Chiang et al. make a notable contribution in Alzheimer disease (AD) therapeutics in a thorough and rigorous study demonstrating superior efficacy of dual therapy against Aβ in a mouse model of amyloid β deposition.
Abstract
In this issue, Chiang et al. (https://doi.org/10.1084/jem.20171484) make a notable contribution to Alzheimer disease (AD) therapeutics in a thorough and rigorous study demonstrating superior efficacy of dual therapy against Aβ in a mouse model of amyloid β deposition.

Insight from David M. Holtzman and Tirth K. Patel.
Extracellular plaques of amyloid β (Aβ) protein form one of the neuropathological hallmarks of Alzheimer disease (AD). Genetic, cellular, animal model, and human data suggest that Aβ deposition in the brain is an initial trigger that sets off a cascade of events that ultimately leads to cognitive decline and AD (Musiek and Holtzman, 2014). As such, this makes Aβ an attractive therapeutic target, especially if targeted early in the process of AD development. More than two decades of intense research have yet to produce an effective treatment that delays the onset, slows the course, or prevents AD. In an exciting new study, Chiang et al. take on this challenge and demonstrate that combination therapy against Aβ—arresting production by genetic manipulation and removing Aβ plaques by passive immunization—is extremely effective at reversing progression of pathology and importantly, restoring cognitive function in a mouse model of Aβ amyloidosis. Although previous studies had explored the efficacy of dual therapy over a single treatment approach (Chow et al., 2010; Wang et al., 2011; Jacobsen et al., 2014; Devi and Ohno, 2015), this study is noteworthy because it provides a putative mechanism (and thereby furthers our thinking about therapeutic targeting) and names a likely culprit for Aβ-induced neuronal damage in this mouse model (i.e., soluble amyloid species in the presence of amyloid deposits).
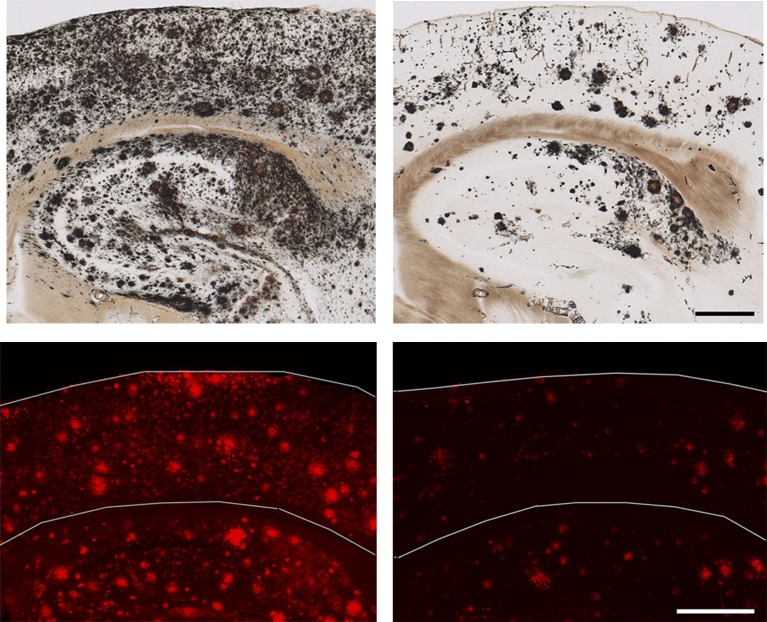
Dual therapy with dox + Ab9 (right) to simultaneously suppress production of Aβ and remove plaques successfully reduces cortical plaque load (top) and oligomeric Aβ (bottom) when compared with vehicle-treated mice (left, top and bottom). Bars: (top) 2,000 µm; (bottom) 500 µm.
Chiang et al. (2018) created a rigorous and thorough experimental framework to directly compare the efficacy of two different types of monotherapy (i.e., suppression of Aβ production and passive immunization) and dual therapy. To suppress the production of Aβ, they used an APP/TTA double transgenic mouse line in which the expression of APP and thus one of its products, Aβ, can be regulated by the tet-off system, where sustained administration of doxycycline (dox) can turn APP and Aβ production off. For passive immunization against Aβ, they used a previously well-characterized monoclonal anti-Aβ antibody, Ab9 (Levites et al., 2006).
Chiang et al. (2018) found that dual therapy was significantly more effective at reducing plaques and soluble Aβ levels than either of the two treatments alone, thus providing a nice proof of concept about the success of this approach. In an encouraging sign, dendritic spine loss was reversed around plaques and neuritic dystrophy was mitigated as well. Only the dual therapy group saw significant improvement in cognitive function as measured by a battery of behavioral and memory tests. Encouraged by these striking findings, the authors looked for an underlying mechanism and asked whether they could link the biochemical/histological reduction in Aβ to improved behavioral outcomes. Through computer modeling and in vivo microdialysis, they concluded that reduction in soluble oligomeric Aβ species, on top of plaque clearance, was the most likely reason for the positive changes seen in the dual therapy group. Their work also implicated aberrant autophagy as a key mediator of neuritic dystrophy, thus marking a major cellular process as a target for future therapy, which has also been suggested in other studies.
Production of Aβ can now be strongly suppressed by inhibitors of the β-secretase-1 enzyme (BACE-1; Vassar et al., 2009) as well as potentially via γ-secretase modulators (Wagner et al., 2017). There are now anti-Aβ antibodies that have been shown to clear Aβ plaques in humans. Although it is likely that targeting Aβ will require starting before symptom onset to have its greatest effects, this combination effect seems much more likely to have a greater impact than either monotherapy alone. The first such approach like this has just moved into the clinic in a trial being conducted by Eli Lilly. A variety of other targets distinct from or downstream from Aβ is still needed. This study also suggests the involvement of mTOR signaling, autophagy and lysosomal function in exacerbating neuritic dystrophy, and other forms of damage. It further expands the availability of a rich list of other potential targets for the future such as tau and neuroinflammation.
In summary, the major implication of this study is that targeting soluble Aβ species at the same time as targeting aggregated Aβ (plaques) might yield better results than targeting either species alone. Collectively, the results of this study are encouraging and will certainly spur more exciting research toward developing Aβ-related therapeutics with treatments that affect both Aβ production and Aβ clearance.
References
- Chiang A.C.A., et al. J. Exp. Med. 2018 doi: 10.1084/jem.20171484. [DOI] [Google Scholar]
- Chow V.W., et al. Sci. Transl. Med. 2010 doi: 10.1126/scitranslmed.3000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L., and Ohno M. Mol. Brain. 2015 doi: 10.1186/s13041-015-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen H., et al. J. Neurosci. 2014 doi: 10.1523/JNEUROSCI.1405-14.2014. [DOI] [Google Scholar]
- Levites Y., et al. J. Clin. Invest. 2006 doi: 10.1172/JCI25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek E.S., and Holtzman D.M. Nat. Neurosci. 2014 doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R., et al. J. Neurosci. 2009 doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [Google Scholar]
- Wagner S.L., et al. J. Pharmacol. Exp. Ther. 2017 doi: 10.1124/jpet.117.240861. [DOI] [Google Scholar]
- Wang A., et al. J. Neurosci. 2011 doi: 10.1523/JNEUROSCI.5077-10.2011. [DOI] [Google Scholar]