Abstract
This study evaluated Mn concentration in the seeds of 120 RILs of lentil developed from the cross “CDC Redberry” × “ILL7502”. Micronutrient analysis using atomic absorption spectrometry indicated mean seed manganese (Mn) concentrations ranging from 8.5 to 26.8 mg/kg, based on replicated field trials grown at three locations in Turkey in 2012 and 2013. A linkage map of lentil was constructed and consisted of seven linkage groups with 5,385 DNA markers. The total map length was 973.1 cM, with an average distance between markers of 0.18 cM. A total of 6 QTL for Mn concentration were identified using composite interval mapping (CIM). All QTL were statistically significant and explained 15.3–24.1% of the phenotypic variation, with LOD scores ranging from 3.00 to 4.42. The high-density genetic map reported in this study will increase fundamental knowledge of the genome structure of lentil, and will be the basis for the development of micronutrient-enriched lentil genotypes to support biofortification efforts.
Keywords: DArT, SNP, QTL mapping, manganese, lentil, Genome Report
Lentil originated in southwestern Asia and its seeds have been consumed since prehistoric times. The origin of cultivated lentil is the Near East Arc and Asia Minor (Muehlbauer and McPhee 2005). Lentils are grown throughout the world, with production from Canada, India, Turkey, and Australia providing most of the world’s supply (Ford et al. 2007). Lentil seeds are an excellent source of manganese (Mn), yet deficiency of this micronutrient affects 35% of children worldwide (Qaim et al. 2007).
Mn is an essential micronutrient with a recommended daily intake of 0.7 to 22.0 mg for adults (Santos et al. 2004). Mn health benefits include development of normal bone structure, metabolism of bones, and promotion of the necessary enzymes for bone health (Price et al. 2012). Mn acts as a co-enzyme to assist metabolic activity in the body (Finkelstein et al. 2007) and is involved in the synthesis of RNA, DNA, and proteins (Xu et al. 2002). Other health benefits associated with adequate Mn intake include the formation of joint tissues (Kannus 2000), proper functioning of the thyroid gland and sex hormones (Soldin and Aschner 2007), metabolism of carbohydrates and fats, and regulation of blood sugar levels (Van den Berghe 2004). Adequate Mn is important for brain function (Takeda 2003) and nervous system activity throughout the body (Erikson and Aschner 2003). Symptoms of Mn deficiency include skeletal abnormality, heart ailments (Witte et al. 2001), high cholesterol (Davis et al. 1990), muscular contraction, poor visual and auditory function, high blood pressure, tremors and shivers (Komaki et al. 1999), severe memory loss, and bone malformation (Bourre 2006).
Plant-based dietary habits are the leading cause of Mn deficiency due to the low micronutrient density of foods, a problem that can be addressed by biofortification, dietary diversification, or supplementation. For the past two decades, researchers have focused on biofortification strategies (Zhu et al. 2007; Mayer et al. 2008; Blancquaert et al. 2014; Zou et al. 2014; Ates et al. 2016; Nakandalage et al. 2016; Aldemir et al. 2017; Garcia-Casal et al. 2017; Giuliano 2017; Sharma et al. 2017). Biofortification is a means of increasing the daily micronutrient consumption of individuals who suffer from micronutrient malnutrition (Bouis 2003). The goal of biofortification is to increase the concentration of micronutrients in the edible part of crop plants through plant breeding (Carvalho and Vasconcelos 2013). Research on increasing the concentration of minerals in seeds has largely focused on crops such as maize (Oikeh et al. 2003; Gupta et al. 2015; Prasad et al. 2015), rice (Gregorio et al. 2000; Gupta et al. 2015; Prasad et al. 2015; Nakandalage et al. 2016), wheat (Monasterio and Graham 2000; Garvin et al. 2006; Gupta et al. 2015; Prasad et al. 2015), barley (Ma et al. 2004; Rodrigo et al. 2013), and lentil (Ates et al. 2016; Aldemir et al. 2017). Identifying the quantitative trait loci (QTL) that control the concentration of Mn in lentils would aid in the development of biofortified cultivars through the use of closely linked molecular markers, which would allow breeders to screen and select for micronutrient dense genotypes. QTL controlling Mn uptake and identified using QTL analysis have been published for cabbage (Wu et al. 2008), Lotus japonicus (Klein and Grusak 2009), clover (Sankaran et al. 2009), canola (Ding et al. 2010), and Arabidopsis (Willems et al. 2010). To date, however, no QTL controlling the concentration of Mn in lentil seeds have been identified. The objectives of this study were to (i) determine the Mn concentration of lentil seeds from recombinant inbred lines (RILs), (ii) calculate genetic variation of Mn concentration among RILs, locations, and years, and (iii) identify QTL controlling Mn concentration in lentil seeds.
Materials And Methods
Soil analysis
Soil samples were collected from experimental fields in three locations in Turkey (Izmir, Adana, and Sanliurfa) to determine the physical and chemical properties of each soil. Soil pH analysis (Black 1965), total soluble salt analysis (Richards 1954), texture analysis (Bouyoucos 1955), organic matter analysis (Black 1965), CaCO3 analysis (Schlicting and Blume 1966), and macro- and micro-nutrient analysis (Bingham 1949; Pratt 1965; Lindsay and Norvell 1978) were carried out at the Department of Plant and Soil Science at Ege University in Izmir, Turkey.
Plant materials
A population of 120 RILs was developed from the cross “CDC Redberry” (P1) × “ILL7502” (P2) and designated LR-8. P1 was developed from a cross made in 1997 between CDC breeding lines 1049F3 / 819-5R. Line 1049F3 was derived from the cross 567-16 / 545-8. Line 819-5R was derived from the cross 86-360 / (458-258G / (458-122 / C8L27- RC // Precoz) F2) F1 (Vandenberg et al. 2006). P2 is a lentil cultivar released in Bangladesh (Sarker et al. 1999). The LR-8 population was generated by advancing F1 plants from the simple cross to the F2 generation, and the RILs developed by single seed descent from the F2 to the F7 generation. The RILs were produced at the University of Saskatchewan, Canada where resources for genetic and genomic studies of lentil have been developed since 2001.
Micronutrient analysis and heritability
The RILs were grown in 2 years (2012 and 2013) in three different locations in Turkey—Ege University Izmir (27°09’ E, 38°25’ N), Cukurova University in Adana (35°18’ E, 37°01’ N), and Harran University in Sanliurfa (38°46’ E, 37°08’ N)—and placed with three replications in a randomized complete block design (RCBD) with three factors (year, location, genotype) for micronutrient analysis. An atomic absortion spectrometer (AAS) (Varian, SpectrAA 220/ FS, California, USA) was used to estimate Mn concentrations in all seed samples. Samples were prepared for analysis as per a previous study (Kacar 1972). Seed samples (2 g) were first washed with tap water and then with pure water to remove surface contaminants. The washed seeds were dried in a hot air oven at 65°. The dried samples were ground using an analytic mill (IKA, A11, Staufen, Germany) and then each 2-g ground sample placed into a 150-mL flask to which 24 mL of 4:1 nitric:percholoric acid were added to decompose the samples. All procedures were performed with three replications. Spectrophometric readings for total Mn concentrations were converted to mg/kg concentration in seed (Kacar 1972; Kacar and Inal 2008). To confirm the accuracy of the AAS, standard Mn solutions (1.0, 2.0, 3.0, 4.0, 5.0 ppm) were analyzed to form a calibration curve (r2 = 0.9999). Heritability (H) based on lentil population means was calculated with the formula H = [MSamong families – MSyear*family]/MSamong families, where MS is the mean square (Courtney et al. 2008).
Variance analysis
Analysis of variance (ANOVA) was used to determine variation in Mn concentrations of the LR-8 RIL population grown in different years and locations using TOTEMSTAT software (Acikgoz et al. 2004). Genotypes were accepted as fixed while year and location were random. Variation of year (Y) × location (L), Y × genotype (G), L × G, and Y × L × G interactions were calculated and significance was accepted at the P ≤ 0.01 and ≤ 0.05 levels.
DNA isolation
Young leaves from 4- to 6-week-old seedling of all lentil genotypes grown at the Izmir location were harvested and placed in labeled aluminum foil containers and then immediately placed in liquid nitrogen. Frozen leaf samples were kept in a deep freezer (-86°) until analysis. Genomic DNA from 120 RILs and the parents were extracted from frozen leaf tissue using the Fermentas DNA Isolation Kit (Thermo Scientific, Hanover, MD, USA). Purity of the DNA was confirmed on 1% agarose gel and the purified DNA then quantified with a Qubit2.0 Fluorometer (Life Technologies, US).
DArT analysis
DArT analyses were carried out following Aldemir et al. (2017). The raw data for SNP discovery are presented as supplemental file 1 (File S1).
Linkage mapping and QTL analysis
JoinMap4.0 software described by Van Ooijen and Voorrips (2004) was used for linkage mapping analysis. A maximum recombination frequency of 0.50 and the kosambi function were used as options in linkage mapping. Distorted markers were eliminated. MapQTL version 6.0 (Van Ooijen 2009) was used for QTL analysis. The effects and positions of QTL were determined following composite interval mapping (CIM). The significant threshold was calculated based on 1000 permutations at the P ≤ 0.01 and ≤ 0.05 levels (Van Ooijen and Voorrips 2004), and QTL that passed the threshold significance are reported. The proportion of observed phenotypic variation explained due to a particular QTL was estimated by the coefficient of determination (R2) using maximum likehood for CIM.
Data Availability
File S1 contains SNP data. File S2 contains Mn phenotyping data.
Results
Soil properties
The physico-chemical properties of soil samples from Izmir, Adana, and Sanliurfa locations are presented in Table 1. Soil samples from all locations were slighty alkaline, non-saline, and calcareous. Soil from Adana had a loamy clay texture and soils from the other two locations had a loamy texture. The bioavailability of Mn by plants from soil is the degree to which an extractable solid-phase quantity is correlated with measured tissue concentration, which is called available Mn (Lindsay and Norvell, 1978). Available Mn contents were low for all three soils.
Table 1. Physico-chemical properties of soil samples from Izmir, Adana, and Sanliurfa.
| Soil properties | Izmir | Adana | Sanliurfa |
|---|---|---|---|
| pH | 7.82 | 7.74 | 7.76 |
| Total salt (%) | 0.04 | 0.03 | 0.04 |
| CaCo3 (%) | 29.4 | 48.0 | 34.5 |
| Organic matter (%) | 1.86 | 1.29 | 1.96 |
| Fine sand (%) | 50.24 | 44.24 | 44.24 |
| Silt (%) | 28.00 | 26.00 | 32.00 |
| Clay (%) | 21.76 | 29.76 | 23.76 |
| Texture | Loamy | Loamy clay | Loamy |
| Total N (%) | 0.06 | 0.05 | 0.05 |
| Available P (mg/kg) | 3.29 | 2.62 | 1.87 |
| Available K (mg/kg) | 417 | 116 | 485 |
| Available Ca (mg/kg) | 6,272 | 6,762 | 7,252 |
| Available Mg (mg/kg) | 554 | 170 | 430 |
| Available Na (mg/kg) | 220 | 307 | 20 |
| Available Fe (mg/kg) | 4.93 | 5.01 | 6.52 |
| Available Zn (mg/kg) | 0.73 | 0.45 | 1.05 |
| Available Cu (mg/kg) | 1.13 | 0.19 | 0.65 |
| Available Mn (mg/kg) | 5.30 | 4.61 | 8.07 |
Mn concentration in seeds of the LR-8 population
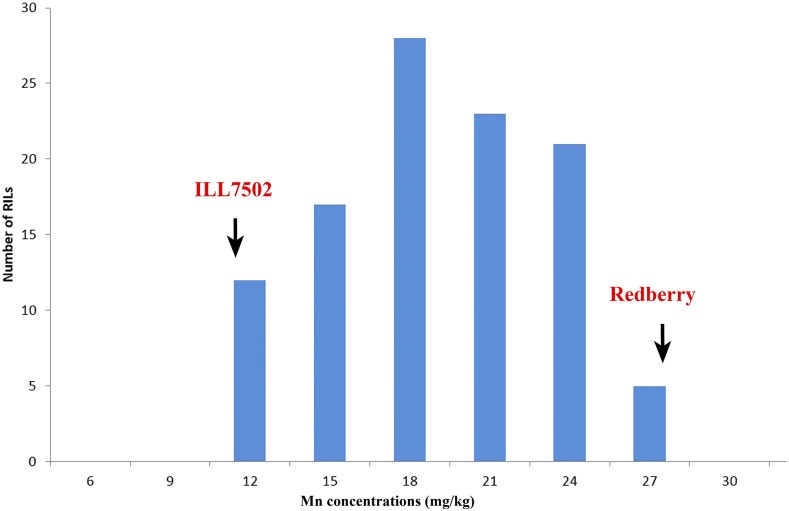
Mn concentrations in P1 and P2 of the LR-8 lentil population are shown in Table 2. The overall mean Mn concentration in seeds of the parents was 9.6 mg/kg for P2 and 27.6 mg/kg for P1. Mn concentrations in seeds of the RILs of the LR-8 population varied from 8.5 to 26.8 mg/kg with a mean of 17.6 mg/kg. The highest concentration of Mn was detected in RIL LR8-113. Heritability for Mn concentrations was detected as 0.76 and 0.74 for 2012 and 2013, respectively (Table 2). This means that Mn accumulation in the seed is affected by genetics rather than the environment. The frequency distribution of Mn concentrations in seeds of the LR-8 population as a mean across three locations and 2 years (Figure 1) shows that concentrations for the RIL population were also continuous but with a near to normal binomial distribution.
Table 2. Minimum, maximum, and mean Mn concentration in seeds of the LR-8 lentil population grown at Izmir, Adana, and Sanliurfa in 2012 and 2013.
| Mn concentration (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|
| Location | Izmir | Adana | Sanliurfa | Mean | |||
| Year | 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | |
| P1 | 25.5 | 29.3 | 24.0 | 32.1 | 27.5 | 27.1 | 27.6 |
| P2 | 7.7 | 8.8 | 9.5 | 10.6 | 8.0 | 12.7 | 9.6 |
| Minimum | 7.7 | 10.8 | 7.5 | 9.2 | 8.0 | 9.0 | 8.5 |
| Maximum | 27.8 | 31.9 | 27.5 | 26.3 | 28.5 | 25.1 | 26.8 |
| Mean | 17.8 | 20.4 | 17.5 | 17.3 | 17.8 | 17.3 | 17.6 |
| Mn heritability | 2012 | 2013 | |||||
| 0.76 | 0.74 | ||||||
| Std dev | 4.2 | 4.0 | |||||
Figure 1.
Frequency distribution of Mn concentration in lentil seeds of 120 RILs and their parents, averaged over three locations (Izmir, Adana, and Sanliurfa) and 2 years (2012 and 2013).
Variance analysis showed that Mn concentration in seeds among RILs was significant at the P ≤ 0.01 level. It was also statistically significant among locations (Table 3). The effects of Y × L, Y × G, L × G, and Y × L × G interactions were statistically significant. Genotypes accumulated Mn in seed at different levels according to year and location.
Table 3. ANOVA for Mn concentrations in seeds of LR-8 lentil RILs grown at three different locations for two years.
| Mn concentration | |||||
|---|---|---|---|---|---|
| Source of Variation | df | Mean Square | F | F prob. 5% | F prob. 1% |
| Block | 2 | 4.1 | 2.4 ns | 3.1 | 4.8 |
| Year (Y) | 1 | 55.7 | 31.6 ** | 3.9 | 6.9 |
| Location (L) | 2 | 14.1 | 8.0 ** | 3.1 | 4.8 |
| Genoype (G) | 119 | 306.0 | 173.4 ** | 1.4 | 1.5 |
| Y × L | 2 | 18.5 | 10.5 ** | 3.1 | 4.8 |
| Y × G | 119 | 3.1 | 1.8 ** | 1.4 | 1.5 |
| L × G | 238 | 4.0 | 2.3 ** | 1.3 | 1.4 |
| Y × L × G | 238 | 3.8 | 2.2 ** | 1.3 | 1.4 |
| Error | 1246 | 1.765 | |||
| General | 1871 | ||||
| Coefficient of variation (CV)= 7.71% | |||||
df: degree of freedom. ns: not significant.
: Significant at P ≤ 0.01.
Construction of linkage maps
Missing data and the data showing segregation distortion were filtered. After filtering, a total of 10,552 SNPs were developed through DArT. Among them, 5,385 SNPs could be mapped in the lentil genome. The LR-8 population was genotyped using the 5,385 SNP markers that covered seven linkage groups (LG). LG1 had the highest number (1,102) of SNPs and LG6 the lowest (439) (Table 4).
Table 4. Characteristics of the linkage groups of the LR-8 lentil population.
| Linkage group | Length (cM) | Number of SNP markers | Number of SNP markers (%) | Average distance between markers (cM) |
|---|---|---|---|---|
| LG1 | 151.8 | 1,102 | 20.5 | 0.13 |
| LG2 | 175.2 | 676 | 12.5 | 0.25 |
| LG3 | 167.7 | 940 | 17.4 | 0.17 |
| LG4 | 169.2 | 835 | 15.5 | 0.20 |
| LG5 | 102.5 | 849 | 15.8 | 0.12 |
| LG6 | 117.9 | 439 | 8.1 | 0.26 |
| LG7 | 88.8 | 544 | 10.1 | 0.16 |
| Total | 973.1 | 5,385 | Average: 0.18 |
Linkage group size and average distance between adjacent markers are shown in Table 4. The smallest LG was LG7 (88.8 cM) and the largest was LG2 (175.2 cM). The total map length was 973.1 cM with an average distance of 0.18 cM between adjacent markers (Figure 2).
Figure 2.
Genetic linkage map for lentil derived from the cross P1 × P2. Left bar of the LGs is cM and the right bar is marker names. QTL for Mn marked with red.
QTL analysis of Mn
Six QTL, with LOD scores ranging from 3.02 to 4.42 and distributed across three linkage groups (LG1, LG3, and LG7) (Figure 2), were associated with seed Mn concentration and explained between 16.1 and 24.1% of the phenotypic variation (Table 5). The largest number of QTL regions for Mn concentration (3 QTL regions) were identified on LG3.
Table 5. Characteristics and locations of QTL regions for Mn concentrations in seeds of the LR-8 lentil population.
| QTL region | LG | Position (cM) | Number of SNPs in the QTL region | % explanation | Additive effect* | LOD | Year/location |
|---|---|---|---|---|---|---|---|
| MnQTL1.1 | LG1 | 26.0-26.3 | 72 | 24.1 | + | 3.25 | 2012 Adana |
| MnQTL1.2 | LG1 | 37.0-37.7 | 87 | 16.1 | — | 3.02 | 2012 Adana |
| MnQTL3.1 | LG3 | 27.0-27.6 | 24 | 18.0 | — | 3.70 | 2013 Sanliurfa |
| MnQTL3.2 | LG3 | 56.6-57.7 | 10 | 22.4 | — | 4.38 | 2012 Izmir; 2013 Izmir, Sanliurfa |
| MnQTL3.3 | LG3 | 114.6-124.1 | 103 | 21.6 | — | 4.22 | 2012 Izmir, Sanliurfa; 2013 Izmir, Sanliurfa |
| MnQTL7.1 | LG7 | 2.3-7.7 | 14 | 16.1 | + | 4.42 | 2012 Adana, Izmir, Sanliurfa; 2013 Adana, Izmir, Sanliurfa |
Positive (+) values of additive effect mean that the positive allele comes from parent P1, while negative (-) values mean that the positive allele comes from parent P2.
MnQTL3.1, located between 27.0-27.6 cM, was clustered with 24 SNP markers and explained 18.0% of the phenotypic variance. MnQTL3.2 and MnQTL3.3, located between 56.6-57.7 cM and 114.6-124.1 cM on LG3, explained 22.4 and 21.6% of the phenotypic variance, respectively. MnQTL3.2 clustered with 10 SNPs and MnQTL3.3 contained 103 SNPs. LG7 contained only one QTL region for Mn concentration. MnQTL7.1 was located between 2.3 and 7.7 cM on LG7 and explained 16.1% of the phenotypic variance. Additive effects of QTL regions of Mn are presented in Table 5.
Discussion
Micronutrient malnutrition affects more than one-half of the total human population, with children and women at the highest risk (Ahmed et al. 2016). Biofortification aims to increase the total amount of minerals in the edible parts of crops by increasing the concentration of compounds, such as Mn, thus promoting their uptake by humans (Graham and Welch 1996). The biofortification strategy for alleviating this form of malnutrition is to increase the consistent daily intake of food staples by all family members, especially children and women, and to target the bridge between human nutrition and agriculture (Graham et al. 1999).
Mn accumulation in seed was determined to be quantitatively inherited in lentil. Supporting our results, previous QTL studies show that Mn concentration of seeds is quantitatively inherited in cabbage (Wu et al. 2008), Lotus japonicus (Klein and Grusak 2009), clover (Sankaran et al. 2009), canola (Ding et al. 2010), and Arabidopsis (Willems et al. 2010). Therefore, this study is also important with respect to understanding the genetic nature of Mn accumulation in seed. To date, no studies have identified QTL for Mn concentration in lentil. Identification of the QTL associated with high Mn concentration in lentil seeds could help select lines containing high Mn concentration in lentil breeding programs. This type of knowledge can be used to develop genetic strategies for molecular breeding to help increase the micronutrient content of edible parts of the lentil plant. Increased consumption of lentil with elevated levels of micronutrients could help to overcome micronutrient deficiency (Cichy et al. 2009), and the large variation in Mn concentration among the RILs could be the basis for developing such a strategy (Beebe et al. 2000).
Mn variation
Mean Mn concentrations of seeds of RILs in the LR-8 population grown at three locations in 2 years varied from 8.5 to 26.8 mg/kg and represented a ∼threefold variation (Table 2). Previous reports indicate Mn concentrations range from 11.5 to 16.2 mg/kg for lentil landraces and from 11.5 to 15.4 mg/kg for lentil cultivars (Karaköy et al. 2012). Mn concentrations reported by Karaköy et al. (2012) are lower than those from the current study, which could be due to the different genotypes they used as well as different soil chemical properties of their experimental field. Per capita global lentil consumption is being increased rapidly and lentil fortification is a simple and promising approach to help decreased Mn deficiency (Podder et al. 2018). The data show that the Mn concentrations we observed could provide a significant amount of the required daily Mn from lentil in a given meal. For example, daily cooked lentil dal (50g/day) contains approximately 1 mg Mn (Mn concentrations in the current research found as a mean of 17.6 mg/kg, Table 2) which falls into recommended daily allowance (RDA) indicated by Santos et al. (2004) (0.7 to 22.0 mg for adults). In previous studies, Mn concentration was detected as a mean of 14 mg/kg in common bean seeds (Pinheiro et al. 2010). The Mn concentration was ranged between 9.2 -14.6 mg/kg in pea, between 4.4 and 12.6 mg/kg in buckwheat (Beitane and Krumina-Zemture 2017) and 16.8 mg/kg in seeds of chickpea (Kahraman et al. 2017). Mn value detected in the currrent study was higher as compared to other legumes.
The ANOVA for Mn concentration shows that location, year, and genotype interactions are statistically significant (Table 3). Interactions among genotypes, locations, and years are likely due to different environmental conditions affecting the availability of Mn in the pool of soil micronutrients available for plant uptake (Sankaran et al. 2009).
Linkage mapping
DArT analysis allowed the construction of high-density linkage maps with a very large number of SNPs. In the current study, the DArT method generated 10,552 SNPs. Using this DArT approach on the parental RIL populations, a total of 5,385 SNPs were mapped (Table 4). The amount of data used for mapping purposes was similar to previous DArT analysis studies (Poland et al. 2012; Li et al. 2014; Han et al. 2016).
In this study, the linkage map of lentil consisted of seven linkage groups with 5,385 SNP markers. The total map length (973.1 cM) in the current study is shorter than for many previous lentil mapping studies, e.g., 1,073 cM (Eujayl et al. 1997), 1,868 cM (Tullu et al. 2008), 1,396.3 cM (Tanyolac et al. 2010), 3,843 cM (Gupta et al. 2012), and 834.7 cM (Sharpe et al. 2013). Recently, the lentil genome was mapped with 1,784 markers (including SNP and SSR) covering a genome size of 4,060.6 cM using genotype by sequencing (GBS) in RILs developed from “PI 320937” × “Eston” parents (Ates et al. 2016). On the other hand, a total map length of 784.1 cM, which is close to our map length, was detected using a few markers [100 Random Amplified Polymorphic DNA (RAPDs), 11 Inter Simple Sequence Repeats (ISSRs) and 3 Resistance Gene Analogs (RGAs)] (Rubeena et al. 2003). Another study constructed a genetic linkage map using 6 SSRs and 537 contigs covering a genome size of 834.7 cM (Sharpe et al. 2013). Overall, the genetic map in this study is more robust compared to previous QTL mapping studies in lentil (Eujayl et al. 1997; Tullu et al. 2008; Tanyolac et al. 2010; Gupta et al. 2012; Sharpe et al. 2013).
The seven major LGs constructed in the current study correspond to the seven haploid chromosome number of lentil (Sharpe et al. 2013; Ates et al. 2016). Differences in the estimated distances of both parental maps may reflect differences in the recombination frequencies of both parents. Putative causes for the difference between the two estimated parental genome maps include marker distribution along the chromosome that varies between parents, and male and female gametes that probably display different recombination frequencies (Khadari et al. 2010).
QTL analysis of Mn
This study is the first to map QTL for Mn concentration in lentil seeds and uses a larger number of SNPs than previous studies mapping the lentil genome. P1 (CDC Redberry) is adapted to the northern temperate zone, and was the parent that had the highest seed Mn concentration. A total of 6 QTL for Mn concentration in seeds of the LR-8 population were identified using CIM. These were distributed across three linkage groups in the LR-8 lentil population (Table 5).
In a study of QTL associated with Mn concentration in Lotus japonicus (Klein and Grusak 2009) two QTL explaining 35.2% of the phenotypic variation were identified on chromosomes 1 and 2. Ten QTL for Mn concentration were distributed across 8 chromosomes, with LOD scores ranging between 3.34-6.55, explaining between 9.06 and 16.43% of the phenotypic variation in Brassica napa (Ding et al. 2010). In other similar studies, six QTL for Mn concentration were found in one of two wheat populations (Pu et al. 2013), two QTL were mapped in soybean (Ramamurthy et al. 2014), and four QTL associated with Mn concentration were identified in rice (Yu et al. 2015). Here, the number of QTL detected was high; micronutrient accumulation in seeds continues to be a complex process controlled by poly-genes (Grusak and DellaPenna 1999).
For Mn in lentil seeds, six QTL were statistically significant, and the phenotypic variation ranged from 16.1 to 24.1% with LOD scores of 3.02-4.42. QTL analysis of nutrient element accumulation in seeds of other crops shows that the value for explaining phenotypic variation typically ranges between 9.06 and 35.2% (Ding et al. 2010; Klein and Grusak 2009; Yu et al. 2015). The value we found falls within the same range, and our estimates of phenotypic variation of seed Mn concentration in lentil were similar to those for canola (Ding et al. 2010) and wheat (Pu et al. 2013).
Conclusions
The LR-8 lentil population studied here demonstrated large phenotypic variation in terms of Mn concentrations in seeds. Mn concentrations in lentil seeds were observed to be quantitatively inherited. DArT analysis allowed the construction of high-density linkage maps with a large number of SNPs. The QTL that were stable across 2 years and three locations were unaffected by environmental conditions, and therefore could be used in marker-assisted selection in lentil breeding programs. We believe that this work is the first to map QTL for Mn concentrations in lentil seeds. The discovery of QTL for seed Mn concentration could have significant implications for biofortification breeding strategies. The QTL analysis might help to resolve some of the complexity with respect to Mn accumulation in lentil grain. RIL LR8-113, which contained the highest Mn concentration, could be used as a parent in breeding programs. The results of this study can be applied to the development of lentil genotypes with higher Mn concentrations. The high-density maps could increase fundamental knowledge of the genome structure of lentil, help in future construction of physical maps, and serve as a basis for map-based cloning in lentil.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.118.200259/-/DC1.
Acknowledgments
The study was supported by The Scientific and Technological Research Council of Turkey (TUBITAK), project no. COST-111O446. The authors declare that they have no conflicts of interest.
Footnotes
Communicating editor: E. Akhunov
Literature Cited
- Acikgoz N., Ilker E., Gokcol A., 2004. Computer evaluations of biological research. Ege University Press, TOTEM, No: 2, Izmir, Turkey. [Google Scholar]
- Ahmed F., Prendiville N., Narayan A., 2016. Micronutrient deficiencies among children and women in Bangladesh: progress and challenges. J. Nutr. Sci. 5: e46 10.1017/jns.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldemir S., Ates D., Temel H. Y., Yagmur B., Alsaleh A., et al. , 2017. QTLs for iron concentration in seeds of the cultivated lentil (Lens culinaris Medic.) via genotyping by sequencing. Turk. J. Agric. For. 41: 243–255. 10.3906/tar-1610-33 [DOI] [Google Scholar]
- Ates D., Sever T., Aldemir S., Yagmur B., Temel H. Y., et al. , 2016. Identification QTLs controlling genes for Se uptake in lentil seeds. PLoS One. 10.1371/journal.pone.0149210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe S., Gonzalez A. V., Rengifo J., 2000. Research on trace minerals in the common bean. Food Nutr. Bull. 21(4): 387–391. 10.1177/156482650002100408 [DOI] [Google Scholar]
- Beitane I., Krumina-Zemture G., 2017. Dietary micronutrient content in pea (Pisum sativum L.) and buckwheat (Fagopyrum esculentum M.) flour, Foodbalt. [Google Scholar]
- Bingham F. T., 1949. Soil test for phosphate. Calif. Agric. 3(7): 11–14. [Google Scholar]
- Black C. A. (Editor), 1965. Methods of soil analysis, pp. 1372–1376. American Society of Agronomy Inc., Madison, Wisconsin. [Google Scholar]
- Blancquaert D., De Steur H., Gellynck X., Van Der Straeten D., 2014. Present and future of folate biofortification of crop plants. J. Exp. Bot. 65(4): 895–906. 10.1093/jxb/ert483 [DOI] [PubMed] [Google Scholar]
- Bouis H. E., 2003. Micronutrient fortification of plants through plant breeding: can it improve nutrition in man at low cost? Proc. Nutr. Soc. 62(2): 403–411. 10.1079/PNS2003262 [DOI] [PubMed] [Google Scholar]
- Bourre J. M., 2006. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: Micronutrients. J. Nutr. Health Aging 10(5): 377–385. [PubMed] [Google Scholar]
- Bouyoucos G. J., 1955. A recalibration of the hydrometer method for making mechanical analysis of the soils. Agron. J. 4(9): 419–434. [Google Scholar]
- Carvalho S. M. P., Vasconcelos M. W., 2013. Producing more with less: Strategies and novel technologies for plant-based food biofortification. Food Res. Int. 2013 10.1016/j.foodres.2012.12.021 [DOI] [Google Scholar]
- Cichy K. A., Caldas G. V., Snapp S. S., Blair M. W., 2009. QTL analysis of seed iron, zinc, and phosphorus levels in an Andean bean population. Crop Sci. 49(5): 1742–1750. 10.2135/cropsci2008.10.0605 [DOI] [Google Scholar]
- Courtney M., Mcharo M., La Bonte D., 2008. Heritability estimates for micronutrient composition of sweetpotato storage roots. HortScience 43(5): 1382–1384. [Google Scholar]
- Davis C. D., Ney D. M., Greger J. L., 1990. Manganese, iron and lipid interactions in rats. Nutrition 120(5): 507–513. [DOI] [PubMed] [Google Scholar]
- Ding G., Yang M., Hu Y., Liao Y., Shi L., et al. , 2010. Quantitative trait loci affecting seed mineral concentrations in Brassica napus grown with contrasting phosphorus supplies. Ann. Bot. 105(7): 1221–1234. 10.1093/aob/mcq050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson K. M., Aschner M., 2003. Manganese neurotoxicity and glutamate-GABA interaction. Neurochem. Int. 43(4–5): 475–480. 10.1016/S0197-0186(03)00037-8 [DOI] [PubMed] [Google Scholar]
- Eujayl I., Baum M., Erskine W., Pehu E., Muehlbauer F. J., 1997. Use of RAPD markers for genetic mapping and evaluation of segregation distortion in lentil (Lens culinaris Medik.). Euphytica 96(3): 405–412. 10.1023/A:1003045000568 [DOI] [Google Scholar]
- Finkelstein Y., Milatovic D., Aschner M., 2007. Modulation of cholinergic systems by manganese. Neurotoxicology 28(5): 1003–1014. 10.1016/j.neuro.2007.08.006 [DOI] [PubMed] [Google Scholar]
- Ford R., Rubeena, Redden R. J., Materne M., Taylor P. W. J., 2007. Lentil. pp. 91–108 in Genome Mapping and Molecular Breeding in Plants, Volume 3: Pulses, Sugar and Tuber Crops. Edited by Kole C. Springer, Berlin. [Google Scholar]
- Garcia-Casal M. N., Pena-Rosas J. P., Giyose P., Consultation Working Groups , 2017. Staple crops biofortified with increased vitamins and minerals: Considerations for a public health strategy. Ann. N. Y. Acad. Sci. 1390(1): 3–13. 10.1111/nyas.13293 [DOI] [PubMed] [Google Scholar]
- Garvin D. F., Welch R. M., Finley J. W., 2006. Historical shifts in the seed mineral micronutrient concentration of US hard red winter wheat germplasm. J. Sci. Food Agric. 86(13): 2213–2220. 10.1002/jsfa.2601 [DOI] [Google Scholar]
- Giuliano G., 2017. Provitamin A biofortification of crop plants: a gold rush with many miners. Curr. Opin. Biotechnol. 44: 169–180. 10.1016/j.copbio.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Graham, R. D., and R. Welch, 1996 Breeding for staple food crops with high micronutrient density, agricultural strategies for micronutrients. Working paper 3. International Food Policy Research Institute, Washington DC, USA.
- Graham R. D., Senadhira D., Beebe S., Iglesias C., Monasterio I., 1999. Breeding for micronutrient density in edible portions of staple food crops: Conventional approaches. Field Crops Res. 60(1-2): 57–80. 10.1016/S0378-4290(98)00133-6 [DOI] [Google Scholar]
- Gregorio G. B., Senadhira D., Htut H., Graham R. D., 2000. Breeding for trace mineral density in rice. Food Nutr. Bull. 21(4): 382–386. 10.1177/156482650002100407 [DOI] [Google Scholar]
- Grusak M. A., DellaPenna D., 1999. Improving the nutrient composition of plants to enhance human nutrition and health. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50(1): 133–161. 10.1146/annurev.arplant.50.1.133 [DOI] [PubMed] [Google Scholar]
- Gupta D. S., Thavarajah D., Ekanayake L. J., Johnson C., Amarakoon D., et al. , 2015. Rice, wheat and maize biofortification, Sustainable Agriculture Reviews, Vol. 16, edited by Lichtfouse E., Goyal A. Springer, Cham: 10.1007/978-3-319-16988-0_6 [DOI] [Google Scholar]
- Gupta M., Verma B., Kumar N., Chahota R. K., Rathour R., et al. , 2012. Construction of intersubspecific molecular genetic map of lentil based on ISSR, RAPD and SSR markers. J. Genet. 91(3): 279–287. 10.1007/s12041-012-0180-4 [DOI] [PubMed] [Google Scholar]
- Han K., Jeong H. J., Yang H. B., Sung-Min K., Jin-Kyung K., et al. , 2016. An ultra-high-density bin map facilitates high-throughput QTL mapping of horticultural traits in pepper (Capsicum annuum). DNA Res. 23(2): 81–91. 10.1093/dnares/dsv038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacar, B., 1972 Chemical Analysis of Plant and Soil: II. Plant Analysis, Ankara University Press, Publication no: 453, Application book no: 155, Ankara, Turkey. [Google Scholar]
- Kacar, B., and A. Inal, 2008 Plant analyses, Nobel Press, First edition, No: 1241, Natural Applied Sciences No: 63, ISBN 978–605–395–036–3 Ankara, Turkey. [Google Scholar]
- Kahraman A., Pandey A., Khan M. K., 2017. Nutritional diversity assessment in chickpea-A prospect for nutrient deprived world. Harran Journal of Agricultural and Food Science 21(3): 357–363. [Google Scholar]
- Kannus P., 2000. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 10(6): 312–320. 10.1034/j.1600-0838.2000.010006312.x [DOI] [PubMed] [Google Scholar]
- Karaköy T., Erdem H., Baloch F. S., Toklu F., Eker S., et al. , 2012. Diversity of macro- and micronutrients in the seeds of lentil landraces. Sci. World J. 2012: 710412 10.1100/2012/710412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadari B., El Aabidine A. Z., Grout C., 2010. A genetic linkage map of olive based on amplified fragment length polymorphism, inter simple sequence repeat and simple sequence repeat markers. J. Am. Soc. Hortic. Sci. 135(6): 548–555. [Google Scholar]
- Klein M. A., Grusak M. A., 2009. Identification of nutrient and physical seed trait QTL in the model legume Lotus japonicus. Genome 52(8): 677–691. 10.1139/G09-039 [DOI] [PubMed] [Google Scholar]
- Komaki H., Maisawa S., Sugai K., Kobayashi Y., Hashimoto T., 1999. Tremor and seizures associated with chronic manganese intoxication. Brain Dev. 21(2): 122–124. 10.1016/S0387-7604(98)00074-6 [DOI] [PubMed] [Google Scholar]
- Li X., Wei Y., Acharya A., Jiang Q., Kang J., Brummer E. C., 2014. A saturated genetic linkage map of autotetraploid alfalfa (Medicago sativa L.) developed using Genotyping-by-Sequencing is highly syntenous with the Medicago truncatula genome. Genes/Genomes/Genetics 4(10): 1971–1979. 10.1534/g3.114.012245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay W. L., Norvell W. A., 1978. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 42(3): 421–428. 10.2136/sssaj1978.03615995004200030009x [DOI] [Google Scholar]
- Ma J. F., Higashitani A., Sato K., Takeda K., 2004. Genotypic variation in Fe concentration of barley grain. Soil Sci. Plant Nutr. 50(7): 1115–1117. 10.1080/00380768.2004.10408583 [DOI] [Google Scholar]
- Mayer J. E., Pfeiffer W. H., Beyer P., 2008. Biofortified crops to alleviate micronutrient malnutrition. Curr. Opin. Plant Biol. 11(2): 166–170. 10.1016/j.pbi.2008.01.007 [DOI] [PubMed] [Google Scholar]
- Monasterio I., Graham R. D., 2000. Breeding for trace minerals in wheat. Food Nutr. Bull. 21(4): 392–396. 10.1177/156482650002100409 [DOI] [Google Scholar]
- Muehlbauer F. J., McPhee K. E., 2005. Lentil (Lens culinaris Medik.), pp. 219–230 in Genetic Resources, Chromosome Engineering and Crop Improvement, edited by Singh R. J., Jauhar P. P. CRC Press, New York: 10.1201/9780203489284.ch8 [DOI] [Google Scholar]
- Nakandalage N., Nicolas M., Norton R. M., Hirotsu N., Milham P. J., et al. , 2016. Improving rice zinc biofortification success rates through genetic and crop management approaches in a changing environment. Front. Plant Sci. 7: 764 10.3389/fpls.2016.00764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikeh S. O., Menkir A., Maziya Dixon B., Welch R., Glahn R. P., 2003. Genotypic differences in concentration and bioavailability of kernel-iron in tropical maize varieties grown under field conditions. J. Plant Nutr. 26(10-11): 2307–2319. 10.1081/PLN-120024283 [DOI] [Google Scholar]
- Pinheiro C., Baeta J. P., Pereira A. M., Domingues H., Ricardo C. P., 2010. Diversity of seed mineral composition of Phaseolus vulgaris L. germplasm. J. Food Compos. Anal. 23(4): 319–325. 10.1016/j.jfca.2010.01.005 [DOI] [Google Scholar]
- Poland J. A., Brown P. J., Sorrells M. E., Jannink J. L., 2012. Development of high-density genetic maps for barley and wheat using a novel two-enzyme Genotyping-by-Sequencing approach. PLoS One 7(2): e32253 10.1371/journal.pone.0032253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podder R., DellaValle D. M., Tyler R. T., Glahn R. P., Tako E., et al. , 2018. Relative bioavailability of iron in Bangladeshi traditional meals prepared with iron-fortified lentil dal. Nutrients 10: 354 10.3390/nu10030354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B. V. G., Mohanta S., Rahaman S., Bareily P., 2015. Bio-fortification in horticultural crops. J. Agric. Eng. Food Technol. 2(2): 95–99. [Google Scholar]
- Pratt P. F., 1965, pp. 1010–1022 in Methods of Soil Analysis, edited by Black C. A., American Society of Agronomy Inc., Wisconsin, USA. [Google Scholar]
- Price C. T., Langford J. R., Liporace F. A., 2012. Essential nutrients for bone health and a review of their availability in the average north american diet. Open Orthop. J. 6: 143–149. 10.2174/1874325001206010143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Z. E., Yu M., He Q. Y., Chen G., Wang J., et al. , 2013. Quantitative trait loci associated with micronutrient concentrations in two recombinant ınbred wheat lines. J. Integr. Agric. 10.1016/S2095-3119(13)60640-1 [DOI] [Google Scholar]
- Qaim M., Stein A. J., Meenakshi J. V., 2007. Economics of biofortification. Agric. Econ. 37(s1): 119–133. 10.1111/j.1574-0862.2007.00239.x [DOI] [Google Scholar]
- Ramamurthy R. K., Jedlicka J., Graef G. L., Waters B., 2014. Identification of new QTLs for seed mineral, cysteine, and methionine concentrations in soybean. [Glycine max (L.) Merr.] Mol. Breed. 34(2): 431–445. 10.1007/s11032-014-0045-z [DOI] [Google Scholar]
- Richards L. A., 1954. 1954 Diagnosis and improvement of saline and alkali soils, United States Department of Agriculture Handbook, USA. [Google Scholar]
- Rodrigo S., Santamaría O., López-Bellido F. J., Poblaciones M. J., 2013. Agronomic selenium biofortification of two-rowed barley under Mediterranean conditions. Plant Soil Environ. 59(3): 115–120. 10.17221/691/2012-PSE [DOI] [Google Scholar]
- Rubeena R., Ford, Taylor P. W. J., 2003. Construction of an intraspecific linkage map of lentil (Lens culinaris ssp. culinaris). Theor. Appl. Genet. 107(5): 910–916. 10.1007/s00122-003-1326-9 [DOI] [PubMed] [Google Scholar]
- Sankaran R. P., Huguet T., Grusak M. A., 2009. Identification of QTL affecting seed mineral concentrations and content in the model legume Medicago truncatula. Theor. Appl. Genet. 119(2): 241–253. 10.1007/s00122-009-1033-2 [DOI] [PubMed] [Google Scholar]
- Santos E. E., Lauria D. C., Porto da Silveira C. L., 2004. Assessment of daily intake of trace elements due to consumption of foodstuffs by adult inhabitants of Rio de Janeiro city. Sci. Total Environ. 327(1–3): 67–69. 10.1016/j.scitotenv.2004.01.016 [DOI] [PubMed] [Google Scholar]
- Sarker A., Erskine W., Hassan M. S., Afzal M. A., Murshed A. N. M. M., 1999. Registration of “Barimasur-4” lentil. Crop Sci. 39(3): 876 10.2135/cropsci1999.0011183X003900030054x [DOI] [Google Scholar]
- Schlicting E., Blume H. P., 1966, p. 94 in Bodenkundliches practicum, edited by Parey V. P., Hamburg: 209. [Google Scholar]
- Sharma P., Aggarwal P., Kaur A., 2017. Biofortification: A new approach to eradicate hidden hunger. Food Rev. Int. 33(1): 1–21. 10.1080/87559129.2015.1137309 [DOI] [Google Scholar]
- Sharpe A. G., Ramsay L., Sanderson L. A., Fedoruk M., Clarke W. E., et al. , 2013. Ancient orphan crop joins modern era: Gene-based SNP discovery and mapping in lentil. BMC Genomics 14(1): 192 10.1186/1471-2164-14-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldin O. P., Aschner M., 2007. Effects of manganese on thyroid hormone homeostasis: Potential links. Neurotoxicology 28(5): 951–956. 10.1016/j.neuro.2007.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A., 2003. Manganese action in brain function. Brain Res. Brain Res. Rev. 41(1): 79–87. 10.1016/S0165-0173(02)00234-5 [DOI] [PubMed] [Google Scholar]
- Tanyolac B., Ozatay S., Kahraman A., Muehlbauer F., 2010. Linkage mapping of lentil (Lens culinaris L.) genome using recombinant inbred lines revealed by AFLP, ISSR, RAPD and some morphologic markers. J. Agric. Biotechnol. Sustain. Dev. 2(1): 1–6. [Google Scholar]
- Tullu A., Tar’an B., Warkentin T., Vandenberg A., 2008. Construction of an intraspecific linkage map and QTL analysis for earliness and plant height in lentil. Crop Sci. 48(6): 2254–2264. 10.2135/cropsci2007.11.0628 [DOI] [Google Scholar]
- Van den Berghe G., 2004. How does blood glucose control with insulin save lives in intensive care? J. Clin. Invest. 114(9): 1187–1195. 10.1172/JCI23506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ooijen J. W., 2009. MapQTL 6, Software for the mapping of quantitative trait loci in experimental populations of diploid species, Kyazma BV, Wageningen, Netherlands. [Google Scholar]
- Van Ooijen J. W., Voorrips R. E., 2004. JoinMap 4.0, software for the calculation of genetic linkage maps in experimental populations, Plant Research International, Wageningen, Netherlands. [Google Scholar]
- Vandenberg A., Banniza S., Warkentin T. D., Ife S., Barlow B., et al. , 2006. CDC Redberry lentil. Can. J. Plant Sci. 86(2): 497–498. 10.4141/P05-071 [DOI] [Google Scholar]
- Willems G., Fre’rot H., Gennen J., Salis P., Saumitou-Lapradeand P., et al. , 2010. Quantitative trait loci analysis of mineral element concentrations in an Arabidopsis halleri x Arabidopsis lyrata petraea F2 progeny grown on cadmium-contaminated soil. New Phytol. 187(2): 368–379. 10.1111/j.1469-8137.2010.03294.x [DOI] [PubMed] [Google Scholar]
- Witte K. K. A., Clark A. L., Cleland J. G. F., 2001. Chronic heart failure and micronutrients. J. Am. Coll. Cardiol. 37(7): 1765–1774. 10.1016/S0735-1097(01)01227-X [DOI] [PubMed] [Google Scholar]
- Wu J., Yuan Y. X., Zhang X. W., Zhao J., Song X., et al. , 2008. Mapping QTLs for mineral accumulation and shoot dry biomass under different Zn nutritional conditions in chinese cabbage (Brassica rapa L. ssp. pekinensis). Plant Soil 310(1-2): 25–40. 10.1007/s11104-008-9625-1 [DOI] [Google Scholar]
- Xu Y., Porntadavity S., St. Clair D. K., 2002. Transcriptional regulation of the human manganese superoxide dismutase gene: The role of specificity protein 1 (Sp1) and activating protein-2 (AP-2). Biochem. J. 362(2): 401–412. 10.1042/bj3620401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. H., Shao Y. F., Liu J., Fan Y. Y., Sun C. X., et al. , 2015. Mapping of quantitative trait loci for contents of macro- and microelements in milled rice (Oryza sativa L.). J. Agric. Food Chem. 63(35): 7813–7818. 10.1021/acs.jafc.5b02882 [DOI] [PubMed] [Google Scholar]
- Zhu C. F., Naqvi S., Gomez-Galera S., Pelacho A. M., Capell T., et al. , 2007. Transgenic strategies for the nutritional enhancement of plants. Trends Plant Sci. 12(12): 548–555. 10.1016/j.tplants.2007.09.007 [DOI] [PubMed] [Google Scholar]
- Zou T., Xu N., Hu G., Pang J., Xu H., 2014. Biofortification of soybean sprouts with zinc and bioaccessibility of zinc in the sprouts. J. Sci. Food Agric. 94(14): 3053–3060. 10.1002/jsfa.6658 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
File S1 contains SNP data. File S2 contains Mn phenotyping data.