Abstract
The fetal basis of adult disease is poorly understood on a molecular level and cannot be solely attributed to genetic mutations or a single etiology. Embryonic exposure to environmental compounds has been shown to promote various disease states or lesions in the first generation (F1). The current study used the endocrine disruptor vinclozolin (antiandrogenic compound) in a transient embryonic exposure at the time of gonadal sex determination in rats. Adult animals from the F1 generation and all subsequent generations examined (F1–F4) developed a number of disease states or tissue abnormalities including prostate disease, kidney disease, immune system abnormalities, testis abnormalities, and tumor development (e.g. breast). In addition, a number of blood abnormalities developed including hypercholesterolemia. The incidence or prevalence of these transgenerational disease states was high and consistent across all generations (F1–F4) and, based on data from a previous study, appears to be due in part to epigenetic alterations in the male germ line. The observations demonstrate that an environmental compound, endocrine disruptor, can induce transgenerational disease states or abnormalities, and this suggests a potential epigenetic etiology and molecular basis of adult onset disease.
The etiology of disease involves genetic, nutritional, and environmental factors. The fetal basis of adult onset disease has been demonstrated (1), but the molecular mechanisms involved are poorly understood. Epigenetic mechanisms involving DNA methylation have been shown to influence several disease states (2, 3), including cardiovascular and intestinal abnormalities (4, 5). Endocrine disruptors are an example of a class of environmental toxicants that interfere with specific endocrine signaling and, after fetal or postnatal exposure, promote disease states in the adult (6, 7). Recently, we have made the observation that a transient embryonic exposure to endocrine disruptors at the time of gonadal sex determination can cause an epigenetic transgenerational disease state of subfertility and spermatogenic defects in F1 through F4 generations (8). Altered DNA methylation was observed in two different genes in F1–F4 generations after endocrine disruptor exposure (8). Several recent observations suggest that abnormal fetal conditions (e.g. caloric restriction) (9) and fetal exposure to therapies (e.g. diethylstilbesterol) (10) can cause abnormalities in the F2 generation. This includes the development of an abnormal reproductive tract (11) and a diabetes-like condition (9, 12). The previous observations (8) led to the hypothesis tested in the current study that a transient embryonic exposure to an endocrine disruptor at the time of gonadal sex determination leads to transgenerational disease states in adults.
The endocrine disruptor used in the current study was vinclozolin, which is a fungicide used in agricultural crops such as grapes grown for the wine industry (13, 14). Vinclozolin is an antiandrogenic compound (13) that is metabolized into more active (i.e. higher affinity binding to the androgen receptor) compounds (13). Embryonic exposure to vinclozolin can influence sexual differentiation, gondal formation, and reproductive functions in the F1 generation (13, 15–18). Vinclozolin also promotes a transgenerational phenotype in the testis that affects male reproduction (8). Although steroid production in the developing fetal gonad is negligible, androstenedione is produced by the fetal adrenal. The androgen and estrogen receptors appear to be expressed in germ cells, Sertoli cells, and precursor peritubular cells in the embryo, such that the fetal gonad may be responsive to endocrine disruptors (19, 20). However, potential toxicology of these environmental compounds also needs to be considered. Vinclozolin has been shown to promote an epigenetic alteration in the germ line that appears to transmit a trans-generational disease state (8). The previous study euthanized the majority of animals at less than 120 d of age to assess tissue abnormalities and the primary lesion identified was a spermatogenic defect and male infertility phenotype (8). In the current study, the progeny of previously treated F0 mothers (8) were analyzed together with progeny from new sets of treated F0 mothers. All progeny (F1–F4) were maintained for 6 –12 months of age. The current study confirms the original observations but also documents a more extensive disease phenotype in the older adult animals. The ability of an environmental factor to promote a variety of different disease states or abnormalities at high frequency for multiple generations suggests a novel mechanism for disease etiology involving epigenetic transmission through the germ line.
Materials and Methods
In vivo procedures
Gestating outbred Sprague Dawley rats from timed pregnant colonies housed at the Washington State University Vivarium were given ip daily morning injections of vinclozolin (100 mg/kg·d) from embryonic d 8–14 (E8–E14) of gestation (F0 generation) as previously described (21). A previous study has demonstrated that 100 vs. 200 mg/kg·d doses induced a similar phenotype, but a 50 mg/kg·d dose was more variable (18), such that 100 mg/kg·d was selected for the current study. The sperm-positive vaginal smear date was designated embryonic d 0. Gestating control mothers received vehicle alone (i.e. dimethylsulfoxide and sesame oil). At least six different lines (individual F0 injected gestating females) were generated for both controls and vinclozolin generation groups for these analyses. The majority of animals from a previous study (8) were used by 120 d of age, and all by 180 d of age. The current study used the progeny from four F0 mother lines from the previous study (8) and two completely new sets of F0 mothers progeny for a total of six F0 control and six F0 vinclozolin-treated mothers. Male and female rats from control and vinclozolin generations were collected at 6–14 months of age for analyses. Some animals were euthanized at earlier ages (e.g. 6–14 months) due to the development of a clinical disease or abnormality requiring euthanasia. F1 vinclozolin generation males at postnatal d 60 (P60) were bred to P60 F1 vinclozolin generation females to generate the F2 vinclozolin generation; F2 vinclozolin males were bred to F2 vinclozolin females to generate the F3 generation; and the F3 generation rats were bred in the same manner to generate the F4 generation. Rats for the control groups were bred in the same manner for all the generations. No inbreeding or sibling crosses were generated. A vinclozolin outcross (VOC) experiment involved breeding an F2 vinclozolin generation male with a wild-type female to generate an F3 generation VOC. Wild-type is defined as the same Sprague Dawley strain but not from the control generation population. A reverse VOC (RVOC) experiment involved breeding an F2 vinclozolin generation female with a wild-type male to generate an F3 generation RVOC. All procedures were approved by the Washington State University Animal Care and Use Committee. The number of male animals used for replicates in the experiments (i.e. n value) for vinclozolin treatment are as follows: F1 (control 6, vinclozolin 9); F2 (control 5, vinclozolin 6); F3 (control 4, vinclozolin 16); and F4 (control 13, vinclozolin 10 and VOC 19). The total number of female animals used was as follows: RVOC, 6; F1–F4 (control, 12; and vinclozolin, 13).
Histology
Tissues were fixed in 10% neutral buffered formalin or Bouin’s (Sigma, St. Louis, MO), embedded in paraffin, sectioned, and then stained with hematoxylin and eosin according to standard procedures. Multiple sections were obtained for each tissue for comparison to allow a representative histology to be selected. The Center for Reproductive Biology, Histology Core Laboratory, and the Washington Animal Disease Diagnostic Laboratory assisted with these procedures.
Pathology
Disease diagnoses were identified by the Washington Animal Disease Diagnostic Laboratory (WADDL) located at Washington State University. All animals submitted to WADDL had a complete necropsy with histopathology and bacteriological analyses. Animal identification and treatment group were blinded to all pathologists for analysis. Data were tabulated for each abnormality based on the percentage of tissue with pathological changes per total tissue per cross-section in two to five tissue cross-sections. Rats developing tumors were submitted as whole animals or excised formalin-fixed tissue for tumor identification. All tissue cross-sections were stained with hematoxylin and eosin for analyses. The testis cross-sections were determined to be abnormal if the number of tubules with atrophy, vacuoles or germ cell agenesis was greater than 20% of the total tubules present in the testis cross section, examining a minimum of 100 tubules. Renal lesions were diagnosed by an increase in morphologically identified tubular damage. The kidney was considered abnormal if more than 30% of the tissue contained tubular lesions. Kidney tubular changes involved extreme dilation with protein-rich fluids, fluid-filled cystic tubules, thickening of the Bowman’s capsule surrounding the glomerulus, as well as reduced glomerular area. Ventral prostate tissue was considered abnormal if more than 30% of the prostatic ducts were atrophic and contained no columnar secretory epithelial cells. Cross-sectional views of the ventral prostate samples were sectioned so that distal, intermediate, and proximal regions were visible. Ventral prostate lesions were not region specific in the samples analyzed as previously described (23). Lateral and dorsal prostatic lobes were analyzed as well, but no gross morphological changes between control and vinclozolin generations were found in the animals analyzed in this study. Immune-related abnormalities were defined as rats having excessive macrophage and lymphocyte invasion into multiple organs and was generally accompanied by bacterial infection. The immune-related abnormalities involved several types of inflammation of the inner ear (otitis), inflammation in the lower limbs, inflammation in the lower respiratory tract (pneumonia), and development of subdermal abscesses, which grew in size and caused septicemia (widespread infection). Immune-related abnormalities were defined as increased macrophage and lymphocyte invasions into multiple tissues and at least one form of inflammation. Occasionally, vinclozolin generation rats did not have widespread inflammation but did have increased macrophage and lymphocyte invasion into a single organ such as the lung, spleen, seminal vesicles, or ventral prostate. These rats were not included as an immune-related abnormality due to variability between animals. Premature aging-related abnormalities were defined as rats developing poor grooming behavior, causing hair to become discolored and coarse, along with reduced mobility and some weight loss. All blood analyses were performed by the Clinical Pathology Laboratory at Washington State University, Veterinary Teaching Hospital with standard procedures previously described. For the blood counts (red and white) data were collected on a Horiba ABX Hematology Analyzer System 910 + CP, with all differentials analysis done manually. The metabolic panel/profile was done on a Cobras MIRA Plus Analyzer (Roche, Indianapolis, IN). The testosterone serum concentrations were determined by the Center for Reproductive Biology Assay Core Laboratory. Body and tissue (i.e. prostate, kidney, spleen, and testis) weights were monitored in age-matched adults.
Statistical analysis
When indicated, the values were expressed as the mean ± sem and data were analyzed using a SAS program (JMP version 3.1.6; SAS Institute Inc., Cary, NC). Statistical analysis was performed and the difference between the means of treatments and respective controls was determined using a Student’s t test. Statistical analysis of the disease prevalence in the total population of control vs. vinclozolin F1–F4 generation animals used a Fisher’s exact test analysis for a 2 × 2 tables using Minitab (Minitab Inc., State College, PA) and was performed by the Statistics Consulting Service of the Department of Statistics at Washington State University. In vivo experiments were repeated with six to 16 individuals for each data point. A statistically significant difference was defined at P < 0.05.
Results
Gestating Sprague Dawley rats at the time of gonadal sex determination, E8–E14, were transiently exposed to vinclozolin (100 mg/kg·d) and compared with a vehicle (dimethylsulfoxide buffer)-treated control animals. The F0 generation gestating mothers were the only animals exposed, although this implies that the F1 generation embryo and germ cells generating the F2 generation are also exposed. F1 generation progeny at 60 d of age were bred to generate an F2 generation and breeding continued out to the F4 generation. No sibling breeding was used to avoid any inbreeding issues. In addition, littermates were used for control and treated F0 mothers to reduce any genetic variation. As previously reported (8, 18), no effects were observed on litter size, pup weights, or gross developmental defects of any of the F1–F4 generation progeny. Both males and females examined between 20 and 120 d of age showed no weight differences or gross abnormalities in any tissue (i.e. prostate, kidney, spleen) examined except the testis (8). In animals older than 6 months of age, no differences were observed in body weights (data not shown), and tissue weights were only different if disease was detected (data not shown). As previously reported, the testis had increased spermatogenic cell apoptosis and subfertility (8). The F1–F4 generation progeny from control and vinclozolin-treated F0 mothers were aged 6–14 months to assess effects on aging adults. In the event, a clinical disease state developed (i.e. tumor or infection), the animal was euthanized and a complete necropsy and blood analysis were performed. All remaining animals were euthanized by 10–14 months of age according to the Washington State University Animal Care and Use Committee guidelines, followed by complete necropsies for both control and vinclozolin generation F1–F4 animals. A number of disease states or tissue abnormalities developed, as discussed below, for all generations.
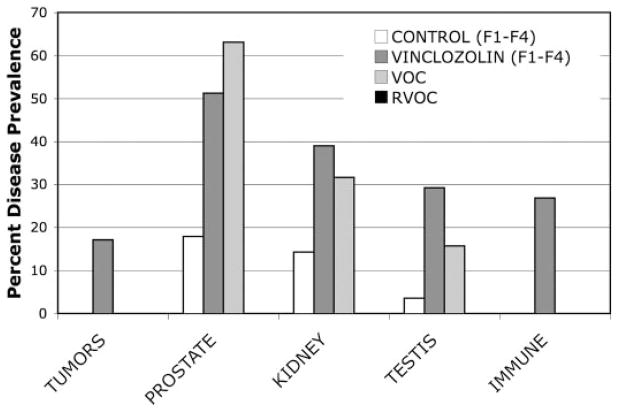
Tumors developed in 12–33% of the animals (Fig. 1), in only the vinclozolin F1–F4 generation animals and not the controls. From the total tumors identified, four were breast adenomas, one was a breast carcinoma, one was a lung sarcoma, and one was a skin (i.e. Merkel cell) melanoma, with the breast adenoma presented (Fig. 2, A and B). Only the lung sarcoma, Merkel cell tumor and breast carcinoma were malignant. The numbers of animals at each generation are shown in Tables 1 and 2. Animals euthanized due to tumor development are indicated in Table 1. The frequency of tumor development in the males was similar among the F1–F4 vinclozolin generation animals (Fig. 1B). Tumors developed in females (i.e. breast) at approximately a 10% frequency for vinclozolin generations (Fig. 1C). No tumors developed in control F1–F4 generation animals.
Fig. 1.

Disease states or abnormalities in vinclozolin and control generation (F1–F4) animals. A, Males from combined (F1–F4) generation animals. B, Males from individual (F1–F4) generation animals. C, Females from combined (F1–F4) generation animals. The percentage disease prevalence is presented for tumors, prostate disease, kidney disease, testis (i.e. spermatogenesis) abnormalities and immune abnormalities. The total number of animals for each generation (F1–F4) is listed in Materials and Methods. The absence of a control bar indicates zero in the control population of animals. All the comparative (A) vinclozolin generation animal disease and abnormality prevalence data provided were statistically different from controls with P < 0.05 using a Fisher’s exact test analysis as described in Materials and Methods.
Fig. 2.
Histology of representative vinclozolin generation breast fibroadenoma. A representative ×400 magnification (A) and ×200 magnification (B) micrograph is presented from a minimum of five different animals with breast adenoma analyzed. A closed arrow indicates a neoplastic glandular epithelial cell population.
TABLE 1.
Individual male animal disease states (F1 and F2)
| F1 generation | Tumor | Prostate | Kidney | Testis | Immune | Pre-aging | Euthanized (months) |
|---|---|---|---|---|---|---|---|
| Control generation | |||||||
| 1 | − | + | + | − | − | − | 14 |
| 2 | − | + | − | − | − | − | 14 |
| 3 | − | − | − | − | − | − | 14 |
| 4 | − | − | − | − | − | − | 14 |
| 5 | − | − | − | − | − | − | 14 |
| 6 | − | − | − | − | − | − | 14 |
| Vinclozolin generation | |||||||
| 1 | − | + | + | + | + | + | 10(I) |
| 2 | + | + | − | − | − | − | 6(T) |
| 3 | − | + | + | + | − | + | 14 |
| 4 | − | + | − | − | − | − | 14 |
| 5 | − | + | − | − | − | − | 14 |
| 6 | − | − | − | − | − | + | 14 |
| 7 | − | − | − | − | − | − | 14 |
| 8 | − | − | − | − | − | − | 14 |
| 9 | − | − | − | − | − | − | 14 |
| F2 generation | |||||||
| 1 | − | − | − | − | − | − | 14 |
| 2 | − | − | − | − | − | − | 14 |
| 3 | − | − | − | − | − | − | 14 |
| 4 | − | − | + | − | − | − | 14 |
| 5 | − | − | − | − | − | − | 14 |
| Vinclozolin generation | |||||||
| 1 | − | − | − | − | − | − | 12 |
| 2 | − | + | + | − | − | + | 12 |
| 3 | − | + | + | + | − | + | 12 |
| 4 | + | − | − | − | − | + | 10(T) |
| 5 | − | + | + | − | + | + | 12(I) |
| 6 | + | − | − | − | + | + | 12(T) |
The absence (−) or presence (+) of a disease or lesion is indicated for individual male animals for F1–F2 control and vinclozolin generation animals. The age in months the specific animal was euthanized is indicated. If the animal was euthanized due to a clinical condition [i.e. tumor (T) or infection (I)] is presented respectively with the age of euthanization. Pathology criteria are described in Materials and Methods.
TABLE 2.
Individual male animal disease states (F3 and F4)
| F3 generation | Tumor | Prostate | Kidney | Testis | Immune | Pre-aging | Euthanized (months) |
|---|---|---|---|---|---|---|---|
| Control generation | |||||||
| 1 | − | + | − | − | − | − | 14 |
| 2 | − | − | − | − | − | − | 14 |
| 3 | − | − | − | − | − | − | 14 |
| 4 | − | − | + | − | − | − | 14 |
| Vinclozolin Generation | |||||||
| 1 | − | + | + | + | − | + | 14 |
| 2 | − | + | + | + | − | − | 14 |
| 3 | − | − | − | − | − | − | 14 |
| 4 | − | − | − | − | − | − | 14 |
| 5 | − | + | − | + | − | + | 14 |
| 6 | − | + | − | − | − | + | 14 |
| 7 | − | − | − | − | − | − | 14 |
| 8 | − | + | − | + | + | − | 14(I) |
| 9 | − | + | + | − | − | − | 14 |
| 10 | − | − | − | − | − | − | 14 |
| 11 | − | − | + | − | + | + | 10(I) |
| 12 | − | + | + | − | + | + | 12(I) |
| 13 | − | + | + | + | + | + | 12(I) |
| 14 | + | − | − | − | − | + | 12(T) |
| 15 | − | − | − | + | + | + | 10(I) |
| 16 | + | − | − | − | − | + | 14(T) |
| F4 generation | |||||||
| 1 | − | − | − | − | − | − | 14 |
| 2 | − | − | − | − | − | − | 14 |
| 3 | − | − | − | − | − | − | 14 |
| 4 | − | − | + | − | − | − | 14 |
| 5 | − | − | − | − | − | − | 14 |
| 6 | − | + | − | − | − | + | 14 |
| 7 | − | + | − | + | − | − | 14 |
| 8 | − | − | − | − | − | − | 14 |
| 9 | − | − | − | − | − | − | 14 |
| 10 | − | − | − | − | − | − | 12 |
| 11 | − | − | − | − | − | − | 12 |
| 12 | − | − | − | − | − | − | 12 |
| 13 | − | − | − | − | − | − | 12 |
| Vinclozolin generation | |||||||
| 1 | − | + | + | + | − | − | 14 |
| 2 | + | + | + | − | − | + | 14(T) |
| 3 | + | − | − | − | + | + | 12(T) |
| 4 | − | − | − | − | + | − | 10(I) |
| 5 | − | + | − | − | − | + | 14 |
| 6 | − | + | + | − | − | − | 14 |
| 7 | − | − | − | − | − | − | 14 |
| 8 | − | + | + | − | − | + | 14 |
| 9 | − | − | − | + | − | + | 14 |
| 10 | − | − | + | + | + | − | 14(I) |
The absence (−) or presence (+) of a disease or lesion is indicated for individual male animals for F3–F4 control and vinclozolin generation animals. The age in months the specific anima was euthanized is indicated. If the animal was euthanized due to a clinical condition [i.e. tumor (T) or infection (I)] is presented respectively with the age of euthanization. Pathology criteria are described in Materials and Methods.
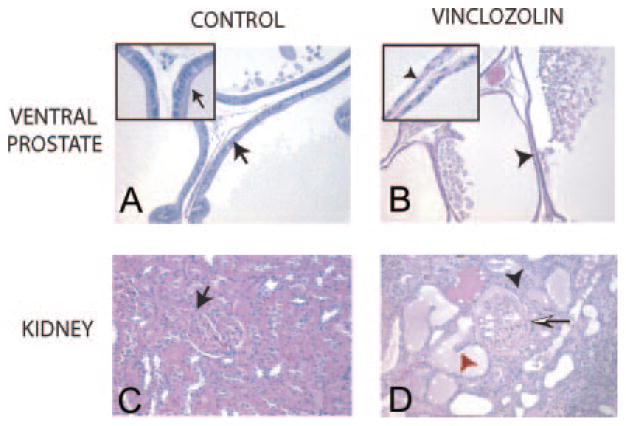
Prostatic lesions were detected in 45–55% of F1–F4 vinclozolin generation males (Fig. 1). A regression of prostatic secretory epithelium involving atrophic glands or ducts were observed (51%), along with prostatitis in selected animals (10%) (Fig. 3, A and B). Abnormal prostate histology was observed in the distal, intermediate, and proximal regions of the ventral prostate, but no morphological effects were observed in the lateral or dorsal prostatic lobes (data not shown). The range of ventral prostate pathology was from atrophic defects to cystic hyperplasia and focal prostatitis, with cystic changes as the most severe pathology. A similar percentage of affected animals in all the F1–F4 vinclozolin generations were observed with prostate abnormalities (Fig. 1B). The numbers of animals at each generation are shown in Tables 1 and 2. Ventral prostatic hyperplasia was observed in three rats from vinclozolin generations and one control animal. Serum testosterone concentrations in the male F1–F4 vinclozolin generation progeny, 1.20 ± 0.71 ng/ml, were similar to controls, 1.57 ± 0.86 ng/ml, such that the prostatic abnormalities cannot be attributed to low serum testosterone.
Fig. 3.
Histology of representative control (A and C) and vincozolin (B and D) F2 or F3 generation tissues are presented, ventral prostate (A and B), and kidney (C and D). A representative ×200 magnification micrograph is presented from a minimum of five animals analyzed. Insets in A and B are ×1000 magnification. A closed arrow indicates a normal epithelial cell and a red arrowhead an abnormal epithelial cell, and a closed arrowhead an abnormal tubule and a half-arrow an increased width of Bowman’s capsule.
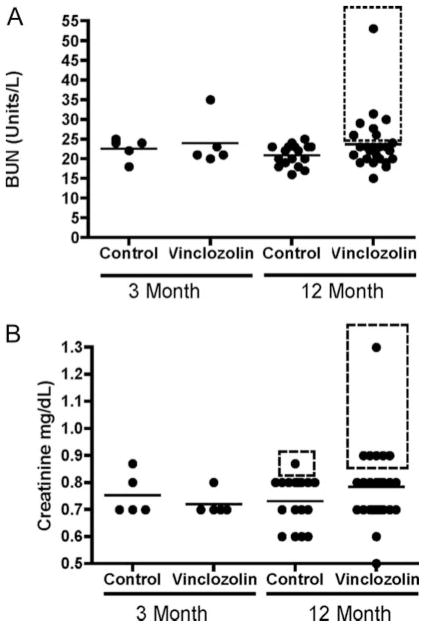
Renal lesions appeared in 20–50% of males of all F1–F4 vinclozolin generations (Fig. 1B). These kidney abnormalities also were observed in female F1–F4 vinclozolin generation animals (Fig. 1C). Tubular nephropathy with protein casts, degenerated ductal epithelium, and sclerotic glomeruli were the principal histologic changes observed in 39% of the vinclozolin generation animals with 20% being severe (Fig. 3, C and D). The numbers of animals at each generation are shown in Tables 1 and 2. Blood analysis demonstrated an increased blood-urea nitrogen (BUN) and creatinine in several animals with renal lesions (Fig. 4). These increased blood markers for renal lesions correlated to the animals with kidney abnormalities as shown in the boxed values in Fig. 4.
Fig. 4.
Blood analysis for BUN (A) and creatinine (B) from control and vinclozolin (F1–F4) generation animals. Scatter plots are presented with a mean line indicated. Samples in the dashed boxes correlate with animals with diagnosed morphological kidney abnormalities and corresponding renal blood marker increases.
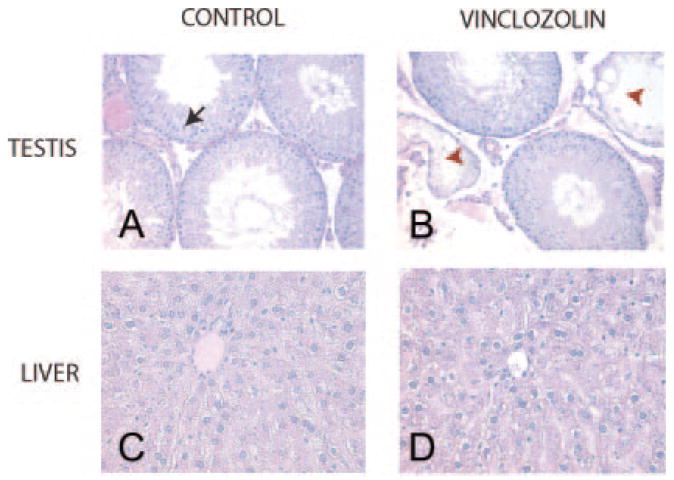
As previously reported (8), abnormal testis function and abnormalities were observed in 15–38% of the F1–F4 generation animals (Fig. 1B). The numbers of animals affected at each generation are shown in Tables 1 and 2. The pathology includes an increased spermatogenic cell apoptosis, gross morphological defects in spermatogenesis, and complete lack of spermatogenesis (8). The testis abnormalities were compiled from animals with histologic defects in spermatogenesis in greater than 20% of the seminiferous tubules and those with a complete lack of spermatogenesis (Fig. 5).
Fig. 5.
Histology of representative control (A, C) and vincozolin (B and D) F2 or F3 generation tissues are presented for normal testis (A), abnormal testis (B), and liver (C and D). A representative ×200 (A and B) or ×400 (C and D) magnification micrograph is presented from a minimum of five animals analyzed. A closed arrow indicates a normal epithelial cell and tubule, where as a redarrow head an abnormal epithelial cell and tubule. No histologic abnormalities were observed in the liver.
Liver histology was normal in vinclozolin generation animals (Fig. 5), and serum concentrations of liver markers, alanine transferase, and alkaline phosphatase, were similar to the control (Fig. 6). Observations suggest no major hepatic defects. In addition, no significant lesions were seen in other tissues including the adrenal glands, epididymis, seminal vesicles, lungs, heart, spleen, ovary, and brain of F1–F4 control or vinclozolin generation animals (data not shown).
Fig. 6.
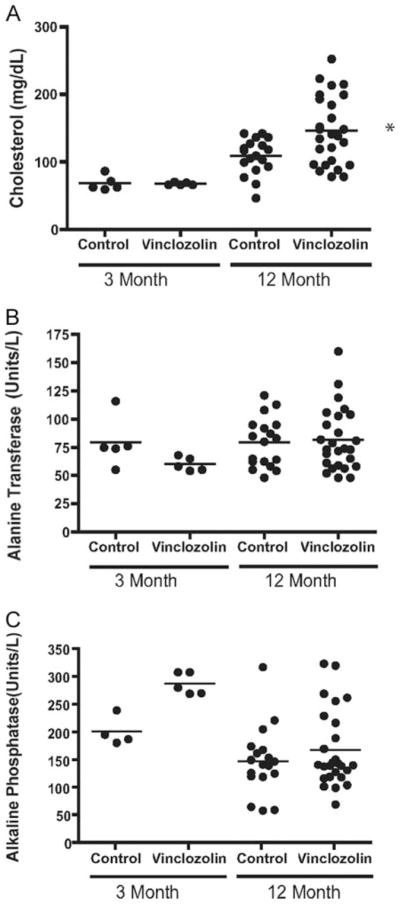
Blood analysis for combined F1–F4 control and vinclozolin generation animals. A, Cholesterol concentrations and liver serum markers of (B) alanine transferase and (C) alkaline phosphatase are presented (units/liter). Scatter plots are indicated for 3-month-old and 6- to 12-month-old animals with the bar representing the mean. *, Mean values are statistically different (P < 0.05) from control values for cholesterol. No statistical differences were detected in B or C means.
Complete blood cell counts (i.e. white blood cell and red blood cell counts) were not different between vinclozolin and control generation animals (data not shown). Inflammation including inner ear (otitis), subdermal abscesses and bacterial infection (e.g. respiratory infection) were observed in 12–33% of F1–F4 vinclozolin generation animals, but no inflammation was present in control animals (Fig. 1B). The numbers of animals affected at each generation are shown in Tables 1 and 2. Animals euthanized due to infection are indicated in Tables 1 and 2. Although immune abnormalities are observed, the immune status of vinclozolin generation animals requires further characterization on a molecular level.
Several blood analyses (e.g. glucose, serum protein, sodium, potassium) were not different between F1–F4 control and vinclozolin generation animals (Table 3). The total samples analyzed were combined and had a composition of 5% F1, 26% F2, 59% F3, and 10% F4 from each generation and showed no difference. Although serum chloride and magnesium concentrations were statistically different between aged control and vinclozolin generations, the slight changes are likely not physiologically relevant. No major metabolic or physiological effects were observed from the blood analysis. Interestingly, a significant increase in serum cholesterol was observed in approximately 35% of 6- to 14-month-old F1–F4 vinclozolin generation animals, compared with controls (Fig. 6). None of these changes were observed at 3 months of age and only developed in older animals. Basal serum cholesterol in the control animals did rise between 3 and 12 months but increased more dramatically in vinclozolin generation animals (Fig. 6). Fasting state of the animals did not alter the cholesterol differences between control and vinclozolin generation animals (data not shown). Serum cholesterol levels were also elevated in the aged female vinclozolin generation animals compared with control female animals (data not shown).
TABLE 3.
Blood chemistry and analysis
| 3 Months
|
12 Months
|
|||
|---|---|---|---|---|
| Control (n = 5) | Vinclozolin (n = 5) | Control (n = 12) | Vinclozolin (n = 19) | |
| Glucose | 157 ± 9 | 192 ± 25 | 124 ± 4 | 131 ± 9 |
| Total protein | 6.5 ± 0.2 | 7.0 ± 0.2 | 6.8 ± 0.2 | 6.9 ± 0.1 |
| Abumin | 3.6 ± 0.1 | 3.7 ± 0.1 | 3.3 ± 0.1 | 3.2 ± 0.1 |
| Globulin | 2.9 ± 0.1 | 3.2 ± 0.1 | 3.5 ± 0.1 | 3.7 ± 0.1 |
| Calcium | 10.2 ± 0.1 | 10.6 ± 0.2 | 10.5 ± 0.3 | 10.8 ± 0.2 |
| Phosphorous | 6.0 ± 0.6 | 8.9 ± 0.5 | 7.2 ± 0.4 | 7.5 ± 0.3 |
| Magnesium | 2.9 ± 0.1 | 3.2 ± 0.1 | 2.80 ± 0.09 | 3.00 ± 0.08a |
| Sodium | 152 ± 1 | 153 ± 2 | 153 ± 0.1 | 152 ± 0.8 |
| Potassium | 7.7 ± 0.5 | 7.5 ± 0.4 | 7.0 ± 0.2 | 7.4 ± 0.2 |
| Chloride | 105 ± 1 | 103 ± 1 | 107.0 ± 0.8 | 104.0 ± 0.6a |
Statistical difference with P < 0.05 between control and vinclozolin generation animals. The number of F1–F4 generation animal combined for the analysis (n value) is indicated.
Animals from F1–F4 vinclozolin generations also developed an apparent premature aging phenotype. This was characterized as decreased grooming behavior resulting in color distortion, decreased mobility and movement, increased skin abnormalities, and periodic weight loss. These same characteristics are observed in control animals greater than 18 months old (23), but none of the 6- to 14-month-old control animals had any of these characteristics (data not shown). In contrast, 50% of the F1–F4 vinclozolin generation animals developed this premature aging phenotype. Although scoring the presence or absence was done, these premature aging characteristics were difficult to measure in degrees or quantitate. These conditions also are present in diseased animals (Tables 1 and 2). Therefore, the premature aging phenotype could not exclude the possibility that it was simply associated with diseased animals. Therefore, the premature aging phenotype is only presented as a subjective measure requiring further investigation. Because neoplasms, renal lesions, and prostate lesions have been observed in aged (24 months) rats (23, 24), one interpretation of the data is that the endocrine disruptor induced a potential trans-generational premature aging phenomena.
The data presented demonstrate that in utero exposure to an environmental compound, the endocrine disruptor vinclozolin, has the ability to induce multiple transgenerational disease states. The prevalence of the disease states or abnormalities described are consistent among F1–F4 generations with no apparent decline (Fig. 1). However, the F1 generation disease prevalence was often less than subsequent generations. Most other organs examined had no gross abnormalities or lesions such that the effects observed appear to be specific to the prostate, kidney, breast, testis, and skin. Analysis of individual animals and specific disease states demonstrated that many animals had multiple abnormalities and 85% of all F1–F4 vinclozolin generation animals developed a transgenerational disease state (Tables 1 and 2). The females did have a high prevalence of tumors and kidney disease in the F1–F4 vincozolin generations (Fig. 1). Vinclozolin generation females also had increased serum cholesterol concentrations and when renal defects were present, increased BUN (data not shown).
The previous study (8) demonstrated that the transgenerational disease state (e.g. testis abnormality) was transmitted only through the male germ line. In the current study, a vinclozolin outcross (VOC) experiment was performed with an F2 vinclozolin generation male bred to a wild-type female, along with a reverse vinclozolin outcross (RVOC) experiment involving an F2 vinclozolin female bred to a wild-type male. The RVOC demonstrated no significant increase in disease frequency over control, Fig. 7. As a comparison the F1–F4 vinclozolin and control generation-treated cross is presented. The VOC animals had an increase in disease prevalence over control in ventral prostate, testis, and kidney disease, but at a reduced incidence to the treated F3 generation cross (Fig. 7). No tumors or immune abnormalities were detected in the VOC animals. Therefore, the transgenerational disease phenotype was primarily transmitted through the male germ line, but the increased prevalence of disease when the vinclozolin generation female was used in a treated cross suggests a potential contribution of the female germ line. The influence of the female germ line now needs to be investigated more thoroughly.
Fig. 7.
Comparison of outcross disease states or abnormalities using F1–F4 control and vinclozolin generation-treated cross compared with VOC (F2 vinclozolin generation male and wild-type female) and a RVOC (F2 vinclozolin generation female and wild-type male). The percentage disease prevalence is presented for tumors, prostate disease, kidney disease, testis abnormalities, and immune abnormalities. The absence of a bar indicates zero in the population of animals. The total number of animals for VOC and RVOC were n = 19 and 6, respectively. The VOC was statistically different from control with P < 0.05.
Discussion
The frequencies of the disease states or abnormalities observed in the current study are consistent for four generations. Although neoplasms, renal lesions, and prostate lesions are observed in aged (24 months) rats (23, 24), none of these pathologies were observed in the 6- to 14-month-old control rats. As a comparison, the frequencies observed are similar to that seen in the human population. Prostatic lesions occur in 50% of men over the age of 50 yr, compared with the 51% observed in the current study. The progression of human prostatic disease has been suggested to involve an initial atrophy of epithelium and glands followed by prostititis, as observed in the current study (25). Renal lesions occur at frequencies in specific human subpopulations (26, 27), similar to the 30% observed in the current study. The abnormal kidney morphology observed corresponded to changes in serum BUN and creatinine levels, as is seen in the human population. Testis abnormalities occur in approximately 10–15% of the human male population (28) compared with the 30% prevalence observed in the current study. The morphological changes and spermatogenic cell defects are similar to the reported human defects (28). The tumor rates for breast cancer are approximately 15% in the human population, but less than 1% in males (27, 29, 30). In contrast, the male rats in the F1–F4 vinclozolin generations had approximately a 10% frequency. As with human tumors, rat tumors observed were primarily of epithelial cell origin with a low frequency of metastasis. Overall, several similarities in frequency and etiology were made with the abnormalities observed in the current study with those found in humans. Future studies are now required to allow a comparison of the rat observations to human disease. This transgenerational phenotype provides a useful experimental animal model to help elucidate the diseases of a variety of tissues with potential application to human disease.
The ability of the endocrine disruptor vinclozolin to induce an epigenetic transgenerational disease state or abnormality suggests fetal exposure to environmental toxicants may be a significant factor in the molecular basis of disease. Previously, both the antiandrogenic compound vinclozolin and the estrogenic compound methoxychlor were found to induce a trans-generational phenotype (8). The concentration of vinclozolin used in the current study is higher than anticipated in the environment. For vinclozolin, the lowest observed adverse effect level recommended is 11 mg/kg·d, but doses at the 1 mg/kg·d have biological effects (31). The environmental levels of vinclozolin have not been rigorously determined, such that no conclusions regarding the toxicology of this compound can currently be made. Toxicology studies to determine whether environmental levels of the compound can induce these disease states are now needed. The mechanism of vinclozolin actions could involve androgen receptor-mediated events and/or toxicity. The androgen receptor has been shown to be present in the embryonic testis at the time of gonadal sex determination in the germ cells, Sertoli cells, and precursor peritubular cells (19, 20). Although the embryonic gonadal steroid production is minimal at this time, androstenedione is produced by the fetal adrenal. Therefore, endocrine disruptors have the capacity to influence embryonic androgen receptor actions (32, 33). Alternatively, vinclozolin actions could involve toxicologic actions on the developing gonad to subsequently influence germ cell development (17, 34, 35). Further studies are needed to elucidate the endocrine vs. toxicologic actions of vinclozolin on the embryonic testis.
Previous studies have shown that an embryonic exposure during gonadal sex determination (E8–E14) can induce onset of disease in the F1 generation, but later embryonic exposure (E15–E20) had no effect (18, 21). The actual sex determination event for the testis occurs from E10–E13.5, with cord formation complete at E14 and initial transcriptional events likely at E8–E10, such that E8–E14 covers the entire period. The primordial germ cells undergo an erasure (i.e. demethylation) of DNA methylation during migration down the genital ridge before colonizing the gonad (36, 37). During sex determination, the germ cells undergo a remethylation in a sex-specific manner (38). Endocrine disruptor exposure during this period appears to cause an epigenetic reprogramming of the germ line that is permanent and is transferred transgenerationally to subsequent generations (8). The male germ line is critical in the transmission of the transgenerational disease phenotype; however, the female germ line appears to influence the phenotype and remains to be investigated. The current study describes the ability of a variety of disease states to be induced through this apparent epigenetic transgenerational effect on the germ line. Correlation of specific changes in DNA methylation of imprinted-like genes (39) with specific tissue abnormalities will be important to elucidate in the future and may identify valuable diagnostic and therapeutic markers.
A previous report demonstrated that transient embryonic exposure to vinclozolin at the time of gonadal sex determination induces an apparent epigenetic effect on the programming of the male germ line (8). An epigenetic transgenerational phenotype is likely responsible for the disease states or abnormalities observed in the current study. The frequency of the abnormal phenotypes observed ranges from 12–50%, as shown in Fig. 1. The frequency of a hot spot DNA sequence mutational event has been shown to be approximately 5% at its highest and generally is less than 1% (22, 40). A genetic DNA sequence mutation also involves segregation with reduced frequency in subsequent generations. Therefore, the high frequency of the disease states and absence of normal Mendelian transmission observed in the current study suggests the transgenerational nature of the phenotype appears to be epigenetic through the germ line. The previous study demonstrated the presence of two genes with altered methylation in the germ line (8), and preliminary studies have revealed the presence of over 15 new imprinted-like genes/DNA sequences with alterations in methylation involving reprogramming of the male germ line (39). Therefore, the molecular basis for the transgenerational disease states observed appears to be epigenetic and due in part to a permanent reprogramming of the germ line.
The potential that an epigenetic (i.e. DNA methylation) trans-generational background may influence disease susceptibility, premature disease onset, and/or development of disease, is a factor in disease etiology not previously appreciated (1). This transgenerational phenomenon could explain how some sub-populations may have differences in the frequency of disease. Due to the ability of an environmental factor to alter this epigenetic transgenerational background, variability in environmental exposures could explain alterations in disease prevalence in different populations and regions. The most sensitive exposure period is at the time of gonadal sex determination, which is early to midgestation in humans. Therefore, the fetal basis of disease will likely in part involve the epigenetic trans-generational mechanism described. The specific genes that have altered methylation states and are transmitted through the germ line (39) remain to be fully elucidated. These genes may provide diagnostic and/or therapeutic markers to better understand specific diseases. Risk assessment for environmental toxicant exposure could consider the use of these genes as biomarkers. The current study describes the phenomena that an environmental compound, the endocrine disruptor vinclozolin, can induce epigenetic transgenerational diseases. This is a novel molecular mechanism to consider for disease etiology.
Acknowledgments
We acknowledge the technical contributions of Dr. Mushtaq Memon, Dr. Eric Nilsson, Dr. Ingrid Sadler-Riggleman, Mr. Shane Rekow, and Ms. Bethanni Johnston. We also acknowledge the assistance of Ms. Jill Griffin in preparation of this manuscript. The gross and microscopic tissue evaluations were performed at the Washington Animal Disease Diagnostic Laboratory and we acknowledge the assistance of Drs. Sushan Han, Seth Paul Harris, Tanya LeRoith, and Patrick H. Caplazi.
This research was supported in part by a grant from the National Institute of Environmental Health Sciences, National Institutes of Health (to M.K.S.).
Abbreviations
- BUN
Blood-urea nitrogen
- E
embryonic day
- RVOC
reverse VOC
- VOC
vinclozolin outcross
Footnotes
Disclosure Statement: The authors have nothing to disclose. The authors declare that they have no competing financial interests.
References
- 1.Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004;56:311–317. doi: 10.1203/01.PDR.0000135998.08025.FB. [DOI] [PubMed] [Google Scholar]
- 2.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 3.Bjornsson HT, Fallin MD, Feinberg AP. An integrated epigenetic and genetic approach to common human disease. Trends Genet. 2004;20:350–358. doi: 10.1016/j.tig.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Corwin EJ. The concept of epigenetics and its role in the development of cardiovascular disease: commentary on “new and emerging theories of cardiovascular disease. Biol Res Nurs. 2004;6:11–16. doi: 10.1177/1099800404264779. discussion 21–23. [DOI] [PubMed] [Google Scholar]
- 5.Sakatani T, Kaneda A, Iacobuzio-Donahue CA, Carter MG, de Boom Witzel S, Okano H, Ko MS, Ohlsson R, Longo DL, Feinberg AP. Loss of imprinting of Igf2 alters intestinal maturation and tumorigenesis in mice. Science. 2005;307:1976–1978. doi: 10.1126/science.1108080. [DOI] [PubMed] [Google Scholar]
- 6.Heindel JJ. The fetal basis of adult disease: Role of environmental exposures—introduction. Birth Defects Res A Clin Mol Teratol. 2005;73:131–132. doi: 10.1002/bdra.20119. [DOI] [PubMed] [Google Scholar]
- 7.Foran CM, Peterson BN, Benson WH. Transgenerational and developmental exposure of Japanese medaka (Oryzias latipes) to ethinylestradiol results in endocrine and reproductive differences in the response to ethinylestradiol as adults. Toxicol Sci. 2002;68:389–402. doi: 10.1093/toxsci/68.2.389. [DOI] [PubMed] [Google Scholar]
- 8.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors on male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz P. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005;566:225–236. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blatt J, Van Le L, Weiner T, Sailer S. Ovarian carcinoma in an adolescent with transgenerational exposure to diethylstilbestrol. J Pediatr Hematol Oncol. 2003;25:635–636. doi: 10.1097/00043426-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Increased tumors but uncompromised fertility in the female descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 1998;19:1655–1663. doi: 10.1093/carcin/19.9.1655. [DOI] [PubMed] [Google Scholar]
- 12.Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- 13.Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE., Jr Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol. 1994;126:276–285. doi: 10.1006/taap.1994.1117. [DOI] [PubMed] [Google Scholar]
- 14.Fisher JS. Environmental antiandrogens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- 15.Gray LE, Jr, Ostby J, Monosson E, Kelce WR. Environmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol Ind Health. 1999;15:48–64. doi: 10.1177/074823379901500106. [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss AK, Ostby JS, Vandenburgh JG, Gray LE., Jr Androgens and environmental antiandrogens affect reproductive development and play behavior in the Sprague-Dawley rat. Environ Health Perspect. 2002;110(Suppl 3):435–439. doi: 10.1289/ehp.02110s3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf CJ, LeBlanc GA, Ostby JS, Gray LE., Jr Characterization of the period of sensitivity of fetal male sexual development to vinclozolin. Toxicol Sci. 2000;55:152–161. doi: 10.1093/toxsci/55.1.152. [DOI] [PubMed] [Google Scholar]
- 18.Uzumcu M, Suzuki H, Skinner MK. Effect of the antiandrogenic endocrine disruptor vinclozolin on embryonic testis cord formation and post-natal testis development and function. Reprod Toxicol. 2004;18:765–774. doi: 10.1016/j.reprotox.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Majdic G, Millar MR, Saunders PT. Immunolocalisation of androgen receptor to interstitial cells in fetal rat testes and to mesenchymal and epithelial cells of associated ducts. J Endocrinol. 1995;147:285–293. doi: 10.1677/joe.0.1470285. [DOI] [PubMed] [Google Scholar]
- 20.Goyal HO, Hutto V, Robinson DD. Reexamination of the morphology of the extratesticular rete and ductuli efferentes in the goat. Anat Rec. 1992;233:53–60. doi: 10.1002/ar.1092330108. [DOI] [PubMed] [Google Scholar]
- 21.Cupp AS, Uzumcu M, Suzuki H, Dirks K, Phillips B, Skinner MK. Effect of transient embryonic in vivo exposure to the endocrine disruptor methoxychlor on embryonic and postnatal testis development. J Androl. 2003;24:736–745. doi: 10.1002/j.1939-4640.2003.tb02736.x. [DOI] [PubMed] [Google Scholar]
- 22.Dong H, Bonala RR, Suzuki N, Johnson F, Grollman AP, Shibutani S. Mutagenic potential of benzo[a]pyrene-derived DNA adducts positioned in codon 273 of the human P53 gene. Biochemistry. 2004;43:15922–15928. doi: 10.1021/bi0482194. [DOI] [PubMed] [Google Scholar]
- 23.Bauck L, Bihun C. Basic anatomy, physiology, husbandry, and clinical techniques (of small rodents) In: Quesenberry K, Hillyer E, editors. Ferrets, rabbits, and rodents: clinical medicine and surgery. Philadelphia: W. B. Saunders; 1997. p. 297. [Google Scholar]
- 24.Percy DH, Barthold SW, editors. Pathology of laboratory rodents, rabbits. 2. Ames, IA: Iowa State University Press; 2001. [Google Scholar]
- 25.Ku JH, Kim SW, Paick JS. Epidemiologic risk factors for chronic prostatitis. Int J Androl. 2005;28:317–327. doi: 10.1111/j.1365-2605.2005.00560.x. [DOI] [PubMed] [Google Scholar]
- 26.Gill N, Nally JV, Jr, Fatica RA. Renal failure secondary to acute tubular necrosis: epidemiology, diagnosis, and management. Chest. 2005;128:2847–2863. doi: 10.1378/chest.128.4.2847. [DOI] [PubMed] [Google Scholar]
- 27.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman JM, Vermeulen A. Declining gonadal function in elderly men. Baillieres Clin Endocrinol Metab. 1997;11:289–309. doi: 10.1016/s0950-351x(97)80302-3. [DOI] [PubMed] [Google Scholar]
- 29.Boyle P. Breast cancer control: signs of progress, but more work required. Breast. 2005;14:429–438. doi: 10.1016/j.breast.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Weiss JR, Moysich KB, Swede H. Epidemiology of male breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:20–26. [PubMed] [Google Scholar]
- 31.Colbert NK, Pelletier NC, Cote JM, Concannon JB, Jurdak NA, Minott SB, Markowski VP. Perinatal exposure to low levels of the environmental antiandrogen vinclozolin alters sex-differentiated social play and sexual behaviors in the rat. Environ Health Perspect. 2005;113:700–707. doi: 10.1289/ehp.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sajjad Y, Quenby SM, Nickson P, Lewis-Jones DI, Vince G. Expression of androgen receptors in upper human fetal reproductive tract. Hum Reprod. 2004;19:1659–1665. doi: 10.1093/humrep/deh295. [DOI] [PubMed] [Google Scholar]
- 33.Turner KJ, McIntyre BS, Phillips SL, Barlow NJ, Bowman CJ, Foster PM. Altered gene expression during rat Wolffian duct development in response to in utero exposure to the antiandrogen linuron. Toxicol Sci. 2003;74:114–128. doi: 10.1093/toxsci/kfg096. [DOI] [PubMed] [Google Scholar]
- 34.Euling SY, Kimmel CA. Developmental stage sensitivity and mode of action information for androgen agonists and antagonists. Sci Total Environ. 2001;274:103–113. doi: 10.1016/s0048-9697(01)00736-7. [DOI] [PubMed] [Google Scholar]
- 35.Hellwig J, van Ravenzwaay B, Mayer M, Gembardt C. Pre- and postnatal oral toxicity of vinclozolin in Wistar and Long-Evans rats. Regul Toxicol Pharmacol. 2000;32:42–50. doi: 10.1006/rtph.2000.1400. [DOI] [PubMed] [Google Scholar]
- 36.Hajkova P, Erhardt S, Lane N, Haaf T, El-Maarri O, Reik W, Walter J, Surani MA. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117:15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 37.Durcova-Hills G, Ainscough J, McLaren A. Pluripotential stem cells derived from migrating primordial germ cells. Differentiation. 2001;68:220–226. doi: 10.1046/j.1432-0436.2001.680409.x. [DOI] [PubMed] [Google Scholar]
- 38.Reik W, Walter J. Genomic imprinting: parental influence on the genome. Nat Rev Genet. 2001;2:21–32. doi: 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- 39.Chang H-S, Anway MD, Rekow SS, Skinner MK. Transgenerational epigenetic imprinting of the male germline by endocrine disruptor exposure during gonadal sex determination. Endocrinology. 2006;147:5524–5541. doi: 10.1210/en.2006-0987. [DOI] [PubMed] [Google Scholar]
- 40.Barber R, Plumb MA, Boulton E, Roux I, Dubrova YE. Elevated mutation rates in the germ line of first- and second-generation offspring of irradiated male mice. Proc Natl Acad Sci USA. 2002;99:6877–6882. doi: 10.1073/pnas.102015399. [DOI] [PMC free article] [PubMed] [Google Scholar]