10.1021/nn103265h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hau HH, Gralnick JA. Ecology and biotechnology of the genus Shewanella. Annu. Rev. Microbiol. 2007;61:237–258. doi: 10.1146/annurev.micro.61.080706.093257. [DOI] [PubMed] [Google Scholar]
- 205.Sotirelis NP, Chrysikopoulos CV. Interaction between graphene oxide nanoparticles and quartz sand. Environ. Sci. Technol. 2015;49(22):13413–13421. doi: 10.1021/acs.est.5b03496. [DOI] [PubMed] [Google Scholar]
- 206.Chowdhury I, Duch MC, Mansukhani ND, Hersam MC, Bouchard D. Deposition and release of graphene oxide nanomaterials using a quartz crystal microbalance. Environ. Sci. Technol. 2014;48(2):961–969. doi: 10.1021/es403247k. [DOI] [PubMed] [Google Scholar]
- 207.Zhang LW, Petersen EJ, Huang QG. Phase Distribution of C-14-Labeled Multiwalled Carbon Nanotubes in Aqueous Systems Containing Model Solids: Peat. Environ. Sci. Technol. 2011;45(4):1356–1362. doi: 10.1021/es1026097. [DOI] [PubMed] [Google Scholar]
- 208.Zhang LW, Petersen EJ, Zhang W, Chen YS, Cabrera M, Huang QG. Interactions of C-14-labeled multi-walled carbon nanotubes with soil minerals in water. Environ. Pollut. 2012;166:75–81. doi: 10.1016/j.envpol.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 209.Zhao Q, Petersen EJ, Cornelis G, Wang XL, Guo XY, Tao S, Xing BS. Retention of C-14-labeled multiwall carbon nanotubes by humic acid and polymers: Roles of macromolecule properties. Carbon. 2016;99:229–237. doi: 10.1016/j.carbon.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gogos A, Kaegi R, Zenobi R, Bucheli TD. Capabilities of asymmetric flow field-flow fractionation coupled to multi-angle light scattering to detect carbon nanotubes in soot and soil. Environ. Sci.: Nano. 2014;1(6):584–594. [Google Scholar]
- 211.Doudrick K, Corson N, Oberdörster Gn, Eder AC, Herckes P, Halden RU, Westerhoff P. Extraction and quantification of carbon nanotubes in biological matrices with application to rat lung tissue. ACS Nano. 2013;7(10):8849–8856. doi: 10.1021/nn403302s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tagmatarchis N, Zattoni A, Reschiglian P, Prato M. Separation and purification of functionalised water-soluble multi-walled carbon nanotubes by flow field-flow fractionation. Carbon. 2005;43(9):1984–1989. [Google Scholar]
- 213.Niyogi S, Hu H, Hamon M, Bhowmik P, Zhao B, Rozenzhak S, Chen J, Itkis M, Meier M, Haddon R. Chromatographic purification of soluble single-walled carbon nanotubes (s-SWNTs) J. Am. Chem. Soc. 2001;123(4):733–734. doi: 10.1021/ja0024439. [DOI] [PubMed] [Google Scholar]
- 214.Fagan JA, Khripin CY, Silvera Batista CA, Simpson JR, Hároz EH, Hight Walker AR, Zheng M. Isolation of Specific Small-Diameter Single-Wall Carbon Nanotube Species via Aqueous Two-Phase Extraction. Adv. Mater. 2014;26(18):2800–2804. doi: 10.1002/adma.201304873. [DOI] [PubMed] [Google Scholar]
- 215.Pycke BF, Halden RU, Benn TM, Westerhoff P, Herckes P. Strategies for quantifying C 60 fullerenes in environmental and biological samples and implications for studies in environmental health and ecotoxicology. TrAC, Trends Anal. Chem. 2011;30(1):44–57. doi: 10.1016/j.trac.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pakarinen K, Petersen E, Leppänen M, Akkanen J, Kukkonen J. Adverse effects of fullerenes (nC 60) spiked to sediments on Lumbriculus variegatus (Oligochaeta) Environ. Pollut. 2011;159(12):3750–3756. doi: 10.1016/j.envpol.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 217.Wang J, Cai Q, Fang Y, Anderson TA, Cobb GP. Determination of fullerenes (C60) in artificial sediments by liquid chromatography. Talanta. 2011;87:35–39. doi: 10.1016/j.talanta.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 218.Carboni A, Helmus R, Emke E, van den Brink N, Parsons JR, Kalbitz K, de Voogt P. Analysis of fullerenes in soils samples collected in The Netherlands. Environ. Pollut. 2016;219(Supplement C):47–55. doi: 10.1016/j.envpol.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 219.Sanchís J, Božović D, Al-Harbi NA, Silva LF, Farré M, Barceló D. Quantitative trace analysis of fullerenes in river sediment from Spain and soils from Saudi Arabia. Anal. Bioanal. Chem. 2013;405(18):5915–5923. doi: 10.1007/s00216-013-6924-z. [DOI] [PubMed] [Google Scholar]
- 220.Pakarinen K, Petersen EJ, Alvila L, Waissi-Leinonen GC, Akkanen J, Leppänen MT, Kukkonen JV. A screening study on the fate of fullerenes (nC60) and their toxic implications in natural freshwaters. Environ. Toxicol. Chem. 2013;32(6):1224–1232. doi: 10.1002/etc.2175. [DOI] [PubMed] [Google Scholar]
- 221.Waissi GC, Bold S, Pakarinen K, Akkanen J, Leppänen MT, Petersen EJ, Kukkonen JVK. Chironomus riparius exposure to fullerene-contaminated sediment results in oxidative stress and may impact life cycle parameters. J. Hazard. Mater. 2017;322(Part A):301–309. doi: 10.1016/j.jhazmat.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tiwari B, Zhang D, Winslow D, Lee CH, Hao B, Yap YK. A Simple and Universal Technique To Extract One- and Two-Dimensional Nanomaterials from Contaminated Water. ACS Appl. Mater. Interfaces. 2015;7(47):26108–26116. doi: 10.1021/acsami.5b07542. [DOI] [PubMed] [Google Scholar]
- 223.Creighton MA, Zhu W, van Krieken F, Petteruti RA, Gao H, Hurt RH. Three-dimensional graphene-based microbarriers for controlling release and reactivity in colloidal liquid phases. ACS Nano. 2016;10(2):2268–2276. doi: 10.1021/acsnano.5b06963. [DOI] [PubMed] [Google Scholar]
- 224.Song W, Kinloch IA, Windle AH. Nematic Liquid Crystallinity of Multiwall Carbon Nanotubes. Science. 2003;302(5649):1363–1363. doi: 10.1126/science.1089764. [DOI] [PubMed] [Google Scholar]
- 225.Kim JY, Kim SO. Electric fields line up graphene oxide. Nat. Mater. 2014;13:325. doi: 10.1038/nmat3929. [DOI] [PubMed] [Google Scholar]
- 226.Cerrillo C, Barandika G, Igartua A, Areitioaurtena O, Marcaide A, Mendoza G. Ecotoxicity of multiwalled carbon nanotubes: Standardization of the dispersion methods and concentration measurements. Environ. Toxicol. Chem. 2015;34(8):1854–1862. doi: 10.1002/etc.2999. [DOI] [PubMed] [Google Scholar]
- 227.Nguyen T, Petersen EJ, Pellegrin B, Gorham JM, Lam T, Zhao M, Sung L. Impact of UV irradiation on multiwall carbon nanotubes in nanocomposites: Formation of entangled surface layer and mechanisms of release resistance. Carbon. 2017;116:191–200. doi: 10.1016/j.carbon.2017.01.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.He Y, Al-Abed SR, Dionysiou DD. Quantification of carbon nanotubes in different environmental matrices by a microwave induced heating method. Sci. Tot. Environ. 2017;580:509–517. doi: 10.1016/j.scitotenv.2016.11.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Herrero-Latorre C, Álvarez-Méndez J, Barciela-García J, García-Martín S, Peña-Crecente RM. Characterization of carbon nanotubes and analytical methods for their determination in environmental and biological samples: A review. Anal Chim Acta. 2015;853:77–94. doi: 10.1016/j.aca.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 230.Wohlleben W, Neubauer N. Quantitative rates of release from weathered nanocomposites are determined across 5 orders of magnitude by the matrix, modulated by the embedded nanomaterial. NanoImpact. 2016;1:39–45. [Google Scholar]
- 231.Bounos G, Andrikopoulos KS, Karachalios TK, Voyiatzis GA. Evaluation of multi-walled carbon nanotube concentrations in polymer nanocomposites by Raman spectroscopy. Carbon. 2014;76:301–309. [Google Scholar]
- 232.Chipara DM, Macossay J, Ybarra AV, Chipara A, Eubanks TM, Chipara M. Raman spectroscopy of polystyrene nanofibers—Multiwalled carbon nanotubes composites. Appl. Surf. Sci. 2013;275:23–27. [Google Scholar]
- 233.de la Chapelle ML, Stéphan C, Nguyen TP, Lefrant S, Journet C, Bernier P, Munoz E, Benito A, Maser WK, Martinez MT, de la Fuente GF, Guillard T, Flamant G, Alvarez L, Laplaze D. Raman characterization of singlewalled carbon nanotubes and PMMA-nanotubes composites. Synth. Met. 1999;103(1):2510–2512. [Google Scholar]
- 234.Becerril HA, Mao J, Liu Z, Stoltenberg RM, Bao Z, Chen Y. Evaluation of Solution-Processed Reduced Graphene Oxide Films as Transparent Conductors. ACS Nano. 2008;2(3):463–470. doi: 10.1021/nn700375n. [DOI] [PubMed] [Google Scholar]
- 235.Gorham JM, Osborn WA, Woodcock JW, Scott KC, Heddleston JM, Walker ARH, Gilman JW. Detecting carbon in carbon: Exploiting differential charging to obtain information on the chemical identity and spatial location of carbon nanotube aggregates in composites by imaging X-ray photoelectron spectroscopy. Carbon. 2016;96:1208–1216. doi: 10.1016/j.carbon.2015.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Gorham JM, Woodcock JW, Scott KC. Challenges, Strategies and Opportunities for Measuring Carbon Nanotubes within a Polymer Composite by X-ray Photoelectron Spectroscopy. NIST Special Publication. 2015;1200:10. [Google Scholar]
- 237.Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS. Graphene-based composite materials. Nature. 2006;442(7100):282–286. doi: 10.1038/nature04969. [DOI] [PubMed] [Google Scholar]
- 238.McAllister MJ, Li J-L, Adamson DH, Schniepp HC, Abdala AA, Liu J, Herrera-Alonso M, Milius DL, Car R, Prud'homme RK, Aksay IA. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007;19(18):4396–4404. [Google Scholar]
- 239.Schniepp HC, Li J-L, McAllister MJ, Sai H, Herrera-Alonso M, Adamson DH, Prud'homme RK, Car R, Saville DA, Aksay IA. Functionalized Single Graphene Sheets Derived from Splitting Graphite Oxide. J. Phys. Chem. B. 2006;110(17):8535–8539. doi: 10.1021/jp060936f. [DOI] [PubMed] [Google Scholar]
- 240.Paredes JI, Villar-Rodil S, Martínez-Alonso A, Tascón JMD. Graphene Oxide Dispersions in Organic Solvents. Langmuir. 2008;24(19):10560–10564. doi: 10.1021/la801744a. [DOI] [PubMed] [Google Scholar]
- 241.Romero-Vargas Castrillón S, Perreault F, de Faria AF, Elimelech M. Interaction of Graphene Oxide with Bacterial Cell Membranes: Insights from Force Spectroscopy. Environ. Sci. Technol. Lett. 2015;2(4):112–117. [Google Scholar]
- 242.Badireddy AR, Wiesner MR, Liu J. Detection, Characterization, and Abundance of Engineered Nanoparticles in Complex Waters by Hyperspectral Imagery with Enhanced Darkfield Microscopy. Environ. Sci. Technol. 2012;46(18):10081–10088. doi: 10.1021/es204140s. [DOI] [PubMed] [Google Scholar]
- 243.Mortimer M, Gogos A, Bartolome N, Kahru A, Bucheli TD, Slaveykova VI. Potential of Hyperspectral Imaging Microscopy for Semi-quantitative Analysis of Nanoparticle Uptake by Protozoa. Environ. Sci. Technol. 2014;48(15):8760–8767. doi: 10.1021/es500898j. [DOI] [PubMed] [Google Scholar]
- 244.Mittal V, Krauss L. Compatibilized polyethylene—thermally reduced graphene nanocomposites: Interfacial interactions and hyperspectral mapping for component distribution. Colloid Polym. Sci. 2014;292(10):2509–2518. [Google Scholar]
- 245.Kim JK, Shin JH, Lee JS, Hwang JH, Lee JH, Baek JE, Kim TG, Kim BW, Kim JS, Lee GH, Ahn K, Han SG, Bello D, Yu IJ. 28-Day inhalation toxicity of graphene nanoplatelets in Sprague-Dawley rats. Nanotoxicol. 2016;10(7):891–901. doi: 10.3109/17435390.2015.1133865. [DOI] [PubMed] [Google Scholar]
- 246.Webb S. Lighting Up Nanoparticles in Complex Samples: ES&T's Top Technology Article 2012. Environ. Sci. Technol. 2013;47(7):3021–3022. doi: 10.1021/es400922g. [DOI] [PubMed] [Google Scholar]
- 247.Pena MDPS, Gottipati A, Tahiliani S, Neu-Baker NM, Frame MD, Friedman AJ, Brenner SA. Hyperspectral imaging of nanoparticles in biological samples: Simultaneous visualization and elemental identification. Microsc. Res. Tech. 2016;79(5):349–358. doi: 10.1002/jemt.22637. [DOI] [PubMed] [Google Scholar]
- 248.CytoViva Hyperspectral Microscopy: Visible Near Infrared (VNIR) and Short Wave Infrared (SWIR) http://cytoviva.com/products/hyperspectral-imaging-2/hyperspectral-imaging/ (February 10, 2017)
- 249.Begum P, Ikhtiari R, Fugetsu B. Graphene phytotoxicity in the seedling stage of cabbage, tomato, red spinach, and lettuce. Carbon. 2011;49(12):3907–3919. [Google Scholar]
- 250.Lammel T, Boisseaux P, Fernández-Cruz M-L, Navas JM. Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Part. Fibre Toxicol. 2013;10(1):27. doi: 10.1186/1743-8977-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Huang L, Hartland GV, Chu L-Q, Luxmi, Feenstra RM, Lian C, Tahy K, Xing H. Ultrafast Transient Absorption Microscopy Studies of Carrier Dynamics in Epitaxial Graphene. Nano Lett. 2010;10(4):1308–1313. doi: 10.1021/nl904106t. [DOI] [PubMed] [Google Scholar]
- 252.Murphy S, Huang L. Transient absorption microscopy studies of energy relaxation in graphene oxide thin film. J. Phys. Condens. Matter. 2013;25(14):144203. doi: 10.1088/0953-8984/25/14/144203. [DOI] [PubMed] [Google Scholar]
- 253.Tong L, Liu Y, Dolash BD, Jung Y, Slipchenko MN, Bergstrom DE, Cheng J-X. Label-free imaging of semiconducting and metallic carbon nanotubes in cells and mice using transient absorption microscopy. Nat. Nano. 2012;7(1):56–61. doi: 10.1038/nnano.2011.210. [DOI] [PubMed] [Google Scholar]
- 254.Davydova Dy, de la Cadena A, Akimov D, Dietzek B. Transient absorption microscopy: advances in chemical imaging of photoinduced dynamics. Laser Photonics Rev. 2016;10(1):62–81. [Google Scholar]
- 255.Schwab F, Bucheli TD, Lukhele LP, Magrez A, Nowack B, Sigg L, Knauer K. Are Carbon Nanotube Effects on Green Algae Caused by Shading and Agglomeration? Environ. Sci. Technol. 2011;45(14):6136–6144. doi: 10.1021/es200506b. [DOI] [PubMed] [Google Scholar]
- 256.Tervonen K, Waissi G, Petersen EJ, Akkanen J, Kukkonen JV. Analysis of fullerene-C60 and kinetic measurements for its accumulation and depuration in Daphnia magna. Environ. Toxicol. Chem. 2010;29(5):1072–1078. doi: 10.1002/etc.124. [DOI] [PubMed] [Google Scholar]
- 257.Saheli P, Rowe R, Petersen E, O'Carroll D. Diffusion of multiwall carbon nanotubes through a high-density polyethylene geomembrane. Geosynth. Int. 2016:1–14. doi: 10.1680/jgein.16.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Elemental Analysis, I. Thermal-Optical Analysis for Organic & Elemental Carbon. http://www.elementalanalysis.com/services/thermal-optical-analysis-ocec/
- 259.Kim KT, Edgington AJ, Klaine SJ, Cho JW, Kim SD. Influence of multiwalled carbon nanotubes dispersed in natural organic matter on speciation and bioavailability of copper. Environ. Sci. Technol. 2009;43(23):8979–8984. doi: 10.1021/es900647f. [DOI] [PubMed] [Google Scholar]
- 260.Spinazzè A, Cattaneo A, Campagnolo D, Bollati V, Bertazzi PA, Cavallo DM. Engineered nanomaterials exposure in the production of graphene. Aerosol Sci. Technol. 2016;50(8):812–821. [Google Scholar]
- 261.Koo J, Lao S, Lee J, Chen D, Lam C, Yong W, Londa M, Pilato L. Morphology and thermal characterization of nanographene platelets. J. Mater. Sci. 2011;46(10):3583–3589. [Google Scholar]
- 262.Park S, An J, Potts JR, Velamakanni A, Murali S, Ruoff RS. Hydrazine-reduction of graphite-and graphene oxide. Carbon. 2011;49(9):3019–3023. [Google Scholar]
- 263.Moon IK, Lee J, Ruoff RS, Lee H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010;1:73. doi: 10.1038/ncomms1067. [DOI] [PubMed] [Google Scholar]
- 264.Chin J, Hunston D, Forster A. Thermo-viscoelastic Analysis of Ambient Cure Epoxy Adhesives Used in Construction Applications. In. [Google Scholar]
- 265.Pang LS, Saxby JD, Chatfield SP. Thermogravimetric analysis of carbon nanotubes and nanoparticles. J. Phys. Chem. 1993;97(27):6941–6942. [Google Scholar]
- 266.Guo F, Wu F, Mu Y, Hu Y, Zhao X, Meng W, Giesy JP, Lin Y. Characterization of organic matter of plants from lakes by thermal analysis in a N2 atmosphere. Sci. Rep. 2016;6 doi: 10.1038/srep22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wang Q, Odlyha M, Cohen NS. Thermal analyses of selected soil samples from the tombs at the Tianma-Qucun site, Shanxi, China. Thermochimica Acta. 2000;365(1–2):189–195. [Google Scholar]
- 268.Fernández JM, Plante AF, Leifeld J, Rasmussen C. Methodological considerations for using thermal analysis in the characterization of soil organic matter. J. Therm. Anal. Calorim. 2011;104(1):389–398. [Google Scholar]
- 269.Dell'Abate MT, Benedetti A, Brookes PC. Hyphenated techniques of thermal analysis for characterisation of soil humic substances. J. Sep. Sci. 2003;26(5):433–440. [Google Scholar]
- 270.Oudghiri F, Allali N, Quiroga JM, Rodriguez-Barroso MR. TG-FTIR analysis on pyrolysis and combustion of marine sediment. Infrared Phys. Technol. 2016;78:268–274. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.