Abstract
Introduction
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is the most common enzymopathy in the human population affecting an estimated 8% of the world population, especially those living in areas of past and present malaria endemicity. Decreased G6PD enzymatic activity is associated with drug-induced hemolysis and increased risk of severe neonatal hyperbilirubinemia leading to brain damage. The G6PD gene is on the X chromosome therefore mutations cause enzymatic deficiency in hemizygote males and homozygote females while the majority of heterozygous females have an intermediate activity (between 30–80% of normal) with a large distribution into the range of deficiency and normality. Current G6PD qualitative tests are unable to diagnose G6PD intermediate activities which could hinder wide use of 8-aminoquinolines for Plasmodium vivax elimination. The aim of the study was to assess the diagnostic performances of the new Carestart G6PD quantitative biosensor.
Methods
A total of 150 samples of venous blood with G6PD deficient, intermediate and normal phenotypes were collected among healthy volunteers living along the north-western Thailand-Myanmar border. Samples were analyzed by complete blood count, by gold standard spectrophotometric assay using Trinity kits and by the latest model of Carestart G6PD biosensor which analyzes both G6PD and hemoglobin.
Results
Bland-Altman comparison of the CareStart normalized G6PD values to that of the gold standard assay showed a strong bias in values resulting in poor area under-the-curve values for both 30% and 80% thresholds. Performing a receiver operator curve identified threshold values for the CareStart product equivalent to the 30% and 80% gold standard values with good sensitivity and specificity values, 100% and 92% (for 30% G6PD activity) and 92% and 94% (for 80% activity) respectively.
Conclusion
The Carestart G6PD biosensor represents a significant improvement for quantitative diagnosis of G6PD deficiency over previous versions. Further improvements and validation studies are required to assess its utility for informing radical cure decisions in malaria endemic settings.
Introduction
8-aminoquinolines (such as primaquine and tafenoquine) are the only drugs able to kill the dormant liver stages (hypnozoites) of Plasmodium vivax. Hypnozoites can be activated and cause new infections (relapses) without the need of new mosquito bites. In vivax endemic areas of South-East Asia, relapses are considered to cause up to 80% of the overall malaria episodes contributing enormously to malaria morbidity and mortality [1–4]. Wide use of 8-aminoquinolines in malarious areas is hindered by the associated hemolytic risk in glucose-6-phosphate dehydrogenase (G6PD) deficient patients [5–7] and by the lack of suitable field testing. G6PD deficiency is the most common enzymopathy in the human population with an estimated 8% prevalence worldwide and up to >30% prevalence in populations living in areas of past and present malaria transmission [8]. G6PD is an essential enzyme for maintaining the redox equilibrium in all cells and especially in red blood cells where a nucleus is lacking. G6PD deficiency can be caused by several mutations in the G6PD gene which cause protein instability and decreased enzymatic activity [9, 10]. Since the G6PD gene is on the X chromosome, a strict correlation between phenotype and genotype only exists for the hemizygous and homozygous genotypes. In heterozygous females, due to early random inactivation of the X-chromosome, only one of the genes is expressed in each cell. In the blood, a variable proportion of G6PD normal and G6PD deficient red blood cells can be found giving rise to a range of phenotypes [11]. The majority of heterozygous females have an intermediate activity between 30% and 80% of normal; in the case of one of the major South-East Asian variants, Mahidol, around 7% of heterozygous women have an activity <30% of normal and 18% have an activity >80% of normal [12]. Current G6PD qualitative rapid tests only diagnose deficiency under the 30% threshold [13–16] and women with intermediate activity are diagnosed as G6PD normal by these tests. At the Thai-Myanmar border and in most of the South-East Asian countries where P. vivax malaria is still endemic, the prevalence of G6PD deficiency among males ranges from 10 to 20% [17] with a correspondent percentage of heterozygous women ranging from 20 to 30%. As a consequence, primaquine is currently either administered in many women with a relatively high risk of hemolysis (in countries where treatment is dependent on RDT testing) [5], or it is not given at all due to safety concerns [18] in a large proportion of the population. At the same time, with the currently available qualitative tests, Tafenoquine cannot be administered safely to any woman because it should only be prescribed in patients with >70% G6PD activity [7].
In populations with high prevalence of G6PD deficiency, quantitative G6PD testing can have a broader use; identifying G6PD deficient persons who have common clinical conditions requiring antibiotic treatment so that oxidative drugs such as nitrofurantoin, nalidixic acid, ciprofloxacin and rasburicase can be avoided [19, 20]. Furthermore, G6PD deficiency is associated with increased susceptibility to neonatal hyperbilirubinemia and bilirubin-induced brain damage [21, 22]; testing for G6PD deficiency on umbilical cord blood or very early in the neonatal period using qualitative rapid tests has been shown to be unreliable [23] because of reticulocytosis [24]. Thus, quantitative G6PD tests in these populations can play a role in diagnosing G6PD deficiency more accurately in neonates.
Laboratory based quantitative testing for G6PD is carried out using a relatively expensive spectrophotometric assay [25] that requires a well equipped laboratory, skilled technicians and reliable source of material. The majority of populations with high prevalence of G6PD deficiency live in tropical countries [8] where this test is available only in specialized hospitals or not available at all. Quantitative G6PD point-of-care (POC) tests are an important tool for wider and safer use of 8-aminoquinolines which are needed for malaria elimination, for safe prescription of oxidative drugs in common clinical conditions, and for a more accurate diagnosis of G6PD deficiency at birth to improve the diagnosis and management of neonatal hyperbilirubinemia. These devices have been developed in recent years [26]. The aim of the study was to assess in a laboratory setting the diagnostic performances of the latest Carestart G6PD quantitative biosensor against the gold standard spectrophotometric assay in blood samples collected from healthy volunteers.
Materials and methods
Study site and population
The study was conducted along the Thai-Myanmar border (Tak Province) at Shoklo Malaria Research Unit (SMRU); SMRU serves a migrant population composed of Burman and Karen ethnic groups through two clinical sites located north and south to Mae Sot. Volunteers aged >18 years were recruited at the clinics and, after giving written informed consent, their blood was collected; samples were refrigerated and shipped in cool boxes to the central hematology laboratory in Mae Sot where analyses were performed.
Study design
A targeted enrollment strategy was used as in Bancone et al. 2015. In brief, individuals with known G6PD status were invited to join the study in order to achieve a convenience sample of approximately 50 G6PD deficient volunteers (male and female), approximately 50 G6PD heterozygous female volunteers, and 50 G6PD-normal volunteers. Targeted enrollment allowed for comparing the new device against the gold standard spectrophotometric assay across the entire range of enzymatic activities without the need to screen a large number of subjects.
Blood sampling
Five hundred microliters of venous blood were drawn from the arm, transferred to a K2 EDTA tube (BD Vacutainer™, USA) and inverted 10 times; samples were kept at 4°C until analysis.
Laboratory procedures
All laboratory procedures were conducted in air-conditioned room.
A complete blood count was performed on samples within 8 hours from blood collection. The G6PD quantitative spectrophotometric assay and the quantitative POC CareStart assay were performed the following day (within 30 hours from blood collection).
The CBC was performed using a CeltacF MEK-8222K hematology analyzer (Nihon Kohden, Japan). The CBC included white blood cells (WBC) total and differential count, red blood cells number (RBC), red blood cells size (MCV), hemoglobin content (MCH and MCHC), total hemoglobin concentration (HGB), hematocrit (HCT) and platelets count (PCT). Quality controls were run every day before analysis of samples.
The G6PD spectrophotometric assay (Trinity Biotech, Ireland) was performed in duplicate using 10μL of whole blood per replicate; instructions from supplier were followed for reagents preparation. An UV-1800 SHIMADZU (Japan) spectrophotometer with an electronically controlled temperature compartment was used to detect the absorbance at 340 nm during 10 minutes at 30°C. G6PD activity was calculated as IU/gHb using the results of the complete blood count on the same blood.
The latest CareStart biosensor model (WellsBio, USA), which analyses both G6PD and Hb, was used (Fig 1). The tests were performed according to the manufacturer’s instructions; both Hb and G6PD activity controls were run each day before samples. Seven μL of blood were transferred to the Hb strip using a professional pipette while 7 μL of blood were spotted on parafilm prior to be transferred to the G6PD strip. After 5 minutes the CareStart biosensor reported both the concentration of Hb in g/dL and the activity of G6PD as U/gHb. Each sample was run in duplicate.
Fig 1. CareStart G6PD biosensor.
The device consolidates a hemoglobin measurement device (on the left) with a G6PD activity measurement device (on the right). Two disposables and samples are therefore required, one of each analyte measurement. The device then calculates the G6PD activity normalized for hemoglobin concentration based on the two measurements.
Notes from the laboratory operators who used the CareStart Biosensor during the study were collected to describe some aspects of usability. Prices for the biosensor and for the associated strips at the time of manuscript submission were not available, therefore no cost analysis was performed during the study.
Statistical methods
The G6PD quantitative spectrophotometric assay was used as the reference assay. Population median was calculated on normal males as already reported before [14]. Correlation between the two replicates of CareStart biosensor was analyzed by linear regression. Area under the curve (AUC) of the receiving operator characteristic (ROC) curve [27] was calculated at different activity thresholds to analyze clinical performances of the Biosensor. Youden’s Index for CareStart biosensor was calculated [27]. All statistical analyses were conducted in Stata 13.0 (Statacorp, College Station, TX).
Ethical approval
Ethical approval was obtained by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University (FTMEC MO/15/259) and by Oxford Tropical Research Ethics Committee (OXTREC 563–15).
Results
Study population
A total of 150 subjects were recruited in the study, 42 males and 108 females. Mean age (±SD) was 37.1 (12.4) in males and 31.0(9.3) in females. The majority of participants were of Sgaw Karen (89; 59.4%) and Poe Karen (28; 18.7%) ethnicity; the remaining were Burman (22; 14.7%) and individual of other ethnicities (11; 7.3%). Participants were healthy; none of them had malaria, 5 (3.3%) had medical complaints and 15 women (13.9%) were pregnant. Blood characteristics for the study sample are reported in Table 1.
Table 1. Hematologic characteristics of study population by gender.
| Sex | WBC (103/μL) | RBC (106/μL) |
HCT (%) |
HGB (g/dL) |
PLT (103/μL) |
|
|---|---|---|---|---|---|---|
| Males (N = 42) |
Mean | 6.5 | 4.92 | 43.6 | 14.1 | 257 |
| SD | 1.5 | 0.45 | 3.3 | 1.2 | 70 | |
| Females (N = 108) |
Mean | 7.6 | 4.49 | 39.3 | 12.8 | 294 |
| SD | 2.2 | 0.52 | 3.4 | 1.2 | 67 | |
| Total (N = 150) |
Mean | 7.3 | 4.61 | 40.5 | 13.2 | 284 |
| SD | 2.1 | 0.54 | 3.9 | 1.3 | 70 |
Use of the CareStart G6PD biosensor
The device was run on power supply in air-conditioned laboratory conditions; testing strips were kept at room temperature in the same laboratory. Blood samples were handled by laboratory technicians and locally trained laboratory staff and blood transfer was carried out using precision pipettes; manual instructions were followed without a previous training. The machine carried out simultaneous analysis of Hb levels and G6PD activity (Fig 1) using two different methods for blood transfer: for the Hb the blood needed to be transferred through a pipette on the Hb strip membrane while for the G6PD the sample could be transferred directly from the finger prick. When transferring the samples from a collection tube, as in our validation study, the blood needed to be spotted first on parafilm and then transferred to the G6PD strip. Since the direct transfer from finger prick would not allow for volume measurement and the instruction manual did not specify the required volume, we tested the transfer of volumes of blood between 4μl and 9μl. Results with blood volumes lower than <5μl were not correct but the machine did not show any alarm for insufficient sample volume.
Results were displayed within five minutes and could be printed or stored in the machine (up to 5,000 results including date, time and measured temperature). No error messages were encountered during the whole validation study and operators had not trouble running the device.
Clinical performances of the CareStart G6PD biosensor
Correlation in G6PD activity and Hb levels between the two replicates of the same sample using the CareStart biosensor were R2 = 0.83 and R2 = 0.94, therefore the average for each sample could be used to compare the performances of the new device against the gold standard.
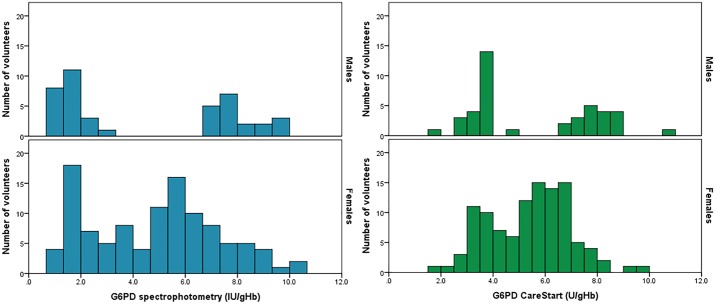
Furthermore, hemoglobin concentration assessed by the biosensor was in good agreement with the results from the complete blood count analysis (R2 = 0.88) so the enzymatic activity expressed as IU/gHb was used for comparisons between the two techniques. Distributions of G6PD activity assessed by the gold standard and by the CareStart biosensor are reported in Fig 2 by gender; both tests showed a clear bimodal distribution for samples from male volunteers and a continuous distribution for samples from female volunteers; the whole population had a similar range of activities by both tests, minimum activities assessed by the gold standard and by the CareStart biosensor were 1.85 IU/gHb and 0.84 IU/gHb respectively and maximum activities were 10.50 IU/gHb and 10.23 IU/gHb respectively.
Fig 2. Distribution of G6PD activity by gender and diagnostic device.
The left pane shows the results of the gold standard spectrophotometric assay, the right pane shows the results of the CareStart biosensor.
Median activity by spectrophotometry in normal males was 7.91IU/gHb; based on this value, the 30% threshold corresponded to 2.37IU/gHb and 80% threshold corresponded to 6.33IU/gHb. Median activity assessed by the CareStart biosensor in normal males was 7.85IU/gHb; based on this value, the 30% threshold corresponded to 2.36IU/gHb and 80% threshold corresponded to 6.28IU/gHb.
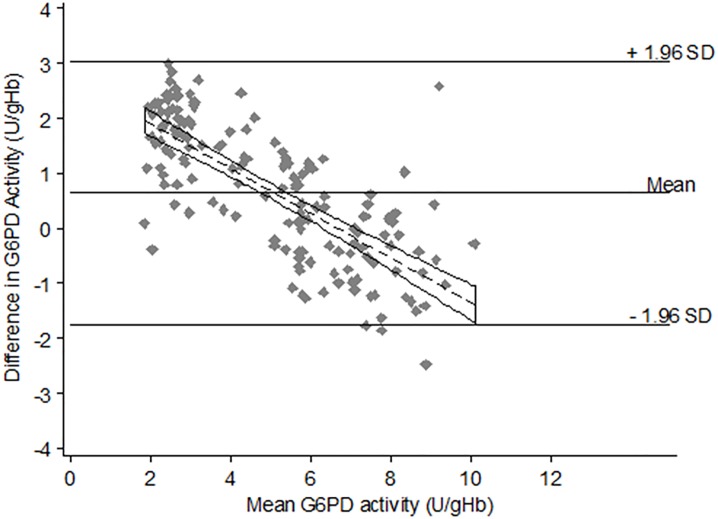
The Bland-Altman plot for comparison of G6PD activity by the CareStart biosensor against the gold standard (Fig 3) showed a large confidence interval with the majority of samples falling within the 1.96SD from the mean difference. Data were not scattered around the mean difference particularly in samples with low G6PD activity (left side of the plot) where the data were all above the mean showing that the CareStart biosensor overestimated activity as compared to the spectrophotometry.
Fig 3. Bland-Altman plot for G6PD activity with regression line.
Assuming equivalency in percent thresholds for the spectrophotometric reference assay and the CareStart product and analyzing the AUC for the ROC curve at different activity thresholds (Table 2), CareStart biosensor performed poorly in detecting deficient subjects at the 30% spectrophotometric activity threshold (<2.37IU/gHb) with an AUC of 0.52. Using Youden’s Index (Table 3), the optimal activity threshold of CareStart for diagnosis of samples with <30% spectrophotometric activity was estimated to be 4.55IU/gHb; at this threshold the test had 100% sensitivity and 92% specificity. The optimal threshold of CareStart for diagnosis of samples with <80% activity was 6.10 IU/gHb, with a sensitivity of 92% and a specificity of 94%.
Table 2. Area under the curve for ROC analysis at different activity thresholds for the reference test and CareStart biosensor.
| Threshold* | Gold Standard G6PD value |
CareStart G6PD value |
All samples | Only females |
|---|---|---|---|---|
| IU/g Hb | IU/g Hb | AUC (95%CI) | AUC (95%CI) | |
| 30% | 2.37 | 2.36 | 0.52 (0.49–0.55) | 0.52 (0.48–0.55) |
| 40% | 3.16 | 3.14 | 0.64 (0.58–0.70) | 0.68 (0.60–0.77) |
| 70% | 5.54 | 5.50 | 0.85 (0.79–0.90) | 0.79 (0.71–0.86) |
| 80% | 6.33 | 6.28 | 0.91 (0.86–0.96) | 0.86 (0.78–0.94) |
*Assuming equivalency in G6PD activity thresholds in terms of % of normal activity
Table 3. Threshold normalized G6PD activity values on the CareStart biosensor optimized to maximize sensitivity and specificity using Youden’s Index.
| 30% Threshold on Gold Standard assay (2.37 IU/g Hb) | |
| Optimal Threshold G6PD activity (IU/g Hb) | 4.55 |
| Sensitivity at Threshold (95%CI) | 1.00 (0.89–1.00) |
| Specificity at Threshold (95%CI) | 0.92 (0.86–0.97) |
| Youden’s Index | 0.92 |
| 40% Threshold on Gold Standard assay (3.16 IU/g Hb) | |
| Optimal Threshold G6PD activity (IU/g Hb) | 4.55 |
| Sensitivity at Threshold (95%CI) | 0.96 (0.83–1.00) |
| Specificity at Threshold (95%CI) | 0.97 (0.91–0.99) |
| Youden’s Index | 0.93 |
| 70% Threshold on Gold Standard assay (5.54 IU/g Hb) | |
| Optimal Threshold G6PD activity (IU/g Hb) | 5.15 |
| Sensitivity at Threshold (95%CI) | 0.79 (0.67–0.86) |
| Specificity at Threshold (95%CI) | 0.98 (0.92–1.00) |
| Youden’s Index | 0.77 |
| 80% Threshold on Gold Standard assay (6.33 IU/g Hb) | |
| Optimal Threshold G6PD activity (IU/g Hb) | 6.1 |
| Sensitivity at Threshold (95%CI) | 0.92 (0.85–0.97) |
| Specificity at Threshold (95%CI) | 0.94 (0.83–0.99) |
| Youden’s Index | 0.86 |
Discussion
Use of high dose primaquine and tafenoquine for radical cure of P. vivax malaria is safe in G6PD normal patients. While G6PD deficiency can be diagnosed by qualitative tests in both males and females, female patients with intermediate G6PD activity who are at risk of hemolysis [5] cannot be diagnosed reliably by current qualitative tests. New devices for the quantitative assessment of G6PD activity in P. vivax patients are urgently needed for elimination of the disease. Furthermore, quantitative POC instruments might give a faster assessment of G6PD status and facilitate the safe prescription of oxidative antibiotic treatment in remote clinical settings. A more reliable diagnosis of G6PD deficiency at birth [28] can improve the diagnosis and management of neonatal hyperbilirubinemia [22]. Ideally, the absolute results from a quantitative G6PD POC test can be compared with the gold standard reference test. More importantly, there should be high sensitivity and specificity at defined threshold points for activity calculated from the relative adjusted male median [29, 30]. Moreover, the test should be able to provide simultaneous results for G6PD activity and hemoglobin levels for quantification of enzyme in terms of IU/gHb.
The CareStart biosensor was recently developed; while previous models would only allow for quantification of G6PD activity [26, 31], a new relatively compact machine was developed recently and was tested in this study. This model was designed to perform both G6PD enzyme and Hb analyses in the same device (Fig 1) from blood samples collected by finger prick or venipuncture. The biosensor was easily operated by laboratory technicians and locally trained laboratory staff following the instruction manual. Analysis of Hb could be performed as part of the G6PD testing or as a stand-alone test, showing a good flexibility for use in clinical settings. A few improvements in the design of the blood transfer would be beneficial, including using the same blood transfer method and the possibility to operate the device with smaller sample volumes (currently a total blood volume of at least 14μl is required).
When compared to the gold standard spectrophotometric assay, the results by the CareStart biosensor were very similar in G6PD normal males but much higher in deficient volunteers, both males and females. Nevertheless, using a higher activity threshold (in this case 4.55IU/gHb) maximized the sensitivity and specificity of the test for diagnosis of deficient samples with <30% activity by spectrophotometry. Using this threshold gave a good diagnostic power in both male and female population to discriminate deficient subjects from the others.
For the female population only, where discrimination of intermediate and normal activities is paramount for correct deployment of primaquine and especially tafenoquine, the sensitivity and specificity were acceptable (90% and 92.5% respectively) when a threshold of 6.10IU/gHb for 80% activity was used. Thus, the device showed improved performances compared to previous versions but the ideal threshold points for discriminating samples with activity between 30% to 80% normal were alarmingly narrow, with a range of only 1.55IU/gHb (4.55–6.10).
Since the quantification of Hb levels was good, the observed differences between the enzymatic activities assessed by the Biosensor as compared to the spectrophotometric assay were most probably dependent on the detection of changes in absorbance or in the algorithm used for calculation of activity as IU/gHb.
It is important that any quantitative G6PD Biosensor machine is extensively validated before use in clinical trials to avoid non-reproducible data [32] or, worse, misdiagnosis in G6PD deficient patients who are at risk of hemolysis from oxidative treatments. This study was performed in a laboratory setting with highly regulated environmental temperature and humidiy settings. Testing the device over multiple environmental conditions more reflective of the intended use in hot tropical climates will be critical to further validate its performance and robustness. Specific validation studies will be needed to assess the performances of the machine in diagnosing G6PD deficiency in newborns using cord blood samples.
Conclusions
The current data shows that the performances of the CareStart biosensor have improved significantly over previous evaluations. Careful considerations need to be made to set threshold G6PD values that inform case management when oxidative drugs will be prescribed. Further validations studies are required to replicate the performance of the test under multiple environmental conditions, different specimens and with different end-users. Considering the slightly more complex steps needed for using a biosensor, only a device with comparable performances to the gold standard spectrophotometry (i.e. superior performances as compared to qualitative test) would be a suitable candidate for replacing currently available G6PD rapid diagnostic tests.
Supporting information
(XLSX)
Acknowledgments
The authors wish to thank all the study participants for their collaboration and understanding and the staff of SMRU clinics for their hard work and dedication. The authors thank WellsBio for the donation of the Carestart Biosensor and strips.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by the Bill & Melinda Gates Foundation, grant number OPP1034534; the UK Department for International Development (DFID), grant number 204139 to PATH. GB, GG, CSC, PP and FN work at the Shoklo Malaria Research Unit, part of the Mahidol Oxford University Research Unit supported by the Wellcome Trust Mahidol Major Overseas Programme–Thailand Unit (Grant Number 106698). The findings and conclusions contained within are those of the authors and do not necessarily reflect positions of the Bill & Melinda Gates Foundation, DFID and Wellcome Trust. PATH is an international nonprofit organization. PATH supports a portfolio of G6PD tests development efforts. PATH has no financial interests in the commercialization of any G6PD related products. PATH does not have any agreements or financial relations with WellsBio. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Betuela I, Rosanas-Urgell A, Kiniboro B, Stanisic DI, Samol L, de Lazzari E, et al. Relapses contribute significantly to the risk of Plasmodium vivax infection and disease in Papua New Guinean children 1–5 years of age. The Journal of infectious diseases. 2012;206(11):1771–80. Epub 2012/09/12. doi: 10.1093/infdis/jis580 . [DOI] [PubMed] [Google Scholar]
- 2.Chu CS, White NJ. Management of relapsing Plasmodium vivax malaria. Expert review of anti-infective therapy. 2016;14(10):885–900. Epub 2016/08/18. doi: 10.1080/14787210.2016.1220304 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross A, Koepfli C, Schoepflin S, Timinao L, Siba P, Smith T, et al. The Incidence and Differential Seasonal Patterns of Plasmodium vivax Primary Infections and Relapses in a Cohort of Children in Papua New Guinea. PLoS neglected tropical diseases. 2016;10(5):e0004582 Epub 2016/05/06. doi: 10.1371/journal.pntd.0004582 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutanto I, Tjahjono B, Basri H, Taylor WR, Putri FA, Meilia RA, et al. Randomized, open-label trial of primaquine against vivax malaria relapse in Indonesia. Antimicrobial agents and chemotherapy. 2013;57(3):1128–35. Epub 2012/12/21. doi: 10.1128/AAC.01879-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu CS, Bancone G, Moore KA, Win HH, Thitipanawan N, Po C, et al. Haemolysis in G6PD Heterozygous Females Treated with Primaquine for Plasmodium vivax Malaria: A Nested Cohort in a Trial of Radical Curative Regimens. PLoS medicine. 2017;14(2):e1002224 Epub 2017/02/09. doi: 10.1371/journal.pmed.1002224 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pamba A, Richardson ND, Carter N, Duparc S, Premji Z, Tiono AB, et al. Clinical spectrum and severity of hemolytic anemia in glucose 6-phosphate dehydrogenase-deficient children receiving dapsone. Blood. 2012;120(20):4123–33. Epub 2012/09/21. doi: 10.1182/blood-2012-03-416032 . [DOI] [PubMed] [Google Scholar]
- 7.Rueangweerayut R, Bancone G, Harrell EJ, Beelen AP, Kongpatanakul S, Mohrle JJ, et al. Hemolytic Potential of Tafenoquine in Female Volunteers Heterozygous for Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency (G6PD Mahidol Variant) versus G6PD-Normal Volunteers. The American journal of tropical medicine and hygiene. 2017. Epub 2017/07/28. doi: 10.4269/ajtmh.16-0779 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howes RE, Piel FB, Patil AP, Nyangiri OA, Gething PW, Dewi M, et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS medicine. 2012;9(11):e1001339 Epub 2012/11/16. doi: 10.1371/journal.pmed.1001339 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham AD, Colavin A, Huang KC, Mochly-Rosen D. Coupling between Protein Stability and Catalytic Activity Determines Pathogenicity of G6PD Variants. Cell reports. 2017;18(11):2592–9. Epub 2017/03/16. doi: 10.1016/j.celrep.2017.02.048 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boonyuen U, Chamchoy K, Swangsri T, Junkree T, Day NPJ, White NJ, et al. A trade off between catalytic activity and protein stability determines the clinical manifestations of glucose-6-phosphate dehydrogenase (G6PD) deficiency. International journal of biological macromolecules. 2017;104(Pt A):145–56. Epub 2017/06/07. doi: 10.1016/j.ijbiomac.2017.06.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371(9606):64–74. Epub 2008/01/08. doi: 10.1016/S0140-6736(08)60073-2 . [DOI] [PubMed] [Google Scholar]
- 12.Bancone G, Gilder ME, Chowwiwat N, Gornsawun G, Win E, Cho WW, et al. Prevalences of inherited red blood cell disorders in pregnant women of different ethnicities living along the Thailand-Myanmar border. Wellcome open research. 2017;2:72 Epub 2017/11/29. doi: 10.12688/wellcomeopenres.12338.2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baird JK, Dewi M, Subekti D, Elyazar I, Satyagraha AW. Noninferiority of glucose-6-phosphate dehydrogenase deficiency diagnosis by a point-of-care rapid test vs the laboratory fluorescent spot test demonstrated by copper inhibition in normal human red blood cells. Translational research: the journal of laboratory and clinical medicine. 2015;165(6):677–88. Epub 2014/10/15. doi: 10.1016/j.trsl.2014.09.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bancone G, Chu CS, Chowwiwat N, Somsakchaicharoen R, Wilaisrisak P, Charunwatthana P, et al. Suitability of capillary blood for quantitative assessment of G6PD activity and performances of G6PD point-of-care tests. The American journal of tropical medicine and hygiene. 2015;92(4):818–24. Epub 2015/02/04. doi: 10.4269/ajtmh.14-0696 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espino FE, Bibit JA, Sornillo JB, Tan A, von Seidlein L, Ley B. Comparison of Three Screening Test Kits for G6PD Enzyme Deficiency: Implications for Its Use in the Radical Cure of Vivax Malaria in Remote and Resource-Poor Areas in the Philippines. PloS one. 2016;11(2):e0148172 Epub 2016/02/06. doi: 10.1371/journal.pone.0148172 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roca-Feltrer A, Khim N, Kim S, Chy S, Canier L, Kerleguer A, et al. Field trial evaluation of the performances of point-of-care tests for screening G6PD deficiency in Cambodia. PloS one. 2014;9(12):e116143 Epub 2014/12/30. doi: 10.1371/journal.pone.0116143 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bancone G, Chu CS, Somsakchaicharoen R, Chowwiwat N, Parker DM, Charunwatthana P, et al. Characterization of G6PD Genotypes and Phenotypes on the Northwestern Thailand-Myanmar Border. PloS one. 2014;9(12):e116063 Epub 2014/12/24. doi: 10.1371/journal.pone.0116063 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen IT, Gosling RD. Targeting Plasmodium falciparum transmission with primaquine: same efficacy, improved safety with a lower dose? Expert review of clinical pharmacology. 2014;7(6):681–6. Epub 2014/08/15. doi: 10.1586/17512433.2014.948421 . [DOI] [PubMed] [Google Scholar]
- 19.Luzzatto L, Nannelli C, Notaro R. Glucose-6-Phosphate Dehydrogenase Deficiency. Hematology/oncology clinics of North America. 2016;30(2):373–93. Epub 2016/04/05. doi: 10.1016/j.hoc.2015.11.006 . [DOI] [PubMed] [Google Scholar]
- 20.Youngster I, Arcavi L, Schechmaster R, Akayzen Y, Popliski H, Shimonov J, et al. Medications and glucose-6-phosphate dehydrogenase deficiency: an evidence-based review. Drug safety. 2010;33(9):713–26. Epub 2010/08/13. doi: 10.2165/11536520-000000000-00000 . [DOI] [PubMed] [Google Scholar]
- 21.Kaplan M, Abramov A. Neonatal hyperbilirubinemia associated with glucose-6-phosphate dehydrogenase deficiency in Sephardic-Jewish neonates: incidence, severity, and the effect of phototherapy. Pediatrics. 1992;90(3):401–5. Epub 1992/09/01. . [PubMed] [Google Scholar]
- 22.Turner C, Carrara V, Aye Mya Thein N, Chit Mo Mo Win N, Turner P, Bancone G, et al. Neonatal intensive care in a Karen refugee camp: a 4 year descriptive study. PloS one. 2013;8(8):e72721 Epub 2013/08/31. doi: 10.1371/journal.pone.0072721 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thielemans L, Gornsawun G, Hanboonkunupakarn B, Paw MK, Porn P, Moo PK, et al. Diagnostic performances of the fluorescent spot test for G6PD deficiency in newborns along the Thailand-Myanmar border: A cohort study. Wellcome open research. 2018;3:1 Epub 2018/03/20. doi: 10.12688/wellcomeopenres.13373.1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paterakis GS, Lykopoulou L, Papassotiriou J, Stamulakatou A, Kattamis C, Loukopoulos D. Flow-cytometric analysis of reticulocytes in normal cord blood. Acta haematologica. 1993;90(4):182–5. Epub 1993/01/01. doi: 10.1159/000204454 . [DOI] [PubMed] [Google Scholar]
- 25.Beutler E, Blume KG, Kaplan JC, Lohr GW, Ramot B, Valentine WN. International Committee for Standardization in Haematology: recommended methods for red-cell enzyme analysis. British journal of haematology. 1977;35(2):331–40. Epub 1977/02/01. . [DOI] [PubMed] [Google Scholar]
- 26.Ley B, Alam MS, O’Donnell JJ, Hossain MS, Kibria MG, Jahan N, et al. A Comparison of Three Quantitative Methods to Estimate G6PD Activity in the Chittagong Hill Tracts, Bangladesh. PloS one. 2017;12(1):e0169930 Epub 2017/01/26. doi: 10.1371/journal.pone.0169930 "Screening assay" was filed with IP Australia (application number 2016900333) on the 3 February 2016. They hold no other conflicts of interest related to employment, consultancies, or other products related to this work. This does not alter our adherence to PLOS ONE policies on sharing data and materials. We declare that there is no other conflict of interest for all other authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar R, Indrayan A. Receiver operating characteristic (ROC) curve for medical researchers. Indian pediatrics. 2011;48(4):277–87. Epub 2011/05/03. . [DOI] [PubMed] [Google Scholar]
- 28.Bhutani VK, Kaplan M, Glader B, Cotten M, Kleinert J, Pamula V. Point-of-Care Quantitative Measure of Glucose-6-Phosphate Dehydrogenase Enzyme Deficiency. Pediatrics. 2015;136(5):e1268–75. Epub 2015/10/16. doi: 10.1542/peds.2015-2122 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ley B, Bancone G, von Seidlein L, Thriemer K, Richards JS, Domingo GJ, et al. Methods for the field evaluation of quantitative G6PD diagnostics: a review. Malaria journal. 2017;16(1):361 Epub 2017/09/13. doi: 10.1186/s12936-017-2017-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domingo GJ, Satyagraha AW, Anvikar A, Baird K, Bancone G, Bansil P, et al. G6PD testing in support of treatment and elimination of malaria: recommendations for evaluation of G6PD tests. Malaria journal. 2013;12:391 Epub 2013/11/06. doi: 10.1186/1475-2875-12-391 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weppelmann TA, von Fricken ME, Wilfong TD, Aguenza E, Philippe TT, Okech BA. Field Trial of the CareStart Biosensor Analyzer for the Determination of Glucose-6-Phosphate Dehydrogenase Activity in Haiti. The American journal of tropical medicine and hygiene. 2017;97(4):1262–70. Epub 2017/08/19. doi: 10.4269/ajtmh.16-0714 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dombrowski JG, Souza RM, Curry J, Hinton L, Silva NRM, Grignard L, et al. G6PD deficiency alleles in a malaria-endemic region in the Western Brazilian Amazon. Malaria journal. 2017;16(1):253 Epub 2017/06/18. doi: 10.1186/s12936-017-1889-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.