Abstract
The fat-1 gene from Caenorhabditis elegans encodes a fatty acid desaturase which was widely studied due to its beneficial function of converting n-6 polyunsaturated fatty acids (n-6PUFAs) to n-3 polyunsaturated fatty acids (n-3PUFAs). To date, many fat-1 transgenic animals have been generated to study disease pathogenesis or improve meat quality. However, all of them were generated using a random integration method with variable transgene expression levels and the introduction of selectable marker genes often raise biosafety concern. To this end, we aimed to generate marker-free fat-1 transgenic pigs in a site-specific manner. The Rosa26 locus, first found in mouse embryonic stem cells, has become one of the most common sites for inserting transgenes due to its safe and ubiquitous expression. In our study, the fat-1 gene was inserted into porcine Rosa 26 (pRosa26) locus via Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/CRISPR-associated 9 (Cas9) system. The Southern blot analysis of our knock-in pigs indicated a single copy of the fat-1 gene at the pRosa26 locus. Furthermore, this single-copy fat-1 gene supported satisfactory expression in a variety of tissues in F1 generation pigs. Importantly, the gas chromatography analysis indicated that these fat-1 knock-in pigs exhibited a significant increase in the level of n-3PUFAs, leading to an obvious decrease in the n-6PUFAs/n-3PUFAs ratio from 9.36 to 2.12 (***P < 0.0001). Altogether, our fat-1 knock-in pigs hold great promise for improving the nutritional value of pork and serving as an animal model to investigate therapeutic effects of n-3PUFAs on various diseases.
Keywords: pig, fat-1, genome editing, omega-3 polyunsaturated fatty acids
The n-6PUFAs and n-3PUFAs have an important role in regulating biological processes. The n-3PUFAs include alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoate acid (DPA). The n-6PUFAs mainly include arachidonic acid (AA) and linoleic acid (LA) (Kim et al. 2016; Zhu et al. 2008). N-3PUFAs provide broad potential health benefits according to previous studies, and thus are increasingly considered as necessary nutrients in a daily diet (Sioutis et al. 2008). It has been showed that cancer cells rich in n-3PUFAs underwent apoptosis whereas control cancer cells proliferated normally (Kim et al. 2018; Ge et al. 2002). Furthermore, a lower n-6PUFAs/n-3PUFAs ratio diet could attenuate disease progression of depressive patients compared to the control group (Beydoun et al. 2015). Recent study provided new insights to the protective effect of n-3 PUFAs against non-alcoholic liver disease (McCormick et al. 2015). Therefore, a lower n-6PUFAs/n-3PUFAs ratio is particularly important for a healthy diet. However, the lack of an enzyme capable of converting n-6PUFAs into n-3PUFAs leads to a high n-6PUFAs/n-3PUFAs ratio in mammals. It has consistently been considered unhealthy when excessive mammalian meat products were included in modern diet (Cheng et al. 2015; Lai et al. 2006).
The fat-1 gene from Caenorhabditis elegans provided a feasible solution to the abovementioned problem because the fatty acid desaturase,it encoded, is capable of converting n-6PUFAs to n-3PUFAs by joining a double bond at the n-3 hydrocarbon position of the n-6PUFAs (Kang 2005). Researchers have developed fat-1 transgenic mouse (Kang et al. 2004), pig (Lai et al. 2006) and cow (Wu et al. 2012) in which the n-6PUFAs were successfully converted into n-3PUFAs. Furthermore, these transgenic animal models have been used to study a wide range of diseases such as arthritis (Woo et al. 2015), allergic reactions (Bilal et al. 2011), cardiovascular diseases (Kris-Etherton et al. 2004), cancers (Han et al. 2016a) and Alzheimer’s disease (Wu et al. 2016a). Indeed, these fat-1 transgenic animals that are rich in n-3 PUFAs alleviate these diseases at different levels. However, all reported fat-1 transgenic animals were obtained by random integration, which may lead to variable expression levels and unstable phenotypes due to multiple-copy integration and uncontrollable insertion sites. Instead, site-specific integration allows the exogenous gene to be inserted into a specific locus, enables stable expression of transgenes at defined sites (Nandy and Srivastava 2011; Obayashi et al. 2012). Furthermore, fat-1 transgenic animals generated by conventional transgenic methods carry selectable marker genes that may disrupt the expression of endogenous genes and increase public concern regarding the release of antibiotic genes into the environment (Yu et al. 2013). Hence, generating selectable marker-free as well as site-specific transgenic animals could ease the potential biosafety problem.
The Rosa26 gene was originally found in mouse embryonic stem cells and subsequently identified in human, rat, pig, sheep and rabbit (Friedrich and Soriano 1991; Irion et al. 2007; Kobayashi et al. 2012; Li et al. 2014; Wu et al. 2016b; Yang et al. 2016). It directs ubiquitous expression of a non-coding RNA in embryonic and adult tissues (Kong et al. 2014). In addition, this locus was proved to be a safe harbor because the transgenes inserted here do not interfere with the function of endogenous genes in mice (Friedrich and Soriano 1991). At present, many Rosa26 targeted animals have been successfully generated. Accordingly, we considered inserting fat-1 gene at this locus so as to achieve controllable expression under the control of endogenous Rosa26 promoter. As a powerful and widely used tool, CRISPR/Cas9 could efficiently produce flexible genome modifications, including deletion, insertion (Wu et al. 2016b; Chu et al. 2016; He et al. 2016), point mutation (Armstrong et al. 2016; Wang et al. 2016) and replacement (Lin and Potter 2016; Luo et al. 2016). It relies on a Cas9/gRNA complex in which the Cas9 protein cleaves at a specific target site under the guidance of a single guide RNA (sgRNA). The cleavage by Cas9 leads to DNA double-strand breaks (DSBs) which could be repaired through either non-homologous end joining (NHEJ) or homology-directed repair (HDR)(Mali et al. 2013). The NHEJ repair pathway often generates indels including non-specific insertions or deletions, whereas the HDR pathway induces precise gene editing at the target site taking advantage of homologous templates (Mali et al. 2013). In this study, we achieved site-specific fat-1 insertion in PFFs (Porcine fetal fibroblasts) via CRISPR/Cas9. The single-copy fat-1 resulted in significant reduction of n-6PUFAs/n-3PUFAs ratio in transgenic pigs, providing a practical reference for further genetic breeding studies.
Materials and Methods
Animals
All pigs were obtained from the Huichang Animal Husbandry Science and Technology Co., Ltd. All animal studies were approved by the Animal Welfare and Research Ethics Committee at Jilin University (ratified ID: 20160601), and all procedures were carried out in strict accordance with The Guide for the Care and Use of Laboratory Animals.
Vector construction
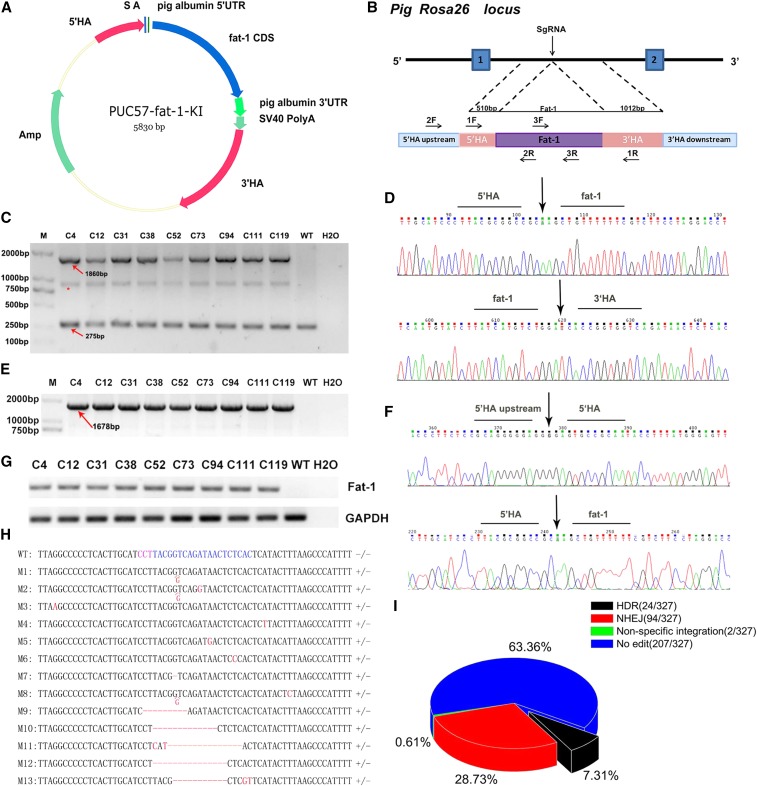
The pROSA26-specific sgRNA was cloned into the PX330 vector (Addgene) to make a functional Cas9/gRNA vector, which is designated as pX330-sgRNA91 hereafter. Pig albumin 5′UTR and 3′UTR synthesized by GENEWIZ (Suzhou, China) were cloned into the psiCheck-2 vector (Promega). The 5′UTR was inserted immediately after the T7 promoter and the 3′UTR was inserted immediately after renilla luciferase gene. The renilla luciferase was reporter gene and the firefly luciferase served as reference gene. We designated this intact vector as psiCheck-2-pig albumin UTR. The codons for the fat-1 gene from C. elegans (GenBank: NM_001028389) were optimized for efficient expression in mammals. The splicing acceptor (SA) sequence, 5′UTR sequence of porcine albumin, optimized fat-1 sequence, 3′UTR sequence of porcine albumin and SV40 PolyA sequence were then synthesized together by GENEWIZ (Suzhou, China) to constitute the transgene fragment for insertion. Based on our previous work, the donor vector showed a higher knock-in efficiency at the pROSA26 locus when it contained a 5′ HA of approximately 0.5 kb and a 3′ HA of approximately 1.0 kb (Xie et al. 2017). Therefore, the optimized HAs were PCR amplified and cloned into the PUC57 vector (Addgene). Finally, the synthesized fragment containing fat-1 was inserted between the 5′ HA and 3′ HA. We designated this intact targeting vector as PUC57-fat-1-KI (Figure 2A).
Figure 2.
Site-specific fat-1 knock-in at the pRosa26 locus. (A) Schematic of the donor plasmid for the fat-1 insertion. 5′HA, 5′ homologous arm (0.5 kb); 3′HA, homologous arm (1.0 kb); (B) Strategy of Cas9-mediated knock-in of fat-1 into the pRosa26 locus; (C) PCR analysis of the PFF clones using primer pair 1F/1R. The 1F primer was designed in 5′ HA and the 1R primer designed in 3′ HA. M: D2000. WT: negative control. H2O: blank control. *: A hybrid band formed by knock-in band and wild-type band in the process of genomic PCR of heterozygous animals; (D) Sanger sequencing results of the PCR products in (C) ; (E) PCR analysis of the PFF clones using primer pair 2F/2R. The 2F primer was designed upstream of 5′ HA and the 2R primer was designed in the fat-1 CDS; (F) Sanger sequencing results of the PCR products in (E) ; The primer pair 1F/1R was used to identify the insertion of the fat-1 gene whereas the primer pair 2F/2R was used to identify the site-specific integration of fat-1; (G) RT-PCR was performed to confirm the transcription of the fat-1 gene in genetically positive clones using the 3F/3R primer (268bp) ; (H) The wild-type sequence is placed on the first line and the mutation sequence of single-cell clones were placed below. The PAM was marked in purple and the target site was marked in blue. −/− means wild-type allele and +/− means NHEJ occurred in one allele; (I) The HDR, NHEJ and non-specific integration ratio of 327 individual cell cones.
Electroporation and luciferase assay
Approximately 30 µg of psiCheck-2-pig albumin UTR plasmid and 30 µg of control psiCheck-2 plasmid were respectively transfected into 3×106 PK-15 cells using the BTX-ECM 2001 Electroporation system. 48 hr later, the cell culture medium was removed and the cells were lysed to detect Firefly and Renilla luminescent according to the protocol of Dual Luciferase Reporter Gene Assay Kit (Beyotime Biotechnology, Shanghai, China) with a Infinite 200 Pro (TECAN).
Electroporation and selection of PFFs
Approximately 30 µg of pX330-sgRNA91 plasmid and 30 µg of donor puc57-fat-1-KI plasmid were cotransfected into 3×106 PFFs. Two days after electroporation, cells were seeded into 100 mm dishes and nine days later individual cell clones were picked and cultured in 24-well plates. When the cells reached approximately 80% confluence, 10% of them were lysed using NP40 lysis buffer (0.45% NP40 plus 0.6% proteinase K). The lysate was used as the PCR template for genotyping. The 1F primer designed in the 5′HA was 5′- GCATTGAGACTGCGTGTTATTAC -3′, and the 1R primer designed in the 3′HA was 5′-ATTCAAAAGACATAAAGGGGAG-3′. The PCR conditions are shown below: 94° for 5 min, followed by 35 cycles of 94° for 30 s, 60° for 30 s, and 72° for 2 min, with a final incubation step at 72° for 5 min. The 2F primer designed outside the 5′HA was 5′-GGTCCCAAATGAGCGAAAC-3′, and the 2R primer designed in the fat-1 CDS was 5′-TGATGACGCACTGCACTCTTT-3′. The PCR conditions for this primer pair are shown below: 94° for 5 min, followed by 35 cycles of 94° for 30 s, 58° for 30 s, and 72° for 1 min 50 s, with a final incubation step at 72° for 5 min. All PCRs were performed using taq DNA polymerase (TIANGEN). The primer pair 1F/1R was used to identify the insertion of the target gene, and the primer pair 2F/2R was used to identify the site-specific integration of fat-1. The PCR amplicons of one individual cell clone were TA cloned using pGM-T Fast Ligation Kit (TIANGEN) and then sequenced. Next, total RNA was extracted from PCR-positive clones using TRNzol-A+ Reagent (TIANGEN) according to the manufacturer’s instructions. An aliquot of 1 μg RNA was used to generate cDNA using a BioRTcDNA First Strand Synthesis Kit (Bioer Technology). The cDNA template was PCR amplified to confirm expression of the fat-1 gene in positive clones. The following primers were used: 3F: 5′-TGTGTGGATTCAGGACAAGG-3′ and 3R: 5′-CCAGTAGTACCAGAACCAGTTG-3′. The PCR conditions are shown below: 94° for 5 min, followed by 35 cycles of 94° for 30 s, 58° for 30 s, and 72° for 30 s, with a final incubation step at 72° for 5 min.
SCNT and embryo transfer
The fat-1-KI-positive PFF cells cultured in 24-well plates were used to perform SCNT, which was carried out according to previous studies (Lai et al. 2002). The positive PFFs were injected into the perivitelline cytoplasm of enucleated oocytes to form reconstructed embryos. The reconstructed embryos were subsequently activated and cultured for approximately 18 h followed by embryo transfer, as described by Lai et al. (Yang et al. 2011).
Genotype analysis of the cloned piglets
To confirm insertion and site-specific integration of the fat-1 gene, genomic DNA extracted from the ears of cloned piglets was used as a template for PCR using the 1F/1R and 2F/2R primer pairs, as described above. Total RNA was extracted from the tails of cloned piglets, and RT-PCR was performed to detect fat-1 mRNA using the 3F/3R primer. The PCR products were subjected to electrophoresis and sequencing.
Southern blot analysis
A Southern blot analysis was performed as described previously (Southern 2006). Briefly, approximately 20 μg of high-quality genomic DNA was digested by BamH I and then subjected to agarose gel electrophoresis. Next, the DNA fragments were transferred to a nylon membrane (Amersham). The probe fragment (729bp) was partial of fat-1 CDS (1212bp) and probe primers specific for fat-1 CDS were F: 5′-TCAACGCCAACACCAAGCA-3′ and R: 5′-GGTAGGTCACGATCACCAGCAT-3′. The PCR product labeled with digoxigenin by a PCR DIG Probe Synthesis Kit (Roche) were tested by gel electrophoresis and finally the purified probe was hybridized with the DNA fragments on the membrane.
Karyotype analysis
Porcine tail fibroblasts isolated from newborn piglets were treated with 10 μg/mL demecolcine for 12 h, incubated in 75 mM KCl at 37° for 30 min, fixed on a glass slide using 3:1 methanol/acetic acid for 20 min, stained with Giemsa for 10 min, and finally imaged under the microscope (Nikon eclipse Ti).
Transcriptional analysis of fat-1 gene in transgenic pigs using real-time PCR
Total RNA from heart, liver, spleen, lung, kidney, skeletal muscle, brain and tongue of fat-1 knock-in piglet were extracted separately using the TRNzol-A+ reagent (Tiangen, Beijing, China). An aliquot of 1 μg RNA was used to generate cDNA using a BioRTcDNA First Strand Synthesis Kit (Bioer Technology) and the resulting samples were used to perform real-time PCR to quantify fat-1 expression at the transcriptional level. Porcine GAPDH served as the reference gene. The primers of fat-1 gene and GAPDH gene were shown as follows: fat-1 forward (5′-TGTGTGGATTCAGGACAAGG-3′) and reverse (5′-CCAGTAGTACCAGAACCAGTTG-3′), GAPDH forward (5′-GCCATCACCATCTTCCAGG-3′) and reverse (5′-TCACGCCCATCACAAACAT-3′).
Fatty acid analysis
To evaluate the activity of fatty acid desaturase in fat-1 transgenic pigs, total muscle fatty acids were extracted as described in previous studies (Han et al. 2016b; Lu et al. 2008). The fatty acid components were then analyzed by gas chromatography spectrometry (7890A, Agilent Technologies, USA) equipped with a SP-2560 capillary column (100 m*0.25 mm*0.2 µm, Sigma). The initial temperature of the column was maintained at 140° for five min, and then raised to 220° for 40 min at a rate of 4° / min. The percentage of each fatty acid was calculated using a peak area normalization method.
Statistical analysis
Statistical analysis was performed using a two tailed Student’s t-test, and p values < 0.05 were considered significant.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
Optimization of fat-1 expression donor vector
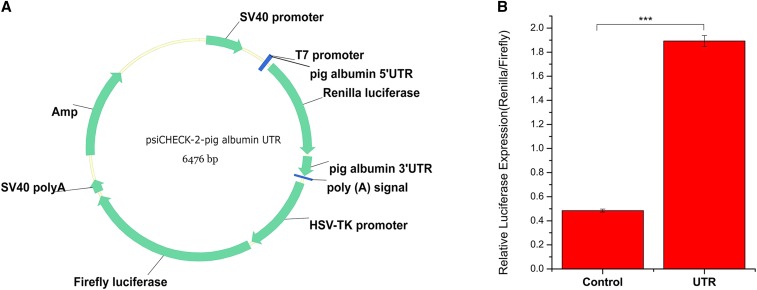
Serum albumin is the richest protein in mammalian plasma (Bujacz 2012; Nishijima et al. 2014). So the 5′UTR and 3′UTR of porcine albumin were thought to have a great potential in regulating gene expression. In our study, the 5′UTR and 3′UTR of porcine albumin were cloned into priCheck-2 and then tansfected into PK-15 cells to verify the up-regulation. Results showed the presence of porcine albumin UTR significantly increased the relative luciferase expression (P < 0.0001) (Figure 1). Therefore, the porcine albumin 5′UTR was added in the upstream of fat-1 CDS and the porcine albumin 3′UTR was added in the downstream of fat-1 CDS for site specific knock-in (Figure 2 A, B).
Figure 1.
Dual luciferase assays in PK-15 cells. (A) Schematic of the psiCheck-2-pig albumin UTR plasmid; (B) The relative luciferase expression between psiCheck-2-pig albumin UTR plasmid transfected cells and control psiCheck-2 plasmid transfected cells. N = 3, *** P < 0.0001.
CRISPR/Cas9-mediated integration of fat-1 in PFFs
To insert the fat-1 CDS at the first intron of the pRosa26 locus, pX330-sgRNA91 and PUC57-fat-1-KI plasmids were cotransfected in PFFs by electroporation. As the donor vector for HDR, PUC57-fat-1-KI plasmid contained no selectable markers or exogenous promoters (Figure 2A). Instead, the endogenous pRosa26 promoter was utilized to drive the expression of fat-1. Two days after electroporation, cells were seeded into 100 mm dishes, and nine days later individual cell clones were collected. PCR products (2F/2R) spanning the junction regions were sequenced to determine the existence of the site-specific insertion of the fat-1 gene. PCR amplicons (1F/1R) covering the integration site were TA cloned and sequenced to assess the NHEJ events. PCR products (3F/3R) covering a smaller fragment of fat-1 were used to assess non-specific integration. A total of 24 cell clones out of 327 single-cell clones exhibited the intended bands following both HA-PCR and junction-PCR (Figure 2C, E). The subsequent sequencing results further confirmed the precise integration of fat-1 at the pRosa26 locus (Figure 2D, F) but all of the positive clones were heterozygous. NHEJ events occurred in 94 individual cell clones including deletions, point mutation and single base insertion (Figure 2H, I and Figure S4 in File S1). The 1F/1R and 3F/3R PCR products of clone No. 74 and clone No. 187 showed expected bands whereas 2F/2R PCR products did not showed expected bands. This result indicated that fat-1 gene was non-specific integrated into pig genome in clone No. 74 and clone No. 187 (Figures S1–S3 in File S1). Overall, the HDR, NHEJ and non-specific integration ratio was 7.31%, 0.61% and 28.73% respectively (Figure 2I). Finally, total RNA was extracted from these KI clones and the RT-PCR results confirmed transcription of the fat-1 gene (Figure 2G).
Generation and genotyping of cloned piglets
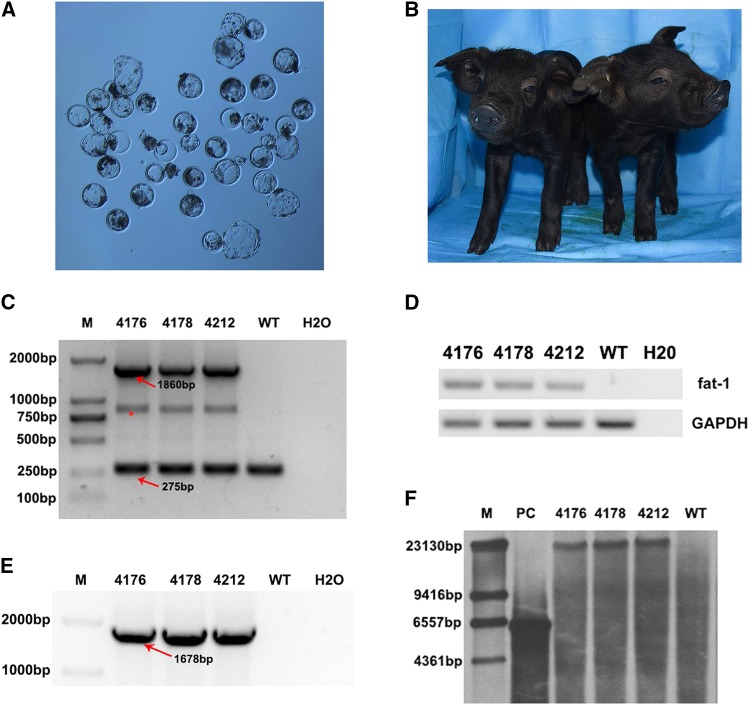
Prior to the reconstructed embryo transfer, wild-type and fat-1 knock-in PFFs were used as donor cells to perform SCNT and examine the developmental potency of reconstructed embryos. Our results demonstrated that wild type and fat-1 knock in PFFs donor cells shared a similar rate of blastocyst development (22.57 ± 0.1122% vs. 22.44 ± 0.2323%, P > 0.05, n = 3) (Figure 3A and Table S1 in File S1) Hence, the fat-1 gene insertion has no adverse effects on the development of reconstructed embryos. A total of 500 reconstructed embryos were surgically transferred to the uterus of five recipients, and two recipients were pregnant (Table S2 in File S1). After approximately 114 days of gestation, three piglets were born by eutocia with an average birth weight of 1.17 kg (Figure 3B). All of them grew and developed normally. The PCR and sequencing results showed that all piglets contained the site-specific fat-1 knock-in at the pRosa26 locus (Figure 3C, D); RT-PCR also confirmed the expression of fat-1 in the tails of these cloned pigs. Chromosome karyotype analysis showed that the positive pigs had normal chromosome numbers (Figure S5 in File S1). Furthermore, Southern blotting results showed an intended band, indicating a single insertion site for fat-1 in the porcine genome (Figure 3F). In summary, we obtained cloned pigs that harbored a site-specific integration of fat-1 at the pRosa26 locus.
Figure 3.
Generation and genotyping of cloned piglets. (A) The reconstructed embryos were cultured in vitro for approximately 6 days until the blastocyst stage; (B) Photo of site-specific fat-1 knock-in piglets at seven days after birth; (C, E) PCR analysis of fat-1 knock-in pigs using primer 1F/1R and primer 2F/2R respectively. *: A hybrid band formed by knock-in band and wild-type band in the process of genomic PCR of heterozygous animals; (D) RT-PCR analysis of fat-1 positive pigs using primer 3F/3R; (F) The Southern blot result of fat-1 knock-in pigs using the digoxigenin labled fat-1 specific probe.
F1 generation of fat-1 knock-in pigs and transcriptional analysis of fat-1 gene
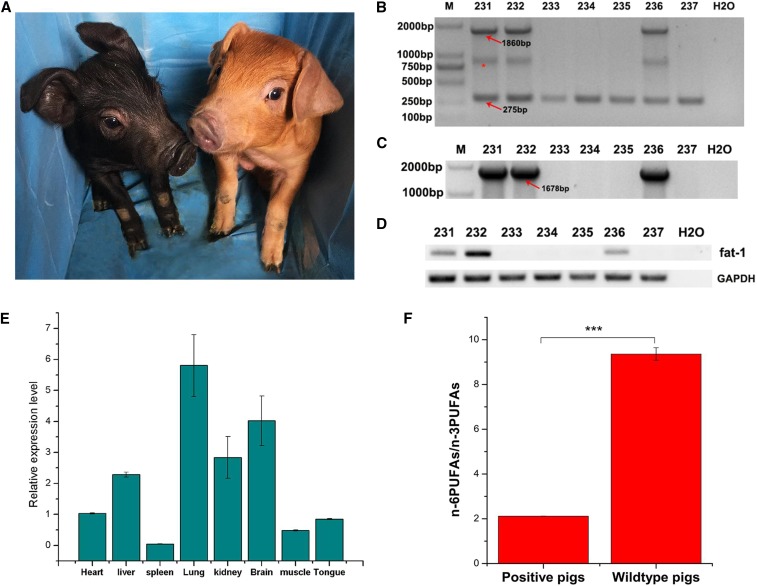
One of the fat-1 knock-in pigs was mated with Duroc boars, and 7 F1 generation piglets were born (Figure 4A). Three piglets are heterozygous for fat-1gene insertion at the pRosa26 locus as evidenced by RT-PCR and sequencing (Figure 4B, C and D). To quantify fat-1 expression at the transcriptional level, total RNA from the heart, liver, spleen, lung, kidney, skeletal muscle, brain and tongue of fat-1 knock-in piglets were subjected to real-time PCR. Results show that fat-1 gene expressed in a wide variety of tissues and has the highest expression in the lung whereas the lowest expression in the spleen among all the tissues detected. In addition, the fat-1 expression level in the kidney and brain was also relatively high (Figure 4E).
Figure 4.
Transcriptional analysis of fat-1 gene and fatty acid analysis of muscle tissues of F1 generation. (A) Photo of F1 generation fat-1 knock-in piglets at ten days after birth; The F1 generation was the offspring of Songliao Black sow and Duroc boars. (B, C) PCR analysis of F1 generation using primer 1F/1R and primer 2F/2R. *: A hybrid band formed by knock-in band and wild-type band in the process of genomic PCR of heterozygous animals; (D) RT-PCR analysis of F1 generation using primer 3F/3R; (E) Transcriptional level analysis of fat-1 gene in a wide variety of tissues as determined by real-time PCR; (F) The n-6PUFAs/n-3PUFAs ratio of fat-1 knock-in pigs and wild-type pigs. There are seven F1 generations and three of them were fat-1 knock-in pigs. The three positive piglets and three littermate wild-type piglets were killed and muscle fatty acids were extracted to evaluate the n-6PUFAs/n-3PUFAs ratio. Values are denoted as the Mean± SEM, n = 3, *** P < 0.0001.
Analysis of fatty acids in the fat-1 knock-in pigs
To further determine whether the fat-1 knock-in pigs exhibit a beneficial phenotype, fatty acids extracted from the muscle tissue of fat-1 and wild-type pigs were measured using gas chromatography. The level of LA and AA in fat-1 pigs decreased dramatically compared with wild-type pigs. In contrast, the level of n-3PUFAs including ALA, DHA and DPA in fat-1 pigs were significantly higher than those in wild-type pigs, leading to an obvious decrease in the n-6PUFAs/n-3PUFAs ratio of fat-1 pigs from 9.36 to 2.12 (Figure 4F and Table 1). Collectively, these results demonstrate that the single copy knock-in of fat-1 driven by Rosa26 promoter is sufficient to convert n-6PUFAs to n-3PUFAs so as to reduce the n-6PUFAs/n-3PUFAs ratio in these pigs.
Table 1. Quantification of PUFAs in fat-1 knock-in and wild-type pigs.
| Fatty acids | Fat-1 knock-in pigs | Wild-type pigs |
|---|---|---|
| n-6 PUFAs | ||
| C18:2(LA) | 4.719 ± 0.04711*** | 7.781 ± 0.002225 |
| C20:4(AA) | 3.374 ± 0.03778*** | 4.518 ± 0.01559 |
| Total n6 | 8.094 ± 0.01791*** | 12.30 ± 0.01623 |
| n-3 PUFAs | 0.4084 ± 0.005407*** | 0.2510 ± 0.002812 |
| C18:3(ALA) | 0.06893 ± 0.008027 | 0.08027 ± 0.00256 |
| C20:5(EPA) | 0.6380 ± 0.007916*** | 0.5159 ± 0.006721 |
| C22:6(DHA) | 2.693 ± 0.01361*** | 0.4692 ± 0.03188 |
| C22:5(DPA) | 3.817 ± 0.008717*** | 1.316 ± 0.03965 |
| Total n3 | 2.120 ± 0.00111*** | 9.360 ± 0.2790 |
| n-6/n-3 ratio |
Each fatty acid is presented as the percentage in total fatty acid. Values are denoted as the Mean± SEM, n = 3, *** P < 0.0001.
Discussion
N-3PUFAs mainly derived from marine products have beneficial health effects related to many diseases such as cardiovascular diseases, inflammatory disease and cancer. As the most eaten meat in the world, pork can be an important source of n-3PUFAs especially for populations that barely consumed marine products (Dugan et al. 2015). But high n-6PUFAs/n-3PUFAs ratio of regular pork makes it a poor source of n-3PUFAs. Transgenic technologies give us a new sight to solve the problem and thus n-3PUFAs-rich transgenic pigs were generated. In the studies of Lai et al., the concentration of n-3PUFAs in fat-1 transgenic pigs tails were significantly increased, leading to a drop in the n-6PUFAs/ n-3PUFAs ratio from 8.52 to 1.69 (Lai et al. 2006). Zhang et al. reported that the ratio of n-6PUFAs/n-3PUFAs in the fat-1 transgenic pig muscle decreased from 48.85 to 10.91 (Zhang et al. 2012). However, all previous fat-1 transgenic pigs were generated using random integration which introduced the exogenous CAG promoter and selectable marker genes. Although the CAG promoter is a powerful synthetic promoter constantly used to induce the high level of gene expression in mammals (Sakaguchi et al. 2014), the methylation of it often leads to unstable expression (Zhou et al. 2014) even silence of transgenes (Duan et al. 2012). The introduction of selectable marker genes would increase public concern on biosafety of transgenic animals, thus limiting the commercial application. Therefore, it is essential to generate site-specific integration as well as selectable marker-free transgenic animals. In our study, we implemented precise genome editing in PFFs, whereby fat-1 was integrated in a site-specific fashion to the pRosa26 locus based on CRISPR/cas9-mediated HDR. Three heterozygous fat-1 knock-in pigs were obtained via SCNT. The Southern blot result indicated no random integration was found in any of the three positive pigs. Furthermore, karyotype analysis indicated they had normal chromosome numbers (Figure S5 in File S1). Additionally, we obtained three F1 piglets in which the fat-1gene was inserted at pRosa26 locus and expressed in a wide variety of tissues. Based on our fatty acid analysis of F1 muscle tissue, the proportion of LA dropped by 1.65 times in fat-1 pigs compared with wild-type pigs and the AA dropped by 1.34 times. The proportion of n-3PUFAs including ALA, DHA and DPA have risen by 1.63 times, 1.24 times and 5.74 times in the fat-1 pigs, respectively. As a result, the ratio of n-6PUFAs/n-3PUFAs in fat-1 pigs decreased dramatically from 9.36 to 2.12.
In summary, we successfully generated site-specific fat-1 transgenic pigs whose genotype could be transmitted to the next generation and enables obvious decrease of n-6PUFAs/n-3PUFAs with a single-copy of fat-1 gene. Importantly, this kind of n-3PUFA-rich pork would bring health benefits to consumers compared to regular pork products. At the same time, the site-specific fat-1 transgenic pigs can serve as an animal model to investigate therapeutic effects of n-3PUFAs on various diseases.
Supplementary Material
Supplemental Material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.118.200114/-/DC1.
ACKNOWLEDGMENTS
The authors thank the members of the Animal Biotechnology Laboratory, College of Animal Science, Jilin University. This work was financially supported through a grant from the Special Funds for Cultivation and Breeding of New Transgenic Organisms (2018ZX0801012B), the Program for JLU Science and Technology Innovative Research Team (2017TD-28) and the Program for Changjiang Scholars and Innovative Research Team in University in China (No.IRT16R32).
Footnotes
Communicating editor: M. Watson
Literature Cited
- Armstrong G. A., Liao M., You Z., Lissouba A., Chen B. E., et al. , 2016. Homology Directed Knockin of Point Mutations in the Zebrafish tardbp and fus Genes in ALS Using the CRISPR/Cas9 System. PLoS One 11(3): e0150188 10.1371/journal.pone.0150188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun M. A., Fanelli Kuczmarski M. T., Beydoun H. A., Rostant O. S., Evans M. K., et al. , 2015. Associations of the Ratios of n-3 to n-6 Dietary Fatty Acids With Longitudinal Changes in Depressive Symptoms Among US Women. Am. J. Epidemiol. 181(9): 691–705. 10.1093/aje/kwu334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal S., Haworth O., Wu L.J., Weylandt K.H., Levy B.D., et al. , 2011. Fat-1 transgenic mice with elevated omega-3 fatty acids are protected from allergic airway responses. Biochimica Et Biophysica Acta-Molecular Basis of Disease 1812(9): 1164–1169. 10.1016/j.bbadis.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujacz A., 2012. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr. D Biol. Crystallogr. 68(Pt 10): 1278–1289. 10.1107/S0907444912027047 [DOI] [PubMed] [Google Scholar]
- Cheng G., Fu C., Wang H., Adoligbe C., Wei S., et al. , 2015. Production of transgenic beef cattle rich in n-3 PUFAs by somatic cell nuclear transfer. Biotechnol. Lett. 37(8): 1565–1571. 10.1007/s10529-015-1827-z [DOI] [PubMed] [Google Scholar]
- Chu V., Weber T., Graf R., Sommermann T., Petsch K., et al. , 2016. Efficient generation of Rosa26 knock-in mice using CRISPR/Cas9 in C57BL/6 zygotes. BMC Biotechnol. 16: 4 10.1186/s12896-016-0234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B., Cheng L., Gao Y., Yin F. X., Su G. H., et al. , 2012. Silencing of fat-1 transgene expression in sheep may result from hypermethylation of its driven cytomegalovirus (CMV) promoter. Theriogenology 78(4): 793–802. 10.1016/j.theriogenology.2012.03.027 [DOI] [PubMed] [Google Scholar]
- Dugan M. E. R., Vahmani P., Turner T. D., Mapiye C., Juarez M., et al. , 2015. Pork as a Source of Omega-3 (n-3) Fatty Acids. J. Clin. Med. 4(12): 1999–2011. 10.3390/jcm4121956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich G., Soriano P., 1991. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5(9): 1513–1523. 10.1101/gad.5.9.1513 [DOI] [PubMed] [Google Scholar]
- Ge Y., Chen Z., Kang Z. B., Cluette-Brown J., Laposata M., et al. , 2002. Effects of adenoviral gene transfer of C. elegans n-3 fatty acid desaturase on the lipid profile and growth of human breast cancer cells. Anticancer Res. 22(2A): 537–543. [PubMed] [Google Scholar]
- Han Y. M., Kim K. J., Jeong M., Park J. M., Go E. J., et al. , 2016a Suppressed Helicobacter pylori-associated gastric tumorigenesis in Fat-1 transgenic mice producing endogenous omega-3 polyunsaturated fatty acids. Oncotarget 7(41): 66606–66622. 10.18632/oncotarget.11261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. M., Park J. M., Cha J. Y., Jeong M., Go E. J., et al. , 2016b Endogenous conversion of ω-6 to ω-3 polyunsaturated fatty acids in fat-1 mice attenuated intestinal polyposis by either inhibiting COX-2/β-catenin signaling or activating 15-PGDH/IL-18. Int. J. Cancer 138(9): 2247–2256. 10.1002/ijc.29956 [DOI] [PubMed] [Google Scholar]
- He X., Tan C., Wang F., Wang Y., Zhou R., et al. , 2016. Knock-in of large reporter genes in human cells via CRISPR/Cas9-induced homology-dependent and independent DNA repair. Nucleic Acids Res. 44(9): e85 10.1093/nar/gkw064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irion S., Luche H., Gadue P., Fehling H. J., Kennedy M., et al. , 2007. Identification and targeting of the ROSA26 locus in human embryonic stem cells. Nat. Biotechnol. 25(12): 1477–1482. 10.1038/nbt1362 [DOI] [PubMed] [Google Scholar]
- Kang J. X., 2005. From fat to fat-1: A tale of omega-3 fatty acids. J. Membr. Biol. 206(2): 165–172. 10.1007/s00232-005-0790-3 [DOI] [PubMed] [Google Scholar]
- Kang J. X., Wang J., Wu L., Kang Z. B., 2004. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature 427(6974): 504 (erratum: Nature 427: 698) 10.1038/427504a [DOI] [PubMed] [Google Scholar]
- Kim D. H., Lee H. J., Amanullah S. M., Adesogan A. T., Kim S. C., 2016. Effects of dietary n-6/n-3 fatty acid ratio on nutrient digestibility and blood metabolites of Hanwoo heifers. Anim. Sci. J. 87(1): 46–53. 10.1111/asj.12401 [DOI] [PubMed] [Google Scholar]
- Kim S., Jing K., Shin S., Jeong S., Han S. H., et al. , 2018. omega3-polyunsaturated fatty acids induce cell death through apoptosis and autophagy in glioblastoma cells: In vitro and in vivo. Oncol. Rep. 39(1): 239–246. 10.3892/or.2017.6101 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Kato-Itoh M., Yamaguchi T., Tamura C., Sanbo M., et al. , 2012. Identification of rat Rosa26 locus enables generation of knock-in rat lines ubiquitously expressing tdTomato. Stem Cells Dev. 21(16): 2981–2986. 10.1089/scd.2012.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q., Hai T., Ma J., Huang T., Jiang D., et al. , 2014. Rosa26 locus supports tissue-specific promoter driving transgene expression specifically in pig. PLoS One 9(9): e107945 10.1371/journal.pone.0107945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris-Etherton P. M., Hecker K. D., Binkoski A. E., 2004. Polyunsaturated fatty acids and cardiovascular health. Nutr. Rev. 62(11): 414–426. 10.1111/j.1753-4887.2004.tb00013.x [DOI] [PubMed] [Google Scholar]
- Lai L., Kang J. X., Li R., Wang J., Witt W. T., et al. , 2006. Generation of cloned transgenic pigs rich in omega-3 fatty acids. Nat. Biotechnol. 24(4): 435–436. 10.1038/nbt1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L., Kolber-Simonds D., Park K. W., Cheong H. T., Greenstein J. L., et al. , 2002. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295(5557): 1089–1092. 10.1126/science.1068228 [DOI] [PubMed] [Google Scholar]
- Li X. P., Yang Y., Bu L., Guo X. G., Tang C. C., et al. , 2014. Rosa26-targeted swine models for stable gene over-expression and Cre-mediated lineage tracing. Cell Res. 24(4): 501–504. 10.1038/cr.2014.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. C., Potter C. J., 2016. Editing Transgenic DNA Components by Inducible Gene Replacement in Drosophila melanogaster. Genetics 203(4): 1613–1628. 10.1534/genetics.116.191783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Nie D., Witt W. T., Chen Q., Shen M., et al. , 2008. Expression of the fat-1 gene diminishes prostate cancer growth in vivo through enhancing apoptosis and inhibiting GSK-3 beta phosphorylation. Mol. Cancer Ther. 7(10): 3203–3211. 10.1158/1535-7163.MCT-08-0494 [DOI] [PubMed] [Google Scholar]
- Luo M., Gilbert B., Ayliffe M., 2016. Applications of CRISPR/Cas9 technology for targeted mutagenesis, gene replacement and stacking of genes in higher plants. Plant Cell Rep. 35(7): 1439–1450. 10.1007/s00299-016-1989-8 [DOI] [PubMed] [Google Scholar]
- Mali P., Yang L., Esvelt K. M., Aach J., Guell M., et al. , 2013. RNA-guided human genome engineering via Cas9. Science 339(6121): 823–826. 10.1126/science.1232033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick K. G., Scorletti E., Bhatia L., Calder P. C., Griffin M. J., et al. , 2015. Impact of high dose n-3 polyunsaturated fatty acid treatment on measures of microvascular function and vibration perception in non-alcoholic fatty liver disease: results from the randomised WELCOME trial. Diabetologia 58(8): 1916–1925. 10.1007/s00125-015-3628-2 [DOI] [PubMed] [Google Scholar]
- Nandy S., Srivastava V., 2011. Site-specific gene integration in rice genome mediated by the FLP-FRT recombination system. Plant Biotechnol. J. 9(6): 713–721. 10.1111/j.1467-7652.2010.00577.x [DOI] [PubMed] [Google Scholar]
- Nishijima M., Goto M., Fujikawa M., Yang C., Mori T., et al. , 2014. Mammalian serum albumins as a chiral mediator library for bio-supramolecular photochirogenesis: optimizing enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate. Chem. Commun. (Camb.) 50(91): 14082–14085. 10.1039/C4CC04818K [DOI] [PubMed] [Google Scholar]
- Obayashi H., Kawabe Y., Makitsubo H., Watanabe R., Kameyama Y., et al. , 2012. Accumulative gene integration into a pre-determined site using Cre/loxP. J. Biosci. Bioeng. 113(3): 381–388. 10.1016/j.jbiosc.2011.10.027 [DOI] [PubMed] [Google Scholar]
- Sakaguchi M., Watanabe M., Kinoshita R., Kaku H., Ueki H., et al. , 2014. Dramatic increase in expression of a transgene by insertion of promoters downstream of the cargo gene. Mol. Biotechnol. 56(7): 621–630. 10.1007/s12033-014-9738-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioutis S., Coates A. M., Buckley J. D., Murphy T. W., Channon H. A., et al. , 2008. N-3 enrichment of pork with fishmeal: Effects on production and consumer acceptability. Eur. J. Lipid Sci. Technol. 110(8): 701–706. 10.1002/ejlt.200700253 [DOI] [Google Scholar]
- Southern E., 2006. Southern blotting. Nat. Protoc. 1(2): 518–525. 10.1038/nprot.2006.73 [DOI] [PubMed] [Google Scholar]
- Wang K., Tang X., Liu Y., Xie Z., Zou X., et al. , 2016. Efficient Generation of Orthologous Point Mutations in Pigs via CRISPR-assisted ssODN-mediated Homology-directed Repair. Mol. Ther. Nucleic Acids 5(11): e396 10.1038/mtna.2016.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S. J., Lim K., Park S. Y., Jung M. Y., Lim H. S., et al. , 2015. Endogenous conversion of n-6 to n-3 polyunsaturated fatty acids attenuates K/BxN serum-transfer arthritis in fat-1 mice. J. Nutr. Biochem. 26(7): 713–720. 10.1016/j.jnutbio.2015.01.011 [DOI] [PubMed] [Google Scholar]
- Wu K. F., Gao X., Shi B. Y., Chen S. Y., Zhou X., et al. , 2016a Enriched endogenous n-3 polyunsaturated fatty acids alleviate cognitive and behavioral deficits in a mice model of Alzheimer’s disease. Neuroscience 333: 345–355. 10.1016/j.neuroscience.2016.07.038 [DOI] [PubMed] [Google Scholar]
- Wu M., Wei C., Lian Z., Liu R., Zhu C., et al. , 2016b Rosa26-targeted sheep gene knock-in via CRISPR-Cas9 system. Sci. Rep. 6(1): 24360 10.1038/srep24360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Ouyang H., Duan B., Pang D., Zhang L., et al. , 2012. Production of cloned transgenic cow expressing omega-3 fatty acids. Transgenic Res. 21(3): 537–543. 10.1007/s11248-011-9554-2 [DOI] [PubMed] [Google Scholar]
- Xie Z., Pang D., Wang K., Li M., Guo N., et al. , 2017. Optimization of a CRISPR/Cas9-mediated Knock-in Strategy at the Porcine Rosa26 Locus in Porcine Foetal Fibroblasts. Sci. Rep. 7(1): 3036 10.1038/s41598-017-02785-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Song J., Zhang J., Xu J., Zhu T., et al. , 2016. Identification and characterization of rabbit ROSA26 for gene knock-in and stable reporter gene expression. Sci. Rep. 6(1): 25161 10.1038/srep25161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Yang H., Li W., Zhao B., Ouyang Z., et al. , 2011. Generation of PPARgamma mono-allelic knockout pigs via zinc-finger nucleases and nuclear transfer cloning. Cell Res. 21(6): 979–982. 10.1038/cr.2011.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Wang Y., Tong Q., Liu X., Su F., et al. , 2013. A site-specific recombinase-based method to produce antibiotic selectable marker free transgenic cattle. PLoS One 8(5): e62457 10.1371/journal.pone.0062457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Zhang Y. D., Dou H. W., Yin J. D., Chen Y., et al. , 2012. Handmade Cloned Transgenic Piglets Expressing the Nematode Fat-1 Gene. Cell. Reprogram. 14(3): 258–266. 10.1089/cell.2011.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhang T., Zhang Q. K., Jiang Y., Xu D. G., et al. , 2014. Unstable expression of transgene is associated with the methylation of CAG promoter in the offspring from the same litter of homozygous transgenic mice. Mol. Biol. Rep. 41(8): 5177–5186. 10.1007/s11033-014-3385-1 [DOI] [PubMed] [Google Scholar]
- Zhu G. M., Chen H. X., Wu X. J., Zhou Y. R., Lu J. S., et al. , 2008. A modified n-3 fatty acid desaturase gene from Caenorhabditis briggsae produced high proportion of DHA and DPA in transgenic mice. Transgenic Res. 17(4): 717–725. 10.1007/s11248-008-9171-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.