Abstract
Percutaneous balloon pulmonary valvuloplasty is the preferred therapy for pulmonary valve stenosis. However, the designs of the cylindrical balloons historically used for valvuloplasty have limitations, especially in patients who have large pulmonary annular diameters. The hourglass-shaped V8 Aortic Valvuloplasty Balloon may prove to be an effective alternative. The balloon has 2 large bulbous segments that are separated by a narrowed waist. The geometric shape is maintained throughout inflation, improving fixation and enabling broader leaflet opening. We present our first experience with the V8 balloon in 3 adults who had severe, symptomatic pulmonary valve stenosis. In addition to describing their cases, we detail our sizing technique for pulmonary valvuloplasty with the V8 balloon. Our successful results suggest that the V8 balloon is efficient and safe for balloon pulmonary valvuloplasty in adults with severe pulmonary valve stenosis.
Keywords: Balloon valvuloplasty/instrumentation/methods, cardiac catheterization, dilatation/instrumentation/methods, equipment design, equipment safety, heart valve disease/therapy, hemodynamics/physiology, pulmonary valve stenosis/physiopathology/therapy, treatment outcome
Percutaneous balloon pulmonary valvuloplasty (BPV), first described by Kan and colleagues1 in 1982, has replaced surgical valvotomy as the preferred treatment for congenital and acquired pulmonary valve stenosis (PVS).2,3 Many patients have had satisfactory long-term results after BPV, but conventional balloon designs have limited effectiveness when pulmonary valve (PV) annular diameters range from 18 to 30 mm; in some cases, effective balloon diameters >30 mm are needed. Cylindrical balloons as large as 30 mm in diameter are commercially available; however, they have several drawbacks, including slow inflation times, which can prolong systemic hypotension; difficulty maintaining catheter position during systole with the potential for intracardiac trauma; and low nominal burst pressures.4 Attempts to minimize these problems through the use of double balloons, triple balloons, and the Inoue Balloon™ (Toray Industries, Inc.) have been described, but these procedures are cumbersome, time-consuming, and expensive.4–8
The V8™ Aortic Valvuloplasty Balloon Catheter (InterValve Medical, Inc.; acquired by Venus Medtech in 2017)9 is an hourglass-shaped balloon with 2 bulbous segments, separated by a narrowed waist. During balloon aortic valvuloplasty (BAV), the design improves fixation and enables better leaflet opening without compromising the annulus. The Inoue balloon also has an hourglass shape, and investigators have reported better BAV outcomes with it than with cylindrical balloons.7,8,10 However, it has a large sheath and rigid properties.11,12 Moreover, the Inoue is a compliant, low-inflation pressure balloon, and its hourglass shape disappears during peak inflation. In contrast, the V8 balloon is semicompliant and retains its shape throughout inflation. The waist and bulbous segments reach predetermined diameters at specified volume-driven inflations. The waist diameter increases through incremental stepwise inflations.
Although the V8 balloon has provided good results in BAV procedures, its use has not been reported for BPV.9 We describe our successful experience with the V8 balloon in 3 patients with PVS, and we detail our balloon sizing technique.
Device Design
The distal end of the V8 balloon catheter system features an hourglass-shaped dilation balloon (Fig. 1). Three radiopaque markers on the catheter shaft correspond to the center of the waist segment and to the distal and proximal shoulders of the bulbous balloon segments (Fig. 2). The length of the fully inflated balloon is 32 mm: the waist segment is 12 mm, and each of the bulbous segments is 10 mm. The waist segment is more compliant than the bulbous segments, so it expands to a greater degree. The distance between the central marker and each of the shoulders is 16 mm. The distal and proximal ends of the balloon segments taper sharply so that they resemble cones. The V8 balloon is short and has a larger inflation lumen in the 10F shaft; this feature enables rapid inflation and deflation times. The waist segment usually locks into the annulus, and this capability reduces the need for rapid ventricular pacing.
Fig. 1.

Illustrations compare the profile of A) a conventional cylindrical balloon catheter with that of B) the hourglass-shaped V8 balloon catheter.
Fig. 2.

Radiopaque markers indicate the longitudinal dimensions of the V8 balloon.
The V8 balloon is available in 4 sizes (17, 19, 21, and 23 mm) that correspond to the waist diameter when inflated at nominal volume. The diameters of the bulbous segments are 5 mm larger (respectively, 22, 24, 26, and 28 mm) than those at the waist. The waist diameter can be precisely increased by inflating the balloon at predetermined incremental volumes.
Techniques
Balloon Sizing. The balloon sizing technique that we used for BPV in our patients differed from our BAV technique. Unlike stenotic aortic valve anatomy, stenotic pulmonary leaflet tips lie adjacent to the waistlike narrowing at the sinotubular junction (STJ).13 Therefore, our sizing strategy was based on positioning the balloon waist within the waistlike narrowing at the STJ, at the level of the domed stenotic leaflets, to ensure a consistent lock between the 2 during BPV.
We measured 4 segments of the PV anatomy: the annulus; the waistlike narrowing at the STJ at the level of the leaflet tips (the reference diameter); the right ventricular outflow tract (RVOT), 16 mm proximal to the STJ at the leaflet tips; and the pulmonary trunk, 16 mm distal to the STJ at the leaflet tips. The measurements above and below the STJ at the leaflet tips corresponded to the 16-mm lengths between the balloon's central marker and the markers at each shoulder.
All measurements were made preoperatively by using pulmonary computed tomographic (CT) angiography (coronal and sagittal sections) (Fig. 3), and again during cardiac catheterization by using right ventricular (RV) angiography (straight and anteroposterior projections and 90° lateral projection) (Fig. 4). The PV annulus, STJ, and pulmonary trunk were measured in systole, and the RVOT was measured in diastole.
Fig. 3.

Patient 1. Computed tomographic angiograms (coronal views) show the dimensions of the pulmonary valve annulus (26.4 mm) and the waistlike narrowing of the sinotubular junction (20.1 mm) in systole, B) the pulmonary trunk 16 mm distal to the waistlike narrowing of the sinotubular junction (39.4 mm) in systole, and C) the right ventricular outflow tract 16 mm below the waistlike narrowing of the sinotubular junction (27.1 mm) in diastole.
Fig. 4.
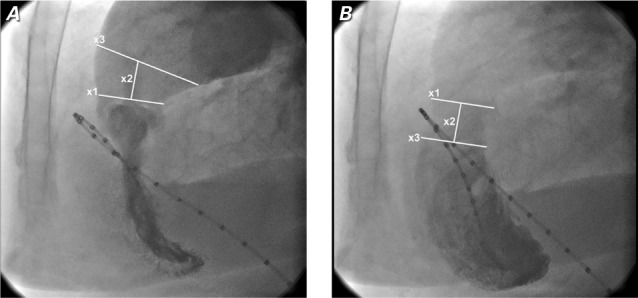
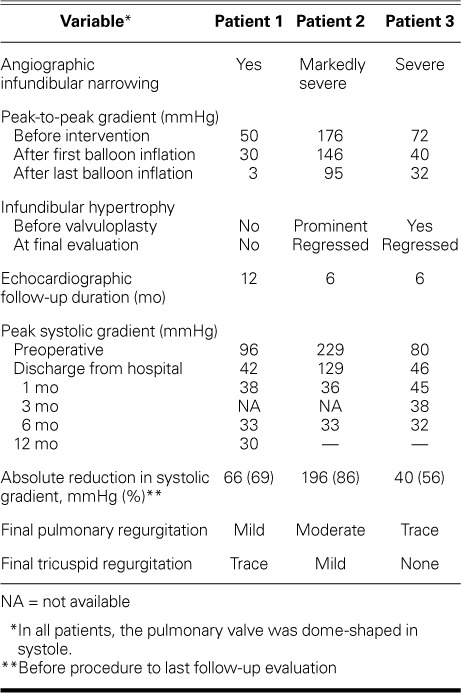
Patient 1. Preoperative right ventricular angiograms show the dimensions of A) the waistlike narrowing of the sinotubular junction (STJ) (x1=26 mm), the distance between the STJ and the distal pulmonary trunk (x2=16 mm), and the pulmonary trunk (x;3=35 mm) in systole, and B) the STJ cross-section mark (x;1), the distance between the STJ and proximal right ventricular outflow tract (RVOT) (x2=16 mm), and the RVOT (x3=28 mm) in diastole.
We used a stepwise dilation approach, beginning with the smallest balloon appropriate for the patient. Subsequent incremental inflations were performed until the desired hemodynamic endpoints were achieved. Maximal balloon sizing was based on these criteria: the bulbous segments were not to exceed the diameters of the RVOT and pulmonary trunk, and the ratio of the balloon waist diameter to the STJ diameter was not to exceed 1.1 to 1.2.
Balloon Pulmonary Valvuloplasty. The BPV procedures were performed at Kocaeli University Faculty of Medicine's Invasive Cardiology Research and Application Unit. Written informed consent was obtained from all the patients, and the local ethics committee approved the study protocol. The study was conducted in accordance with the latest version of the Declaration of Helsinki. During the procedures, the patients were under conscious sedation.
Two 6F venous sheaths were inserted into the right and left transfemoral veins and a 5F arterial sheath was inserted into the right femoral artery. A pigtail catheter with radiopaque markers was positioned in the RV. Anteroposterior and straight lateral ventriculograms were obtained to measure the baseline diameters of the RVOT and pulmonary trunk, as detailed above. A balloon-tipped right-sided heart catheter or 5F multipurpose catheter was used to cross the PV with use of a 0.035-in angled-tip guidewire (Terumo Inc.). Simultaneous RV and pulmonary artery (PA) pressures were recorded, and peak systolic gradients were measured. A 0.035-in extra-stiff guidewire was then positioned in a peripheral PA branch via the PA catheter. The 6F right femoral venous sheath was exchanged for a 12F sheath over the extra-stiff guidewire positioned in the PA. Then the V8 balloon catheter was advanced to the PA over the guidewire.
Next, the central balloon marker was aligned with the stenotic PV leaflets at the level of the STJ. The V8 balloon was rapidly inflated with 1:4 dilute-contrast at the volume specified to achieve the desired waist diameter. Balloon inflation volumes were increased incrementally until the predetermined maximal hemodynamic endpoints were reached. To achieve the incremental increases, we removed the initial V8 balloon and inserted a larger one. Procedures were performed without ventricular pacing.
The balloon was then removed, and a pigtail or multipurpose catheter was positioned to obtain simultaneous RV and PA pressure recordings. Success was defined as a 50% reduction in the baseline peak-to-peak gradient with minimal increase in pulmonary insufficiency.
Case Summaries
Patient 1
A 54-year-old woman was admitted to the clinic with shortness of breath, chest pain, and general fatigue. She had a history of hypertension, chronic obstructive pulmonary disease due to asthma, diabetes mellitus, and chronic renal disease. Transthoracic echocardiograms revealed RV wall hypertrophy, thickened and doming PV leaflets with severe PVS, a peak gradient of 96 mmHg (mean, 58.5 mmHg), and mild pulmonary regurgitation (Tables I and II). Her EuroScore II risk was 12%.
TABLE I.
Baseline Characteristics of the 3 Patients

TABLE II.
Hemodynamic Measurements Before and After Valvuloplasty
Preoperatively, pulmonary CT angiograms and RV angiograms were obtained to measure the patient's pulmonary annulus, STJ, RVOT, and pulmonary trunk (Figs. 3 and 4), after which the BPV procedure was started (Fig. 5). The patient's initial peak-to-peak gradient was 50 mmHg. After the first balloon inflation, it decreased to 30 mmHg, and after the second inflation, it was 3 mmHg. The ratios of the balloon waist diameter to the STJ diameter for each of 3 inflations were 0.7, 0.8, and 0.9 (Table III).
Fig. 5.

Patient 1. Right ventriculogram (straight lateral view) shows the initial 24/19-mm V8 balloon inflated to 22-cc volume to achieve a 21-mm waist diameter.
Supplemental motion image is available for Figure 5.
TABLE III.
Intraprocedural Anatomic Measurements, Balloon Sizing, and Valvuloplasty Procedural Details

Although the patient's peak-to-peak gradient had decreased to 3 mmHg intraoperatively, her peak systolic gradient was 42 mmHg on an echocardiogram at the time of discharge. The gradient was thought to be subvalvular. The patient was prescribed β-adrenergic blocker therapy. At her 12-month follow-up evaluation, echocardiograms showed a substantially reduced systolic gradient of 30 mmHg. The PV regurgitation remained mild throughout follow-up monitoring (Table II).
Patient 2
A 21-year-old woman with PVS was referred to our clinic for evaluation. Her symptoms included dyspnea, fatigue, and syncopal episodes. Transthoracic echocardiograms revealed severe RV wall and infundibular hypertrophy, thickened and doming PV leaflets with severe PVS, a peak gradient of 229 mmHg (mean, 140 mmHg), and mild pulmonary regurgitation (Tables I and II).
During the intraprocedural evaluation, angiograms revealed that the patient had critical PVS and infundibular stenosis. Because the pressure gradient between the RV and PA was extremely high, the smallest V8 balloon was initially selected, and incremental inflations were performed in stepwise fashion to avoid acute pulmonary edema due to an abrupt, marked increase in pulmonary blood flow (Table II). Her systolic peak-to-peak gradient decreased from 176 to 95 mmHg intraoperatively.
At discharge, the patient's peak systolic gradient was 129 mmHg on echocardiography. The residual gradient most likely occurred across the hypertrophic RVOT. The final inflation, performed with a size 26/21 balloon at an inflation volume of 26 cc, resulted in a balloon waist diameter of 23.5 mm. The final ratio of the balloon waist diameter to the STJ diameter was 0.92 (Table III). The patient began β-adrenergic blocker therapy, and after 6 months, echocardiograms showed a substantially reduced systolic gradient of 33 mmHg. Her PV regurgitation, which had been mild, was moderate throughout follow-up monitoring (Table II).
Patient 3
Seven years before presenting at our clinic, a 21-year-old man with mild PVS and a perimembranous ventricular septal defect (VSD) had undergone surgical closure of the VSD. At the current presentation, he reported dyspnea, palpitations, and exertional fatigue. Transthoracic echocardiograms revealed RV hypertrophy, severe PVS, a peak gradient of 80 mmHg (mean, 58 mmHg), and mild pulmonary regurgitation (Tables I and II). A small left-to-right color-flow jet across the suture line of the VSD patch repair was consistent with a small left-to-right shunt.
During BPV, the patient's peak-to-peak systolic gradient decreased from 72 to 32 mmHg upon stepwise balloon dilation increases. The final inflation, performed with a size 26/21 balloon at an inflation volume of 26 cc, resulted in a waist diameter of 23.5 mm. The ratio of the balloon waist diameter to the STJ diameter was 0.94 (Table III). At the time of discharge, an echocardiogram revealed a systolic gradient of 46 mmHg, predominantly across the infundibulum. After 6 months of β-adrenergic blocker therapy, the patient's peak systolic gradient was 32 mmHg. Trace PV regurgitation remained throughout follow-up monitoring (Table II).
Discussion
Our experience suggests that the hourglass shape of the V8 aortic valvuloplasty balloon increases the effectiveness of BPV in patients with PVS. Echocardiographic studies in our 3 patients showed markedly improved gradients immediately after BPV, and these results were sustained on follow-up studies 6 to 12 months later. The decreases in our patients' gradients were similar to those after BPV with other types of balloons.5–8,10 Early after treatment, all of our patients had residual systolic gradients, but these continued to improve during the follow-up period. All 3 patients were prescribed β-adrenergic blocker therapy after intervention; it was useful in each case, but was especially beneficial to Patients 1 and 2 because of their infundibular hypertrophy and presumed systolic gradient contributing to RV and PA stenosis. Finally, pulmonary insufficiency improved or was unchanged at the patients' last follow-up evaluation.
The various catheters used for BPV have had fixed-size cylindrical balloons with imprecise diameters and no labels stating the appropriate inflation volume to achieve a specific dimension.1 Lower-profile balloon catheter systems with noncompliant balloons, such as the Tyshak® II (NuMED, Inc.), are now more widely available for BPV. However, some pediatric and adult patients have a large PV annulus, with diameters ranging from 18 to 30 mm. In these cases, the effective balloon diameter can range from 22 to >30 mm.4 Cylindrical balloons as large as 30 mm are commercially available; however, they have some disadvantages. These include large sheaths; slow inflation times, which make it difficult to maintain catheter position during systole; unpredictable balloon diameters and low nominal burst pressures; and balloon material redundancy, which may be associated with an increased risk of clot formation.4
To minimize these problems, double-balloon, triple-balloon, and Inoue-balloon techniques have been developed, but they have ultimately offered little benefit at increased costs.4–8 Using smaller balloons for double-balloon valvuloplasty theoretically facilitates insertion, manipulation, and removal of the balloon catheters while reducing vascular trauma at the insertion site. However, the technique necessitates additional femoral venous access and increases the risk of associated complications. In addition, these balloons are mounted on relatively small shafts, so they have narrowed inflation lumens, resulting in prolonged inflation times and an increased potential for systolic disengagement.4–6 A triple-balloon technique can be effective, but positioning 3 balloons side-by-side makes the procedure cumbersome.4 The Inoue balloon reportedly improves hemodynamics; however, it is compliant, and it loses its hourglass shape during maximal inflation, which places the PV annulus, the STJ, and the leaflets at risk of rupture.7,8,10–12 Moreover, it can be difficult to manipulate.
The shape and special characteristics of the V8 balloon are markedly different from those of the Inoue and other shaped balloons such as the Nucleus-X™ Balloon and Aortic Pulmonic Catheter (B. Braun Interventional Systems Inc.), which are larger and ultimately assume a full cylindrical shape during the later stages of inflation. These features place a larger area of the perivalvular anatomy at risk of trauma. In contrast, the narrow waist and larger bulbous ends of the V8 balloon are maintained throughout inflation, enabling a consistent lock on the dome-shaped PV leaflets at the level of the narrowed STJ. The differential compliance between the V8 segments also enables a stepwise approach to increasing inflation diameters at the balloon waist, along the fused leaflet tips. Thus, it produces a controlled, iterative improvement in PV hemodynamics.9 In addition, the V8 balloon's rapid inflation and deflation times reduce the need for rapid ventricular pacing. None of our patients underwent pacing, nor did they experience hemodynamic compromise.
Balloon size and geometric shape are factors that contributed to the favorable results in our patients. Historically, the best results with conventional balloons have been obtained when the balloon diameter is larger than the PV annulus,14 generally at a ratio of 1.2 to 1.3. Ratios that exceed 1.3 are associated with a greater likelihood of substantial pulmonary insufficiency in the long term. The valve annulus is usually measured at the base of the PV leaflet hinge points.7,14 However, unlike the leaflets of the aortic valve, the leaflets of the PV have no direct fibrous support other than that provided by the valve sinuses.13 Stamm and colleagues13 showed that dome-shaped stenotic PVs have a relatively circular formation of leaflets, with 3 raphes tethered to the arterial wall at the STJ that produce a waistlike narrowing. The stenotic leaflet tips usually lie adjacent to the STJ narrowing. Because the mechanism of BPV involves applying a measured force to the fused leaflet tips, our sizing technique for the V8 balloon is based on positioning the balloon waist on the leaflet tips at the level of the STJ, not on the PV annulus. In our patients, the balloon waist was consistently locked on the waistlike narrowing of the STJ as inflation volumes were incrementally increased to achieve the optimal waist diameter.
It is well known that the traditional balloon sizing rule for BPV uses the formula of PV annulus diameter × 1.2.1 However, instead of the annulus, we used the waistlike narrowing of the STJ as a reference point. Table IV compares our patients' PV anatomic dimensions and V8 balloon sizes with those associated with conventional balloons. Using the conventional balloon-to-annulus formula would result in similar conventional balloon-to-STJ and V8-to-STJ ratios. However, applying this formula to the V8 balloon would potentially cause the bulbous segments, which are larger than the waist, to damage the RVOT 16 mm proximal to the leaflet tips. Thus, our results show that basing the V8 size on the STJ diameter, instead of the PV annulus, provided optimal hemodynamic benefits by achieving similar balloon-to-STJ ratios without causing substantial complications. Historically, in adults, systolic PV gradients are reduced by 60%, and severe complications occur in less than 1% of patients.15 In our patients, the PV gradients were reduced by more than 60%, without complications.
TABLE IV.
Comparison of V8 Balloon Ratios with Conventional Balloon Ratios

Our early experience with the V8 balloon catheter for BPV, coupled with our novel method for balloon sizing, proved successful in 3 adults with PVS. Thus, the V8 balloon catheter may offer an alternative, safe, effective, and efficient approach to BPV in adults.
Supplementary Material
Acknowledgments
We thank Halil Ibrahim Ada, MD, and Hasan Tahsin Sarisoy, MD, for the radiologic multidetector CT measurements of the pulmonary diameters; Mrs. Abbie Young for preparation and editing of the manuscript; and Dirk Segers, Mark Ungs, and Onur Kircali for their technical support during the procedures.
References
- 1. Kan JS, White RI Jr, Mitchell SE, Gardner TJ.. Percutaneous balloon valvuloplasty: a new method for treating congenital pulmonary-valve stenosis. N Engl J Med 1982; 307 9: 540–2. [DOI] [PubMed] [Google Scholar]
- 2. Rocchini AP, Kveselis DA, Crowley D, Dick M, Rosenthal A.. Percutaneous balloon valvuloplasty for treatment of congenital pulmonary valvular stenosis in children. J Am Coll Cardiol 1984; 3 4: 1005–12. [DOI] [PubMed] [Google Scholar]
- 3. Pepine CJ, Gessner IH, Feldman RL.. Percutaneous balloon valvuloplasty for pulmonic valve stenosis in the adult. Am J Cardiol 1982; 50 6: 1442–5. [DOI] [PubMed] [Google Scholar]
- 4. Escalera RB 2nd, Chase TJ, Owada CY.. Triple-balloon pulmonary valvuloplasty: an advantageous technique for percutaneous repair of pulmonary valve stenosis in the large pediatric and adult patients. Catheter Cardiovasc Interv 2005; 66 3: 446–51. [DOI] [PubMed] [Google Scholar]
- 5. Mullins CE, Nihill MR, Vick GW 3rd, Ludomirsky A, O'Laughlin MP, Bricker JT, Judd VE.. Double balloon technique for dilation of valvular or vessel stenosis in congenital and acquired heart disease. J Am Coll Cardiol 1987; 10 1: 107–14. [DOI] [PubMed] [Google Scholar]
- 6. Park JH, Yoon YS, Yeon KM, Han MC, Kim CW, Oh BH, Lee YW.. Percutaneous pulmonary valvuloplasty with a double-balloon technique. Radiology 1987; 164 3: 715–8. [DOI] [PubMed] [Google Scholar]
- 7. Lau KW, Hung JS, Wu JJ, Chern MS, Yeh KH, Fu M.. Pulmonary valvuloplasty in adults using the Inoue balloon catheter. Cathet Cardiovasc Diagn 1993; 29 2: 99–104. [DOI] [PubMed] [Google Scholar]
- 8. Bahl VK, Chandra S, Wasir HS.. Pulmonary valvuloplasty using Inoue balloon catheter. Int J Cardiol 1994; 45 2: 141–3. [DOI] [PubMed] [Google Scholar]
- 9. Pedersen WR, Mooney MR, Ungs D, Pedersen C, Sorajja P, Poulose AK, . et al. Improvement in aortic valve area using a new “hour glass” shaped valvuloplasty balloon compared with standard cylindrical balloons in severe aortic stenosis patients. Minerva Cardioangiol 2014; 62 3: 243–9. [PubMed] [Google Scholar]
- 10. Liu S, Xu X, Liu G, Ding X, Zhao X, Qin Y.. Comparison of immediate and long-term results between the single balloon and Inoue balloon techniques for percutaneous pulmonary valvuloplasty. Heart Lung Circ 2015; 24 1: 40–5. [DOI] [PubMed] [Google Scholar]
- 11. Sharma R, Rajbhandari R, Limbu Y, Singh S, Bhatt YKD, KC MB.. Balloon pulmonary valvuloplasty in patients with congenital valvular pulmonary stenosis. Nepal Heart J 2012; 9: 1 Available from: https://www.nepjol.info/index.php/NHJ/article/view/8340. [Google Scholar]
- 12. Lin SC, Hwang JJ, Hsu KL, Lee CM, Wang JK, Tseng CD, . et al. Balloon pulmonary valvuloplasty in adults with congenital valvular pulmonary stenosis. Acta Cardiol Sin 2004; 20: 147–53. Available from: http://www.tsoc.org.tw/upload/journal/1/200410/3.pdf. [Google Scholar]
- 13. Stamm C, Anderson RH, Ho SY.. Clinical anatomy of the normal pulmonary root compared with that in isolated pulmonary valvular stenosis. J Am Coll Cardiol 1998; 31 6: 1420–5. [DOI] [PubMed] [Google Scholar]
- 14. Rao PS. Percutaneous balloon pulmonary valvuloplasty: state of the art. Catheter Cardiovasc Interv 2007; 69 5: 747–63. [DOI] [PubMed] [Google Scholar]
- 15. Pedersen WR, Goldenberg IF, Ben-Dor I, Feldman TE.. Aortic and pulmonic balloon valvuloplasty. : Lasala JM, Rogers JH, . Interventional procedures for adult structural heart disease. Philadelphia: Saunders Elsevier; 2014. p 50–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


