Abstract
Cardiovascular surgeons have long debated the safe duration of deep hypothermic circulatory arrest during thoracic aortic aneurysm surgery. The rationale for using adjunctive cerebral perfusion (or not) is to achieve the best technical aortic repair with the lowest risk of morbidity and death. In this literature review, we highlight the debates surrounding these issues, evaluate the disparate findings on deep hypothermic circulatory arrest durations and temperatures, and consider the usefulness of adjunctive perfusion.
Keywords: Aorta, thoracic/surgery; brain ischemia/pathology/prevention & control; cardiac surgical procedures; cerebrovascular circulation; circulatory arrest, deep hypothermia-induced/adverse effects/methods; cognition disorders/etiology/prevention & control; perfusion/adverse effects/methods; postoperative complications/prevention & control; risk assessment; time factors
One of the most debated topics in aortic surgery is the safe duration of deep hypothermic circulatory arrest (DHCA). In this literature review, we present the highly variable findings on DHCA duration at various temperatures, and we discuss whether antegrade cerebral perfusion (ACP) and retrograde cerebral perfusion (RCP) are useful in tandem with straight DHCA.
Early Use of Hypothermia during Cardiac Surgery
In 1952, Lewis and Taufic performed the first successful cardiac operation with the use of hypothermia in a human being.1 Armed with the knowledge of Bigelow and associates' experiments with hypothermia2 and his own experience with several hundred dogs, Lewis successfully closed a secundum atrial septal defect in a 5-year-old girl.3 He then performed hypothermia-aided surgery in 29 more patients, only 3 of whom died. Before cardiopulmonary bypass (CPB) was introduced, the risk of ventricular fibrillation was intrinsic to hypothermia,2 so early practitioners used cardiac massage, intracardiac adrenaline, and electrical shock to restore sinus rhythm in more than 90% of patients.2,3 These operative successes attracted worldwide recognition as milestones in cardiac surgery at that time. However, after the CPB machine became available, cardiac surgeons initially abandoned the sole use of hypothermia. In 1955, Cooley and colleagues4 used hypothermia to achieve cerebral protection during total replacement of the aortic arch in a 49-year-old patient who had a syphilitic aneurysm involving the arch and descending aorta. Surface cooling lowered the patient's temperature to 33 °C, and temporary shunts were placed for blood flow to the carotid arteries and distal aorta. However, the patient had a stroke, attributed to an 8-min occlusion of the right carotid shunt. Thus was hypothermia reintroduced in aortic arch surgery.
Deep Hypothermic Circulatory Arrest
In 1975, Griepp and colleagues5 reported the cases of 4 patients who underwent aortic arch replacement with the use of DHCA and cerebral protection during major, complex aortic operations. The patients' brain activity and energy demand were minimized while the surgeons were able to excise distal clamp sites, completely view the aortic anatomy in bloodless fields, and perform distal anastomoses without leaving clamp-compromised tissue. Despite these advantages, practitioners have continually expressed concerns about the optimal temperature for DHCA and the longest safe interval for repair without risking adverse neurologic sequelae.
The safe cutoff duration for DHCA was generally considered to be 35 to 40 min at a temperature of 20 °C,6 but experience suggests that this interval may be extended.6
The main complications of DHCA are postischemic hypothermia, impaired autoregulatory mechanisms, and damage to the blood-brain barrier evidenced by increased cerebrovascular resistance during the rewarming phase after aortic arch surgery.7,8 To prevent such problems, reperfusion and rewarming are established gradually: the gradient between the perfusate and core temperatures should not exceed 10 °C. Concomitant metabolic management is also crucial, so pharmacologic agents such as mannitol are used to prevent cerebral edema, lower intracranial pressure, and eliminate free radicals.7
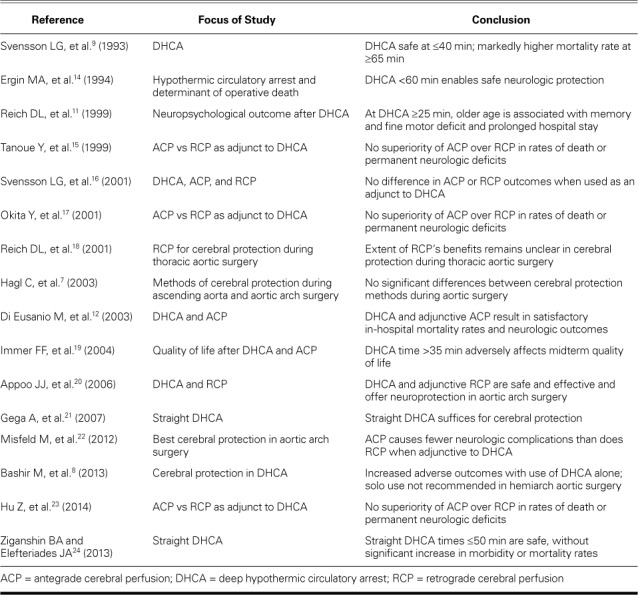
In 656 patients who underwent aortic arch surgery,9 the median DHCA time was 31 min (range, 7–120 min). The overall rates of stroke and early death were 7% and 10%, respectively. Periods of circulatory arrest longer than 45 and 60 min were independent predictors of those respective outcomes. Subsequently, it was shown that the human cerebral metabolic rate is 17% of baseline at a temperature of 15 °C, and that safe concomitant circulatory arrest lasts ≤29 min.10 Circulatory arrest lasting only 25 min was similarly associated with higher risks of transient neurologic, memory, and fine-motor deficits.11–13 Accordingly, in patients undergoing aortic arch repair, surgeons have tried to keep circulatory arrest times <30 min. Table I summarizes studies on DHCA intervals, appropriate temperatures, and evidence supporting the authors' conclusions.7–9,11,14–24
TABLE I.
Summaries and Conclusions in Studies of DHCA during Aortic Arch Surgery
The Physiology of Hypothermia
Hypothermia provides neuroprotection by substantially decreasing the global cerebral metabolism of glucose and oxygen.25 Hypothermia also substantially reduces the temperature-dependent release and extracellular levels of glutamate and other excitatory neurotransmitters, inhibits proapoptotic activity, and lowers levels of free radicals and inflammatory cytokines.26 The central nervous system is vulnerable to ischemia and has a huge metabolic demand with limited energy stores27,28; mean cerebral blood flow, which is autoregulated at 750 mL/min at 37 °C, is 16% of total cardiac output.
McCullough and associates10 concluded that cerebral autoregulation is maintained in hypothermia and stops only at 6 to 12 °C. They also showed that oxygen consumption was 2.9 mL/g/min at 37 °C, 0.9 mL/g/min at 25 °C, and 5% of normal metabolic demand at 8 °C. Although the mechanism is poorly understood, DHCA might provide some cerebral protection by reducing cellular metabolic rates.26 One would expect that the brain's metabolic demands are reduced in hypothermia just as in other vital organs. After studying human cerebral metabolic suppression, McCullough and associates10 predicted a safe duration of 29 min for hypothermic circulatory arrest at a temperature of 15 °C. In a large clinical series, Svensson and colleagues16 showed DHCA to be safe in aortic arch surgery and suggested larger-scale investigation of ACP and RCP.
How Long Should Deep Hypothermic Circulatory Arrest Last?
In short, there is no consensus about the safe duration of DHCA.29 Some authors have suggested that DHCA durations as short as 20 to 25 min will cause adverse outcomes and poor quality of life after thoracic aortic surgery19,30; in contrast, Elefteriades31 reported that most aortic arch interventions can be performed safely at DHCA durations <50 min.
The incidence of postoperative stroke increased in patients who needed >40 min of DHCA9; however, in a different study involving other patients, a duration of ≤60 min of DHCA was safe.14 Ziganshin and Elefteriades24 associated DHCA times >49 min with a 16.7% stroke rate, versus only 2% at times of 40 to 49 min. Upon multivariable analysis, DHCA times ≥50 min significantly predicted adverse outcomes and early death. The authors24 commented that the 50-min threshold should be “taken with a grain of salt.” A DHCA interval safely lasting up to 50 min may add a 10-min surgical “cushion period.”
Nevertheless, the sole use of DHCA for cerebral protection has limitations: the time to cool the patient, the even longer rewarming time, and—of importance—the risk of neurologic injury. Griepp and associates5 confirmed diffuse cerebral injury and higher mortality rates during circulatory arrest periods >60 min.
Neurologic and Cognitive Issues
Neurologic outcome after DHCA was typically defined by whether postoperative stroke occurred; however, this criterion by itself is insufficient for reliable evaluation.32 Another type of neurologic injury that can occur after DHCA is complex temporary neurologic dysfunction (TND).
As a functional manifestation of transient brain injury,6,7,9 TND presents as postoperative confusion, agitation, and delirium. It is not typically associated with structural abnormalities detectable on conventional images. Symptoms of TND usually resolve before a patient's discharge from the hospital.30,33 It is thought that TND occurs rather often, that it is directly related to inadequate brain protection, and that its incidence corresponds with the duration of circulatory arrest. Supplemental cerebral perfusion does not seem to change its incidence or severity. Ergin and colleagues14 studied 3 issues: the incidence of TND in patients who perform poorly on early neuropsychological tests, the correlation between poor performance and TND severity, and whether supplementary brain perfusion prevents TND. The prevalence of TND was higher in patients who performed poorly on early postoperative tests (63% vs 12% in better performers) and that 50% had severe TND. They proposed that TND might be a clinical marker for chronic neuropsychological deficit after DHCA. A clear temporal relationship existed: one third of patients exhibited severe TND after 50 min of DHCA.
Upon performing neuropsychological evaluations, Reich and associates11 showed that circulatory arrest times >25 min predicted long-term functional deficits at temperatures of 28 to 30 °C, whereas Ziganshin and Elefteriades24 reported safe extension of DHCA to 50 min at temperatures of 18 to 19 °C. Such differences in temperature and DHCA duration persist among surgeons performing open aortic arch reconstruction,34 and most brain-preservation methods are complex and difficult to predict and study. In fact, TND and the cognitive changes after DHCA in major and complex aortic surgery are also seen after straightforward coronary or valve surgery. Hence, disruptive neurocognitive mechanisms have been attributed to the extended use of CPB—yet it is difficult to separate the effects of CPB from those of DHCA in complex aortic surgery. To cloud matters further, inadequately perfused brain tissue and ischemic strokes can have similar radiographic appearances.35
Therapeutic hypothermia-induced cellular protection against anoxic brain injury is a global process that affects multiple molecular and cellular mechanisms.35 Ischemic brain injury, reperfusion injury, and secondary brain damage are the 3 main temperature-dependent injury processes.36 The degree of hypothermia affects apoptosis and mitochondrial dysfunction, inflammation, the blood-brain barrier, and free-radical production. The apoptotic mechanism is disrupted dramatically, and altered blood flow to the brain lowers adenosine triphosphate levels and initiates anaerobic glycolysis.37 Ischemia-reperfusion injury stimulates innate immune responses that can lead to secondary brain insult, and the process triggers releases of proinflammatory cytokines, chemokine monocyte chemoattractant protein-1, and proinflammatory mediators in microglia and circulating leukocytes.38–40 Injury further includes the variable formation of free radicals such as superoxide, peroxynitrite, hydrogen peroxide, and hydroxyl.41
Straight versus Adjunctive Arrest
Authors have discussed using DHCA with or without adjunctive cerebral protection. Operative death, morbidity, and long-term survival rates vary widely in reports of DHCA with ACP, RCP, or both in patients who have undergone aortic surgery. Meaningful data are lacking because of too few randomized studies. Variables affecting adjunctive protection are flow rate and temperature, embolization risk, use of unilateral or bilateral perfusion (and the perfusion site itself), and trauma to cranial vessels.24 Times of intensive care unit and total hospital stay have been similar in studies of DHCA alone versus DHCA with adjunctive support,24 and this finding seems to support straight DHCA as adequate cerebral protection during aortic surgery. However, aortic aneurysm involvement complicates surgery and can lengthen operative time. Anticipating this and similar challenges, Griepp and Di Luozzo42 calculated safe intervals for the preservation of tissue integrity during interrupted cerebral perfusion at various times and temperatures.
Given the wide disagreement about which cerebral protection approach is superior for aortic arch surgery and at what temperature, one might speculate that emerging evidence is heavily biased, due to patient selection and other factors. The neurologic dysfunction associated with straight DHCA has elicited criticism from advocates of adjunctive cerebral protection methods.
Antegrade versus Retrograde Perfusion
Cannulation of the axillary or brachiocephalic artery for ACP is cumbersome and risky. In 65 patients, Schachner and colleagues43 reported technical problems in 14% of right axillary cannulation attempts, and a complication rate of 11%. Another disadvantage of using ACP is no standardization of appropriate flow rates44: hyperperfusion can lead to cerebral edema, and hypoperfusion can cause cerebral damage. Further concerns are particulate embolism and vascular injury during cannulation. An intact circle of Willis is thought to be important during unilateral ACP, to enable perfusion of the contralateral cerebral hemisphere; however, the circle of Willis might be complete in only 42% to 47% of the population.45,46 Disagreeing with other authors, Urbanski and colleagues47 concluded that an anatomically incomplete circle of Willis does not correlate with insufficient brain protection during unilateral ACP alone. The circle of Willis is best viewed on computed tomographic angiograms, and flow through the circle can be evaluated on magnetic resonance images.48,49
Retrograde cerebral perfusion lost popularity when evidence showed less oxygen reaching the brain and more blood flowing into collateral vessels than into the brain.18 Okita,17 Tanoue,15 and their respective colleagues separately compared RCP use with ACP use during DHCA and found no differences in rates of death or permanent neurologic deficits. Hu and colleagues23 also reported no difference in cerebral protection between adjunctive methods.
Investigators in a small randomized controlled study16 (10 patients in each arm) compared ACP use, RCP use, and straight DHCA in aortic arch surgery and found straight DHCA to be superior in postoperative long-term neurocognitive test results. Some authors20 concluded that RCP during short-term DHCA was safe; others21 considered RCP to be unnecessary. Investigators who retrospectively associated straight DHCA with increased neurologic dysfunction22,49,50 included hemiarch and total arch surgery in their analyses. In the International Registry for Aortic Dissection multicenter experience,51 which included applications of DHCA with and without ACP and RCP, brain function frequently returned in comatose and stroke-afflicted aortic surgical patients. The authors mentioned no specific superiority of any protective mechanism.
Pediatric Population. Elassal and colleagues52 concluded that straight DHCA was safe in 29 pediatric patients (mean age, 20.6 ± 8.2 mo) at a temperature of 18 °C and a median DHCA time of 20.03 min (range, 3–52 min). Other authors53–56 reported similar outcomes at DHCA durations <60 min.
Conclusion
Defining a clinically useful and safe duration of DHCA remains daunting and might be achieved only through appropriate patient selection and data randomization. This will surely necessitate being able to determine prospectively, in an individual patient, the DHCA duration that causes morbidity equal to or less than that of the alternative perfusion strategy with use of continuous CPB. It is easy to contend that we are not close to an answer because of sparse data, disparate outcome measures, and biological variability, such as patients' morbidity and surgical complexity. Given the basic pathophysiology of DHCA, many uncertainties are inherent in defining safe DHCA duration, and we observe that DHCA largely adds to, rather than replaces, CPB-related risk.
Relatively good reasons should justify the routine choice of straight DHCA or DHCA plus ACP or RCP. However, compelling justification does not exist, so investigators must continue to explore such potentials.
Acknowledgments
We thank Neil Roberts, Alex Shipolini, Prof. Rakesh Uppal, and Prof. Aung Oo for contributing to this article.
References
- 1. Lewis FJ, Taufic M.. Closure of atrial septal defects with the aid of hypothermia; experimental accomplishments and the report of one successful case. Surgery 1953; 33 1: 52–9. [PubMed] [Google Scholar]
- 2. Bigelow WG, Callaghan JC, Hopps JA.. General hypothermia for experimental intracardiac surgery; the use of electrophrenic respirations, an artificial pacemaker for cardiac standstill and radio-frequency rewarming in general hypothermia. Ann Surg 1950; 132 3: 531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewis FJ, Taufic M, Varco RL, Niazi S.. The surgical anatomy of atrial septal defects: experiences with repair under direct vision. Ann Surg 1955; 142 3: 401–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooley DA, Mahaffey DE, DeBakey ME.. Total excision of the aortic arch for aneurysm. Surg Gynecol Obstet 1955; 101 6: 667–72. [PubMed] [Google Scholar]
- 5. Griepp RB, Stinson EB, Hollingsworth JF, Buehler D.. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975; 70 6: 1051–63. [PubMed] [Google Scholar]
- 6. Rimmer L, Fok M, Bashir M.. The history of deep hypothermic circulatory arrest in thoracic aortic surgery. Aorta (Stamford) 2014; 2 4: 129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hagl C, Khaladj N, Karck M, Kallenbach K, Leyh R, Winterhalter M, Haverich A.. Hypothermic circulatory arrest during ascending and aortic arch surgery: the theoretical impact of different cerebral perfusion techniques and other methods of cerebral protection. Eur J Cardiothorac Surg 2003; 24 3: 371–8. [DOI] [PubMed] [Google Scholar]
- 8. Bashir M, Shaw M, Desmond M, Kuduvalli M, Field M, Oo A.. Cerebral protection in hemi-aortic arch surgery. Ann Cardiothorac Surg 2013; 2 2: 239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Svensson LG, Crawford ES, Hess KR, Coselli JS, Raskin S, Shenaq SA, Safi HJ.. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg 1993; 106 1: 19–31. [PubMed] [Google Scholar]
- 10. McCullough JN, Zhang N, Reich DL, Juvonen TS, Klein JJ, Spielvogel D, . et al. Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Ann Thorac Surg 1999; 67 6: 1895–9. [DOI] [PubMed] [Google Scholar]
- 11. Reich DL, Uysal S, Sliwinski M, Ergin MA, Kahn RA, Konstadt SN, . et al. Neuropsychologic outcome after deep hypothermic circulatory arrest in adults. J Thorac Cardiovasc Surg 1999; 117 1: 156–63. [DOI] [PubMed] [Google Scholar]
- 12. Di Eusanio M, Wesselink RM, Morshuis WJ, Dossche KM, Schepens MA.. Deep hypothermic circulatory arrest and antegrade selective cerebral perfusion during ascending aorta-hemiarch replacement: a retrospective comparative study. J Thorac Cardiovasc Surg 2003; 125 4: 849–54. [DOI] [PubMed] [Google Scholar]
- 13. Sakamoto T, Zurakowski D, Duebener LF, Hatsouka S, Lidov HG, Holmes GL, . et al. Combination of alpha-stat strategy and hemodilution exacerbates neurologic injury in a survival piglet model with deep hypothermic circulatory arrest. Ann Thorac Surg 2002; 73 1: 180–90. [DOI] [PubMed] [Google Scholar]
- 14. Ergin MA, Galla JD, Lansman SL, Quintana C, Bodian C, Griepp RB.. Hypothermic circulatory arrest in operations on the thoracic aorta. Determinants of operative mortality and neurologic outcome. J Thorac Cardiovasc Surg 1994; 107 3: 788–99. [PubMed] [Google Scholar]
- 15. Tanoue Y, Tominaga R, Ochiai Y, Fukae K, Morita S, Kawachi Y, Yasui H.. Comparative study of retrograde and selective cerebral perfusion with transcranial Doppler. Ann Thorac Surg 1999; 67 3: 672–5. [DOI] [PubMed] [Google Scholar]
- 16. Svensson LG, Nadolny EM, Penney DL, Jacobson J, Kimmel WA, Entrup MH, D'Agostino RS.. Prospective randomized neurocognitive and S-100 study of hypothermic circulatory arrest, retrograde brain perfusion, and antegrade brain perfusion for aortic arch operations. Ann Thorac Surg 2001; 71 6: 1905–12. [DOI] [PubMed] [Google Scholar]
- 17. Okita Y, Minatoya K, Tagusari O, Ando M, Nagatsuka K, Kitamura S.. Prospective comparative study of brain protection in total aortic arch replacement: deep hypothermic circulatory arrest with retrograde cerebral perfusion or selective antegrade cerebral perfusion. Ann Thorac Surg 2001; 72 1: 72–9. [DOI] [PubMed] [Google Scholar]
- 18. Reich DL, Uysal S, Ergin MA, Griepp RB.. Retrograde cerebral perfusion as a method of neuroprotection during thoracic aortic surgery. Ann Thorac Surg 2001; 72 5: 1774–82. [DOI] [PubMed] [Google Scholar]
- 19. Immer FF, Lippeck C, Barmettler H, Berdat PA, Eckstein FS, Kipfer B, . et al. Improvement of quality of life after surgery on the thoracic aorta: effect of antegrade cerebral perfusion and short duration of deep hypothermic circulatory arrest. Circulation 2004; 110 11 Suppl 1: II250–5. [DOI] [PubMed] [Google Scholar]
- 20. Appoo JJ, Augoustides JG, Pochettino A, Savino JS, McGarvey ML, Cowie DC, . et al. Perioperative outcome in adults undergoing elective deep hypothermic circulatory arrest with retrograde cerebral perfusion in proximal aortic arch repair: evaluation of protocol-based care. J Cardiothorac Vasc Anesth 2006; 20 1: 3–7. [DOI] [PubMed] [Google Scholar]
- 21. Gega A, Rizzo JA, Johnson MH, Tranquilli M, Farkas EA, Elefteriades JA.. Straight deep hypothermic arrest: experience in 394 patients supports its effectiveness as a sole means of brain preservation. Ann Thorac Surg 2007; 84 3: 759–67. [DOI] [PubMed] [Google Scholar]
- 22. Misfeld M, Leontyev S, Borger MA, Gindensperger O, Lehmann S, Legare JF, Mohr FW.. What is the best strategy for brain protection in patients undergoing aortic arch surgery? A single center experience of 636 patients. Ann Thorac Surg 2012; 93 5; 1502–8. [DOI] [PubMed] [Google Scholar]
- 23. Hu Z, Wang Z, Ren Z, Wu H, Zhang M, Zhang H, Hu X.. Similar cerebral protective effectiveness of antegrade and retrograde cerebral perfusion combined with deep hypothermia circulatory arrest in aortic arch surgery: a meta-analysis and systematic review of 5060 patients. J Thorac Cardiovasc Surg 2014; 148 2: 544–60. [DOI] [PubMed] [Google Scholar]
- 24. Ziganshin BA, Elefteriades JA.. Deep hypothermic circulatory arrest. Ann Cardiothorac Surg 2013; 2 3: 303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karnatovskaia LV, Wartenberg KE, Freeman WD.. Therapeutic hypothermia for neuroprotection: history, mechanisms, risks, and clinical applications. Neurohospitalist 2014; 4 3: 153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gonzalez-Ibarra FP, Varon J, Lopez-Meza EG.. Therapeutic hypothermia: critical review of the molecular mechanisms of action. Front Neurol 2011; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parissis H, Hamid U, Soo A, Al-Alao B.. Brief review on systematic hypothermia for the protection of central nervous system during aortic arch surgery: a double-sword tool? J Cardiothorac Surg 2011; 6: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee JM, Grabb MC, Zipfel GJ, Choi DW.. Brain tissue responses to ischemia. J Clin Invest 2000; 106 6: 723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ziganshin BA, Rajbanshi BG, Tranquilli M, Fang H, Rizzo JA, Elefteriades JA.. Straight deep hypothermic circulatory arrest for cerebral protection during aortic arch surgery: safe and effective. J Thorac Cardiovasc Surg 2014; 148 3: 888–900. [DOI] [PubMed] [Google Scholar]
- 30. Ergin MA, Uysal S, Reich DL, Apaydin A, Lansman SL, McCullough JN, Griepp RB.. Temporary neurological dysfunction after deep hypothermic circulatory arrest: a clinical marker of long-term functional deficit. Ann Thorac Surg 1999; 67 6: 1887–94. [DOI] [PubMed] [Google Scholar]
- 31. Elefteriades JA. What is the best method for brain protection in surgery of the aortic arch? Straight DHCA. Cardiol Clin 2010; 28 2: 381–7. [DOI] [PubMed] [Google Scholar]
- 32. Kumral E, Yuksel M, Buket S, Yagdi T, Atay Y, Guzelant A.. Neurologic complications after deep hypothermic circulatory arrest: types, predictors, and timing. Tex Heart Inst J 2001; 28 2: 83–8. [PMC free article] [PubMed] [Google Scholar]
- 33. Krahenbuhl E, Immer FF, Stalder M, Englberger L, Eckstein FS, Carrel TP.. Temporary neurological dysfunction after surgery of the thoracic aorta: a predictor of poor outcome and impaired quality of life. Eur J Cardiothorac Surg 2008; 33 6: 1025–9. [DOI] [PubMed] [Google Scholar]
- 34. Ullah H. Deep hypothermic circulatory arrest - anesthetic considerations. Anaesth Pain Intens Care 2016; 20 Suppl 1: S115–8. Available from: http://www.apicareonline.com/deep-hypothermic-circulatory-arrest-anesthetic-considerations/ [cited 2018 Mar 26]. [Google Scholar]
- 35. Markus HS. Cerebral perfusion and stroke. J Neurol Neurosurg Psychiatry 2004; 75 3: 353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmitt KR, Tong G, Berger F.. Mechanisms of hypothermia-induced cell protection in the brain. Mol Cell Pediatr 2014; 1 1: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Small DL, Morley P, Buchan AM.. Biology of ischemic cerebral cell death. Prog Cardiovasc Dis 1999; 42 3: 185–207. [DOI] [PubMed] [Google Scholar]
- 38. Wang Q, Tang XN, Yenari MA.. The inflammatory response in stroke. J Neuroimmunol 2007; 184 1–2: 53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meybohm P, Gruenewald M, Zacharowski KD, Albrecht M, Lucius R, Fosel N, . et al. Mild hypothermia alone or in combination with anesthetic post-conditioning reduces expression of inflammatory cytokines in the cerebral cortex of pigs after cardiopulmonary resuscitation. Crit Care 2010; 14 1: R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perrone S, Szabo M, Bellieni CV, Longini M, Bango M, Kelen D, . et al. Whole body hypothermia and oxidative stress in babies with hypoxic-ischemic brain injury. Pediatr Neurol 2010; 43 4: 236–40. [DOI] [PubMed] [Google Scholar]
- 41. Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD.. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem 1995; 65 4: 1704–11. [DOI] [PubMed] [Google Scholar]
- 42. Griepp RB, Di Luozzo G.. Hypothermia for aortic surgery. J Thorac Cardiovasc Surg 2013; 145 3 Suppl: S56–8. [DOI] [PubMed] [Google Scholar]
- 43. Schachner T, Nagiller J, Zimmer A, Laufer G, Bonatti J.. Technical problems and complications of axillary artery cannulation. Eur J Cardiothorac Surg 2005; 27 4: 634–7. [DOI] [PubMed] [Google Scholar]
- 44. Chau KH, Ziganshin BA, Elefteriades JA.. Deep hypothermic circulatory arrest: real-life suspended animation. Prog Cardiovasc Dis 2013; 56 1: 81–91. [DOI] [PubMed] [Google Scholar]
- 45. Macchi C, Catini C, Federico C, Gulisano M, Pacini P, Cecchi F, . et al. Magnetic resonance angiographic evaluation of circulus arteriosus cerebri (circle of Willis): a morphologic study in 100 human healthy subjects. Ital J Anat Embryol 1996; 101 2: 115–23. [PubMed] [Google Scholar]
- 46. Krabbe-Hartkamp MJ, van der Grond J, de Leeuw FE, de Groot JC, Algra A, Hillen B, . et al. Circle of Willis: morphologic variation on three-dimensional time-of-flight MR angiograms. Radiology 1998; 207 1: 103–11. [DOI] [PubMed] [Google Scholar]
- 47. Urbanski PP, Lenos A, Blume JC, Ziegler V, Griewing B, Schmitt R, . et al. Does anatomical completeness of the circle of Willis correlate with sufficient cross-perfusion during unilateral cerebral perfusion? Eur J Cardiothorac Surg 2008; 33 3: 402–8. [DOI] [PubMed] [Google Scholar]
- 48. Edelman RR, Mattle HP, O'Reilly GV, Wentz KU, Liu C, Zhao B.. Magnetic resonance imaging of flow dynamics in the circle of Willis. Stroke 1990; 21 1: 56–65. [DOI] [PubMed] [Google Scholar]
- 49. Shihata M, Mittal R, Senthilselvan A, Ross D, Koshal A, Mullen J, MacArthur R.. Selective antegrade cerebral perfusion during aortic arch surgery confers survival and neuroprotective advantages. J Thorac Cardiovasc Surg 2011; 141 4: 948–52. [DOI] [PubMed] [Google Scholar]
- 50. Czerny M, Fleck T, Zimpfer D, Dworschak M, Hofmann W, Hutschala D, . et al. Risk factors of mortality and permanent neurologic injury in patients undergoing ascending aortic and arch repair. J Thorac Cardiovasc Surg 2003; 126 5: 1296–301. [DOI] [PubMed] [Google Scholar]
- 51. Berretta P, Patel HJ, Gleason TG, Sundt TM, Myrmel T, Desai N, . et al. IRAD experience on surgical type A acute dissection patients: results and predictors of mortality. Ann Cardiothorac Surg 2016; 5 4: 346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Elassal AA, Debis RS, Faden MS, Alqari AA, Abdulaziz MA, Al Radi OO.. Outcomes of deep hypothermic circulatory arrest in pediatric cardiac surgery: a single center experience. J Egypt Soc Cardiothorac Surg 2016; 24 3: 228–31. Available from: https://www.sciencedirect.com/science/article/pii/S1110578X16300700 [cited 2018 Mar 26]. [Google Scholar]
- 53. Conolly S, Arrowsmith JE, Klein AA.. Deep hypothermic circulatory arrest. Contin Educ Anaesth Crit Care Pain 2010; 10 5: 138–42. Available from: https://academic.oup.com/bjaed/article/10/5/138/274654 [cited 2018 Mar 26]. [Google Scholar]
- 54. Tassani P, Barankay A, Haas F, Paek SU, Heilmaier M, Hess J, . et al. Cardiac surgery with deep hypothermic circulatory arrest produces less systemic inflammatory response than low-flow cardiopulmonary bypass in newborns. J Thorac Cardiovasc Surg 2002; 123 4: 648–54. [DOI] [PubMed] [Google Scholar]
- 55. Abdelkhalik M, El-Sawy H.. The use of deep hypothermia & total circulatory arrest in pediatric cardiac surgery (Egyptian experience). J Egypt Soc Cardiothorac Surg 2002; 10 3: 415–21. Available from: http://escts.net/new/483 [cited 2018 Mar 26]. [Google Scholar]
- 56. Fuller S, Rajagopalan R, Jarvik GP, Gerdes M, Bernbaum J, Wernovsky G, . et al. J. Maxwell Chamberlain Memorial Paper for congenital heart surgery. Deep hypothermic circulatory arrest does not impair neurodevelopmental outcome in school-age children after infant cardiac surgery. Ann Thorac Surg 2010; 90 6: 1985–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


