ABSTRACT
Based on analyses of multiple molecular markers (18S rDNA, ITS1, ITS2 rDNA, rbcL), an alga that causes red snow on the melting ice cover of a high-alpine lake in the High Tatras (Slovakia) was shown to be identical with Chlainomonas sp. growing in a similar habitat in the Tyrolean Alps (Austria). Both populations consisted mostly of smooth-walled quadriflagellates. They occurred in slush, and shared similar photosynthetic performances (photoinhibition above 1300 µmol photons m–2 s–1), very high levels of polyunsaturated fatty acids (PUFA, 64% and 74% respectively) and abundant astaxanthin accumulation, comparable to the red spores of Chlamydomonas nivalis (Bauer) Wille. Physiological differences between the Slovak and Austrian populations included higher levels of α-tocopherol and a 13Z-isomer of astaxanthin in the former. High accumulation of secondary pigments in the Slovak population probably reflected harsher environmental conditions, since the collection was made later in the growing season when cells were exposed to higher irradiance at the surface. Using a polyphasic approach, we compared Chlainomonas sp. with Chlamydomonas nivalis. The latter causes ʻconventionalʼ red snow, and shows high photophysiological plasticity, with high efficiency under low irradiance and no photoinhibition up to 2000 µmol photons m–2 s–1. Its PUFA content was significantly lower (50%). An annual cycle of lake-to-snow colonization by Chlainomonas sp. from slush layers deeper in the ice cover is proposed. Our results point to an ecologically highly specialized cryoflora species, whose global distribution is likely to be more widespread than previously assumed.
Keywords: Astaxanthin, Chlainomonas, fatty acids, field sample, High Tatras, morphology, photosynthesis, snow algae, ultrastructure, alpine lake
Introduction
Red snow discolouration in alpine and polar regions is caused by many algal species (Kol, 1968; Lutz et al., 2015; Matsuzaki et al., 2015), which in most cases belong to the genera Chlamydomonas, Chloromonas and Chlainomonas (Chlorophyta) (Novis et al., 2008; Brown et al., 2016; Procházková et al., 2018). The genus Chlainomonas was established by Christen (1959), with the freshwater species Chlainomonas ovalis as the type. Two other species of this genus were described from snow, C. kolii (Hardy & Curl) Hoham and C. rubra (Stein & Brook) Hoham (Hoham, 1974a,b ). Recently, populations of Chlainomonas sp. thriving periodically in slush at a high-alpine lake and at a glacier in the Austrian Alps were described (Remias et al., 2016). Despite morphological similarities to spherical immotile red cells of the common snow alga Chlamydomonas nivalis, these two Chlamydomonadacean genera are not closely phylogenetically related. Blooms of Chlainomonas sp. were restricted to summer snow banks with higher water content, whereas Chlamydomonas nivalis was not found in these specific habitats (Remias et al., 2016). However, several questions concerning the ecology and physiology of Chlainomonas sp. remain unanswered. For example, the photosynthetic activity in a broad range of light conditions has not yet been elucidated. Furthermore, change in fatty acid composition is one of the crucial adaptations to cold habitats (De Maayer et al., 2014). The fatty acid profile has not been investigated for this genus so far.
The aim of this study was to compare the ecophysiology and morphology of two populations of Chlainomonas sp. causing red snow on melting ice sheets in two high-alpine lakes in different European mountain ranges. We hypothesized that the population in the High Tatras (Slovakia) is the same species as the one in the Tyrolean Alps (Austria). Our intention was (1) to confirm their close phylogenetic relationship; (2) to reveal details of their cytological adaptations to the snow habitat; (3) to evaluate if the spatial distribution of the population in the ice-cover correlates with the availability of liquid water in the snow; (4) to compare the rates of photosynthesis; (5) to analyse the composition of secondary pigments; and (6) to test whether there are any differences in adaptation strategies between the populations, in terms of fatty acids. Finally, in order to evaluate differences from the snow alga C. nivalis, we investigated the same parameters and metabolites of field samples of C. nivalis from the Tyrolean Alps. These analyses allowed a comprehensive description of this red-snow species of the genus Chlainomonas, which seems to be restricted to extremophilic habitats, including slush layers in lake-ice and glacier surfaces.
Materials and methods
Sampling and snow characterization
The cryoflora causing red snow on the ice cover on Ľadové Lake (the High Tatras, Slovakia, LP03) and Gossenkӧlle Lake (Tyrolean Alps, Austria, DL06) and near Gossenkӧlle Lake (DL07) was investigated in May and June 2016 (Table 1, Fig. 1). Surface snow was harvested with a sterile shovel, placed in 10 l buckets, and transported the same day to the laboratory. Prior to photosynthesis measurements, samples were slowly melted overnight and kept in the dark at 4–5°C. Electrical conductivity (EC) and pH of the meltwater were obtained with WTW Instruments (Cond 340i and Inolab, Germany) or with HANNA (Combo EC, Romania). Snow water content (SWC) was measured by coring snow with a cylindrical polyvinylchloride corer, according to Procházková et al. (2018). The spatial distribution of SWC and cell densities in snow on the ice cover at Lake Gossenkӧlle were evaluated on 27 May 2016 along a transect from the southern to northern shores (Supplementary fig. S3), at the following distances from the southern shore: 1, 2, 17, 32, 47, 62, 77, 92, 107 and 108 m.
Table 1.
Samples of Chlainomonas sp. from the High Tatras (Slovakia, LP03), Tyrolean Alps (Austria, DL06), and Chlamydomonas nivalis from the latter region (DL07) with sample codes, collection date, sampling site, altitude (m) and geographic position (GPS).
| Sample | Date | Location | Altitude | GPS |
|---|---|---|---|---|
| LP03 | 19 June 2016 | snow on ice cover of Ľadové Lake | 2058 | N49° 11.018 E20° 09.649 |
| DL06 | 27 May 2016 | snow on ice cover of Gossenkӧlle Lake | 2411 | N47° 13.762 E11° 00.885 |
| DL07 | 28 May 2016 | snow on slope close to Gossenkӧlle Lake | 2380 | N47° 13.709 E11° 00.949 |
Fig. 1.
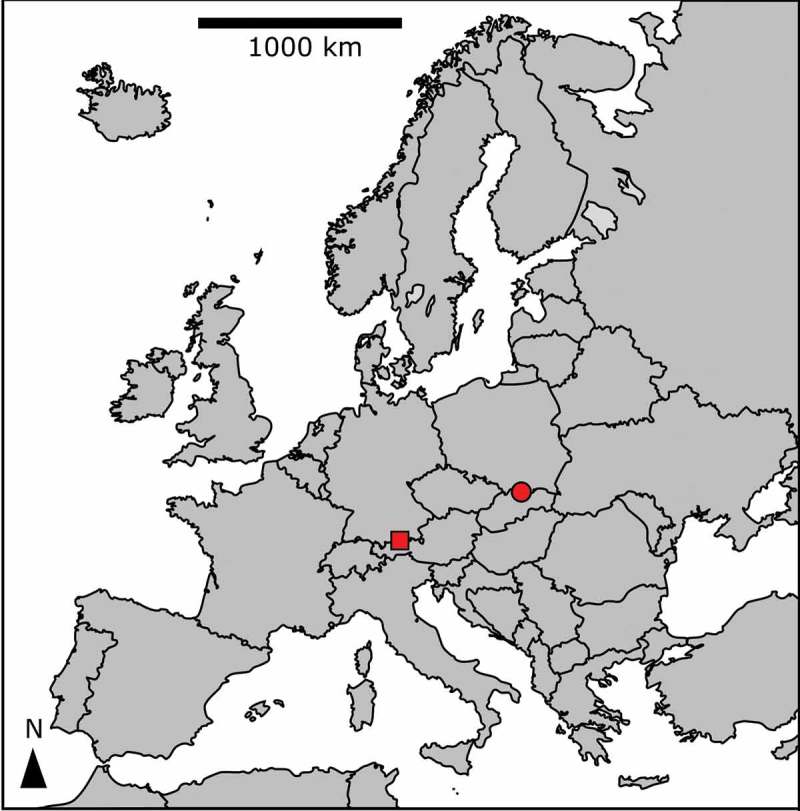
Sample locations at the High Tatras, Slovakia (circle), and the Tyrolean Alps, Austria (square).
Light and electron microscopy
Light microscopy (LM, magnification 1000×) was performed and preparation of samples for scanning and transmission electron microscopy (SEM and TEM) were carried out in the same manner as described by Procházková et al. (2018), with the exception that from the moment of harvest, cells of the thermosensitive Chlainomonas sp. were immediately placed in thermos bottles to keep them cool.
Cell counting
In order to quantify the red-snow colouration in the field, we counted the highest cell concentration. An estimation of the mean cell concentration was not intended since there were enormous differences in cell densities at the spatial scale of a dozen cm along the transect in snow at ice cover of Gossenkӧlle Lake. We took a 10 ml snow subsample from each sampling site (DL06, DL07, LP03) and from these, a further subsample of 0.5 ml snow meltwater was taken and placed in a Kolkwitz plankton-counting chamber (Hydro-Bios, Germany) and processed according to Remias et al. (2016).
Isolation of DNA and sequencing
DNA was isolated as described by Procházková et al. (2018). The 18S small subunit ribosomal RNA gene (18S rDNA), internal transcribed spacer regions 1 and 2 (ITS1, ITS2 rDNA), and ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (rbcL) gene regions were amplified from DNA isolates by polymerase chain reaction (PCR), using existing primers (Supplementary table S1). Amplification and sequencing reactions for these markers were described by Procházková et al. (2018). New sequences were submitted to the NCBI nucleotide sequence database (accession numbers in Supplementary table S2).
Photosynthesis
The light-dependent photosynthesis rates were obtained as the relative electron-transport rate of photosystem II with a fluorometer (PAM 2500, Heinz Walz GmbH, Germany). Cells were exposed to photon flux densities (PFDs) of 5, 34, 67, 104, 201, 366, 622, 984, 1389, 1666 and 2018 μmol photons m–2 s–1 for 30 s each at 2°C in a pre-cooled sample chamber (volume of 1 ml). Five independent biological replicates were measured for LP03, DL06 and DL07. After each light exposure, a saturating pulse was applied to detect the effective photochemical quantum yield of photosystem II. A curve of the relative electron-transport rate (rETR) upon PFD was calculated and fitted by the model according to Walsby (1997), assuming photoinhibition. Relative and maximum electro-transport rate (ETRmax), initial slope (α) and light saturation point (Ik) were determined (Procházková et al., 2018).
Pigment analysis
Chlorophylls, carotenoids and tocopherols were extracted and quantified according to Remias & Lütz (2007). Briefly, cells were freeze-dried on glass-fibre filters, disrupted with a grinding mill and extracted with dimethylformamide. The analysis was performed with an Agilent 1100 HPLC system with a LiChroSpher C18 column and diode array and fluorescence detectors. Carotenoid standards were obtained from CarotNature (Switzerland).
Lipid extraction and analysis of fatty acid methyl esters (FAMEs)
The extraction procedure was based on the method of Bligh & Dyer (1959), and elution was done from a Sep-Pak Vac Silica cartridge 35cc (Waters; 10 g normal-phase silica) by chloroform (neutral lipids), acetone (glycolipids) and methanol (phospholipids) (Saunders & Horrocks, 1984). All classes of lipids were saponified overnight in 10% KOH in methanol at room temperature. The structures of FAMEs were confirmed by comparison with GC/MS retention times, and fragmentation patterns with those of standard FAMEs (Supelco, Prague) (Řezanka, 1990; Dembitsky et al., 1991). Procedures were described in detail by Procházková et al. (2018).
Climatic conditions
For a comparison of the prevailing climate above the snow surface at the sampling localities in the two mountain ranges in the course of a year, radiation, monthly and daily mean air temperature (°C), and monthly cumulative precipitation (mm) were used. For complete description see the Supplementary text.
Results
Collection sites and habitat conditions
Red snow caused by Chlainomonas sp. was found in late spring 2016 on two still partly ice-covered high-alpine lakes, Ľadové Lake in the High Tatras and Gossenkӧlle Lake in the Tyrolean Alps (Table 1). In the High Tatras, the lake was partly ice-free and red snow was visible on all remaining ice-covered parts (Figs 2–5). The texture of this snow was partly slushy and partly frozen (sample LP03). A prominent soft slush layer, which was apparently soaked by lake water, began approximately 10 cm below the surface. At the Tyrolean location, the snow colouration was visible only close to the lake shore, where melting of the underlying ice was advanced (sample DL06; Supplementary figs S1–S4). The majority of the lake surface was still covered with white snow, and red horizontal patches of snow populated by Chlainomonas sp. were hidden several centimetres below the snow surface, close to the interface with the ice cover. One week later, red spots appeared across the entire lake on the surface of the snow. For a comparison ‘terrestrial’ red snow caused by C. nivalis was collected near Gossenkӧlle Lake (sample DL07). This sample was dominated by blood-red mature spores, with a smaller contribution (15%) of young cells in an early stage of development, with green parts of the chloroplasts visible. The habitat conditions of all localities are summarized in Table 2.
Figs 2–5.

Overview of the sampling site of Chlainomonas sp. at Ľadové Lake (the High Tatras, Slovakia). Fig. 2. Red colouration was visible at nearly all ice-covered parts of the lake (mid-June 2016). The harvest spot was close to the southern shore (sample LP03, red arrowhead). Fig. 3. Detailed view of red snow after harvest, Chlainomonas sp. was present at the surface and down to a depth of 2 cm. Fig. 4. Approximately 10 cm below the surface a prominent soft slush layer was noticed, which was apparently soaked by lake water. Fig. 5. Detail view of the interface between the lake margin with red snow close to the shore and lake water with edges of a slush layer.
Figs 19–24.
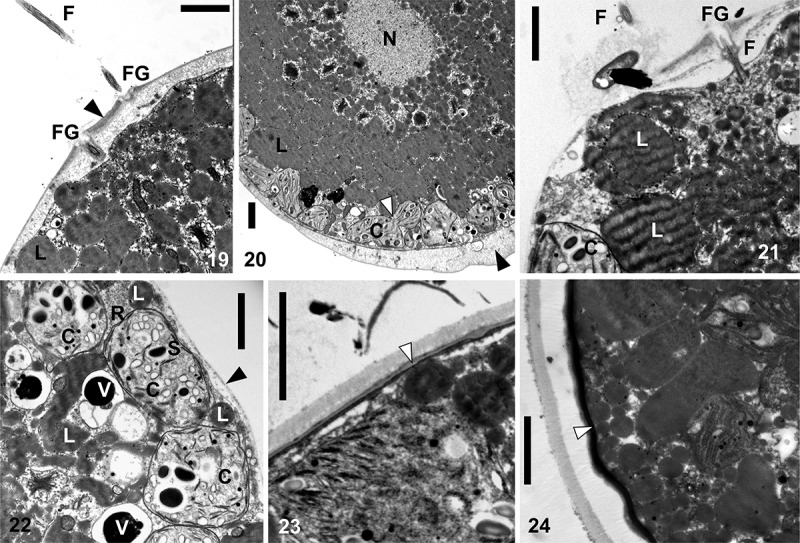
TEM micrographs of Chlainomonas sp. swarmers (Figs 19–22) and spores (Figs 23, 24) from the snow of the Ľadové Lake. Figs 19, 20. A section showing flagellate (F) and two flagella grooves (FG) of a swarmer. Cell wall thickened at the anterior and the posterior of the cell (black arrows). Note small chloroplasts (C) located parietally and a putative process of plastid division (white arrow). Centrally located nucleus (N), likely surrounded by many lipid bodies (L). Figs 21, 22. A swarmer with a single, equally thin cell wall (black arrow) with papilla. Section showing one flagella (F) and the flagella groove (FG). Note lipid bodies (L), starch grains (S) in chloroplasts (C), electron dense vacuoles (V) containing a crystalline content and a ribosome rich region (R) close to the cell wall. Figs 23, 24. Spherical spore with the trilaminar sheath (secondary cell wall, white arrow). Later outer layers of extracellular matrix are developed. Scale = 2 µm.
Table 2.
Abiotic habitat parameters and cell sizes of snow algae in field samples from the High Tatras (LP03) and Tyrolean Alps (DL06, DL07).
| Species (sample) | EC | pH | SWC | Population density ml–1 | Cell length | Cell width |
|---|---|---|---|---|---|---|
| Chlainomonas sp. (LP03) | n.a. | 5.8 | 56.4±3.8 | 44150±3091 | 37.7±7.5 | 35.9±7.3 |
| Chlainomonas sp. (DL06) | 2.5 | 5.8 | 57.9±1.6 | 6728±619 | 30.7±6.2 | 29±5.9 |
| Chlamydomonas nivalis (DL07) | 8.4 | 6.2 | 53.9±2.4 | 56036±3867 | 16.3±4.2 | 15.7±4.3 |
Electrical conductivity (EC; μS cm–1), pH of meltwater and snow water content (SWC; %), population density (cells ml–1 meltwater) ± SD (standard deviation), mean sizes of cells in μm ± SD, n.a. – not available.
Morphology and ultrastructure
The morphology of Chlainomonas sp. was characterized by LM (Figs 6–14 for the High Tatras and Supplementary figs S5–S12 for the Tyrolean Alps). The populations at the two locations had very similar morphology. Swarmers of Chlainomonas sp. had four flagella. Each flagellum was about as long as the cell (Table 2). The ellipsoidal to nearly spherical flagellates were morphologically variable: many of them possessed a papilla (thickened cell walls with flagellar openings at the anterior pole) and a pseudo-papilla (thickened posterior cell wall) (Fig. 6, Supplementary fig. S5); rarely, the cell wall was entirely detached from the protoplast (Fig. 7). Additionally, swarmers with an equally thin cell wall and papilla (Figs 8, 9, Supplementary figs S6, S7) and swarmers with a collar-like papilla (Fig. 10, Supplementary figs S8–S11) occurred. The protoplast was almost entirely occupied with red pigment. In a few cases, greenish spots of a parietal chloroplast were visible (Fig. 9).
Fig. 25.
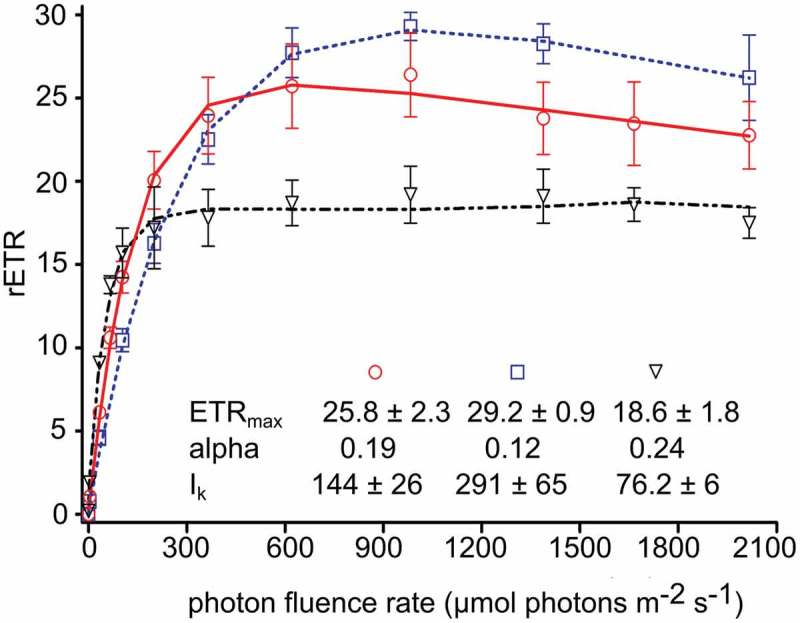
Comparison of the light-dependent relative electron transport rate (rETR) between two genera causing red snow: Chlainomonas sp. swarmers inhabiting ice-covered high alpine lakes (two samples: LP03 (High Tatras) – circles, DL06 (Tyrol Alps) – squares) and Chlamydomonas nivalis spores thriving in terrestrial snow habitats (DL07 – triangles). Values of maximum relative electron transfer rate (rETRmax), initial slope (α) and saturation irradiance (Ik) for both genera are shown. The data points were fitted with the model of photoinhibition according to Walsby model (1997) assuming photoinhibition. Each symbol represents the mean value of five replicate measurements (± SD).
Figs 6–14.
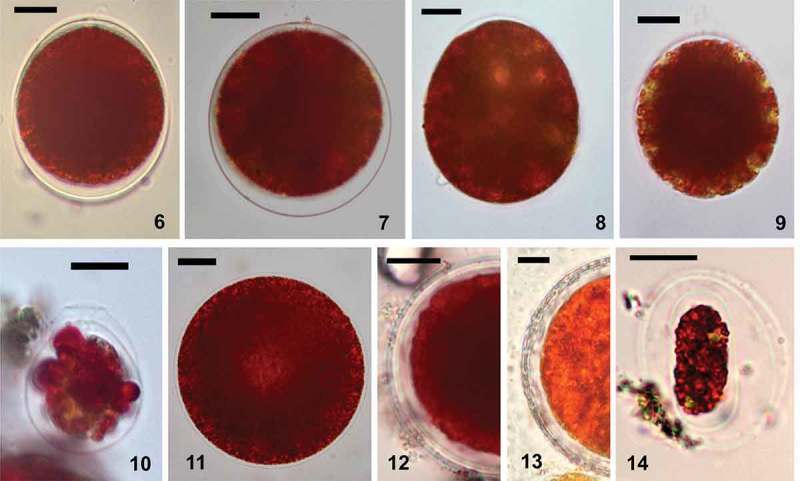
LM micrographs of Chlainomonas sp. showing cells from snow at the ice cover of the Ľadové Lake directly after harvest (Figs 6–11) and after several months at lab conditions (Figs 12–14). Figs 6–10. Morphological variability of swarmers. Fig. 6. Typical swarmer possessed papilla and pseudo-papilla (suggested zygote). Fig. 7. Rarely, the cell wall was more distant from the protoplast all around. Figs 8, 9. Motile cells with a single thin cell wall and a papilla. Note greenish spots of the parietal chloroplasts. Fig. 10. A flagellate with a collared papilla. Fig. 11. Non-motile stage without partially thickened cell wall, note central brighter region most likely representing the position of the nucleus. Figs 12, 13. Mature spores. Fig. 12. Smaller spore with hyaline primary cell wall and multiple layers of secondary cell walls. Fig. 13. During ageing of spores, the secondary cell wall becomes thicker. Fig. 14. Oblong flagellate containing red pigments and a few greenish spots of parietal chloroplasts. It is probably a daughter cell still remaining in the spore. Scale = 10 µm.
The mean cell sizes of the most common, non-collared flagellates of Chlainomonas sp. from both locations are summarized in Table 2. Other life-cycle stages occurred rarely (<5%), and their cell sizes are shown in Supplementary table S3. Flagellates with collared papillae were smaller than the dominant non-collared flagellates. Immotile stages were ovoid to spherical (Fig. 11). Maintaining field-collected material in melt-water at 4°C in the laboratory for several months helped to reveal other life-cycle stages, which were rarely found in the field. First, mature spores developed from quadriflagellate swarmers, their primary cell wall became hyaline, and a new secondary cell wall was formed below. Sometimes, a third cell wall or multiple layers were also present (Figs 12, 13, Supplementary fig. S12). Second, smaller oblong red biflagellates enclosed by two layers of a mother cell wall (likely derived from mature spores) appeared in a subsample kept in the same conditions but in the dark (Fig. 14). Further cellular details such as the stigma, cell-wall surface structures such as spines, cell divisions, or other putative stages in the life cycle were not observed. The cell-wall surface of Chlainomonas sp. was depicted by SEM (Figs 15–18). A smooth surface was characteristic for swarmers (Figs 15, 16). Four spherical flagellar grooves in a subrectangular arrangement were found (Figs 17, 18). Mature spores of Chlainomonas sp. possessed fine structures arranged in such a way as to lend an undulating appearance (Supplementary fig. S15, corresponding to Supplementary fig. S12).
Figs 15–18.
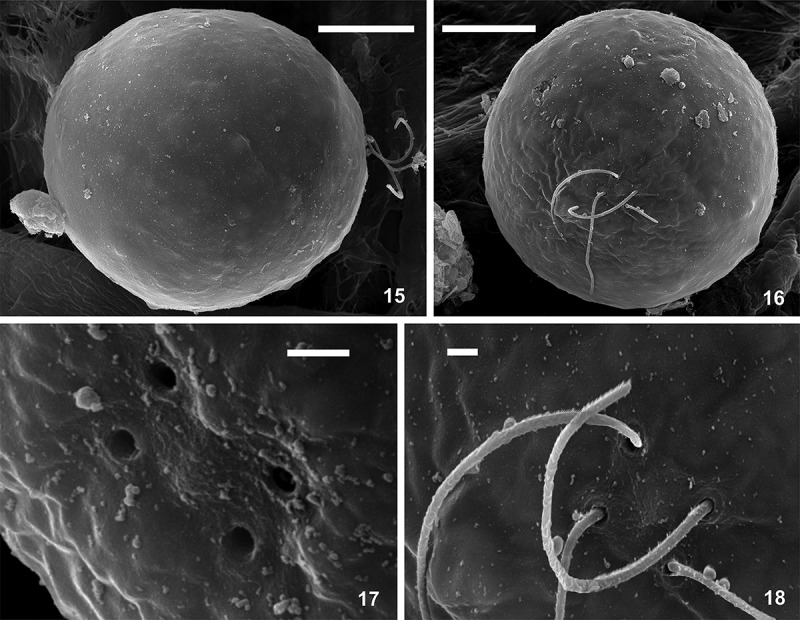
SEM micrographs of Chlainomonas sp. swarmers from the snow of the Ľadové Lake. Figs 15, 16. Side and apical view showing the smooth surface of quadriflagellate cells. Fig. 17. Detail view of four spherical flagellar grooves in slightly rectangular position. Fig. 18. Detail view of two pairs of flagella. Scale = 10 µm (Figs 15, 16) and 1 µm (Figs 17, 18).
The ultrastructure of Chlainomonas sp. was analysed by TEM (Figs 19–24 for LP03; Supplementary figs S14, S16, S17 for DL06). The cell walls of most swarmers were thickened at the anterior and posterior ends (Figs 19, 20). A section showing two flagella is depicted in Fig. 19. Small plastids containing starch grains were located parietally (Figs 20, 22). No pyrenoid was observed. The nucleus was positioned centrally and surrounded by many lipid bodies (Fig. 20). Swarmers with a single, uniformly thin cell wall were also found (Figs 8, 9, 21, 22; Supplementary figs S6, S7, S14). Mature spores possessed a trilaminar sheath (secondary wall) surrounded by outer layer(s) of extracellular matrix (Figs 23, 24, Supplementary figs S16, S17). All observed cell stages contained cytoplasmic electron-dense vacuoles, commonly filled with crystalline structures (Fig. 22).
Population density
The population densities of Chlainomonas sp. were 6728±619 and 44150±3091 cells ml–1 melt-water in the samples from the Tyrolean Alps and the High Tatras, respectively (Table 2). Population densities and SWC were investigated in a 109 m-long south-north transect on the ice cover of Lake Gossenkӧlle (Supplementary figs S18, S19). The highest values of SWC were reached at sampling points closest to both lake shores (89.3±1.5% and 80.8±2.9%). At points more distant from the lake shores, SWC was significantly lower and ranged from 50.5±2.6 to 57.9±1.6% (Supplementary fig. S19). The cells were least abundant in the central part of the transect (<400 cells ml–1 meltwater), and most abundant in the slushy part, in close proximity to the northern lake shore (>10 000 cells ml–1 meltwater) (Supplementary fig. S18). In contrast, the slushy area next to the southern lake shore harboured only one-third as many cells per volume. The spatial variation at the other sampling points of the transect was considerable, ranging from >1500 to <7500 cells ml–1 meltwater.
Snow-algal identity inferred from molecular markers
Analyses of the molecular markers 18S rDNA, ITS1 rDNA, ITS2 rDNA and rbcL showed that the red snow of the ice covers of both lakes (samples LP03 and DL06) was caused by the same species (100% identity between markers of the two populations). Furthermore, 18S rDNA and rbcL sequences were identical to those of Chlainomonas sp. in previous reports (GU117574.1, LN897303; Remias et al., 2010, 2016). The 18S rDNA and ITS2 rDNA for C. nivalis (DL07) causing red snow on slopes neighbouring Lake Gossenkӧlle was, with the exception of one nucleotide change at ITS2 rDNA, identical to a field sample found at a location 40 km south-west (GU117577.1, Remias et al., 2010).
Photosynthesis
Chlainomonas sp. from the High Tatras showed an α value of 0.19±0.02, a relative ETRmax of 25.8±2.3 and an Ik value of 144±26 μmol photons m–2 s–1 (Fig. 25). Chlainomonas sp. from the Tyrolean Alps showed a similar photosynthetic performance (Fig. 25). In both locations, photoinhibition occurred above 1300 μmol photons m–2 s–1. The only significant differences were a one-third lower α value (0.12±0.01), a slightly higher ETRmax (29.2±0.9), and a two-fold higher Ik value (291±65 μmol photons m–2 s–1) for the latter population. In contrast, C. nivalis showed signs of photoinhibition beginning only at much higher irradiances (2000 μmol photons m–2 s–1); it also showed a lower ETRmax (18.6±1.8) and Ik (76.2±6), but a higher α (0.24).
Pigment composition
The reddish colouration of Chlainomonas sp. in the High Tatras and the Austrian Alps was caused by secondary (non-plastidal) carotenoids, which comprised 93% and 88.5% of all pigments, respectively. These pigments were identified as derivatives (likely esters) of the keto-carotenoid astaxanthin (Supplementary figs S20, S21). Chlorophyll-a and -b comprised 5% and 8% of all pigments, primary (plastidal) carotenoids represented 2% and 3.5% in the pigment pool. For astaxanthin, 44.5% (High Tatras) and 31.9% (Alps) occurred as the native 13Z isomer. The overall ratio of astaxanthin to chl-a was 26 and 17 to one, respectively. Other pigment contents are given in Supplementary table S4.
Fatty acid composition
The relative contents of FAs (as % of total lipids and as % of the three major lipid groups) in three snow-algal field samples are shown in Table 3. The Slovak and Austrian populations of Chlainomonas sp. had very high levels of polyunsaturated fatty acids (PUFAs; 64.4% and 74.2% of total lipids), whereas the content of saturated acids (SAFAs) did not exceed 27% or 21% (mainly palmitic acid, 16:0 and stearic acid, 18:0), respectively. The contribution of monounsaturated fatty acids (MUFAs) was low (<10% of total lipids), with oleic acid (18:1 (9Z)) the most abundant. The major PUFAs were α-linolenic acid (18:3 (9Z,12Z,15Z)), followed by stearidonic acid (18:4 (6Z, 9Z,12Z,15Z)) and linoleic acid (18:2 (9Z,12Z)). For comparison, C. nivalis had significantly lower levels of PUFAs (50% of total lipids) (Table 3). The content of SAFAs did not exceed 30%, resulting in a two-fold higher contribution of MUFAs (20% of total lipids) in comparison with Chlainomonas sp. Apart from oleic acid, vaccenic acid (18:1 (11Z)) was the second most abundant MUFA. The major PUFAs for C. nivalis were the same as for Chlainomonas sp. Composition of three major lipid groups differed in saturation of their fatty acids: neutral lipids were composed predominantly of saturated lipids, whereas phospholipids and glycolipids were composed predominantly of PUFAs (Table 3). The total lipid contents of the dry biomass were about 10% for both Chlainomonas sp. populations and for C. nivalis (Table 3). Chlamydomonas nivalis differed in the PUFA profile of its biomembranes (phospholipids and glycolipids) from those of Chlainomonas sp. in the nearly 10-fold higher and 3-fold lower contents of vaccenic acid and linoleic acid in Chlamydomonas nivalis.
Table 3.
Snow algal fatty acid composition in % of total lipids (TL) and in % of the three major lipid groups: neutral lipids (NL), phospholipids (PL) and glycolipids (GL).
|
Chlainomonas sp. LP03 |
Chlainomonas sp. DL06 |
Chlamydomonas nivalis DL07 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TL | NL | PL | GL | TL | NL | PL | GL | TL | NL | PL | GL | |
| 14:0 | 3.0 | 5.0 | 0.9 | 3.0 | 1.5 | 3.9 | 0.8 | 0.8 | 0.7 | 0.9 | 0.6 | 0.5 |
| 16:0 | 19.1 | 37.8 | 13.2 | 7.9 | 13.3 | 37.4 | 4.7 | 3.5 | 19.6 | 53.0 | 7.8 | 6.3 |
| 16:1 (9Z) | 0.7 | 0.4 | 0.9 | 0.9 | 0.9 | 0.8 | 0.9 | 0.9 | 1.2 | 1.5 | 1.0 | 0.9 |
| 16:1 (11Z) | 0.1 | 0.0 | 0.4 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 1.3 | 1.6 | 1.1 | 1.0 |
| 16:2 (7Z,10Z) | 2.0 | 1.0 | 2.3 | 2.3 | 1.8 | 0.9 | 2.1 | 1.7 | 1.6 | 2.0 | 1.4 | 1.3 |
| 16:3 (4Z, 7Z,10Z) | 3.0 | 2.0 | 3.4 | 3.7 | 3.4 | 2.0 | 3.8 | 4.0 | 2.1 | 2.7 | 1.8 | 1.6 |
| 16:3 (7Z, 10Z, 13Z) | 1.4 | 1.0 | 2.5 | 2.2 | 1.8 | 1.0 | 2.7 | 2.0 | 0.9 | 0.4 | 0.9 | 1.1 |
| 16:4 (4Z, 7Z, 11Z, 13Z) | 3.5 | 2.0 | 4.2 | 5.4 | 4.2 | 3.0 | 4.7 | 4.1 | 5.6 | 3.8 | 6.0 | 4.7 |
| 18:0 | 4.2 | 1.0 | 5.5 | 6.6 | 5.7 | 2.0 | 10.4 | 8.5 | 9.7 | 2.5 | 12.1 | 7.9 |
| 18:1 (9Z) | 8.1 | 13.9 | 3.8 | 4.9 | 3.4 | 6.9 | 3.8 | 4.2 | 9.1 | 10.1 | 9.5 | 9.4 |
| 18:1 (11Z) | 0.4 | 1.0 | 0.7 | 0.7 | 0.8 | 0.6 | 1.4 | 1.2 | 8.3 | 7.6 | 11.2 | 11.8 |
| 18:2 (9Z, 12Z) | 14.1 | 12.0 | 15.1 | 14.8 | 11.6 | 8.9 | 12.3 | 14.4 | 4.3 | 2.5 | 4.8 | 4.7 |
| 18:3 (9Z, 12Z, 15Z) | 22.2 | 14.9 | 26.4 | 26.7 | 32.9 | 18.7 | 32.2 | 35.6 | 26.4 | 10.1 | 27.6 | 32.2 |
| 18:4 (6Z, 9Z, 12Z 15Z) | 18.2 | 8.0 | 20.7 | 20.7 | 18.5 | 13.7 | 19.9 | 18.8 | 9.2 | 1.3 | 14.2 | 16.6 |
| SUFA | 26.3 | 43.8 | 19.6 | 17.5 | 20.5 | 43.3 | 15.9 | 12.8 | 30.0 | 56.4 | 20.5 | 14.7 |
| MUFA | 9.3 | 15.3 | 5.8 | 6.7 | 5.3 | 8.5 | 6.4 | 6.6 | 19.9 | 20.8 | 22.8 | 23.1 |
| PUFA | 64.4 | 40.9 | 74.6 | 75.8 | 74.2 | 48.2 | 77.7 | 80.6 | 50.1 | 22.8 | 56.7 | 62.2 |
The table shows only fatty acids that have abundances greater than 0.1%. The relative proportion of saturated (SAFA), monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acids is also given.
Climatic conditions
The air temperature in close proximity to Ľadové Lake and Gossenkӧlle Lake followed the same pattern over the course of a year (Supplementary fig. S23). The precipitation regime in late spring and early summer differed (Supplementary fig. S24).
Discussion
Taxonomy and related species
Melting ice layers of both high-alpine lakes were populated by the same species. Chlainomonas sp. groups among the two other cryoflora species of this genus (see the phylogenetic tree by Remias et al., 2016 in fig. 3). According to their rbcL phylogeny, the populations in this study belong to a lineage independent from any other known Chlainomonas species (Remias et al., 2016), although their cell size ranges overlap (Supplementary table S3). In order to determine their affiliation, a multigene analysis will be necessary, which is not yet possible because no molecular markers (except rbcL) are available for other Chlainomonas species. In addition, the sole use of the rbcL gene in the phylogeny of Chloromonadinia is problematic because unusual gene substitutions, which occur frequently in this group, may result in misleading taxonomic artefacts (Nozaki et al., 2002, 2010). Chlainomonas sp. seems to be in close affinity (99% identity at 18S rDNA) to two algae tentatively assigned as ʻChloromonas sp. TA1ʼ (AB903004.1) and ʻChloromonas sp. TA3ʼ (AB902981.1), which were isolated from red snow at alpine sites in Japan, at an elevation of 2270 m. However, Chlainomonas sp. from Slovakia and Austria represents an independent species from these Japanese snow-algal samples (only 69% similarity at ITS2 rDNA, AB903004.1 and AB902981.1). We refrain from generating a new phylogenetic tree, because for all molecular markers investigated, no new molecular information has become available since the study by Remias et al. (2016).
Cytological adaptations to the snow habitat
At both locations, the dominant stages were non-collared ovoid swarmers with four flagella and abundant red pigmentation. Occurrence of swarmers with collar-like papillae (corresponding to the life-cycle stage shown by Novis, 2001, 2002b ) would morphologically point to a traditional designation as Chlainomonas kolii. However, the proportion of collar-like flagellates in populations is probably a dynamic process (Novis, 2002a ). Thus, the taxonomic value of this feature is subject to discussion. Additionally, cell sizes of dark-red oblong biflagellate swarmers corresponded to the morphotype found in populations of both C. kolii in New Zealand (Novis, 2002b ) and C. rubra in North America (Hoham, 1974a ). Very probably, these small oblong biflagellates are daughter cells released from spores after germination, as shown by Hoham (1974a ) for C. kolii. We hypothesize that the smaller elongate biflagellates represent the vegetative stage, whereas the quadriflagellate swarmers are prolonged planozygotes (as considered by Stein & Brooke, 1964; Hoham, 1974a ), which later become spherical and immotile zygotes. The quadriflagellate swarmers of Chlainomonas sp. observed here share the arrangement of unequal flagella insertion as described for C. kolii (Novis et al., 2008), where the flagellar basal apparatus was organized as two distinct pairs of basal bodies that lack any significant connections. This observation supports the speculation that these stages represent planozygotes originated from a fusion of two biflagellate swarmers. Consequently, the genus Chlainomonas might be invalid, in view of the finding that molecular markers place it within the genus Chloromonas (Novis et al., 2008), where vegetative swarmers are generally biflagellate. These presumed planozygotes described in this study probably do not have well-developed mechanisms of mechanical resistance or thermostability similar to the mature immotile stages. Thus, their protoplast is quickly damaged after being frozen below 0°C (Hoham, 1975; this study) or when suffering heat stress (Remias et al., 2016; this study). The process of plastid reorganization of Chloromonas spp. in the course of their life cycle (Remias et al., 2010; Procházková et al., 2018) seems to be valid also for Chlainomonas, as occasionally we found thin-walled swarmers with one or a few larger plastids close to the central part of a cell. The ‘final seasonal stage’ morphotype for Chlainomonas sp. had a reticulate surface, which is common for some other members of Chlamydomonadaceae (VanWinkle-Swift & Rickoll, 1997; Malmberg & VanWinkle-Swift, 2001). The thick-walled mature spores of Chlainomonas sp. seem to be adapted to survive harsh conditions, including starvation, mechanical abrasion, freezing and desiccation (Holzinger et al., 2016). Cytokinesis, as reported by Hoham (1974b ) and Novis et al. (2008), was not noted in this study. As sexual reproduction was not directly observed and attempts to generate a strain were unsuccessful, further details of the life cycle of Chlainomonas sp. remain unknown.
Spatial distribution and habitat conditions
The blooms of Chlainomonas sp. seem to be an annual phenomenon at Ľadové Lake (tentatively identified as ʻChlamydomonas cf. nivalisʼ by Nedbalová et al., 2006) and at Gossenkӧlle Lake (Remias et al., 2016) – lakes which share several characteristics of morphometry and limnochemistry (Kamenik et al., 2000; Kopáček et al., 2006). Moreover, both lakes are covered with ice and snow for more than seven months per year (Felip et al., 2002; Šporka et al., 2006), and the ice cover reaches a maximum thickness of 2.5 m (former) or up to one-third of the lake volume (latter). Red snow has never been reported from neighbouring lakes in the same valleys, probably as a result of their geomorphological settings: shallow lakes with rather flat beds usually have less-developed ice cover and associated snowpacks (Šporka et al., 2006). In the two lakes inhabited by Chlainomonas, high snow accumulations turn ice covers into complex structures consisting of several ice layers (Sattler et al., 2012). Highly active microbial communities in slush layers of the winter cover (Felip et al., 1999) can be colonizers of the lake water column (Alfreider et al., 1996) and vice versa (Felip et al., 1995). Thus, one may suggest that the Chlainomonas population causing red snow on melting lake ice covers is derived from lake slush layers – the algae are released into the water column after complete snowmelt and enter the phytoplankton (Nedbalová et al., 2006). In fact, several large-sized red-pigmented volvocalean cells (tentatively identified as ʻChlamydomonas sp.ʼ, ʻPteromonasʼ and ʻChlamydomonas nivalisʼ) have been found in the slush layers and small pools on the top of the ice cover of Gossenkölle Lake (Felip et al., 2002). In this lake, some of these cell morphotypes most likely represent stages in the life cycle of Chlainomonas, as proposed by Novis (2001). Comparison of mean population abundances in lake snow and at the lake shore, the latter being significantly lower (>1000 cells ml–1 vs. <50 cells ml–1, Novis, 2002a ), support a concept of annual colonization of the lake ice-cover by Chlainomonas sp. mainly from the water column rather than from contributing surface streams. A patchy distribution of Chlainomonas sp. along the south-north transect observed in this study (Supplementary fig. S18) corresponds to a considerable spatial variation in population densities within the algal bloom, as also shown for C. kolii in New Zealand (Novis, 2002a ). A population from the Austrian Alps reached, in the course of our study, comparable densities to previous years (Remias et al., 2016). However, this population was sparse in comparison with the four-fold higher abundances at Ľadové Lake, which can be attributed partly to the collection later in the season. Generally Chlainomonas sp. was found in slush or nearly completely melted snow on the lake ice cover, but it has also been reported from a glacier in Austria (Remias et al., 2016). In a similar way, C. kolii caused snow discolouration in a slush layer (SWC 69%) over snow with a lower density (SWC 46%) (Hardy & Curl, 1968). In our field samples, no cells in the process of division were found. Despite very rare observations of sporangia reported for C. kolii (Novis, 2002a ), population densities can show significant passive increases, associated partly or even solely with ablation of snow (Novis, 2002a ), which can explain the absence of any correlation between the SWC and population densities in this study. Taking these possibilities into account, we assume that cell division and gamete mating are most likely already taking place in the slush layers prior to their exposure to the surface. This generally corresponds to the finding that sexual stages of Chloromonas snow algae occur if the SWC is lower (39–50%, L Procházková, unpublished observation; 47–54% observed by Hoham & Duval, 2001).
Morphologically similar algae and their habitat preferences
Three species or subspecies of red snow-causing algae are known from the High Tatras, namely Chlamydomonas nivalis (Kol, 1975a , 1975b ), Chloromonas nivalis subsp. tatrae (Kol) Procházková, Remias, Nedbalová & Řezanka (Procházková et al., 2018), and Chlamydomonas sanguinea Lagerheim (Kol, 1969). According to Remias et al. (2016) this last, poorly morphologically described, species looks very similar to Chlainomonas sp. Chlamydomonas sanguinea occurred on the Slovak side (Kol, 1975a , 1975b ) and probably also on the Polish side of the High Tatras (misidentified as Chlamydomonas nivalis; Kawecka, 1981). In the Tyrolean Alps, the first report of Chlainomonas was from the small glacier Gamezkogelferner, not far from Gossenkӧlle Lake (Ettl, 1968), and corresponded to scattered cells found in the course of this study in snow (Supplementary fig. S13). The original description of Chlainomonas rubra included cells with striking spikes on the wall surface (Stein & Brooke, 1964). The second Chlainomonas species living in snow, C. kolii, differs from C. rubra in the presence of swarmers with a collar-like papilla with an outer, ephemeral cell wall consisting of mosaic plates. Similar to our observation of the habitat preference for Chlainomonas sp. in Europe, remarkable blooms of the related C. kolii were recorded in the snow of the ice covers of an alpine lake on Mt Philistine and on Canyon Lake, New Zealand (Novis, 2002a , 2002b ; Novis et al., 2008), and C. rubra in the similar habitats of Squaw Lake and Upper Lena Lake, United States of America (Novis et al., 2008). Therefore, a worldwide distribution of the genus Chlainomonas in snowpacks associated with high-alpine lacustrine ecosystems seems to be more common than previously thought. In addition, C. rubra is known from snowpacks above the timberline on the volcanic Mt Ruapehu in New Zealand (Hardy, 1966), and from the Vitosha Mountains in Bulgaria (fig. 39 in Lukavský et al., 2009). According to other North American reports, C. rubra and C. kolii were found beneath or adjacent to coniferous canopies (Stein & Brooke, 1964; Hardy & Curl, 1968; Hoham, 1974a , 1974b ).
Photosynthesis
The photosynthetic rates of Chlainomonas sp. from both sites were consistent in their relationship to irradiance and in suffering photoinhibition at very high light intensities, from 1300 µmol photons m–2 s–1 upwards, which are common at open, high-alpine sites. Chlainomonas sp. (DL06) appears to tolerate higher light intensities, where it shows very high photosynthetic performance. Under low light, photosynthesis is less efficient; however, both show similar kinetics. In contrast, the ‘terrestrial’ snow alga C. nivalis behaves differently, showing high photophysiological plasticity: high photosynthetic efficiency under low light, but less photoinhibition under high light. As indicated by the Ik, Chlainomonas sp. from the High Tatras needs only half the level of irradiance compared with the Tyrolean population to become saturated. This can be explained by the pronounced temperature stress and associated irradiance stress experienced later in the season for the population sampled at Ľadové Lake (Supplementary figs S25, S26), in comparison to the population sampled earlier at Gossenkölle Lake. An increased accumulation of Chlainomonas sp. at the snow surface is expected as a consequence of rain events (Novis, 2002a ), which are more frequent in the High Tatras (Niedzwiedz, 1992). The pronounced topographic shading of Ľadové Lake may also contribute to the observed photophysiological differences (Supplementary figs S27, S28; Novikmec et al., 2013), although maximum irradiance levels during the day can reach comparable levels of 2500 µmol photons m–2 s–1 (Sommaruga & Psenner, 1997; Procházková et al., 2018).
Pigments
The high levels of astaxanthin causing the red colouration of Chlainomonas sp. are comparable to mature C. nivalis obtained from alpine (Bidigare et al., 1993; Remias et al., 2005) and polar regions (Müller et al., 1998). The higher astaxanthin: chlorophyll ratios for the High Tatras population than for the earlier-sampled Austrian Alps population may be a result of the ongoing season, presuming that astaxanthin accumulation is continuous until complete snowmelt due to an active metabolism; alternatively, the amount of chlorophyll could have decreased. This corresponds to a three-fold increase in α-tocopherol (this study), a major antioxidant of the chloroplasts. Similar α-tocopherol to chl-a ratios were observed in the ʻred phaseʼ of two algal strains isolated from Arctic snow and kept in conditions of low nitrogen and high irradiation (CCCryo 006-99 and 101-99/R2; Leya et al., 2009). Increased accumulation of α-tocopherol during spore maturation was reported for field samples of C. nivalis from the Alps (Remias et al., 2010) and in the stationary-growth phase of strains in a screening study by Mudimu et al. (2017). The harsher environmental conditions later in the season are in good agreement with the doubled amount of 13Z astaxanthin, which provides advanced protection from UV in relation to all-trans-astaxanthin (Supplementary fig. S22). Notably, astaxanthin as a bio-indicator was found in the entire profile of a sediment core (32 cm) taken from the deepest point of Gossenkӧlle Lake, representing the last 800 years (Kamenik et al., 2000). This may be associated not only with zooplankton grazing on algae containing astaxanthin (Tartarotti et al., 2017), but also with the presence of Chlainomonas sp. over time in the lake.
Fatty acid composition
Snow-algal strains are reported as suitable candidates for biotechnological applications (Hulatt et al., 2017). In this study, Chlainomonas sp. had a high proportion of PUFAs in the total FA pool. High levels of PUFAs is quite common for aplanozygotes of snow-inhabiting members of the Chloromonas clade (Řezanka et al., 2008, 2014). α-Linolenic acid has been found as the dominant unsaturated FA of Chlainomonas sp. in other snow algae in this clade (Procházková et al., 2018). On the other hand, stearidonic and linoleic acids were twice as abundant as is typical for all 22 Chloromonas species (mean 7.6% and 6.4%, respectively) screened by Lang et al. (2011). Surprisingly, the FA profile of Chlamydomonas nivalis from the Austrian Alps was more similar to Chlainomonas sp. (e.g. in the dominance of α-linolenic acid) than to arctic field samples associated with the Chlamydomonas nivalis clade (oleic acid dominated >45% in sample 9/10 1b of Spijkerman et al., 2012). This suggests that the stage of spore maturation and differences in abiotic habitat parameters connected with latitude (e.g. irradiance, nutrients, water availability) may play a role in FA profiles in species belonging to the same phylogenetic clade (see fig. 4 in Leya et al., 2004). The culture maturation stage was also critical for the FA composition in Zygnematophycean green algae, where upon pre-akinete formation two unsaturated FAs in particular (oleic acid and linoleic acid) increased drastically (Pichrtová et al., 2016).
In summary, Chlainomonas sp. regularly causes red snow on lake-ice cover in the High Tatras and the Austrian Alps, as reported earlier by Remias et al. (2016). The differences in ecophysiology and morphology between these two populations illustrate well the influence of harsher conditions, prolonged time for spore maturation and population development during the later part of the growing season. The environmental conditions of the two high-alpine habitats seem to be similar. Several cell morphotypes corresponding to life-cycle stages (biflagellates, flagellates with collar-like papillae, quadriflagellates, mature spores) were found and quadriflagellate swarmers were suggested to be prolonged planozygotes. We hypothesize a route of lake-surface colonization by an inoculum originating from sediments, which first incubates in several slush layers prior to the appearance of the visible red colouration at the lake surface. Population patchiness along the spatial transect on the lake-ice cover was shown, no cell division was found, and the SWC varied from slush to nearly completely melted snow. Mating and cell divisions are thus presumed to take place in deeper slush layers and earlier in the season, where the SWC is most likely lower. Both Chlainomonas sp. and C. nivalis exhibited similar photosynthetic rates during high irradiance, suggesting that these snow algae are well adapted to live in open sites, although they have shown differences in their sensitivity to temperature and in the ultrastructural organization of plastids in mature spores (Remias et al., 2016). Both genera share low levels of primary pigments and a high contribution of non-polar astaxanthin esters. Chlamydomonas nivalis had a much lower PUFA content, partly due to the younger spore stages found during the sampling.
Remaining uncertainties about the life cycle of Chlainomonas sp. could be resolved by establishing a strain, which likely requires culturable, vegetative states occurring in deep slush layers early in the season. Polyphasic research at other, similar localities (alpine lakes, flat glaciers) with red-snow blooms is needed to assess the cosmopolitan distribution of this species, the morphology of dispersal cells, and the route(s) of colonization of distant locations. Analysis of multiple molecular markers of the other Chlainomonas cryoflora taxa would help to determine the exact taxonomic position of each species within the clade.
Supplementary Material
Funding Statement
This study was supported by the Austrian Science Fund (FWF) grant P 29959-B29 to D. R. and the Czech Science Foundation (GACR) project 17–00027S to T. Ř. The study was further supported by the Austrian Science Fund (FWF) grants P 24242-B16 and I 1951-B16 to A. H.
Acknowledgements
We are grateful to Juliet Brodie and two anonymous reviewers for their critical comments which helped us to improve the manuscript. We thank Janet Reid for the language correction of the paper. We are grateful to Klaus Herburger for his advice on ETR measurements and evaluating the results, and to Siegfried Aigner for assistance with HPLC analysis (both at the University of Innsbruck, Institute of Botany, Austria; K. H. present address: Institute of Molecular Plant Sciences, The University of Edinburgh, UK). We express our sincere thanks to Birgit Sattler (University of Innsbruck, Institute of Ecology, Austria) for providing access to the limnological field station at Kühtai, and to the Stredisko lavínovej prevencie Horskej záchrannej služby (Liptovský Hrádok, Slovakia) for providing meteorological data from Ľadové Lake. We wish to thank Tomáš Hájek and David Kaftan for their technical support (University of South Bohemia in České Budějovice, Czech Republic) and to Miroslav Hyliš for his logistical assistance (Charles University in Prague, Czech Republic).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi:10.1080/09670262.2018.1426789
Supplementary text. Climatic conditions
Supplementary table S1. List of primers used for amplification of 18S rDNA, ITS1 rDNA, ITS2 rDNA (ITS) and rbcL markers.
Supplementary table S2. List of taxa and GenBank accession numbers of nuclear-encoded 18S ribosomal DNA (rDNA) genes, nuclear rDNA internal transcribed spacer 1 (ITS1) and spacer 2 (ITS2) regions, and the large subunit of RuBisCO (rbcL) genes.
Supplementary table S3. List of Chlainomonas species causing red snow.
Supplementary table S4. Relative content of pigments and α-tocopherol in ratios to chlorophyll-a (=1) in field samples of Chlainomonas sp. from the High Tatras (sample LP03) and the Austrian Alps (sample DL06), determined by HPLC.
Supplementary figs S1–S4. Overview of the sampling site of the snow alga Chlainomonas sp. at Gossenkӧlle Lake (Tyrolean Alps, Austria).
Supplementary figs S5–S12. LM micrographs of Chlainomonas sp. showing cells from the snow on the ice cover of Gossenkӧlle Lake after harvest and after several months in lab conditions.
Supplementary fig. S13. A swarmer related to Chlainomonas rubra found in red snowfields nearby to Gossenkӧlle lake.
Supplementary figs S14–S17. TEM and SEM micrographs of Chlainomonas sp. from the snow on the ice cover of Gossenkӧlle Lake.
Supplementary figs S18, S19. Spatial distribution of Chlainomonas sp. population and snow water content on the southern-northern transect on the ice cover of Gossenkӧlle Lake.
Supplementary figs S20, S21. HPL-chromatogram of Chlainomonas sp. at 480 nm.
Supplementary fig. S22. Spectral absorbance of all-trans-astaxanthin and of the isomer 13Z astaxanthin.
Supplementary figs S23, S24. Prevailing climatic conditions close to habitats of Chlainomonas sp.
Supplementary figs S25, S26. Daily mean air temperature in period when the bloom of Chlainomonas sp. is expected (May and June 2016) at ice covers of Ľadové Lake and Gossenkӧlle Lake.
Supplementary figs S27, S28. Daily course of photosynthetically active radiation (PAR) in a few contrasting days during May–June.
Supplementary fig. S29. Daily course of PAR (photosynthetic active radiation; µmol photons m–2 s–1) during May–June 2016 in the proximity of Gossenkӧlle Lake.
References
- Alfreider A., Pernthaler J., Amann R., Sattler B., Wille A. & Psenner R. (1996). Community analysis of the bacterial assemblages in the winter cover and pelagic layers of a high mountain lake by in situ hybridization. Applied and Environmental Microbiology, 62: 2138–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidigare R.R., Ondrusek M.E., Kennicutt II, Iturriaga M.C., Harvey R., Hoham H.R., W. R. & Macko S.A. (1993). Evidence for a photoprotective function for secondary carotenoids of snow algae. Journal of Phycology, 29: 427–434. [Google Scholar]
- Bligh E.G. & Dyer W.J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37: 911–917. [DOI] [PubMed] [Google Scholar]
- Brown S.P., Ungerer M.C. & Jumpponen A. (2016). A community of clones: snow algae are diverse communities of spatially structured clones. International Journal of Plant Sciences, 177: 432–439. [Google Scholar]
- Christen H.R. (1959). Flagellaten aus dem Schützenweiher bei Veltheim. Mitteilungen der Naturwissenschaftlichen Gesellschaft Winterthur, 29: 167–189. [Google Scholar]
- De Maayer P., Anderson D., Cary C. & Cowan D.A (2014). Some like it cold: understanding the survival strategies of psychrophiles. EMBO Reports, 15: 508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembitsky V.M., Řezanka T., Bychek I.A. & Shustov M.V. (1991). Identification of fatty acids from Cladonia lichens. Phytochemistry, 30: 4015–4018. [Google Scholar]
- Ettl H. (1968). Ein Beitrag zur Kenntnis der Algenflora Tirols. Berichte des Naturwissenschaftlich-Medizinischen Vereins in Innsbruck, 56: 177–354. [Google Scholar]
- Felip M., Camarero L. & Catalan J. (1999). Temporal changes of microbial assemblages in the ice and snow cover of a high mountain lake. Limnology and Oceanography, 44: 973–987. [Google Scholar]
- Felip M., Sattler B., Psenner R., & Catalan J. (1995). Highly active microbial communities in the ice and snow cover of high mountain lakes. Applied and Environmental Microbiology, 61: 2394–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felip M., Wille A., Sattler B. & Psenner R. (2002). Microbial communities in the winter cover and the water column of an alpine lake: system connectivity and uncoupling. Aquatic Microbial Ecology, 29: 123–134. [Google Scholar]
- Hardy J.T. (1966). Identification, culture, and physiological ecology of cryophilic algae. Oregon State University, Corvallis. M.S. thesis. [Google Scholar]
- Hardy J.T. & Curl H.J. (1968). Red snow caused by a new species of Trachelomonas. Journal of Phycology, 4: 9–12. [DOI] [PubMed] [Google Scholar]
- Hoham R.W. (1974a). Chlainomonas kolii (Hardy et Curl) comb. nov. (Chlorophyta, Volvocales), a revision of the snow alga, Trachelomonas kolii Hardy et Curl (Euglenophyta, Euglenales). Journal of Phycology, 10: 392–396. [Google Scholar]
- Hoham R.W. (1974b). New findings in the life history of the snow alga, Chlainomonas rubra (Stein et Brooke) comb. nov. (Chlorophyta, Volvocales). Syesis, 7: 239–247. [Google Scholar]
- Hoham R.W. (1975). Optimum temperatures and temperatures ranges for growth of snow algae. Arctic and Alpine Research, 7: 13–24. [Google Scholar]
- Hoham R.W. & Duval B. (2001). Microbial ecology of snow and freshwater ice with emphasis on snow algae In Snow Ecology: An Interdisciplinary Examination of Snow-Covered Ecosystems (Jones H.G., Pomeroy J.W., Walker D.A. & Hoham R.W., editors), 168–228. Cambridge University Press. [Google Scholar]
- Holzinger A., Allen M.C. & Deheyn D.D. (2016). Hyperspectral imaging of snow algae and green algae from aeroterrestrial habitats. Journal of Photochemistry and Photobiology B: Biology, 162: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulatt C.J., Berecz O., Egeland E.S., Wijffels R.H. & Kiron V. (2017). Polar snow algae as a valuable source of lipids? Bioresource Technology, 235: 338–347. [DOI] [PubMed] [Google Scholar]
- Kamenik C., Koinig K.A., Schmidt R., Appleby P.G., Dearing J.A., & Psenner R. (2000). Eight hundred years of environmental changes in a high alpine lake (gossenkӧllesee, tyrol) inferred from sediment records. Journal of Limnology, 59 (Suppl. 1): 43–52. [Google Scholar]
- Kawecka B. (1981). Biology and ecology of snow algae. 2. Formation of aplanospores in Chlamydomonas nivalis (Bauer) Wille (Chlorophyta, Volvocales). Acta Hydrobiologia, 23: 211 –215. [Google Scholar]
- Kol E. (1969). Chlamydomonas sanguinea Lagerh. in the High Tatra. Annales Historico-Naturales Musei Nationalis Hungarici, 61: 111–115. [Google Scholar]
- Kol E. (1975a). Cryobiological researches in the High Tatra I. Acta Botanica Academiae Scientiarum Hungaricae, 21: 61–75. [Google Scholar]
- Kol E. (1975b). Cryobiological researches in the High Tatra II. Acta Botanica Academiae Scientiarum Hungaricae, 21: 279–287. [Google Scholar]
- Kol E. (1968). Kryobiologie. Biologie und Limnologie des Schnees und Eises. I. Kryovegetation (Elster P. & Ohle W., editors). Die Binnengewässer, Band XXIV Schweizerbart’sche Verlagsbuchhandlung, Stuttgart. [Google Scholar]
- Kopáček J., Stuchlík E. & Hardekopf D. (2006). Chemical composition of the Tatra Mountain lakes: recovery from acidification. Biologia, 61: S21–S33. [Google Scholar]
- Lang I., Hodac L., Friedl T. & Feussner I. (2011). Fatty acid profiles and their distribution patterns in microalgae: a comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biology, 11: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leya T., Müller T., Ling H.U. & Fuhr G.R. (2004). Snow algae from North-Western Spitsbergen (Svalbard) In The coastal ecosystem of Kongsfjorden, Svalbard : Synopsis of biological research performed at the Koldewey Station in the years 1991–2003 (Berichte zur Polar- und Meeresforschung 492) (Wiencke C., editor), 46–54. Bremerhaven. [Google Scholar]
- Leya T., Rahn A., Lütz C. & Remias D. (2009). Response of arctic snow and permafrost algae to high light and nitrogen stress by changes in pigment composition and applied aspects for biotechnology. FEMS Microbiology Ecology, 67: 432–443. [DOI] [PubMed] [Google Scholar]
- Lukavský J., Furnadzhieva S. & Nedbalová L. (2009). First record of cryoseston in the Vitosha Mountains, Bulgaria. Nova Hedwigia, 88: 97–110. [Google Scholar]
- Lutz S., Anesio A.M., Field K. & Benning L.G. (2015). Integrated “Omics”, targeted metabolite and single-cell analyses of arctic snow algae functionality and adaptability. Frontiers in Microbiology, 6: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg A.E. & VanWinkle-Swift K.P. (2001). Zygospore germination in Chlamydomonas monoica (Chlorophyta): timing and pattern of secondary zygospore wall degradation in relation to cytoplasmic events. Journal of Phycology, 37: 86–94. [Google Scholar]
- Matsuzaki R., Kawai-Toyooka H., Hara Y. & Nozaki H. (2015). Revisiting the taxonomic significance of aplanozygote morphologies of two cosmopolitan snow species of the genus Chloromonas (Volvocales, Chlorophyceae). Phycologia, 54: 491–502. [Google Scholar]
- Mudimu P., Koopmann I. K., Rybalka N., Friedl T., Schulz R. & Bilger W. (2017). Screening of microalgae and cyanobacteria strains for α-tocopherol content at different growth phases and the influence of nitrate reduction on α-tocopherol production. Journal of Applied Phycology, 29: 2867–2875. [Google Scholar]
- Müller T., Bleiẞ W., Rogaschewski C.-D.M.S. & Fuhr G. (1998). Snow algae from northwest Svalbard: their identification, distribution, pigment and nutrient content. Polar Biology, 20: 14–32. [Google Scholar]
- Nedbalová L., Stuchlík E. & Strunecký O. (2006). Phytoplankton of a mountain lake (Ľadové pleso, the Tatra Mountains, Slovakia): seasonal development and first indications of a response to decreased acid deposition. Biologia, 61: S91–S100. [Google Scholar]
- Niedzwiedz T. (1992). Climate of the Tatra Mountains. Mountain Research and Development, 12: 131–146. [Google Scholar]
- Novikmec M., Svitok M., Kočický D., Šporka F. & Bitušík P. (2013). Surface water temperature and ice cover of Tatra Mountains lakes depend on altitude, topographic shading, and bathymetry. Arctic, Antarctic, and Alpine Research, 45: 77–87. [Google Scholar]
- Novis P.M. (2001). Ecology and taxonomy of alpine algae, Mt Philistine, Arthur’s Pass National Park, New Zealand. University of Canterbury, Christchurch, New Zealand; PhD thesis. [Google Scholar]
- Novis P.M. (2002a). Ecology of the snow alga Chlainomonas kolii (Chlamydomonadales, Chlorophyta) in New Zealand. Phycologia, 41: 280–292. [Google Scholar]
- Novis P.M. (2002b). New records of snow algae for New Zealand, from Mt Philistine, Arthur’s Pass National Park. New Zealand Journal of Botany, 40: 297–312. [Google Scholar]
- Novis P.M., Hoham R.W., Beer T. & Dawson M. (2008). Two snow species of the quadriflagellate green alga Chlainomonas (Chlorophyta, Volvocales): ultrastructure and phylogenetic position within the Chloromonas clade. Journal of Phycology, 44: 1001–1012. [DOI] [PubMed] [Google Scholar]
- Nozaki H., Nakada T. & Watanabe S. (2010). Evolutionary origin of Gloeomonas (Volvocales, Chlorophyceae), based on ultrastructure of chloroplasts and molecular phylogeny. Journal of Phycology, 46: 195–201. [Google Scholar]
- Nozaki H., Onishi K. & Morita E. (2002). Differences in pyrenoid morphology are correlated with differences in the rbcL genes of members of the Chloromonas lineage (Volvocales, Chlorophyceae). Journal of Molecular Evolution, 55: 414–430. [DOI] [PubMed] [Google Scholar]
- Pichrtová M., Arc E., Stöggl W., Kranner I., Hájek T., Hackl H. & Holzinger A. (2016). Formation of lipid bodies and changes in fatty acid composition upon pre-akinete formation in Arctic and Antarctic Zygnema (Zygnematophyceae, Streptophyta) strains. FEMS Microbiology Ecology, 92: fiw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procházková L., Remias D., Řezanka T. & Nedbalová L. (2018). Chloromonas nivalis subsp. tatrae , subsp. nov. (Chlamydomonadales, Chlorophyta): re-examination of a snow alga from the High Tatra Mountains (Slovakia). Fottea, 18: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remias D., Karsten U., Lütz C. & Leya T. (2010). Physiological and morphological processes in the alpine snow alga Chloromonas nivalis (Chlorophyceae) during cyst formation. Protoplasma, 243: 73–86. [DOI] [PubMed] [Google Scholar]
- Remias D. & Lütz C. (2007). Characterisation of esterified secondary carotenoids and of their isomers in green algae: a HPLC approach. Algological Studies, 124: 85–94. [Google Scholar]
- Remias D., Lütz-Meindl U. & Lütz C. (2005). Photosynthesis, pigments and ultrastructure of the alpine snow alga Chlamydomonas nivalis. European Journal of Phycology, 40: 259–268. [Google Scholar]
- Remias D., Pichrtová M., Pangratz M., Lütz C. & Holzinger A. (2016). Ecophysiology, secondary pigments and ultrastructure of Chlainomonas sp. (Chlorophyta) from the European Alps compared with Chlamydomonas nivalis forming red snow. FEMS Microbiology Ecology, 92: fiw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Řezanka T. (1990). Identification of very long polyenoic acids as picolinyl esters by Ag+ ion-exchange high-performance liquid chromatography, reversed-phase high-performance liquid chromatography and gas chromatography—mass spectrometry. Journal of Chromatography A, 513: 344–348. [Google Scholar]
- Řezanka T., Nedbalová L., Procházková L. & Sigler K. (2014). Lipidomic profiling of snow algae by ESI-MS and silver-LC/APCI-MS. Phytochemistry, 100: 34–42. [DOI] [PubMed] [Google Scholar]
- Řezanka T., Nedbalová L. & Sigler K. (2008). Unusual medium-chain polyunsaturated fatty acids from the snow alga Chloromonas brevispina. Microbiological Research, 163: 373–379. [DOI] [PubMed] [Google Scholar]
- Sattler B., Post B., Remias D., Lutz C., Lettner H. & Psenner R. (2012). Cold Alpine regions In Life at Extremes. Environments, Organisms, and Strategies for Survival (Bell E. editor), 138–154. CABI, Wallingford. [Google Scholar]
- Saunders R. D. & Horrocks L. A. (1984). Simultaneous extraction and preparation for high-performance liquid chromatography of prostaglandins and phospholipids. Analytical Biochemistry, 143: 71–75. [DOI] [PubMed] [Google Scholar]
- Sommaruga R. & Psenner R. (1997). Ultraviolet radiation in a high mountain lake of the Austrian Alps: air and underwater measurements. Photochemistry and Photobiology, 65: 957–963. [Google Scholar]
- Spijkerman E., Wacker A., Weithoff G. & Leya T. (2012). Elemental and fatty acid composition of snow algae in Arctic habitats. Frontiers in Microbiology, 3: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šporka F., Livingstone D.M., Stuchlík E., Turek J. & Galas J. (2006). Water temperatures and ice cover in lakes of the Tatra Mountains. Biologia, 61: S77–S90. [Google Scholar]
- Stein J.R. & Brooke R.C. (1964). Red snow from Mt. Seymour, British Columbia. Canadian Journal of Botany, 42: 1183–1188. [Google Scholar]
- Tartarotti B., Trattner F., Remias D., Saul N., Steinberg C.E.W. & Sommaruga R. (2017). Distribution and UV protection strategies of zooplankton in clear and glacier-fed alpine lakes. Scientific Reports, 7: 4487. doi: 10.1038/s41598-017-04836-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanWinkle-Swift K.P. & Rickoll W.L. (1997). The zygospore wall of Chlamydomonas monoica (Chlorophyceae): morphogenesis and evidence for the presence of sporopollenin. Journal of Phycology, 33: 655–665. [Google Scholar]
- Walsby A.E. (1997). Modelling the daily integral of photosynthesis by phytoplankton: its dependence on the mean depth of the population. Hydrobiologia, 349: 65–74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


