Abstract
Purine and pyrimidine analogues have important uses in chemotherapies against cancer, and a better understanding of the mechanisms that cause resistance to these drugs is therefore of importance in cancer treatment. In the yeast Saccharomyces cerevisiae, overexpression of the HAM1 gene encoding inosine triphosphate pyrophosphatase confers resistance to both the purine analogue 6-N-hydroxylaminopurine (HAP) and the pyrimidine analogue 5-fluorouracil (5-FU) (Carlsson et al., 2013, PLoS One 8, e52094). To find out more about the mechanisms of resistance to nucleotide analogues, and possible interdependencies between purine and pyrimidine analogue resistance mechanisms, we screened a plasmid library in yeast for genes that confer HAP resistance when overexpressed. We cloned four such genes: ADE4, DUT1, APT2, and ATR1. We further looked for genetic interactions between these genes and genes previously found to confer resistance to 5-FU. We found that HMS1, LOG1 (YJL055W), HAM1, and ATR1 confer resistance to both 5-FU and HAP, whereas ADE4, DUT1 and APT2 are specific for HAP resistance, and CPA1 and CPA2 specific for 5-FU resistance. Possible mechanisms for 5-FU and HAP detoxification are discussed based on the observed genetic interactions. Based on the effect of LOG1 against both 5-FU and HAP toxicity, we propose that the original function of the LOG (LONELY GUY) family of proteins likely was to degrade non-canonical nucleotides, and that their role in cytokinin production is a later development in some organisms.
Introduction
Antimetabolite drugs such as purine and pyrimidine analogues play an important role in chemotherapy against cancer. However, tumours may acquire resistance to such drugs by clonal selection of resistant cancer cells. A better understanding of the mechanisms of action of anticancer drugs and in particular the ways by which drug resistance can arise is therefore of importance both for cancer treatment and for the development of new more efficient anticancer drugs. Since nucleotide metabolism is evolutionarily conserved, these mechanisms can be studied in model organisms such as Saccharomyces cerevisiae (baker’s yeast) where advanced methods for molecular genetics are available.
The first antimetabolite drug that was developed to specifically target cancer cells was the pyrimidine analogue 5-fluorouracil, 5-FU [1]. 5-FU targets several cellular mechanisms through its activated metabolites FdUMP, FUTP and FdUTP [2–3]. FdUMP inhibits thymidylate synthase (TYMS), and this is a major mechanism behind both the toxicity and the anticancer effect of 5-FU [4–8]. However, effects on RNA metabolism also play an important role in 5-FU toxicity [9–12]. Such effects result from inhibition of RNA modifications such as methylation and pseudouridylation [11,13], which in turn cause disturbed exosome processing of polyadenylated rRNA [14], interference with spliceosome function [15], and destabilization of tRNAs [12].
We previously carried out a screen in yeast for genes that confer resistance to 5-FU when overexpressed from a high copy number plasmid [16]. Among the cloned resistance genes we found HAM1, which encodes inosine triphosphate pyrophosphatase, an enzyme that dephosphorylates non-canonical purine nucleoside triphosphates ITP, dITP and XTP and thus prevents their incorporation into DNA and RNA [17–18]. This finding suggested that the Ham1 protein may have a broader specificity than originally thought, targeting non-canonical pyrimidines in addition to purines [16]. It also raised questions about interactions between the purine and pyrimidine metabolic pathways and the role of such interactions in acquired resistance to nucleotide analogues.
To look further into these questions, we decided to carry out a screen for yeast genes that confer resistance to purine analogues when overexpressed. For this screen we chose to use 6-N-hydroxylaminopurine (HAP), the drug that was originally used to identify HAM1 as a gene that causes hypersensitivity to purine analogues when disrupted [17]. HAP is a cytotoxic and hemolytic purine analogue that is similar to adenine but with a hydroxylamine group instead of an amino group attached to the number 6 carbon. In contrast to other purine analogues such as azathioprine and mercaptopurine [19–20] it is not used in medicine since it is hemolytic at concentrations below what could have therapeutic potential [21]. However, it has been used in research to study purine analogue toxicity in both human tumor cell lines and in the yeast Saccharomyces cerevisiae [17,22–25].
The screen was carried out both in a wild type strain and in a ham1 knockout mutant that is hypersensitive to HAP. We found four new yeast genes that confer resistance to HAP when overexpressed: ADE4, DUT1, APT2, and ATR1. We proceeded to test these genes for their effects on 5-FU resistance. We also looked for genetic interactions between the genes isolated in the 5-FU and HAP resistance screens as well as interactions with other genes involved in purine metabolism. Based on our findings, we discuss possible roles of the cloned genes in the metabolism and detoxification of HAP, 5-FU and other nucleotide analogues. In particular, we propose that the original function of the widely distributed LOG (LONELY GUY) family of proteins was to facilitate the removal of non-canonical nucleotides from the nucleotide pool, working downstream of Ham1p and other nucleotide phosphatases, and that their role in cytokinin production in plants and some microorganisms [26–28] is a later development that occurred in these organisms.
Materials and methods
Yeast strains and plasmids
Yeast deletion strains in the BY4742 haploid and the isogenic BY4743 diploid background were obtained from Euroscarf (http://www.uni-frankfurt.de/fb15/mikro/euroscarf). The open reading frame in each deletion strain has been replaced by the KanMX selection cassette [29]. The plasmids pCPA1, pCPA2, pHMS1, pYJL055w, pHAM1 have been described previously [16]. Plasmid pYJL055w is referred to as pLOG1 in the present paper in order to make the genetic nomenclature consistent.
PCR cloning
Plasmid pAPT1 was constructed by PCR amplification of a fragment spanning from 430 bp upstream to 150 bp downstream of the APT1 ORF, with primers adding a SacI site at the 3’ end: 5'-GAG CTC GCA CTC CAG AAA CAA CAG CA-3', and a BamHI site at the 5’ end: 5'-GGA TCC TGT GGC ACA AAG CAG AAA AG-3'. The PCR fragment was TA-cloned into pCR2.1 (Invitrogen, US) and subsequently subcloned between the SacI and BamHI sites in the polylinker of the shuttle vector pHR81 [30]. Plasmid pATR2 was constructed by PCR amplification of a fragment spanning from 356 bp upstream to 210 bp downstream of the ATR2 ORF, with primers adding SacI sites at both ends: 5'-GAG CTC ACA GGG GTG CGC ATA AAT AG-3' and 5'-GAG CTC CTT GCG CAA ATG AAG AAC AA-3'. The PCR fragment was TA-cloned into pCR2.1, and subsequently subcloned into the SacI site in the polylinker of pHR81.
Growth media and chemicals
Rich media (YPD) and synthetic complete media (SC) or dropout media based on SC were prepared as previously described [31]. The synthetic media contained either 2% glucose or 2% galactose as carbon source. 6-N-Hydroxylaminopurine (HAP) and 5- fluorocytosine (5-FC) were obtained from Apollo Scientific (Manchester, UK). 5-Fluorouracil (5-FU) and 6-azauracil (6-AzaU) were obtained from Sigma-Aldrich (Stockholm, Sweden). 8-azaguanine (8-AzaG) was obtained from Accel Pharmatech (East Brunswick, US). Boric acid was obtained from Fluka Chemie (Buchs, Switzerland).
Shuttle plasmid library screen
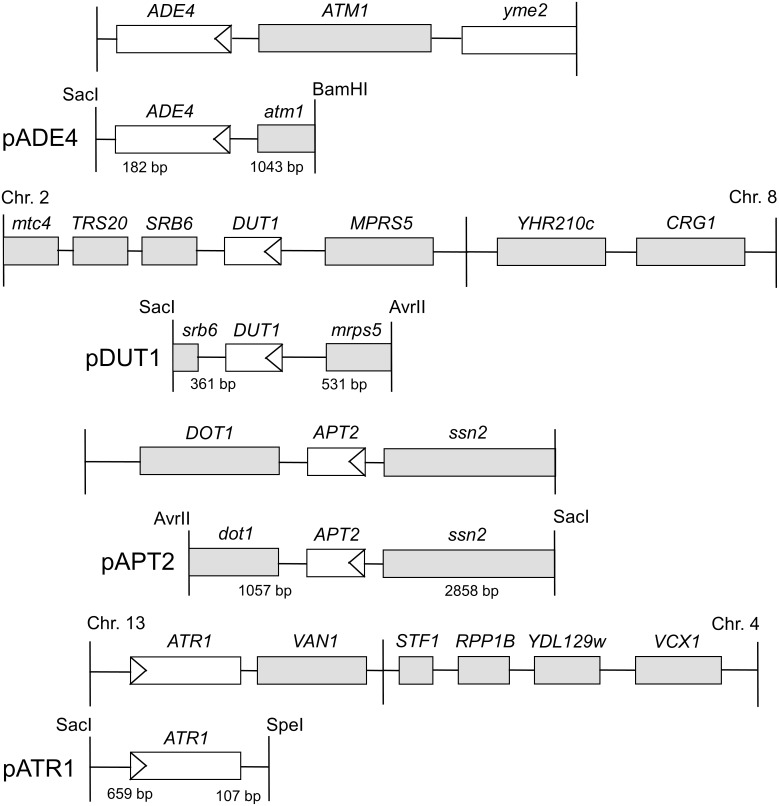
The BY4742 wild type and the ham1 knockout strain were transformed with a yeast genomic library made in the 2 μm URA3 LEU2-d vector pHR81 [30]. The copy number of 2 μm plasmids is 5–50 molecules per cell, and will adjust if the insert is selected for or against. It is therefore possible to recover also weak dosage suppressors in a library screen which may require a higher copy number to be effective. To select for HAP resistance, the wild type transformants were plated on SC galactose media without uracil and adenine containing 50 μg/ml HAP, and the ham1 transformants were plated on SC glucose media without uracil and adenine containing 50 μg/ml HAP. Early emerging colonies and colonies with greater size were picked to grids on drug free media. In order to verify that the picked transformants were HAP resistant, they were sequentially replicated twice to the same HAP containing media that they were initially selected on. Plasmids were rescued from confirmed HAP resistant colonies, retransformed into the wild type BY4742, and retested for HAP resistance. The genes responsible for HAP resistance were mapped by deletions and PCR subcloning (Fig 1), followed by testing of the resulting plasmids after retransformation into yeast. We estimate that in total we screened approximately 120 000 transformants.
Fig 1. Restriction maps of plasmids isolated in the HAP resistance screen.
The shortest subclone that could still confer HAP resistance when overexpressed is shown below each plasmid. Open reading frames are shown as grey boxes, with the mapped resistance gene as a white box. The numbers of included base pairs upstream and downstream of the open reading frame in each minimal subclone are also shown.
Yeast growth and spot assays
To assay drug sensitivity, transformants were grown overnight at 30 °C in SC galactose or glucose media without uracil. These overnight precultures were diluted into fresh media to a final OD600 of 0.1 and grown to late exponential phase. Cultures were diluted to OD600 0.1 in water before spotting. For the overexpression and disruption assays 10-fold serial dilutions were made. A 2.5 μl aliquot of each dilution was spotted onto control plates and drug plates. The drug concentrations used were higher than in the initial screens since we wanted to highlight differences between different suppressor plasmids. Growth was monitored daily starting after two days at 30 °C.
Results
Cloning of genes that confer resistance to HAP when overexpressed
In order to identify yeast genes that confer resistance to purine nucleotide analogues when overexpressed we screened a yeast genomic DNA library in the high copy number shuttle vector pHR81 [30] for plasmids that confer resistance to HAP when overexpressed. The screen was carried out both in a wild type strain and in a ham1 knockout mutant that is hypersensitive to HAP in order to facilitate the recovery of weaker resistance genes. Transformants in the wild type strain were screened on HAP-containing media with galactose as a carbon source since we found that galactose increases the sensitivity to HAP, whereas transformants in the hypersensitive ham1 mutant were screened on HAP-containing media with glucose as a carbon source. The reason for this difference remains to be determined, but it is not unusual to see phenotypic differences between cells grown on glucose and other carbon sources, since glucose repression is a global regulatory response that alters the expression of a large part of the yeast genome [32].
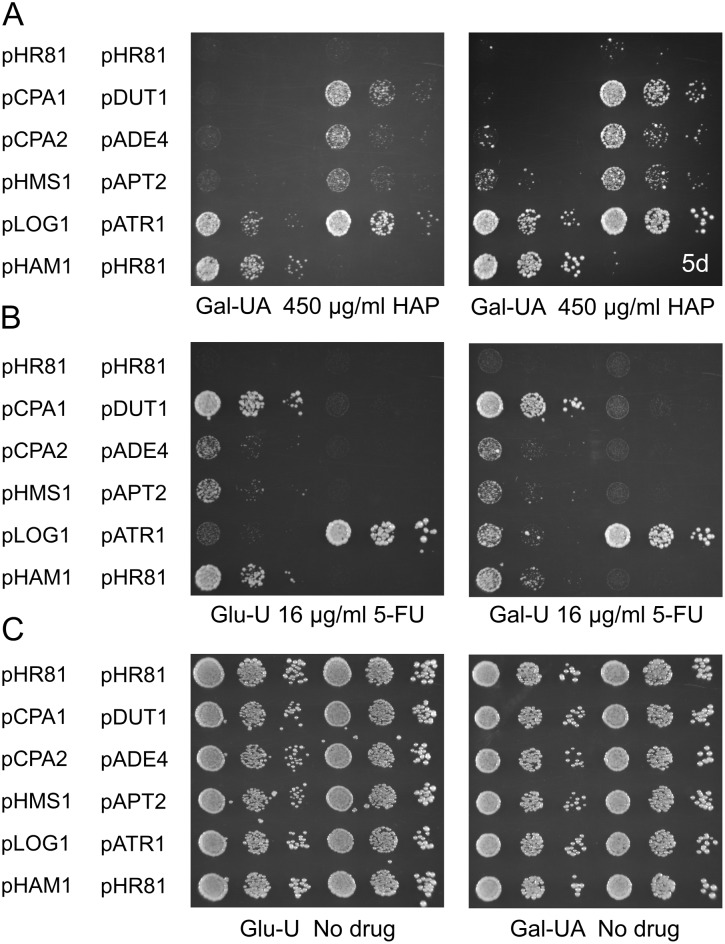
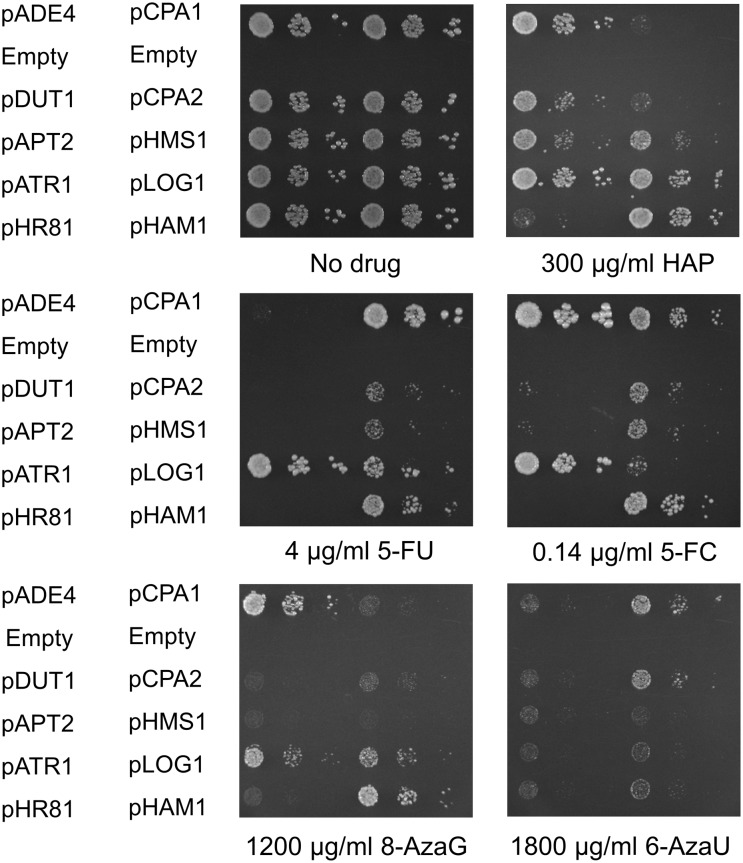
In total, we screened approximately 120 000 transformants, which corresponds to a 30-fold coverage of the yeast genome, assuming an average insert size of 4 kbp. After rescue of the plasmids back into E. coli, retransformation into yeast for confirmation of the HAP resistance phenotype, and mapping of the HAP resistance within the plasmid inserts, we identified four genes, ADE4, DUT1, APT2, and ATR1, that confer resistance to HAP when overexpressed (Fig 1). ADE4, DUT1 and APT2 were cloned from the ham1 strain, whereas ATR1 was cloned from the wild type. In addition, the HAM1 gene was isolated four times from the ham1 strain and once from the wild type. The resistance conferred by each cloned gene to HAP is shown in Fig 2A. As discussed below, some of the genes also confer resistance to 5-FU (Fig 2B).
Fig 2. Resistance to HAP and 5-FU due to overexpression of different genes.
The genes isolated in our HAP resistance screen were tested for resistance to HAP (A) and 5-FU (B). No drug controls are shown in panel C. Also included were the genes isolated in our previous screen for 5-FU resistance [16]. Cells transformed with the empty vector pHR81 [30] were included as a negative control. Transformants were grown in liquid medium to late exponential phase, serially diluted, and then spotted onto uracil- and adenine-less SC galactose (Gal-UA) plates, uracil-less glucose (Glu-U) or uracil-less galactose (Gal-U) plates with or without HAP or 5-FU at the indicated concentrations. Plates were incubated for 3 days except for the second plate containing HAP, which was incubated for 5 days (5d) in order to show the weak effect of HMS1 overexpression.
ADE4 encodes 5-phosphoribosyl-1-pyrophosphate amidotransferase, which catalyzes the first step in the de novo synthesis of purine nucleotides. Overexpression of ADE4 causes increased synthesis of purine nucleotides and has been shown to mediate resistance to the DNA-crosslinker cisplatin, which is used as an anticancer drug [33]. We found that ADE4 overexpression confers resistance to HAP (Fig 2A) but not to 5-FU (Fig 2B), which is consistent with a model where increased de novo synthesis of purines suppresses the toxicity of HAP by diluting the drug with freshly synthesized purines.
DUT1 is an essential gene encoding deoxyuridine triphosphate diphosphatase (dUTPase), an enzyme required both for de novo synthesis of thymidylate, and for genome stability by preventing incorporation of uridylate into DNA [34]. DUT1 is also known to be important for the resistance to antifolates such as aminopterin and the anticancer drug methotrexate [35]. Since Dut1p is thought to act primarily on dUTP, a pyrimidine nucleotide, one might expect it to confer resistance to 5-FU when overexpressed. However, we found that whereas DUT1 clearly confers resistance to HAP (Fig 2A), its effect, if any, on 5-FU resistance is barely detectable (Fig 2B, galactose).
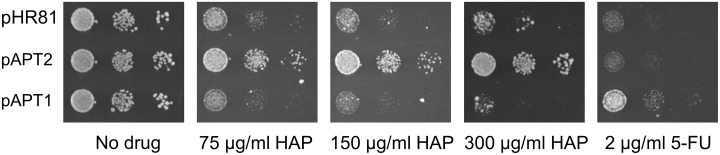
APT2 is a duplicated copy of the APT1 gene encoding adenine phosphoribosyltransferase (APRT). APT1 and APT2 were identified as one of 549 gene pairs that remain as evidence of an ancient whole genome duplication in the Saccharomyces lineage [36]. The retention of both genes suggests that they may have acquired unique and different functions. However, Apt2p lacks APRT activity when expressed in E.coli and a disruption of the APT2 gene has no apparent phenotype in yeast [37]. It is thus not clear what enzymatic activity, if any, that Atp2p possesses. Nevertheless, we found that the APT2 gene confers resistance to HAP (Fig 2A) but not to 5-FU (Fig 2B) when overexpressed. We also tested a PCR clone of APT1 (Fig 3). As expected, it did not confer resistance to HAP, but instead had a slightly poorer growth on HAP. However, it did instead confer some resistance to 5-FU at low concentrations.
Fig 3. Resistance to HAP and 5-FU conferred by overexpression of APT2 and APT1.
The cloned APT2 plasmid and a PCR-amplified APT1 gene cloned into the vector pHR81 were tested for resistance to HAP and 5-FU. Cells transformed with pHR81 [30] were included as a negative control. Transformants were grown in liquid medium to late exponential phase, serially diluted, and then spotted onto uracil- and adenine-less SC galactose (Gal-UA) plates with or without HAP or 5-FU at the indicated concentrations.
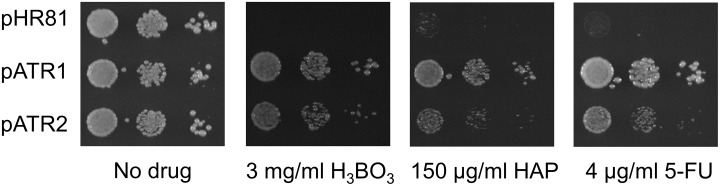
ATR1, finally, encodes a multidrug efflux pump that belongs to the major facilitator superfamily (MFS). Atr1p was originally named from the fact that it is required for resistance to the heterocyclic amine aminotriazole, and ATR1 expression has been found to increase during DNA-replication stress [38–39]. We found that overexpression of ATR1 confers strong resistance to both HAP and 5-FU (Fig 2A and 2B). ATR1 has an unnamed paralogue, YMR279C, which similarly to ATR1 was shown to confer resistance to boric acid [40]. We therefore also tested overexpression of a PCR-cloned copy of that gene (Fig 4). We found that YMR279C confers resistance to boric acid, HAP and 5-FU when overexpressed, though not as strongly as ATR1. We conclude from this that YMR279C has a similar multidrug efflux pump activity as ATR1. We therefore propose the name ATR2 for the open reading frame YMR279C.
Fig 4. Resistance to HAP, 5-FU and boric acid conferred by overexpression of ATR1 and ATR2.
The cloned ATR1 plasmid and a PCR-amplified ATR2 (YMR279C) gene cloned into the vector pHR81 were tested for resistance to HAP, 5-FU and boric acid. Cells transformed with pHR81 [30] were included as a negative control. Transformants were grown in liquid medium to late exponential phase, serially diluted, and then spotted onto uracil- and adenine-less SC galactose (Gal-UA) plates with or without HAP, 5-FU or boric acid at the indicated concentrations.
Some 5-FU resistance genes also mediate resistance to HAP when overexpressed
Our finding that one of the HAP resistance genes, ATR1, also confers resistance to 5-FU prompted us to test if any of our previously identified 5-FU resistance genes [16] would confer resistance to HAP. As shown in Fig 2, we found that overexpression of HAM1 and LOG1 (YJL055W) confers a comparatively strong resistance to HAP and that overexpression of HMS1 confers a very weak but still detectable HAP resistance (Fig 2A). In contrast, overexpression of the carbamoyl phosphate synthetase subunits CPA1 or CPA2 had no apparent effect on the resistance to HAP. Two of the genes that we previously found to confer 5-FU resistance, HMS1 and LOG1 [16], did not appear in our screen even though they do confer HAP resistance when overexpressed (Fig 2A). This might suggest that the screen was not exhaustive, though we did recover HAM1 five times. However, HMS1 has a very weak effect, and may therefore have escaped detection in our screen.
To assess the strength of the different resistance genes, we examined the size of single cell clones at the appropriate dilution in the presence of HAP or 5-FU, since colony size is a sensitive measure of growth rate in yeast. We found that HAM1 and ATR1 have the strongest effect on HAP resistance, followed by LOG1 and DUT1, and then by ADE4, APT2 and HMS1 in decreasing order of resistance (Fig 2A). This was determined on galactose media since HAP resistance is much easier to score on galactose than on glucose. As for 5-FU resistance, we found that CPA1 and ATR1 have the strongest effect, followed by HAM1, and then by HMS1, CPA2 and LOG1 in decreasing order of resistance. This was determined on glucose (Fig 2B). However, on galactose we found that LOG1 has a much stronger effect, comparable to that of HAM1, and that DUT1 also had a barely but still detectable effect (Fig 2B). The reason why LOG1 is much more efficient in conferring resistance against 5-FU on galactose remains to be determined. None of the other 5-FU resistance genes showed a similar effect, so it is likely not due to differences in the uptake or metabolism of 5-FU on galactose compared to glucose. A likely explanation for this effect is that LOG1 is moderately (2–3 fold) repressed by glucose [41].
In the case of HAM1, a likely explanation for the resistance to HAP is suggested by its known enzymatic activity. Ham1p has been found to be a nucleoside triphosphate pyrophosphatase, which does not seem to discriminate between deoxyribonucleotides and ribonucleotides. It has the highest specificity against deaminated purines, e.g. (d)XTP and (d)ITP, amongst the nucleotides assayed, but also showed some residual activity against dATP and dCTP [18]. This suggests that the molecular mechanism by which overexpression of HAM1 confers resistance to HAP, and likely also to 5-FU, is its pyrophosphatase activity.
The function of the YJL055W gene is not known. However, a BLAST search [42] with the Yjl055wp amino acid sequence as probe identified the plant LOG (LONELY GUY) family of proteins as its closest homologues, with E-values of 4e-56 for the rice LOG protein and 2e-52 for the Arabidopsis LOG1 protein. YJL055W is the only yeast gene encoding a protein with strong similarity to the plant LOG proteins. This suggests that YJL055W is an ortholog of the plant LOG genes, and we will therefore refer to it as the yeast LOG1 gene. The plant LOG proteins were originally identified as enzymes that activate adenylate-type pre-cytokinins, a group of plant hormones, by cleaving off the N6-modified adenine nucleobase from the precursor cytokinin nucleoside monophosphate [26,43]. It is therefore conceivable that yeast Log1p may catalyze a similar reaction in the degradation of non-canonical nucleotides (see Discussion).
Sensitivity of knockout mutants lacking resistance genes to HAP and 5-FU
Overexpression and loss of a gene frequently has opposite effects on the affected phenotypes. In order to determine if this is true in our case, we proceeded to test knockout mutants lacking each of the resistance genes for sensitivity to both HAP and 5-FU. These experiments were carried out using strains transformed with the empty vector pHR81 to facilitate comparisons with the overexpression experiments and avoid complications due to the fact that the genetic background used, BY4742, is ura3 and thus deficient for pyrimidine biosynthesis, which in turn affects 5-FU sensitivity [16]. The pHR81 plasmid carries the URA3 marker which restores a functional pyrimidine biosynthesis in these strains. Since the DUT1 gene is essential, the effect of a haploid knockout mutant could not be tested, but we instead included a heterozygous dut1/DUT1 strain and a wild type diploid control in order to examine the effect of haploinsufficiency.
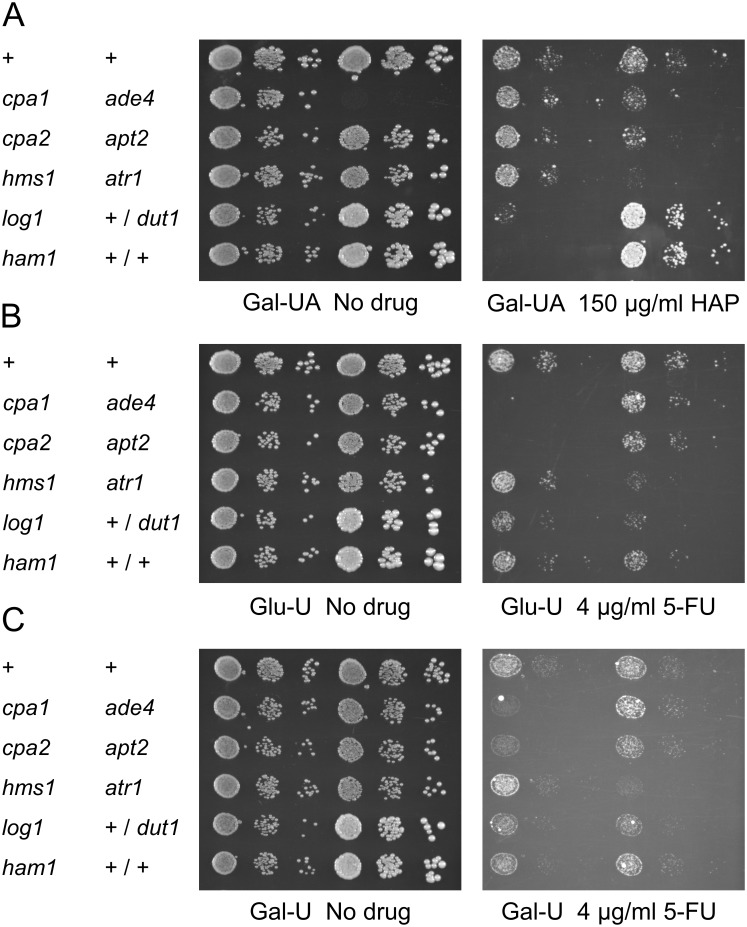
The sensitivity of different strains to 150 μg/ml HAP is shown in Fig 5A. We found that the ham1 and atr1 knockouts are highly sensitive to HAP at this concentration. The sensitivity of the ham1 knockout is consistent with previous findings that ham1 strains are sensitive to purine analogues [17,23] and with the known role of Ham1p in dephosphorylation of HAPTP. The sensitivity of the atr1 knockout to HAP suggests that the Atr1p multidrug efflux pump contributes to HAP detoxification under normal conditions, and not only when overexpressed. We further found that the log1 and apt2 knockouts are sensitive to 150 μg/ml HAP, though not as strongly as the ham1 and atr1 knockouts (Fig 5A). A sensitivity of the log1 knockout to purine analogues has been noted previously [23], and is consistent with a proposed role of Log1p in dephosphorylation of nucleoside monophosphates (see Discussion). The sensitivity of the apt2 knockout to HAP has not been described previously. It suggests that Apt2p contributes to HAP detoxification under normal conditions, and not only when overexpressed. In contrast to these observations, the cpa1, cpa2 and hms1 strains were not sensitive to HAP (Fig 5A). Nor was the dut1/DUT1 heterozygote more sensitive to HAP than the wild type diploid. Finally, we note that the sensitivity of the ade4 strain to HAP could not be assessed since it does not grow in the absence of adenine, a competitive inhibitor of HAP toxicity which must be omitted in order to score sensitivity to HAP. In fact, the ade4 knockout grows weakly in the presence but not the absence of HAP (Fig 5A). A likely explanation is that deamination of HAP to hypoxanthine permits the ade4 strain to grow in the absence of adenine.
Fig 5. Sensitivity to HAP and 5-FU due to disruption of different resistance genes.
Yeast strains disrupted for the genes isolated in our HAP resistance screen and in our previous screen for 5-FU resistance [16] were tested for increased sensitivity to HAP (A) and 5-FU (B and C). All strains were transformed with the empty vector pHR81 in order to complement the ura3 mutation in the genetic background and thus restore a functional pyrimidine biosynthesis. The + sign stands for the haploid wild type control strain BY4742, +/+ stands for the diploid wild type control strain BY4743, and +/dut1 for the diploid DUT1/dut1 heterozygote. Transformants were grown in liquid medium to late exponential phase, serially diluted, and then spotted onto uracil- and adenine-less SC galactose plates (Gal-UA) plates, uracil-less glucose (Glu-U) or uracil-less galactose (Gal-U) plates with or without HAP or 5-FU at the indicated concentrations. The no drug control plates were photographed after 3 days and the drug plates after 5 days.
The sensitivity of different strains to 4 μg/ml 5-FU is shown in Fig 5B (on glucose) and 3C (on galactose). As we previously noted [16], the cpa1 and cpa2 knockouts are highly sensitive to 5-FU, but also the atr1 knockout, which is consistent with a role for the Atr1p multidrug efflux pump in detoxification of 5-FU under normal conditions and not only when overexpressed. Consistent with our previous observations [16], the log1 knockout was weakly sensitive to 5-FU. Finally, we note that the wild type diploid is more sensitive to 5-FU than the haploid strains, and that this sensitivity is further increased in the dut1/DUT1 heterozygote. This is a striking effect since diploid strains normally grow much better than the haploids, as evident from the no drug control (Fig 5B). It suggests that diploids are more sensitive to 5-FU than haploids, and also that Dut1p is important for resistance to 5-FU under normal conditions.
Cross-dependencies between genes that mediate resistance to HAP or 5-FU
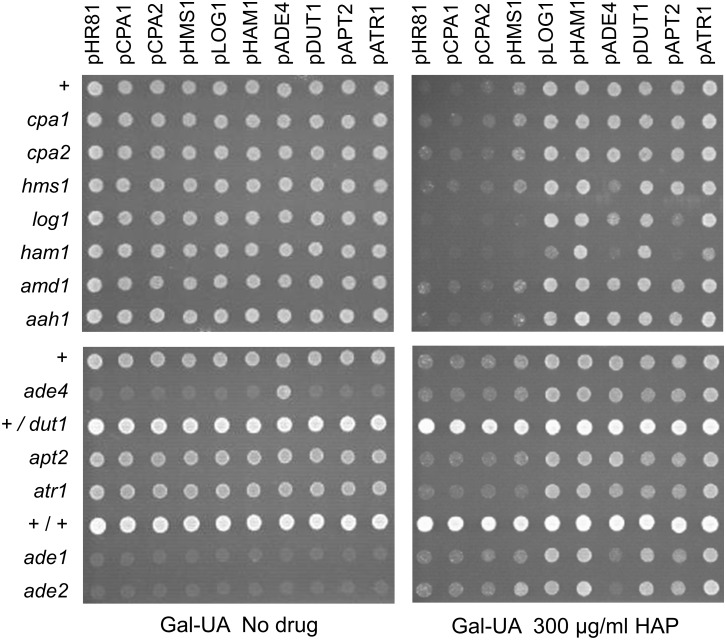
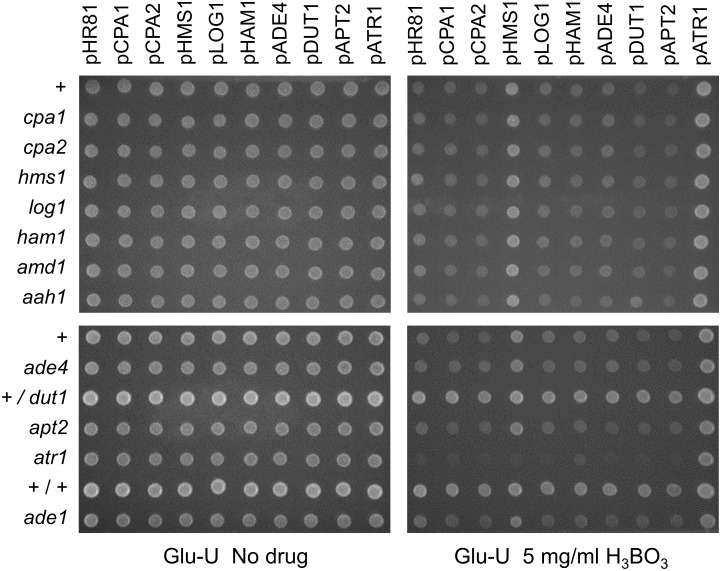
We next tested for genetic interactions and cross-dependencies between the different drug resistance genes by transforming each resistance plasmid into yeast strains where one of the other genes had been knocked out (Figs 6 and 7). The most pronounced genetic interactions affecting HAP sensitivity were seen in the ham1 knockout. Thus, overexpression of ADE4, APT2 and HMS1 failed to cause HAP resistance in the ham1 strain, and the effects of ATR1 and LOG1 overexpression were significantly reduced (Fig 6). This suggests that these genes to some extent are dependent on HAM1 for their resistance phenotype, though it could be argued that the increased HAP sensitivity of the ham1 knockout would make it difficult to detect the effect of other genes, particularly in the case of HMS1 which has a rather weak effect. Perhaps more surprisingly, the ability of DUT1 to confer HAP resistance when overexpressed does not seem to be affected by the ham1 knockout (Fig 6). We conclude that the HAP resistance conferred by DUT1 overexpression is strong enough to fully compensate for the increased sensitivity of the ham1 strain. This somewhat surprising since Ham1p targets both HAPTP and dHAPTP whereas Dut1p is thought to be specific for deoxyribonucleotides. It suggests that the main cytotoxic effect of HAP is mediated by dHAPTP (see Discussion). We further note that ADE4 overexpression was less efficient in conferring HAP resistance also in the hms1 and log1 knockouts. APT2 overexpression, finally, was also less efficient in conferring HAP resistance in the log1 knockout (Fig 6).
Fig 6. Cross-dependencies between different genes for the ability to confer resistance to HAP when overexpressed.
Each plasmid was transformed into yeast knockout strains where one of the other resistance genes had been deleted. Overexpression plasmids (see Fig 1) are shown at the top, yeast strains at the left, and drug concentrations at the bottom in each subfigure. Transformants were grown in liquid medium to late exponential phase, diluted, and then spotted onto uracil- and adenine-less SC galactose (Gal-UA) plates with or without 300 μg/ml HAP. Note that the ade4, ade1, and ade2 strains do not grow on adenine-less media in the absence of the drug, but are able to grow weakly in the presence of HAP which is partially deaminated to adenine. The + sign stands for the haploid wild type control strain BY4742, +/+ stands for the diploid wild type control strain BY4743, and +/dut1 for the diploid DUT1/dut1 heterozygote. The control vector is pHR81 [30]. The no drug control plate was photographed after 3 days and the HAP plate after 5 days.
Fig 7. Cross-dependencies between different genes for the ability to confer resistance to 5-FU when overexpressed.
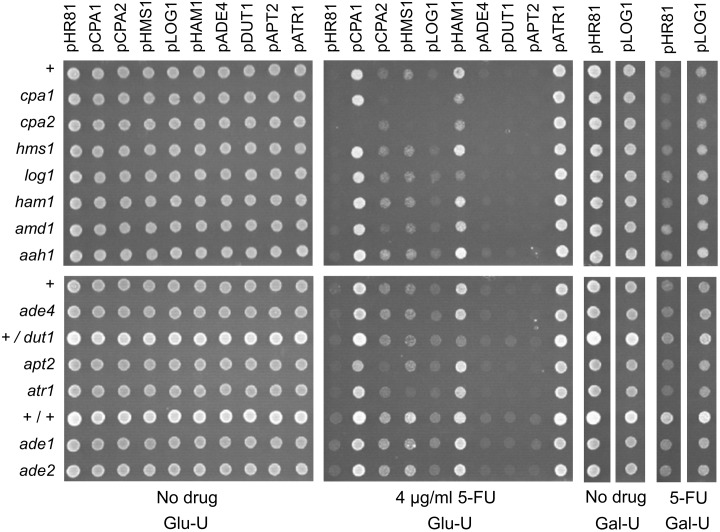
Each plasmid was transformed into yeast knockout strains where one of the other resistance genes had been deleted. Overexpression plasmids (see Fig 1) are shown at the top, yeast strains at the left, and drug concentrations at the bottom in the figure. Transformants were grown in liquid medium to late exponential phase, diluted, and then spotted onto uracil-less SC glucose (Glu-U) plates with or without 4 μg/ml 5-FU. Also shown to the right are results obtained on uracil-less SC galactose (Gal-U) plates for the LOG1 gene and the pHR81 vector control. The + sign stands for the haploid wild type control strain BY4742, +/+ stands for the diploid wild type control strain BY4743, and +/dut1 for the diploid DUT1/dut1 heterozygote. The control vector is pHR81 [30]. The no drug control plate was photographed after 3 days and the 5-FU plate after 5 days.
Resistance to 5-FU was tested on glucose media and also on galactose media for LOG1, since we found that the effect of LOG1 on 5-FU resistance is much stronger on galactose whereas other effects are more easily seen on glucose. We saw that CPA1 depends on CPA2 and CPA2 depends on CPA1 (Fig 7), consistent with our previous findings [16]. Interestingly, HMS1 overexpression depends on ATR1 for its ability to confer 5-FU resistance, and HAM1 depends partially on LOG1 and DUT1, and also more weakly on CPA1 and CPA2 (Fig 7). Furthermore, HMS1 also seems to be dependent on CPA1 and CPA2 (Fig 7). However, it should be noted that the weak 5-FU resistance conferred by HMS1 overexpression could be hard to detect in the cpa1 and cpa2 strains, which are quite sensitive to 5-FU. Surprisingly, CPA1 and CPA2 were also partially dependent on APT2 (Fig 7). This finding was unexpected since APT2 was cloned due to its effect on HAP resistance, and since its sequence similarity to APT1 suggests a role purine metabolism. It is conceivable that this effect may reflect some kind of cross-talk between purine and pyrimidine metabolism, but other explanations are also possible. The observed cross-dependencies for resistance to HAP and 5-FU are summarized in Fig 8.
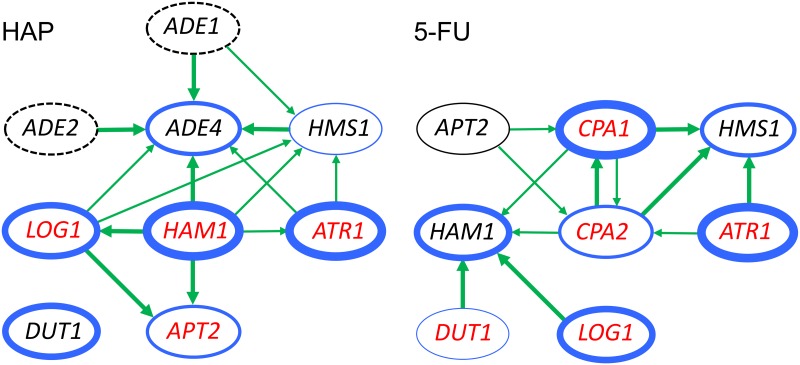
Fig 8. Overview of the genetic interactions observed between different drug resistance genes.
Interactions affecting the sensitivity to HAP are shown at the top and those affecting the sensitivity to 5-FU at the bottom. Genes that cause resistance to the drug when overexpressed are enclosed in blue ovals, the thickness of which indicates the strength of the effect. The dashed ovals around ADE1 and ADE2 means that these genes were not tested for overexpression effects. A green arrow between two genes means that the target needs the source in order to confer full resistance when overexpressed, with thick arrows indicating a strong or complete dependence and thin arrows a weaker effect. Genes for which the knockout mutant shows increased sensitivity to the drug are shown in red.
Dependencies of resistance genes on other genes involved in purine metabolism
We also tested if knockouts of other genes involved in the purine metabolism would affect the ability of the cloned genes to confer resistance to either HAP (Fig 6) or 5-FU (Fig 7). To this end, we transformed all resistance plasmids into the purine deaminase strains amd1 (AMP deaminase), and aah1 (adenine deaminase) to test if the HAP resistance conferred by overexpression of different genes was dependent on the ability to remove, through deamination, the hydroxylamine group of HAP, a reaction that might be performed by adenine deaminases [44]. Finally, we transformed all resistance plasmids into the ade1 and ade2 strains to test if resistance was dependent on a functional purine biosynthesis pathway. This experiment was prompted by our finding that the 5-FU resistance conferred by overexpression of CPA1 and CPA2 is dependent on the pyrimidine biosynthesis pathway [16].
As shown in Fig 6, we found that the sensitivity to HAP was not significantly affected in the amd1 and aah1 knockout strains. Nor were any obvious interactions between amd1 or aah1 and any of the other genes conferring HAP resistance seen. However, the HAP resistance conferred by ADE4 overexpression was abolished in the ade1 and ade2 strains (Fig 6). This is consistent with the known function of ADE4 which encodes phosphoribosylpyrophosphate amidotransferase, the first enzyme in the purine biosynthetic pathway, and supports the notion that ADE4 overexpression reduces the toxicity of HAP by diluting it with freshly synthesized purines. There was also a small effect of the ade1 knockout on the HAP resistance conferred by HMS1 overexpression (Fig 6). The HAP resistance conferred by the LOG1, HAM1, DUT1, APT2 and ATR1 genes were all unaffected by the ade1 and ade2 knockouts, and thus apparently independent of de novo purine biosynthesis. As expected, the amd1, aah1, ade1 and ade2 knockouts had no effects on the resistance to 5-FU in any of the strains (Fig 7).
Effect of resistance genes on the sensitivity to other purine and pyrimidine analogues
In order to test the generality of our findings with HAP and 5-FU, we also tested our cloned resistance genes with several other purine and pyrimidine analogues. A problem with such experiments is that not all drugs are toxic in yeast, due to poor uptake or failure of conversion to the active metabolite. In total, we tested six purine analogues (2-chloroadenine, 6-thioguanine, 6-mercaptopurine, 6-N-hydroxyaminopurine, 2-amino-6-hydroxylaminopurine, and 8-azaguanine) and three pyrimidine analogues (6-azauracil, 5-azacytosine and 5-fluorocytosine). Six of the drugs (2-chloroadenine, 6-thioguanine, 6-mercaptopurine, 6-N-hydroxyaminopurine, 2-amino-6-hydroxylaminopurine, and 5-azacytosine) were not toxic at the highest concentrations that could be tested without precipitation of the drug. The purine analogue 8-azaguanine (8-AzaG) and the pyrimidine analogues 6-azauracil (6-AzaU) and 5-fluorocytosine (5-FC) did show toxicity, and we proceeded to test the effects of the resistance genes on sensitivity to these three drugs. The results are shown in Fig 9, with HAP and 5-FU included as controls.
Fig 9. Resistance to 5-FC, 6-azauracil and 8-azaguanine conferred by overexpression of different genes.
The genes isolated in our HAP resistance screen were tested for resistance to 5-FC, 6-AzaU and 8-AzaG. No drug controls are also shown. Also included were the genes isolated in our previous screen for 5-FU resistance [16]. Cells transformed with the empty vector pHR81 [30] were included as a negative control. Transformants were grown in liquid medium to late exponential phase, serially diluted, and then spotted onto uracil- and adenine-less SC galactose (Gal-UA) plates with or without HAP, 5-FU, 5-FC, 6-AzaU or 8-AzaG at the indicated concentrations. An empty row (Empty) was left below the pADE4 and pCPA1 transformants in order to prevent effects on adjacent strains due to the release of adenine and uracil into the media.
For the purine analogue 8-AzaG, the pattern was similar to that seen with HAP in that overexpression of ADE4, ATR1, LOG1 and HAM1 all caused resistance to the drug. We presume that the mechanisms involved are similar to those for HAP resistance. However, overexpression of DUT1, APT2 or HMS1 did not cause any significant resistance to 8-AzaG (Fig 9). For the pyrimidine analogue 6-AzaU, we saw that overexpression of CPA1 and CPA2 caused resistance, similar to the case with 5-FU. However, the other plasmids did not have much of an effect. The other pyrimidine analogue, 5-FC, was more similar to 5-FU in its pattern of sensitivity. However, a striking difference is that overexpression of ADE4 confers resistance to 5-FC but not to 5-FU (Fig 9). This was an unexpected finding since ADE4 confers resistance to HAP and other purine analogues by boosting the de novo synthesis of purines. (Fig 9).
Discussion
We have performed a screen for genes whose overexpression confer resistance to HAP. To facilitate detection of weak effects, the screen was carried out both in a wild type yeast strain and in a ham1 knockout strain that has increased sensitivity to HAP [17]. In addition to HAM1, we found four genes conferring HAP resistance: ADE4, ATR1, DUT1 and APT2 (Figs 1 and 2). To find out more about the mechanisms by which the genes cause resistance to HAP, we also tested knockout mutants for sensitivity to HAP. Furthermore, we carried out a cross-dependency test in which all plasmids were transformed into strains with knockouts of each one of the other genes, and tested for the abilities to confer HAP resistance (Fig 6). Also included in this experiment were the five genes that we previously found to confer resistance to 5-FU when overexpressed: CPA1, CPA2, HMS1, LOG1 and HAM1 [16], and four genes involved in purine de novo synthesis and salvage: ADE1, ADE2, AMD1, AAH1. All strains and plasmids were also tested for resistance to 5-FU in order to identify common and unique mechanisms of drug resistance. The observed effects and genetic interactions are summarized in Fig 8. We found that four of the genes confer resistance to both HAP and 5-FU: HMS1, LOG1, HAM1, and ATR1. APT2 and ADE4 only confer HAP resistance, whereas CPA1 and CPA2 only confer resistance to 5-FU. DUT1 mainly confers HAP resistance, though a barely detectable effect was also seen on resistance to 5-FU.
The cloned genes represent four different mechanisms by which a cell may acquire resistance to nucleotide analogues. The first resistance mechanism is to promote efflux of the drug, and is exemplified by ATR1, ATR2 and HMS1. The ATR1 gene encodes a multidrug efflux pump, and a likely reason for the strong resistance to both HAP and 5-FU conferred by overexpression of ATR1 is that Atr1p pumps out the non-canonical nucleotides, (d)HAPMP and 5-F(d)UMP, or the corresponding free bases. HMS1 encodes a myc-related transcription factor that was found to activate ATR1 expression in a microarray experiment [45]. This provides a likely explanation for our findings that overexpression of HMS1 confers resistance to both 5-FU and HAP. Consistent with this, we found that HMS1 has no effect on either 5-FU or HAP resistance in the atr1 knockout strain (Figs 6 and 7). In further support of this, we found that HMS1 overexpression confers resistance to boric acid, for which ATR1 has been shown to be important [46], and that this effect also disappears in the atr1 knockout strain (Fig 10). The genetic interactions between ATR1, HMS1, LOG1 and HAM1 in their effects on HAP resistance (Fig 6) supports the notion that Atr1p acts as an efflux sink for the products of Ham1p and Log1p. We interpret these interactions as synergisms between drug efflux (Atr1p and Hms1p) and other detoxification mechanisms (Log1p and Ham1p). Finally, our finding that the ATR1-related gene ATR2 (YMR279C) also confers resistance to HAP and 5-FU (Fig 4) suggests that its gene product acts as a multidrug efflux pump similar to Atr1p.
Fig 10. Boric acid sensitivity of the atr1 knockout strain and boric acid resistance conferred by HMS1 overexpression.
Each plasmid was transformed into yeast knockout strains where one of the resistance genes had been deleted. Overexpression plasmids (see Fig 1) are shown at the top, yeast strains at the left, and drug concentrations at the bottom. Transformants were grown in liquid medium to late exponential phase, diluted, and then spotted onto uracil-less SC glucose (Glu-U) plates with or without 80 mM boric acid (H3BO3). The + sign stands for the haploid wild type control strain BY4742, +/+ stands for the diploid wild type control strain BY4743, and +/dut1 for the diploid DUT1/dut1 heterozygote. The control vector is pHR81 [30]. Note that HMS1 overexpression has no effect in the atr1 knockout strain, indicating that Atr1p functions downstream of Hms1p in conferring resistance to boric acid.
The second resistance mechanism is to dilute the drug or its activated metabolites by boosting the de novo synthesis of nucleotides. This mechanism is exemplified by CPA1, CPA2 and ADE4. CPA1 and CPA2 overexpression boosts de novo pyrimidine synthesis, thus preventing 5-FU from exerting its toxic action [16]. Overexpression of ADE4, which encodes the first enzyme in purine biosynthesis, similarly boosts de novo purine synthesis, which dilutes HAP and prevents it from exerting its toxic action. As expected, we found that these resistance genes are highly specific for the type of nucleotide: CPA1 and CPA2 do not confer resistance to HAP (Fig 6), and ADE4 does not confer resistance to 5-FU (Fig 7). However, CPA1 and CPA2 do confer resistance to other pyrimidine analogues such as 5-fluorocytosine and 6-azauracil (Fig 9).
A third resistance mechanism is to interfere with activation of the drug, and it is possible that APT2 could act in this way. Our finding that APT2 overexpression confers resistance to HAP is surprising since previous studies did not detect a knockout phenotype or enzymatic activity associated with APT2, which was therefore proposed to be a pseudogene [37]. The homology to APT1 which encodes APRT suggests that APT2, if active, should encode an enzyme with similar activity. APRT is needed for activation of HAP into its toxic metabolite HAPMP [24], so overexpression of a protein with APRT activity would be expected to make cells more rather than less sensitive to HAP. Consistent with this, we found that overexpression of APT1 did make the cells slightly more sensitive to HAP (Fig 3). One possible explanation for our finding that overexpression of APT2 confers resistance to HAP could be that it interferes with the expression of Apt1p and thus with its ability to activate HAP. Such interference could occur by promoter competition for an activator of both genes, since APT2 is known to be transcribed [37]. Alternatively, since APRT is a dimeric enzyme [37], overexpression of APT2 might lead to the formation of inactive Apt1p-Apt2p heterodimers. However, a second possible explanation for our finding could be that Apt2p has a previously undetected activity that helps to detoxify HAP. APRT catalyzes a reversible reaction [47], so one possibility is that Apt2p is an APRT that favours the reverse reaction, converting HAPMP to HAP. Yeast isozymes can have opposite favoured directions; one example of this is the alcohol dehydrogenases Adh1p and Adh2p [48]. Our finding that the apt2 knockout is moderately sensitive to HAP (Fig 5A) is consistent with the second explanation, since it suggests that APT2 is not just a pseudogene. Our finding that APT1 overexpression confers resistance to 5-FU (Fig 3) could be due to interference with activation of 5-FU to 5-FUMP (Fig 11), since the 5-FU activating enzyme Fur1p and the adenine activating enzyme Apt1p both use the same substrate, PRPP. Purines have been shown to relieve 5-FU toxicity in a cell line, and it was suggested that this is due to PRPP being depleted during activation by nucleobase phosphoribosyltransferases [49].
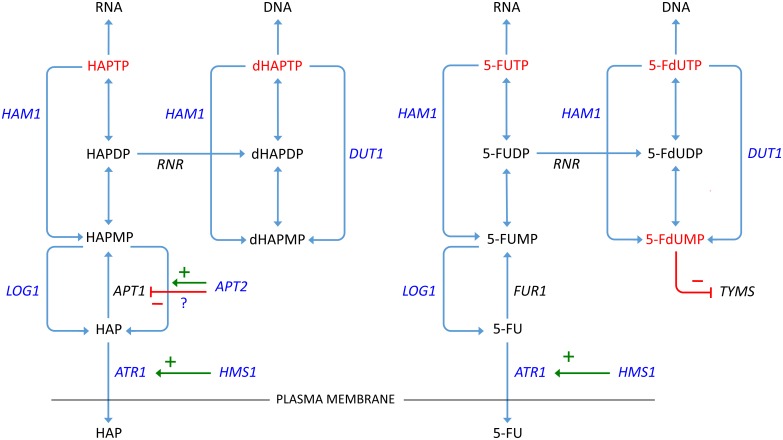
Fig 11. Proposed models for mechanisms of resistance to HAP (left) and 5-FU (right).
Gene symbols are shown adjacent to the metabolic reactions proposed to be catalyzed by the encoded proteins. Genes that cause drug resistance when overexpressed are shown in blue and activated toxic metabolites of HAP and 5-FU in red. Metabolic reactions are shown as blue arrows, positive regulatory effects in green, and negative regulatory effects in red. For APT2, two possible effects on HAP metabolism are indicated: either inhibition of APT1 or catalysis of the opposite reaction, i.e. conversion of HAPMP to HAP. RNR stands for ribonucleotide reductase and TYMS for thymidylate synthase. Not shown in the figure are the effects of overexpressing ADE4 and CPA1 or CPA2, which boost purine and pyrimidine synthesis, and thus dilute HAP and 5-FU, respectively.
The fourth resistance mechanism is to detoxify the drug by degrading it or its activated metabolites. All organisms need to keep their nucleotide pools free from non-canonical nucleotides that might cause damage if incorporated into DNA or RNA. Some non-canonical nucleotides are generated continuously from the metabolism, such as dUTDP produced as an intermediate in TMP synthesis, or IMP and XMP which are intermediates in AMP and GMP synthesis. Other non-canonical nucleotides are generated by oxidation or deamination of canonical nucleotides. Several enzymes have evolved to deal with the threat posed by non-canonical nucleotides [50], and overexpression of such enzymes is expected to confer resistance to nucleotide analogues. This resistance mechanism is exemplified by the DUT1 and HAM1 genes and, as argued below, we also think that LOG1 belongs to this class of genes.
DUT1 encodes dUTP pyrophosphatase, which degrades the genotoxic dUTP generated from the dUDP produced during TMP synthesis [51]. It is an essential enzyme in many organisms including yeast [52]. Dut1p was long thought to be specific for dUTP, but it was more recently found to have significant activity also against the non-canonical purine nucleotide dITP [52]. Our finding that overexpression of DUT1 confers resistance to HAP suggest that Dut1p may have an even broader specificity, including purine analogue triphosphates such as dHAPTP. In this context, our finding that overexpression of DUT1 has little or no effect on the resistance to 5-FU is surprising since 5-FdUTP is more similar to dUTP, and since the dut1/DUT1 heterozygote, as expected, was sensitive to 5-FU but not to HAP (Fig 5). However, dephosphorylation of 5-FdUTP generates 5-FdUMP which is also highly toxic due to its inhibition of thymidylate synthase [4]. It is conceivable that increased production of 5-FdUMP could explain why overexpression of DUT1 has little or no beneficial effect on 5-FU toxicity. Since dHAPMP is not toxic, a similar situation would not exist for HAP toxicity, which could explain why DUT1 overexpression has an effect in that case.
Interestingly, we observed a significantly higher sensitivity to 5-FU in the diploids, both in the wild type and in the dut1/DUT1 heterozygote (Fig 5B). A likely reason for this is that the response to genotoxic stress differs between haploids and diploids. Thus, Li and Tye [53] found that the replication stress induced by a defective mcm4 allele caused a diploid-specific severe genetic instability and reduced viability. It was suggested that this is due to different repair pathways being favoured in haploid and diploid cells. In haploids, replication stress mainly induces Rad6-dependent pathways that resume stalled forks, whereas diploids use the Rad52- and MRX-dependent pathways that repair double strand breaks. Presumably, the latter type of repair events are lethal when massively induced, which may explain both the reduced viability of mcm4 diploids [53] and our finding that diploids are more sensitive to 5-FU than haploids (Fig 5B).
The HAM1 gene was discovered in a screen for yeast genes that cause increased sensitivity to HAP when mutated [17]. Ham1p and its orthologues in other species are purine nucleoside triphosphate phosphatases with specificity for (d)ITP and (d)XTP [18,54–55]. This suggested that Ham1p has evolved to deal with the threat posed by these naturally occurring non-canonical purine nucleotides. However, it was subsequently shown that overexpression of HAM1 also confers resistance to the pyrimidine analogues 5-bromodeoxyuridine [56] and 5-FU [16]. This indicates that the Ham1p enzyme has a broader specificity, being active also against non-canonical pyrimidine nucleotides. Our finding that overexpression of DUT1 is fully able to compensate for the increased HAP sensitivity of the ham1 strain (Fig 6) is interesting, as Dut1p is thought to be specific for deoxyribonucleotides, and would thus presumably target only dHAPTP but not HAPTP. It suggests that the genotoxic effects of dHAPTP are more important for HAP toxicity than the effects of HAPTP incorporation into RNA.
Our finding that LOG1 confers resistance to HAP when overexpressed is consistent with the previous finding that a log1 knockout is sensitive to HAP [23]. The function of the yeast Log1 protein remains to be determined. However, its homology to the plant LOG proteins, which produce free cytokinins by cleaving off the N6-modified adenine from cytokinin nucleoside monophosphates [26,43], is intriguing since it is the same N6-position of adenine that is modified in HAP. It is therefore likely that Log1p confers resistance to HAP by cleaving off the HAP nucleobase from the activated (deoxy)ribonucleoside monophosphate, (d)HAPMP, thus preventing its conversion into (d)HAPTP that can be incorporated into RNA or DNA. This would be consistent with the partial dependence of LOG1 on HAM1 for its ability to confer HAP resistance when overexpressed (Fig 6), since Log1p would then function downstream of Ham1p in the degradation of non-canonical purine nucleotides (Fig 11). The fact that LOG1 confers resistance to 5-FU when overexpressed further suggests that Log1p is active also against non-canonical pyrimidine nucleoside monophosphates such as 5-FUMP. The partial dependence of HAM1 on LOG1 for the ability to confer 5-FU resistance (Fig 7) is consistent with this notion.
In order to assess how general our findings were, we also tested the effects of the cloned resistance genes on the sensitivity to nine additional purine and pyrimidine drugs. Six of the drugs failed to produce any toxicity in yeast, but results were obtained with the pyrimidine analogues 5-FC and 6-AzaU and the purine analogue 8-AzaG, which are shown in Fig 9.
The resistance gene profile of 8-AzaG was similar to that of HAP, except for the fact that overexpression of DUT1, APT2 and HMS1 did not confer any significant resistance to 8-AzaG. The absence of an effect of DUT1 is likely due to 8-AzaG toxicity being mainly caused by its incorporation into RNA, which inhibits protein synthesis [57]. Hence, a reduction in any 8-aza-dGTP formed is not expected to relieve toxicity. The absence of an effect of APT2 is likely due to the fact that activation of guanine and 8-AzaG to ribonucleotides is catalyzed by hypoxanthine-guanine phosphoribosyltransferase, encoded by the yeast HPT1 gene [58], in contrast to HAP, which is mainly activated by APT1 [24]. The lack of a detectable effect of HMS1 on 8-AzaG toxicity could simply be due to the fact that HMS1 was the weakest resistance gene recovered in our screen (Fig 2A).
The resistance gene profile of 6-AzaU was more narrow than that of 5-FU. Thus, we saw a significant effect only with the CPA1 and CPA2 genes (Fig 9). A likely explanation for this is that unlike 5-FU, 6-AzaU does not get incorporated into RNA or DNA, but exerts its toxic effect by inhibiting the pyrimidine biosynthetic enzyme OMP-decarboxylase [57]. It is therefore not surprising that overexpression of CPA1 and CPA2 which boosts pyrimidine biosynthesis and thus provides more substrate for OMP-decarboxylase can relieve the 6-AzaU toxicity.
The resistance gene profiles of 5-FC and 5-FU were similar, but interestingly, overexpression of ADE4, which boosts de novo synthesis of purines, confers resistance to 5-FC but not 5-FU (Fig 9). A likely explanation is that the uptake of 5-FC and 5-FU in yeast is mediated by different transporters. Uracil and 5-FU are taken up by the uracil permease Fur4p, which is feedback-inhibited by intracellular pyrimidines [59–61]. 5-FC is instead taken up by the purine and cytosine permease Fcy2p, which is not inhibited or repressed by cytosine [59], but possibly by an adenine metabolite [62]. Furthermore, ADE4 overexpression has been shown to cause excretion of inosine and hypoxanthine [63], and hypoxanthine acts as a competitive inhibitor of Fcy2p mediated cytosine uptake that can also relieve 5-FC toxicity [60,62]. We conclude that the resistance to 5-FC conferred by ADE4 overexpression most likely is due to its effect on Fcy2p-mediated uptake of 5-FC.
In conclusion, it seems that Ham1p, Log1p and Dut1p all have broader specificities than initially thought, affecting both purines and pyrimidines (Fig 11). Together, they serve as gatekeepers that prevent non-canonical bases from being incorporated into nucleic acids, by dephosphorylating nucleoside triphosphates (Ham1p and Dut1p) and by cleaving the resulting nucleoside monophosphates into free bases and ribose-1-phosphate (Log1p). Ham1p targets both ribo- and deoxyribonucleoside triphosphates, thereby preventing the incorporation of non-canonical bases into RNA and DNA. Dut1p only dephosphorylates deoxyribonucleoside triphosphates. It may have evolved to deal with the special threat posed by the genotoxic dUTP generated during biosynthesis of TTP, but also targets non-canonical purine deoxyribonucleotides such as dITP and dHAPTP. Log1p, finally, acts downstream of Ham1p by cleaving its products, which should facilitate the Ham1p reaction by keeping the concentrations of these products low, and prevent reactivation by phosphorylation. Based on the wide phylogenetic distribution of the LOG (LONELY GUY) family of enzymes, it seems likely that keeping the nucleotide pool free from non-canonical nucleotides is their original function, and that their role in cytokinin production in plants and some microorganisms [26–28] is a more recent development in these organisms.
Acknowledgments
This work was supported by grants to HR from the Swedish Cancer Society (CAN 2014/910) and the Knut and Alice Wallenberg Foundation (KAW 2015.0056).
Abbreviations
- 5-FC
5-fluorocytosine
- 5-FU
5-fluorouracil
- 6-AzaU
6-azauracil
- 8-AzaG
8-azaguanine
- HAP
6-N-hydroxylaminopurine
- ITP
inosine triphosphate
- PRPP
phosphoribosyl pyrophosphate
- SC
synthetic complete media
- TYMS
thymidylate synthase
- XTP
xanthosine 5'-triphosphate
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants to HR from the Swedish Cancer Society (CAN 2014/910), http://www.cancerfonden.se, and the Knut and Alice Wallenberg Foundation (KAW 2015.0056), http://kaw.wallenberg.org. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Scheiner J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 1957;179: 663–666. doi: 10.1038/179663a0 [DOI] [PubMed] [Google Scholar]
- 2.Fukushima M, Nomura H, Murakami Y, Shirasaka T, Aiba K. Estimation of Pathways of 5-Fluorouracil Anabolism in Human Cancer Cells in Vitro and in Vivo. Gan to Kagaku Ryoho. Cancer & Chemotherapy. 1996;23(6): 721–31. [PubMed] [Google Scholar]
- 3.Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat Rev Cancer. 2003;3(5): 330–38. doi: 10.1038/nrc1074 [DOI] [PubMed] [Google Scholar]
- 4.Hoskins J, Butler JS. RNA-Based 5-Fluorouracil Toxicity Requires the Pseudouridylation Activity of Cbf5p. Genetics. 2008;179(1): 323–30. 179/1/323. doi: 10.1534/genetics.107.082727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW, Vogelstein B. Disruption of p53 in Human Cancer Cells Alters the Responses to Therapeutic Agents. J Clin Investig. 1999;104(3): 263–269. doi: 10.1172/JCI6863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladner RD. The Role of dUTPase and Uracil-DNA Repair in Cancer Chemotherapy. Curr Protein Peptide Sci. 2001;2(4): 361–70. doi: 10.2174/1389203013380991 [DOI] [PubMed] [Google Scholar]
- 7.Yoshioka A, Tanaka S, Hiraoka O, Koyama Y, Hirota Y, Ayusawa D, Seno T, Garrett C, Wataya Y. Deoxyribonucleoside Triphosphate Imbalance. 5-Fluorodeoxyuridine-Induced DNA Double Strand Breaks in Mouse FM3A Cells and the Mechanism of Cell Death. J Biol Chem. 1987;262(17): 8235–41. doi: 10.1016/0006-291X(87)90719-4 [PubMed] [Google Scholar]
- 8.Santi DV, McHenry CS, Sommer H. Mechanism of Interaction of Thymidylate Synthetase with 5-Fluorodeoxyuridylate. Biochemistry. 1974;13(3): 471–81. doi: 10.1021/bi00700a012 [DOI] [PubMed] [Google Scholar]
- 9.Giaever, Flaherty P, Kumm J, Proctor M, Nislow C, Jaramillo DF, et al. Chemogenomic profiling: identifying the functional interactions of small molecules in yeast. Proc Natl Acad Sci USA. 2004;101: 793–798. doi: 10.1073/pnas.0307490100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lum PY, Armour CD, Stepaniants SB, Cavet G, Wolf MK, Butler JS, et al. Discovering modes of action for therapeutic compounds using a genome-wide screen of yeast heterozygotes. Cell. 2004;116:121–137. doi: 10.1016/S0092-8674(03)01035-3 [DOI] [PubMed] [Google Scholar]
- 11.Hoskins J, Butler JS. Evidence for Distinct DNA- and RNA-Based Mechanisms of 5-Fluorouracil Cytotoxicity in Saccharomyces cerevisiae. Yeast. 2007;24(10): 861–70. doi: 10.1002/yea.1516 [DOI] [PubMed] [Google Scholar]
- 12.Gustavsson M, Ronne H. Evidence That tRNA Modifying Enzymes Are Important in Vivo Targets for 5-Fluorouracil in Yeast. RNA. 2008;14(4): 666–74. doi: 10.1261/rna.966208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spedaliere CJ, Mueller EG. Not All Pseudouridine Synthases Are Potently Inhibited by RNA Containing 5-Fluorouridine. RNA. 2004;10(2): 192–9. doi: 10.1261/rna.5100104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang F, Hoskins J, Butler JS. 5-Fluorouracil Enhances Exosome-Dependent Accumulation of Polyadenylated rRNAs. Mol Cell Biol. 2004;24(24): 10766–76. doi: 10.1128/MCB.24.24.10766-10776.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao X, Yu Y-T. Incorporation of 5-Fluorouracil into U2 snRNA Blocks Pseudouridylation and Pre-mRNA Splicing in Vivo. Nucl Acids Res. 2007;35(2): 550–58. doi: 10.1093/nar/gkl1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlsson M, Gustavsson M, Hu G-Z, Murén E, Ronne H. A Ham1p-Dependent Mechanism and Modulation of the Pyrimidine Biosynthetic Pathway Can Both Confer Resistance to 5-Fluorouracil in Yeast. PloS One. 2013;8(10): e52094 doi: 10.1371/journal.pone.0052094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noskov VN, Staak K, Shcherbakova PV, Kozmin SG, Negishi K, Ono B-C, et al. HAM1, the Gene Controlling 6-N-Hydroxylaminopurine Sensitivity and Mutagenesis in the Yeast Saccharomyces Cerevisiae. Yeast. 1996;12(1): 17–29. [DOI] [PubMed] [Google Scholar]
- 18.Davies O, Mendes P, Smallbone K, Malys N. Characterisation of Multiple Substrate-Specific (d)ITP/(d)XTPase and Modelling of Deaminated Purine Nucleotide Metabolism. BMB Rep. 2012;45(4): 259–64. doi: 10.5483/BMBRep.2012.45.4.259 [DOI] [PubMed] [Google Scholar]
- 19.von Ahsen N, Armstrong VW, Behrens C, von Tirpitz C, Stallmach A, Herfarth H, et al. Association of Inosine Triphosphatase 94C>A and Thiopurine S-Methyltransferase Deficiency with Adverse Events and Study Drop-Outs under Azathioprine Therapy in a Prospective Crohn Disease Study. Clin Chem. 2005;51(12): 2282–88. doi: 10.1373/clinchem.2005.057158 [DOI] [PubMed] [Google Scholar]
- 20.Stocco G, Cheok MH, Crews KR, Dervieux T, French D, Pei D, et al. Genetic Polymorphism of Inosine Triphosphate Pyrophosphatase Is a Determinant of Mercaptopurine Metabolism and Toxicity during Treatment for Acute Lymphoblastic Leukemia. Clin Pharmacol Therap. 2009;85(2): 164–72. doi: 10.1038/clpt.2008.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dollinger MR, Krakoff IH. Hemolysis Induced by 6-N-Hydroxylaminopurine Riboside, an Adenosine Analogue. Clin Pharmacol Therap. 1975;17(1): 57–65. doi: 10.1002/cpt197517157 [DOI] [PubMed] [Google Scholar]
- 22.Menezes MR, Waisertreiger ISR, Lopez-Bertoni H, Luo X, Pavlov YI. Pivotal Role of Inosine Triphosphate Pyrophosphatase in Maintaining Genome Stability and the Prevention of Apoptosis in Human Cells. PloS One. 2012;7(2): e32313 doi: 10.1371/journal.pone.0032313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stepchenkova E, Kozmin S, Alenin V, Pavlov Y. Genome-Wide Screening for Genes Whose Deletions Confer Sensitivity to Mutagenic Purine Base Analogs in Yeast. BMC Genetics. 2005;6(1): 31 doi: 10.1186/1471-2156-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stepchenkova E, Kozmin S, Alenin V, Pavlov Y. Genetic Control of Metabolism of Mutagenic Purine Base Analogs 6-Hydroxylaminopurine and 2-Amino-6-Hydroxylaminopurine in Yeast Saccharomyces cerevisiae. Genetika. 2009;45: 471–7. doi: 10.1134/S1022795409040048 [PMC free article] [PubMed] [Google Scholar]
- 25.Waisertreiger ISR, Menezes MR, Randazzo J, Pavlov YI. Elevated Levels of DNA Strand Breaks Induced by a Base Analog in the Human Cell Line with the P32T ITPA Variant. J Nucl Acids. 2010. doi: 10.4061/2010/872180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, et al. Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme. Nature. 2007;445 (7128): 652–55. doi: 10.1038/nature05504 [DOI] [PubMed] [Google Scholar]
- 27.Samanovic M, Tu S, Strnad M, Darwin KH. Proteasomal Control of Cytokinin Synthesis Protects Mycobacterium tuberculosis against Nitric Oxide. Mol Cell. 2015;57: 984–94. doi: 10.1016/j.molcel.2015.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naseem M, Sarukhanyan E, Dandekar T. LONELY GUY Knocks Every Door: Crosskingdom Microbial Pathogenesis. Trends Plant Sci. 2015;20: 781–783. doi: 10.1016/j.tplants.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 29.Kelly DE, Lamb DC, Kelly SL. Genome-Wide Generation of Yeast Gene Deletion Strains. Comparat Func Genom. 2001;2(4): 236–42. doi: 10.1002/cfg.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nehlin JO, Carlberg M, Ronne H. Yeast Galactose Permease Is Related to Yeast and Mammalian Glucose Transporters. Gene. 1989;85(2): 313–19. doi: 10.1016/0378-1119(89)90423-X [DOI] [PubMed] [Google Scholar]
- 31.Gustavsson M, Barmark G, Larsson J, Murén E, Ronne H. Functional Genomics of Monensin Sensitivity in Yeast: Implications for Post-Golgi Traffic and Vacuolar H+-ATPase Function. Mol Genet Genom. 2008;280 (3): 233–48. doi: 10.1007/s00438-008-0359-9 [DOI] [PubMed] [Google Scholar]
- 32.Ronne H. Glucose repression in fungi. Trends Genet. 1995;11(1): 12–17. [DOI] [PubMed] [Google Scholar]
- 33.Kowalski D, Pendyala L, Daignan-Fornier B, Howell SB, Huang R-Y. Dysregulation of Purine Nucleotide Biosynthesis Pathways Modulates Cisplatin Cytotoxicity in Saccharomyces Cerevisiae. Mol Pharmacol. 2008;74(4): 1092–1100. doi: 10.1124/mol.108.048256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guillet M, Van Der Kemp PA, Boiteux S. dUTPase Activity Is Critical to Maintain Genetic Stability in Saccharomyces Cerevisiae. Nucl Acids Res. 2006;34(7): 2056–66. doi: 10.1093/nar/gkl139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tinkelenberg BA, Hansbury MJ, Ladner RD. dUTPase and Uracil-DNA Glycosylase Are Central Modulators of Antifolate Toxicity in Saccharomyces Cerevisiae. Cancer Res. 2002;62(17): 4909–15. [PubMed] [Google Scholar]
- 36.Byrne KP, Wolfe KH. The Yeast Gene Order Browser: Combining curated homology and syntenic context reveals gene fate in polyploid species. Genome Res. 2005;15(10): 1456–61. doi: 10.1101/gr.3672305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alfonzo JD, Crother TR, Guetsova ML, Daignan-Fornier B, Taylor MW. APT1, but Not APT2, Codes for a Functional Adenine Phosphoribosyltransferase in Saccharomyces Cerevisiae. J Bacteriol. 1999;181(1): 347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanazawa S, Driscoll M, Struhl K. ATR1, a Saccharomyces Cerevisiae Gene Encoding a Transmembrane Protein Required for Aminotriazole Resistance. Mol Cell Biol. 1988;(2): 664–73. doi: 10.1128/MCB.8.2.664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tkach JM, Yimit A, Lee AY, Riffle M, Costanzo M, Jaschob D, et al. Dissecting DNA Damage Response Pathways by Analysing Protein Localization and Abundance Changes during DNA Replication Stress. Nature Cell Biol. 2012;14(9): 966–76. doi: 10.1038/ncb2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bozdag GO, Uluisik I, Gulculer GS, Karakaya HC, Koc A. Roles of ATR1 Paralogs YMR279c and YOR378w in Boron Stress Tolerance. Biochem Biophys Res Commun. 2011;409(4): 748–51. doi: 10.1016/j.bbrc.2011.05.080 [DOI] [PubMed] [Google Scholar]
- 41.Brauer MJ, Saldanha AJ, Dolinski K, Botstein D. Homeostatic adjustment and metabolic remodeling in glucose-limited yeast cultures. Mol. Biol. Cell 2005;16: 2503–17. doi: 10.1091/mbc.E04-11-0968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez R, Silventoinen V, Robinson S, Kibria A, Gish W. WU-Blast2 Server at the EuropeanBioinformatics Institute. Nucl Acids Res. 2003;31(13): 3795–98. doi: 10.1093/nar/gkg573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuroha T, Tokunaga H, Kojima M, Ueda N, Ishida T, Nagawa S, et al. Functional Analyses of LONELY GUY Cytokinin-Activating Enzymes Reveal the Importance of the Direct Activation Pathway in Arabidopsis. Plant Cell. 2009; 21(10): 3152–69. doi: 10.1105/tpc.109.068676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merkler DJ, Wali AS, Taylor J, Schramm VL. AMP Deaminase from Yeast. Role in AMP Degradation, Large Scale Purification, and Properties of the Native and Proteolyzed Enzyme. J Biol Chem. 1989;264(35): 21422–30. [PubMed] [Google Scholar]
- 45.Chua G, Morris QD, Sopko R, Robinson MD, Ryan O, Chan ET, et al. Identifying Transcription Factor Functions and Targets by Phenotypic Activation. Proc Natl Acad Sci U S A. 2006;103(32): 12045–50. doi: 10.1073/pnas.0605140103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaya A, Karakaya HC, Fomenko DE, Gladyshev VN, Koc A. Identification of a Novel System for Boron Transport: Atr1 Is a Main Boron Exporter in Yeast. Mol Cell Biol. 2009;29(13): 3665–74. doi: 10.1128/MCB.01646-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bashor C, Denu JM, Brennan RG, Ullman B. Kinetic Mechanism of Adenine Phosphoribosyltransferase from Leishmania Donovani. Biochemistry. 2002;41(12): 4020–31. doi: 10.1021/bi0158730 [DOI] [PubMed] [Google Scholar]
- 48.Russell DW, Smith M, Williamson VM, Young ET. Nucleotide sequence of the yeast alcohol dehydrogenase II gene. J Biol Chem. 1983;258: 2674–82. [PubMed] [Google Scholar]
- 49.Yoshida M, Hoshi A. Mechanism of Inhibition of Phosphoribosylation of 5-Fluorouracil by Purines. Biochem. Pharmacol. 1984;33 (18): 2863–67. doi: 10.1016/0006-2952(84)90208-9 [DOI] [PubMed] [Google Scholar]
- 50.Galperin MY, Moroz OV, Wilson KS, Murzin AG. House cleaning, a part of good housekeeping. Mol Microbiol. 2005;59: 5–19. doi: 10.1111/j.1365-2958.2005.04950.x [DOI] [PubMed] [Google Scholar]
- 51.Vertessy BG, Toth J. Keeping uracil out of DNA: physiological role, structure and catalytic mechanism of dUTPases. Acc Chem Res. 2009;42: 97–106. doi: 10.1021/ar800114w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tchigvintsev A, Singer AU, Flick R, Petit P, Brown G, Evdokimova E, et al. Structure and Activity of the Saccharomyces Cerevisiae dUTP Pyrophosphatase DUT1, an Essential Housekeeping Enzyme. Biochem J. 2011;437(2): 243–53. doi: 10.1042/BJ20110304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li XC, Tye BK. Ploidy dictates repair pathway choice under DNA replication stress. Genetics 2011;187:1031–1040. doi: 10.1534/genetics.110.125450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burgis NE, Cunningham RP. Substrate specificity of RdgB protein, a deoxyribonucleoside triphosphate pyrophosphohydrolase. J. Biol. Chem. 2007; 282(6):3531–8. doi: 10.1074/jbc.M608708200 [DOI] [PubMed] [Google Scholar]
- 55.Simone PD, Pavlov YI, Borgstahl GEO. ITPA (inosine triphosphate pyrophosphatase): From surveillance of nucleotide pools to human disease and pharmacogenetics. Mut. Res. 2013;753: 131–46. doi: 10.1016/j.mrrev.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takayama S, Fujii M, Kurosawa A, Adachi N, Ayusawa D. Overexpression of HAM1 gene detoxifies 5-bromodeoxyuridine in the yeast Saccharomyces cerevisiae. Curr. Genet. 2007;52:203–11. doi: 10.1007/s00294-007-0152-z [DOI] [PubMed] [Google Scholar]
- 57.Heidelberger C. Cancer Chemotherapy with Purine and Pyrimidine Analogues. Ann Rev Pharmacol 1967;7(1): 101–24. doi: 10.1146/annurev.pa.07.040167.000533 [DOI] [PubMed] [Google Scholar]
- 58.Guetsova ML, Lecoq K, Daignan-Fornier B. The isolation and characterization of Saccharomyces cerevisiae mutants that constitutively express purine biosynthetic genes. Genetics 1997;147: 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grenson M. The Utilization of Exogenous Pyrimidines and the Recycling of Uridine-5-Phosphate Derivatives in Saccharomyces Cerevisiae, as Studied by Means of Mutants Affected in Pyrimidine Uptake and Metabolism. Eur. J. Biochem. 1969;11 (2): 249–60. doi: 10.1111/j.1432-1033.1969.tb00767.x [DOI] [PubMed] [Google Scholar]
- 60.Jund R, Lacroute F. Genetic and Physiological Aspects of Resistance to 5-Fluoropyrimidines in Saccharomyces Cerevisiae. J. Bacteriol. 1970;102 (3): 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Polak A, Grenson M. Evidence for a Common Transport System for Cytosine, Adenine and Hypoxanthine in Saccharomyces Cerevisiae and Candida Albicans. Eur. J. Biochem. 1973;32 (2): 276–82. doi: 10.1111/j.1432-1033.1973.tb02608.x [DOI] [PubMed] [Google Scholar]
- 62.Chevallier MR, Jund R, Lacroute F. Characterization of Cytosine Permeation in Saccharomyces Cerevisiae. J. Bacteriol. 1975;122 (2): 629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rebora K, Desmoucelles C, Borne F, Pinson B, Daignan-Fornier B. Yeast AMP Pathway Genes Respond to Adenine through Regulated Synthesis of a Metabolic Intermediate. Mol. Cell. Biol. 2001;21 (23): 7901–12. doi: 10.1128/MCB.21.23.7901-7912.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.