Abstract
Heme oxygenase-1 (HO-1) catalyzes the first and rate-limiting step in the metabolism of free heme into equimolar amounts of ferrous iron, carbon monoxide (CO), and biliverdin. Biliverdin is subsequently converted to bilirubin by biliverdin reductase. HO-1 has recently been identified as a promising therapeutic target in the treatment of vascular inflammatory disease, including atherosclerosis. HO-1 represses inflammation by removing the pro-inflammatory molecule heme and by generating CO and the bile pigments, biliverdin and bilirubin. These HO-1 reaction products are capable of blocking innate and adaptive immune responses by modifying the activation, differentiation, maturation, and/or polarization of numerous immune cells, including endothelial cells, monocytes/macrophages, dendritic cells, T lymphocytes, mast cells, and platelets. These cellular actions by CO and bile pigments result in diminished leukocyte recruitment and infiltration, and pro-inflammatory mediator production within atherosclerotic lesions. This review highlights the mechanisms by which HO-1 suppresses vascular inflammation in atherosclerosis, and explores possible therapeutic modalities by which HO-1 and its reaction products can be employed to ameliorate vascular inflammatory disease.
2. Introduction
Atherosclerosis and its cardiovascular complications are the major cause of morbidity and mortality in the developed world, accounting for approximately 50% of all deaths (1). Atherosclerosis involves the formation of arterial lesions known as plaque that are characterized by lipid accumulation, inflammation, cell activation and death, and fibrosis. Over time these lesions grow and mature and can cause blood flow-limiting stenoses. However, more serious clinical conditions arise following the rupture of a plaque, which exposes the pro-thrombotic material within the plaque to the blood resulting in abrupt thrombotic occlusion of the artery at the site of eruption. This can precipitate acute clinical events such as myocardial ischemia and stroke. While reductions in risk factors and improvement in the treatment of atherosclerosis have decreased the number of age-adjusted cardiovascular deaths, the emerging epidemic of obesity and insulin-resistance threatens to reverse the recent gains in life expectancy, emphasizing the need for the development of new therapeutic modalities that target this disease (2,3).
Although long considered a simple lipid storage disorder, atherosclerosis is now viewed as a chronic inflammatory process characterized by the activation of both the innate and adaptive arms of the immune system. Studies in the past decade have implicated endothelial cells, monocytes/macrophages, dendritic cells, T lymphocytes (T cells), mast cells, and platelets along with a host of inflammatory mediators and pathways in the initiation, propagation, and eventual rupture of atherosclerotic plaques (see 4–6). The understanding of atherosclerosis as an inflammatory process has begun to influence clinical diagnosis and prognosis, and raises the potential for novel therapies that target the underlying inflammation. In this respect, recent studies have identified heme oxygenase-1 (HO-1) as a promising therapeutic target in atherosclerosis that possesses potent anti-inflammatory properties. This article will review potential mechanisms by which HO-1 protects against atherosclerosis, focusing on cellular and molecular mechanisms that contribute to the anti-inflammatory actions of this enzyme. In addition, it will highlight potential therapeutic strategies targeting HO-1 or its reaction products in the treatment and prevention of vascular inflammatory disease.
3. Anti-Inflammatory Actions of HO-1
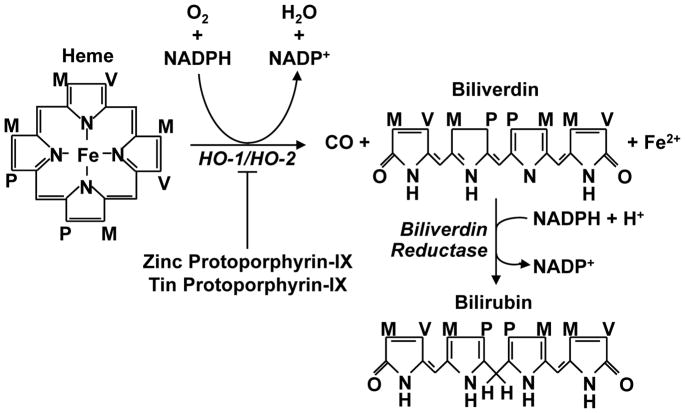
Heme oxygenase (HO) catalyzes the first and rate-limiting step in heme metabolism. HO oxidatively degrades free heme to generate equimolar amounts of carbon monoxide (CO), ferrous iron, and biliverdin (Figure 1). This reaction consumes molecular oxygen and nicotinamide adenine dinucleotide phosphate (NADPH), and requires the concerted action of cytochrome p450 reductase (7). Subsequently, biliverdin is rapidly metabolized to bilirubin by biliverdin reductase while free iron is promptly sequestered by ferritin and recycled for heme synthesis. Two functional HO isoforms, HO-1 and HO-2, are expressed in mammals. These isozymes are products of distinct genes and differ markedly in their distribution and molecular properties (8–11). While HO-2 is constitutively expressed and is present in high concentration in specific organs, such as the brain and testes, HO-1 is ubiquitously distributed and highly inducible. However, both isoforms are inhibited by specific metalloporphrins, including tin and zinc protoporphyrin-IX. These molecules resemble heme in their porphyrin structure and reversibly compete with heme for binding to HO (8).
Figure 1.
The heme oxygenase (HO)-mediated metabolism of heme. HO catalyzes the degradation of heme into equimolar amounts of ferrous heme (Fe2+), carbon monoxide (CO), and biliverdin. This oxidative reaction is blocked by various metalloporphyrins, including tin and zinc protoporphyrin-IX. Biliverdin is subsequently metabolized to bilirubin by biliverdin reductase. M, P, and V represent methyl, propionyl, and vinyl groups, respectively; NADPH, nicotinamide adenine dinucleotide phosphate.
Accumulating evidence indicates that HO-1 plays an important role in protecting tissues from immune-mediated injury. HO-1 is induced by a wide variety of inflammatory mediators, including its own substrate heme, oxidative and nitrosative stress, and cytokines (8–11). In addition, increased HO-1 expression has been demonstrated in several inflammatory states such as atherosclerosis, diabetes, sepsis, ischemia-reperfusion injury, organ failure, and organ transplantation (see 12). In these settings, the induction of HO-1 functions in an adaptive manner to limit the inflammatory process. In a model of pleural inflammation, Willis et al (13) initially reported that pharmacological induction of HO-1 results in a marked decrease in inflammatory cell infiltration and exudates whereas inhibition of HO-1 by tin protoporphyrin-IX enhanced inflammatory exudates, illustrating that HO-1 modulates the inflammatory response. Consistent with these early findings, HO-1 has been shown to retard the inflammatory response in numerous tissues (14–19). The induction of HO-1 also plays a critical role in immune processes associated with transplant rejection where HO-1 positively correlates with transplant survival. Soares et al (20) first demonstrated that cardiac transplants from mice lacking HO-1 into rats were promptly rejected whereas hearts from donors expressing HO-1 survived for up to 60 days. Subsequently the graft-protective properties of HO-1 have been extended to other tissues, including the liver, kidney, thyroid, aorta, and pancreatic islets (21–23).
A beneficial role for HO-1 in inflammation is also supported by findings in HO-1-deficient mice. These animals exhibit chronic inflammation characterized by enlarged lymph nodes, increased blood leukocyte count and serum IgM levels, and accumulation of polymorphonuclear leukocytes and monocytes/macrophages in the spleen as well as non-lymphoid tissues (24–26). In addition, splenocytes obtained from HO-1-deficient animals secrete disproportionately high levels of pro-inflammatory cytokines on stimulation (26). Moreover, mice lacking HO-1 demonstrate vasculitis characterized by the adherence of monocytes to the vessel wall. Significantly, the only diagnosed human with HO-1-deficiency died at a young age due to an inflammatory syndrome associated with a cellular vulnerability to oxidative stress (27,28). An anti-inflammatory role for HO-1 is also provided by clinical studies demonstrating that serum concentrations of the heme metabolite bilirubin are inversely correlated to systemic inflammation as assessed by the circulating level of the inflammatory marker, high-sensitivity C-reactive protein (hsCRP) (29,30).
Multiple mechanisms contribute to the anti-inflammatory action of HO-1. In particular, the ability of HO-1 to catabolize heme may play an important role since free heme has several pro-inflammatory activities. Heme stimulates leukocyte activation and migration, adhesion molecule expression, and the induction of inflammatory cytokines and acute phase proteins (31–33). In addition, heme promotes an increase in vascular permeability and the infiltration of leukocytes into various tissues (34). Significantly, the inflammatory responses mediated by heme are counteracted by HO-1 both in vitro and in vivo (33,34). Aside from negating the pro-inflammatory actions of free heme, HO-1 generates CO and the bile pigments biliverdin and bilirubin, which exert potent anti-inflammatory effects by regulating the synthesis of inflammatory mediators and the differentiation, activation, and function of immune cells (see 35–37).
4. Role of Inflammation in Atherosclerosis
Atherosclerosis is a chronic inflammatory disease that involves multiple cell types, mediators, and pathways (see 4–6). Inflammation mediates all stages of atherosclerosis from initiation, to progression, and eventual plaque rupture. Accumulation and oxidative modification of lipoproteins such as low density lipoprotein (LDL) within the arterial intima represents the initial event in atherogenesis. Oxidized LDL (oxLDL) and other biologically active lipids stimulate endothelial and smooth muscle cell activation and the expression of adhesion molecules most notably vascular cell adhesive molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) (4). In addition, activated vascular cells generate a host of chemokines, including CC-chemokine ligand 2 (CCL2), CCL5, CCL10, CCL11, CXC-chemokine ligand 9 (CXCL9), CXCL10, CXCL11, and CXCL16, that guide the recruitment of monoctyes, T cells, and mast cells to the vessel wall. The combined expression of adhesion molecules and chemokines promotes the recruitment, adherence, and transmigration of immune cells into the subendothelial space. Furthermore, the production of macrophage colony-stimulating factor (M-CSF) by activated vascular cells induces the differentiation of monocytes into macrophages. Macrophages avidly internalize local particles of oxidized LDL via scavenger receptors to form foam cells, which are a salient feature of atherosclerotic plaques.
The vascular inflammatory response is further inflamed by the up-regulation and activation of Toll-like receptors (TLRs) on macrophages and endothelial cells by oxLDL and other ligands, which leads to the activation of nuclear factor-kappa B (NFκB) and the transcription of pro-inflammatory mediators such as tumor necrosis factor-α (TNFα), interleukin (IL)-6, inducible nitric oxide synthase (iNOS), and matrix metalloproteinases (MMPs). In addition, T cells present in the lesion undergo activation after interacting with antigen presenting cells, such as macrophages and dendritic cells, both of which process and present local antigens, including oxLDL, heat shock protein 60, and possibly local microorganisms. A T helper 1 (TH1) cell-dominant response ensues due to the local production of IL-12, IL-15, and IL-18 by macrophages and smooth muscle cells, leading to the production of tumor necrosis factor-α (TNFα), interferon-γ (IFNγ), and CD40 ligand (CD40L). All three products directly accelerate lesion formation by stimulating the expression of pro-inflammatory cytokines, adhesion molecules, proteolytic enzymes, and pro-thrombotic mediators. In addition, they inhibit vascular cell proliferation and collagen production by vascular smooth muscle cells. Alternatively, the production of the anti-inflammatory cytokines IL-10 and transforming growth factor-β (TGF-β) by vascular cells, macrophages, regulatory T cells (TReg), and platelets dampens the atherogenic response by attenuating plaque inflammation and fragility.
5. Protective Role of HO-1 against Inflammation in Atherosclerosis
Substantial evidence indicates that HO-1 plays a beneficial role in atherosclerosis. HO-1 is expressed in the endothelium, macrophage, foam cells, and medial smooth muscle cells of atherosclerotic lesions in both humans and experimental animals (38,39). HO-1 expression is detected throughout the development of lesions from early fatty streaks to advanced complex atherosclerotic lesions and correlates with plaque burden and phenotype (40,41). Elevated HO-1 expression is observed in human vulnerable atherosclerotic plaques that contain large amounts of lipids and macrophages, and high levels of pro-inflammatory cytokines and chemokines, including IL-6 and CCL2. HO-1 is localized predominately in the intimal base of vulnerable lesions and co-localizes extensively with residing macrophages (41). Interestingly, HO-1 expression is more common in atherosclerotic plaques obtained from asymptomatic compared to symptomatic patients, suggesting a probable role for HO-1 in plaque stability (42). Significantly, the only known human case of HO-1-deficiency exhibited marked endothelial cell injury and early atherosclerotic changes in the vasculature, as reflected by the presence of fatty streaks and plaque (27,28). Furthermore, studies assessing polymorphisms in the promoter region of the human HO-1 gene support a favorable role for HO-1 in atherosclerosis. Specifically, promoters that contain a long (GT)n microsatellite polymorphism that is linked to impaired expression is associated with susceptibility to coronary artery disease in certain patient populations (43–45).
Further support for the protective role of HO-1 in atherosclerosis is derived from animal studies. Inhibition of HO activity by metalloporphyrins enhances lesion formation in Watanabe heritable hyperlipidemic rabbits and LDL-receptor-knockout mice fed a high fat diet (46,47). In a similar fashion, deletion of HO-1 in apolipoprotein E (apoE)-null mice fed a western diet results in larger and more advanced lesions, independent of any change in circulating cholesterol levels (48). Alternatively, the administration of the HO-1 inducer, hemin, diminishes lesion size in LDL-receptor deficient mice (47). In addition, systemic delivery of an HO-1 adenovirus results in a significant decrease in lesion area at the aortic root and arch in both young and old apoE-depleted mice relative to control littermates receiving an empty adenoviral vector (49). Similarly, adenoviral-mediated gene transfer of HO-1 ameliorates graft arteriosclerosis following rat cardiac and aortic transplantation (50–52). More recent work using a vulnerable plaque model found that HO-1 may also regulate plaque phenotype (40). Molecular or pharmacological induction of HO-1 retards vulnerable plaque formation in apoE-knockout mice leading to lesions with a reduced lipid concentration and necrotic core, and increased smooth muscle cell content and fibrous cap thickness. In contrast, inhibition of HO-1 activity by zinc protoporphyrin-IX engenders an opposite effect promoting plaque destabilization. Collectively, clinical and experimental studies suggest that HO-1 affords protection against the development of coronary artery disease by reducing plaque size and vulnerability.
Since the oxidation of LDL by reactive oxygen plays a critical role in triggering the inflammatory response in atherosclerosis, the ability of HO-1 to scavenge reactive oxygen species may be particularly relevant. Consistent with an antioxidant role in atherosclerosis, the induction of HO-1 reduces plasma hydroperoxide concentrations in LDL-receptor knockout mice and hyperlipidemic rabbits while HO inhibition elevates circulating and tissue hydroperoxide levels (46,47). The bile pigments biliverdin and bilirubin are likely involved in the antioxidant action of HO-1, since these pigments are highly efficient scavengers of numerous oxidants and, importantly, are able to prevent the oxidation of LDL (53,54). Moreover, bilirubin oxidation products are detected in atherosclerotic lesions (39). However, other actions of HO-1 may also mediate its antioxidant effect in atherosclerosis. In particular, HO-1 reduces the levels of pro-oxidant iron in atherosclerotic lesions of apoE-knockout mice (49). Although the exact mechanism whereby HO-1 diminishes iron overload has not been elucidated, HO-1-mediated increases in iron efflux from cells may be implicated (55). Furthermore, the increase in ferritin expression that accompanies HO-1 induction may sequester free iron and limit its pro-oxidant/pro-inflammatory capacity. Finally, CO may further enhance the antioxidant actions of HO-1 by inducing the expression of antioxidant genes while blocking the activity of pro-oxidant enzymes (56,57).
HO-1 may also impede early lesion formation by blocking immune cell recruitment and infiltration into atherosclerotic lesions. HO-1 inhibits the expression of adhesion molecules and chemokines associated with the activation of endothelial cells. Overexpression of HO-1 using either pharmacological or genetic approaches blocks cytokine-mediated increases in VCAM-1 and E-selectin expression, and CCL2 secretion by cultured human endothelial cells (58–60). The induction of HO-1 has also been shown to attenuate the expression of E- and P-selectin in several vascular beds (61). These anti-inflammatory actions of HO-1 are mimicked by the exogenous administration of bilirubin or by the chelation of iron, and involve the inhibition of the transcription factor NF-κB, which is strictly required for the expression of adhesion molecules and other inflammatory mediators (58,60,62). By blocking the vascular expression of adhesion receptors, bilirubin is able to reduce the rolling, adhesion, and infiltration of monocytes into the vessel wall (47,58,59,61). Significantly, the induction of HO-1 in monocytes suppresses their chemotactic activity by decreasing the expression of chemokine receptors (63). This anti-inflammatory effect of HO-1 involves both CO and bilirubin since the combined application of both heme metabolites is required to mimic the action of HO-1. Interestingly, recent work demonstrates that circulating monocytes display heterogeneity, which commits to specific functions in atherogenesis. In particular, an inflammatory pro-atherogenic subset of monocytes that possesses a distinct repertoire of surface receptors that allows for efficient trafficking to sites of acute inflammation is elevated in hypercholesterolemia (6). Whether HO-1 can influence the expansion of this monocyte subset is currently not known but this would provide an additional mechanism by which this enzyme regulates monocyte infiltration. Nevertheless, the ability of HO-1 to co-ordinately inhibit adhesion molecule, chemokine, and chemokine receptor expression provides a potent mechanism by which HO-1 is able to dampen inflammatory cell recruitment into vascular lesions. In support of this assertion, bone marrow transplantation experiments performed in lethally irradiated LDL-receptor-null mice reveals that animals reconstituted with bone marrow from HO-1-deficient mice display atherosclerotic lesions with greater macrophage content compared to animals reconstituted with bone marrow from wild-type mice (64).
HO-1 may also inhibit atherosclerosis by directly regulating macrophage function. Peritoneal macrophages derived from HO-1-knockout or HO-1 heterozygous mice exhibit distinct properties compared to macrophages isolated from wild-type littermates (64). Both decreased and absent HO-1 expression results in increased lipid uptake and foam cell formation in macrophages exposed to oxLDL. The rise in lipid uptake by macrophages with compromised HO-1 expression correlates with increased reactive oxygen species generation and is attributable, in part, to increased expression of scavenger receptor A. These in vitro findings are consistent with in vivo data demonstrating that ablation of HO-1 increases lipid accumulation in atherosclerotic lesions of apoE-deficient mice (48). Deletion of HO-1 also results in the greater release of IL-6 and CCL2 from activated macrophages while overexpression of HO-1 decreases the synthesis of several pro-inflammatory cytokines, including IL-6, CCL2, and TNFα (64–66). The suppression of pro-inflammatory cytokine production by HO-1 is duplicated by the exogenous administration of CO and requires the activation of p38 mitogen-activated protein kinase (66). More recently, CO has been reported to attenuate macrophage TLR signaling by promoting the interaction between caveolin-1 and TLR4 and repressing the association of TLR4 with MyD88 and the subsequent downstream activation of NF-κB (67). Furthermore, CO may block macrophage differentiation by inhibiting the expression of GM-CSF (68). A recent study also found that HO-1 contributes to an alternative (M2) macrophage activation profile, which promotes the resolution of inflammation (69). Thus, HO-1 may down-regulate vascular inflammation by modulating macrophage activation, differentiation, and polarization.
Significantly, CO stimulates the synthesis of IL-10 by activated macrophages and in mice exposed to lipopolysaccharide (66). The induction of IL-10 production occurs in a NO- and soluble guanylate cyclase-independent manner but is dependent on mitogen-activated protein kinase kinase 3 since CO fails to elicit increases in circulating levels of IL-10 in endotoxin-treated mice deficient in this kinase. Interestingly, biliverdin is also able to up-regulate IL-10 production by macrophages. In this case, the generation of IL-10 by biliverdin is driven through the binding of the bile pigment to biliverdin reductase expressed on the cell surface and the subsequent activation of phosphatidylinositol-3-kinase/Akt signaling pathway (70). The ability of CO and biliverdin to stimulate the formation of IL-10 may further contribute to the anti-inflammatory action of HO-1 since this cytokine inhibits the production of pro-inflammatory cytokines, suppresses leukocyte adhesion and migration, promotes TReg cell development, and inhibits antigen-specific responses (71). Intriguingly, HO-1 may be a downstream effector of IL-10. IL-10 stimulates HO-1 gene expression in a murine macrophage cell line and IL-10-mediated protection against inflammation during septic shock and transplant arteriosclerosis is dependent on the induction of HO-1 (72,73). These studies suggest the presence of a positive feedback loop between HO-1 and IL-10 that may serve to amplify their anti-inflammatory actions.
HO-1 may also protect against atherosclerosis by promoting vascular cell survival. Apoptosis of endothelial cells plays a fundamental role in the progression of atherosclerosis. Atherogenic stimuli such as oxLDL, inflammatory cytokines, and reactive oxygen species are potent inducers of endothelial cell apoptosis, and apoptotic endothelial cells have been observed in atherosclerotic plaques (74). Gene transfer of HO-1 inhibits endothelial apoptosis in response to many atherogenic factors and provides endothelial protection in animal models of transplant arteriosclerosis (23,75). The cytoprotection afforded by HO-1 is principally mediated by CO, which regulates a multitude of steps in the apoptotic cascade (75,76). However, the bile pigments are also capable of blocking endothelial cell death (77). Interestingly, we previously reported that pharmacological induction of HO-1 also prevents the apoptosis of vascular smooth muscle cells through the release of CO (78,79). Since vascular smooth muscle cell apoptosis is a pivotal process in plaque rupture (80), the capacity of HO-1 to retard smooth muscle cell death in the vulnerable shoulder area of plaques may provide a vital mechanism by which HO-1 preserves plaque stability.
Accumulating evidence suggests that HO-1 may attenuate the inflammatory response in atherosclerosis by affecting the function of antigen presenting cells. Dendritic cells are specialized antigen-presenting cells that populate atherosclerotic plaque and regional draining lymph nodes. These cells bridge the innate and adaptive immune responses and present antigens and co-stimulatory molecules to T cells to trigger the cellular immune response. In the presence of inflammation, immature dendritic cells undergo maturation, a process involving up-regulation of surface major histocompatibility complex class II (MHCII) and co-stimulatory molecules, secretion of pro- and anti-inflammatory molecules, and acquire the ability to stimulate the differentiation of naïve T cells into effector cells. Interestingly, immature dendritic cells constitutively express HO-1 and this expression is strongly down-regulated in response to inflammatory mediators, suggesting a biological role for HO-1 in dendritic cell maturation (81). However, this coupling between HO-1 expression and dendritic maturation depends on the culture conditions used to propagate dendritic cells and is not seen in all dendritic cell subpopulations or for all cell surface proteins (82,83). Several studies have reported that the induction of HO-1 inhibits dendritic cell activation and immunogenicity, and this response is mimicked by the pharmacological delivery of CO or biliverdin/bilirubin (81,84–86). More recently, the genetic loss or small interference RNA-mediated silencing of HO-1 in dendritic cells was shown to up-regulate MHCII expression via a MHCII trans-activator-driven mechanism and direct the primary T-cell response preferentially toward a CD4+ T rather than CD8+ T cell reaction in an in vitro mixed lymphocyte response assay (87). These findings suggests that alterations in dendritic cell regulation in the absence of HO-1 may contribute to the increase in CD4+ T/CD8+ T cell ratio detected in aging HO-1-knockout animals (25). In a murine model of transplant arteriosclerosis, adoptive transfer of HO-1-deficient dendritic cells before allograft transplantation is associated with pronounced intragraft CD4+ T cell infiltration and accelerated transplant arteriosclerosis. Similarly, inhibition of HO-1 activity by zinc protoporphyrin-IX in allograft recipients aggravates transplant arteriosclerosis and this is associated with significant CD4+ T cell infiltration in the allograft. Thus, HO-1 plays a central role in regulating dendritic cell function and subsequent T-cell priming in transplant arteriosclerosis.
Several studies indicate that HO-1 may directly affect T cell function. Overexpression of HO-1 or delivery of CO blocks the entry of T cells into the cell cycle and their proliferation. This anti-proliferative effect is independent of the soluble guanylate cyclase/cyclic GMP pathway but is mediated by the suppression of IL-2 production (88,89). Similarly, the exogenous administration bilirubin inhibits T cell proliferation (86). The cytostatic action of bilirubin is mediated via multiple mechanisms, including inhibition of co-stimulator activities, suppression of immune transcription factor activation, and down-regulation of IL-2 synthesis (86,90). Alternatively, HO-1 has been shown to attenuate T cell responses by promoting activation-induced death of alloreactive T cells, possibly through the release of CO (91,92). Since free heme can stimulate T cell proliferation (93), the ability of HO-1 to degrade heme may also contribute to its ability to block T cell activation.
Significantly, HO-1 may also impair plaque progression by favoring a Th2 cell response. In liver transplantation studies, high levels of HO-1 expression within grafts correlate with a shift in the Th1 and Th2 cytokine balance toward a Th2 cytokine profile consisting predominantly of IL-4 and IL-10 secretion (94). A similar cytokine profile change is noted in liver allograft recipients receiving the CO pro-drug methylene chloride (95), suggesting that CO may be responsible for promoting the Th2 response. The association of HO-1 activity with a Th2 cytokine production pattern has been observed in other inflammatory conditions and may also contribute to plaque stability by limiting the synthesis of plaque destabilizing cytokines such as IFNγ (15). Additionally, HO-1 may curb vascular inflammation by modulating TReg cell function. TReg cells dampen immune responses by suppressing the activation of Th1 and Th2 effector cells. They can achieve this indirectly by affecting antigen presenting cell function or directly through T cell interactions. Interestingly, human and mouse TReg cells express HO-1 constitutively and pharmacologic inhibition of HO-1 abolishes their suppressive activity on CD4+ T cells (96). However, TReg cells isolated from HO-1-deficient mice retain normal suppressive capacity both in vitro and in vivo (97), calling into question a direct physiological role for HO-1 in modulating TReg function. In this respect, a recent in vitro study found that TReg cell activity is dependent on the expression of HO-1 in antigen presenting cells (98). Together, these studies indicate that HO-1 exerts multiple actions on T cells that can potentially impact on the progression and stability of atherosclerotic plaques.
HO-1 may also evoke a salutary effect on vascular inflammation by stabilizing mast cells. Although low in abundance relative to other immune cells, mast cells are found in atherosclerotic plaques where they provoke inflammation (99,100). Activation of mast cells result in the release of histamine and other autacoids that increase vascular permeability and alter vascular tone. In addition, mast cells stimulate the release of pro-inflammatory cytokines (TNFα, IL-6, and IFNγ) and proteinases that can activate MMPs. The degranulation of mast cells also releases heparin that can bind growth-regulatory proteins and contribute to foam cell formation. Interestingly, the induction of HO-1 in mast cells blocks their degranulation and their ability to generate inflammatory cytokines (101,102). In addition, HO-1 down-regulates mast cell-dependent leukocyte adhesion on venular endothelium (101). The bile pigments biliverdin and bilirubin mimic the suppressive actions of HO-1 on both mast cell degranulation and leukocyte adhesion, whereas CO is ineffective. Thus, the ability of HO-1 to inhibit mast cell activation through the formation of bile pigments may also contribute to the anti-inflammatory actions of this enzyme in atherosclerosis.
Finally, HO-1 may diminish vascular inflammation by inhibiting platelet function. Beyond their role in hemostasis, platelets exert important inflammatory responses (103). The adhesion and activation of platelets on the vessel wall results in the release pro-inflammatory molecules such as IL-1β, CD40L, and CCL5 that alters the adhesive and chemotactic properties of endothelial cells, supporting the infiltration of monocytes into atherosclerotic lesions. In addition, exposure of P-selectin on the surface of activated platelets permits the binding of platelets to monocytes via P-selectin glycoprotein receptor 1 (PSGL-1) thereby increasing monocyte adhesion to endothelium. Activated platelets also liberate platelet factor-4 that promotes the differentiation of monocytes to macrophages. The release of growth factors and MMPs via the degranulation of platelet α-granulates may also influence the composition of the vascular lesions by affecting the content of smooth muscle cells and extracellular matrix, respectively.
Interestingly, several studies have demonstrated that exogenous administration of CO inhibits platelet aggregation (104,105). Moreover, we previously reported that the endogenous production of CO by HO-1 activity in vascular cells is sufficient to block platelet aggregation (106). The inhibitory effect of CO on platelet aggregation is likely mediated by the activation of soluble guanylate cyclase (105,106); however, a soluble guanylate cyclase-independent mechanism has also been proposed (107). The induction of HO-1 or the application of CO or bilirubin has also been shown to mitigate the adhesion of platelets to the venular endothelium of endotoxin-treated animals (108). Thus, by blocking platelet adhesion and activation, HO-1 may counteract the pro-inflammatory actions of platelets in atherosclerotic lesions. Moreover, the ability of HO-1 to block platelet function may also limit thrombosis following plaque rupture. The induction of HO-1 ameliorates micro and macrovascular thrombus formation following endothelial injury (109,110). In addition, loss of HO-1 accelerates arterial thrombus formation following photochemical injury and augments thrombus size in a murine model of deep vein thrombosis (111,112). HO-1-deficiency in mice also results in thrombosis and early mortality following allogeneic aortic transplantation (113). However, adoptive transfer of wild-type platelets prolongs survival of HO-1-deficient aortic graft recipients. Furthermore, the systemic administration of CO rescues the pro-thrombotic phenotype and significantly improves survival in HO-1-deficient animals. Together, these data suggest that HO-1 and its reaction products reduce platelet-mediated vascular inflammation and the risk of atherothrombosis.
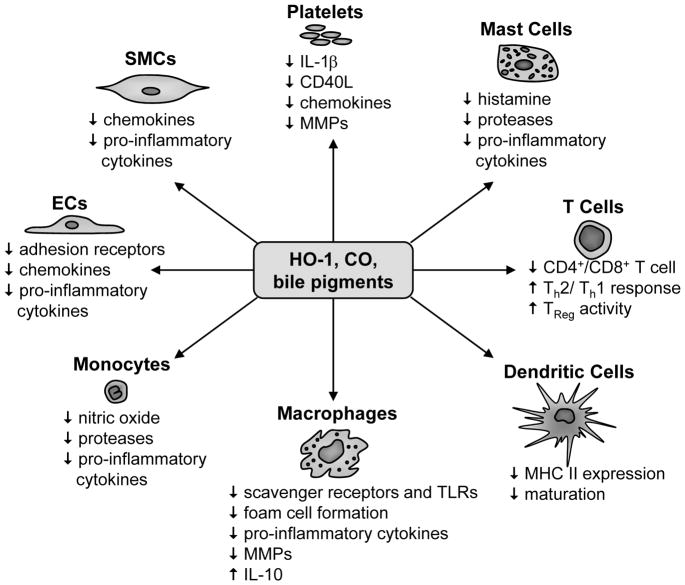
In summary, HO-1 and its reaction products exert potent anti-inflammatory actions on immune cells that regulate the process of atherogenesis (Figure 2). HO-1 inhibits the recruitment and infiltration of immune cells into the vasculature by suppressing adhesion receptor expression and chemokine production by vascular cells. HO-1 also blocks the release of proteases, nitric oxide, and pro-inflammatory cytokines from monocytes and the activation and differentiation of macrophages into foam cells by blocking the expression of scavenger receptors and TLRs while inhibiting MMPs activity and stimulating the synthesis of the anti-inflammatory cytokine, IL-10. In addition, HO-1 may indirectly affect T cell function by blocking surface expression of MHCII and the maturation of dendritic cells, and by promoting the activity of TReg cells. Moreover, HO-1 may drive T cell differentiation toward a CD4+ T phenotype and a Th2-dominant reaction that limits the production of pro-atherogenic cytokines. Finally, HO-1 also prevents the release of inflammatory mediators from mast cells and platelets by inhibiting their activation and degranulation.
Figure 2.
Potential mechanisms by which HO-1 inhibits vascular inflammation in atherosclerosis. HO-1 and its reaction products exert potent anti-inflammatory effects on numerous cell types that may inhibit the process of atherogenesis. HO-1 inhibits the recruitment and infiltration of immune cells into the vasculature by suppressing adhesion receptor expression and chemokine production by vascular endothelial cells (ECs) and smooth muscle cells (SMCs). HO-1 also blocks the release of proteases, nitric oxide and pro-inflammatory cytokines from monocytes and the activation and differentiation of macrophages into foam cells by blocking the expression of scavenger receptors and toll-like receptors (TLRs) while inhibiting matrix metalloproteinase (MMPs) activity and stimulating the synthesis of the anti-inflammatory cytokine, interleukin-10 (IL-10). In addition, HO-1 may indirectly affect T lymphocyte (T cell) function by blocking surface expression of major histocompatibility complex class II (MHCII) and the maturation of dendritic cells, and by promoting the activity of T regulatory cells (TReg). Moreover, HO-1 may drive T cell differentiation toward a CD4+ T cell and a T helper T 2 (Th2) reaction that limits the production of pro-atherogenic cytokines. Finally, HO-1 also prevents the release of inflammatory mediators from mast cells (histamine, proteases, and pro-inflammatory cytokines) and platelets [interleukin-1β (IL-1β), CD40 ligand (CD40L), chemokines, and MMPs] by inhibiting their activation and degranulation.
6. Therapeutic Approaches Targeting HO-1 in Vascular Inflammation
The multiple anti-inflammatory actions of HO-1 that operate on cells found in atherosclerotic plaques makes HO-1 a highly attractive therapeutic target in treating atherosclerosis and other vascular inflammatory disorders. Several strategies can be employed to deliver HO-1. One promising approach involves the use of pharmacological inducers. Heme and it synthetic analogues are potent inducers of HO-1 expression that provides additional substrate to the enzyme for optimal CO and biliverdin production (8,114). These compounds have been shown to protect against the development of vascular disease in diverse animal models, including atherosclerosis (8–11,47). In addition, hemin is already approved by the United States Food and Drug Administration (US FDA) for the treatment of porphyria (115,116) and a small clinical study has documented the efficacy of hemin in stimulating HO-1 expression in healthy volunteers (117). However, possible pro-oxidant and pro-inflammatory actions associated with free heme may limit the use of heme compounds (31–34). A potential less toxic approach to up-regulating HO-1 gene expression may involve the use dietary supplements. A large number of dietary antioxidants have been demonstrated to induce HO-1 in vascular cells and leukocytes, including quercetin, circumin, α-lipoic acid, catechins, carnosol, sulphoraphane, and resveratrol (118). In addition, amino acids such as glutamine, methionine, and alanine are known to increase HO-1 expression, and may mediate their anti-inflammatory action through the induction of HO-1 (119–121). However, clinical studies are needed to establish the efficacy and safety of any nutritional approach that induces HO-1 expression.
Interestingly, the vanguard drugs used to treat atherosclerosis stimulate HO-1 expression. Statins are widely prescribed 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors that reduce various atherosclerotic complications (120). Although lowering of circulating LDL cholesterol levels accounts for much of the clinical benefit, statins exert other anti-atherogenic effects that are independent of LDL reduction (121). Recent evidence suggests that HO-1 may contribute to the beneficial, non-lipid lowering action of statins. Several statins (rosuvastatin, simvastatin, lovastatin, and atorvastatin) are able to stimulate the expression of HO-1 in cultured vascular cells (122–124). Furthermore, the induction of HO-1 mediates the anti-inflammatory and anti-proliferative effect of statins in vascular smooth muscle cells (124). Oral administration of stains in mice results in a statin- and tissue-specific elevation in HO-1 expression (125). A significant statin-mediated increase in HO-1 expression is also noted in murine aortic endothelium, with a lesser induction detected in atheroprone regions of the vessel wall (126). However, the induction of HO-1 in cultured endothelial cells is inconsistent and is usually observed at concentrations that exceed the concentrations found in patient’s plasma during statin therapy, raising the question of therapeutic relevance (127). Of note, a recent study found that laminar shear stress potentiates the induction of HO-1 by statins in cultured endothelial cells (126), suggesting that endothelial cells may be more responsive to statins in their native in vivo environment than in culture. Similarly, probucol, a rarely used cholesterol-lowering agent that retards atherosclerosis in humans and animals (128,129), stimulates HO-1 gene expression in vascular cells (130). Moreover, the induction of HO-1 contributes to the anti-inflammatory and growth-regulatory properties of this compound drug (131).
Drugs used to treat the acute and chronic complications of atherosclerosis also elevate HO-1 expression. Aspirin stimulates HO-1 expression in endothelial cells and this may play a role in the drug’s antioxidant and anti-thrombotic profile (134). In addition, HO-1 may contribute to the effectiveness of drug-eluting stents in preserving blood flow in regions of atherostenosis following percutaneous interventions. Strikingly, both rapamycin and paclitaxel, which are released by stents, are potent inducers of HO-1 in vascular smooth muscle cells, and HO-1 contributes to the anti-proliferative action of these compounds (135–137).
While the above studies validate the use of a pharmacological approach in delivering HO-1, some challenges remain. In particular, the induction and duration of HO-1 expression must be carefully calibrated since prolonged, high level HO-1 expression has the potential of adversely affecting cell viability via the generation of toxic levels of free iron and biliverdin (138,139). Apart from toxicity, the presence of polymorphisms in the promoter that limits the induction of HO-1 may reduce the efficacy of pharmacological inducers in certain patient groups (140). Consequently, the genotyping of patients may be required prior to pharmacological intervention. Alternatively, increasing HO-1 gene expression via viral-mediated gene transfer circumvents this problem and allows for a more selective approach in targeting this gene to all patient populations. In this respect, HO-1 gene transfer approaches in animals have proven highly effective in attenuating atherosclerosis and intimal thickening following arterial injury (49,141,142). Moreover, the recent deployment of inducible and cell-specific vectors may allow for selective and temporal patterns of HO-1 gene expression (143). However, further improvements in safety and efficacy are required before human HO-1 gene therapy becomes a viable option.
Alternatively, the products of the HO-1 reaction may be directly employed to treat atherosclerosis and its complications. Preclinical studies demonstrate that CO is effective in preventing vascular disease. Inhalation of low concentrations of CO affords protection in animal models of transplant arteriosclerosis, intimal hyperplasia, pulmonary hypertension, and ischemia-reperfusion injury (144–147). Based on such positive findings, the US FDA granted orphan drug status for inhaled CO for use in ameliorating the incidence and severity of delayed graft function in patients undergoing solid organ transplantation (148). Presently, several clinical trials are exploring the feasibility of using acute, episodic CO inhalation regimens for the treatment of lung inflammation, obstructive pulmonary disease, and kidney transplantation. Preliminary studies found that the inhalation of low doses of CO for one or two hours is safe and potentially beneficial to patients (149,150).
The use of CO-saturated solutions provides another means for administering CO. We previously reported on the utility of this approach by demonstrating that local application of a saturated solution of CO blocks neointima formation following rat carotid artery balloon injury (151). The recent development of CO-releasing molecules (CORMs) that liberate CO under physiologic conditions offers a promising approach that may allow for a more controlled and targeted delivery of this gas (152,153). Another potential advantage of these compounds is their ability to elevate local levels of CO without significantly increasing blood carboxyhemoglobin levels, which is often observed with CO inhalation therapy (152). Significantly, CORMs inhibit in-stent stenosis in a rat model (154); however, the ability of these molecules to inhibit atherosclerosis in animals remains to be established.
The administration of biliverdin or bilirubin may provide an alternative approach in treating atherosclerotic disease. The delivery of bile pigments inhibits vascular inflammation following organ transplantation and reduces the degree of neointima formation following arterial injury in rodents (90,155,156). Interestingly, the salutary effect observed with the administration of bile pigments in animals occurs with mild increases in circulating bilirubin levels. This corresponds to clinical studies demonstrating that only modest elevations in serum bilirubin reduce inflammation and the attendant risk of coronary and peripheral artery disease (29,30,157,158). Since the anti-atherogenic effect of HO-1 may involve the synergistic interaction between its reaction products, combined therapy with CO and biliverdin may be advantageous over the application of a single HO-1 product.
7. Perspectives
A growing body of evidence indicates that HO-1 protects against atherosclerosis by inhibiting the inflammatory processes that contribute to plaque development, progression, and rupture. Aside from metabolizing pro-inflammatory free heme, HO-1 generates CO and the bile pigments biliverdin and bilirubin which exert potent anti-inflammatory effects by modulating the synthesis of inflammatory modulators as well as the activation, differentiation, function of cells found within atherosclerotic lesions. Several strategies can be used to target HO-1 in atherosclerosis. Pharmacological induction of HO-1 has proven effective in animal models of atherosclerosis and represents an attractive and highly feasible approach. Many inducers of HO-1 have been identified and several are used clinically. In fact, the current drugs used to treat atherosclerosis and its clinical complications elicit their beneficial actions, in part, through the induction of HO-1. However, pharmacological approaches may be compromised in certain segments of the population that possess promoter polymorphisms that limit the inducibility of HO-1. In this situation, HO-1 gene therapy or the direct administration of HO-1 reaction products may be more effective. In this respect, inhalation of CO has been demonstrated to prevent inflammation and transplant arteriosclerosis in several animal models, and initial clinical studies indicate that CO inhalation may be a viable option. The use of CO-releasing molecules provides an additional and perhaps safer alternative in delivering CO to patients. Finally, given experimental and epidemiological studies demonstrating the beneficial actions of bile pigments on coronary artery disease, the administration of bile pigments either alone or in combination with CO may provide another therapeutic modality to treat atherosclerosis. Future clinical studies are needed to establish the efficacy and safety of any of the above approaches targeting HO-1 or its reaction products in the treatment of vascular inflammatory disease.
Acknowledgments
The author acknowledges the support of grants from the National Institutes of Health HL59976 and HL74966, and the American Heart Association Midwest Affiliate.
References
- 1.Faxon DP, Fuster V, Libby P, Beckman JA, Hiatt WR, Thompson RW, Topper JN, Annex BH, Rundback JH, Fabunmi RP, Robertson RM, Loscalzo J. American Heart Association: Atherosclerotic Vascular Disease Conference: Writing Group III: pathophysiology. Circulation. 2004;109:2617–2625. doi: 10.1161/01.CIR.0000128520.37674.EF. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho MM, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. American Heart Association: Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 3.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Ridker PM, Hansson GK for the Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 6.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 7.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maines MD. Heme oxygenase: functions, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1989;2:2557–2568. [PubMed] [Google Scholar]
- 9.Durante W, Johnson FK, Johnson RA. Role of carbon monoxide in cardiovascular function. J Cell Mol Med. 2006;10:672–686. doi: 10.1111/j.1582-4934.2006.tb00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 11.Ryter SW, Alam J, Choi AMK. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 12.Wagener FADTG, Volk H-D, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev. 2003;55:551–571. doi: 10.1124/pr.55.3.5. [DOI] [PubMed] [Google Scholar]
- 13.Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 14.Tamion F, Richard V, Renet S, Thuillez C. Protective effects of heme oxygenase-1 expression against endotoxin shock: inhibition of tumor necrosis factor-alpha and augmentation of interleukin-10. J Trauma. 2006;61:1078–1084. doi: 10.1097/01.ta.0000239359.41464.ef. [DOI] [PubMed] [Google Scholar]
- 15.Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular response to hypoxia. Proc Natl Acad Sci USA. 2001;98:8793–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 gene ablation and expression. J Am Soc Nephrol. 2001;11:965–973. doi: 10.1681/ASN.V115965. [DOI] [PubMed] [Google Scholar]
- 17.Nath KA, Vercellotti GM, Grande JP, Miyoshi H, Paya CV, Manivel JC, Haggard JJ, Croatt AJ, Payne WD, Alam J. Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney Int. 2001;59:106–117. doi: 10.1046/j.1523-1755.2001.00471.x. [DOI] [PubMed] [Google Scholar]
- 18.Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest. 1999;103:1047–1054. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wunder C, Potter RF. The heme oxygenase system: its role in liver inflammation. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:199–208. doi: 10.2174/1568006033481410. [DOI] [PubMed] [Google Scholar]
- 20.Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey ST, Colvin RB, Choi AM, Poss KD, Bach FH. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4:1073–1077. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 21.Soares MP, Bach FH. Heme oxygenase-1 in organ transplantation. Front Biosci. 2007;12:4932–4945. doi: 10.2741/2439. [DOI] [PubMed] [Google Scholar]
- 22.Nakao A, Choi AMK, Murase N. Protective effect of carbon monoxide in transplantation. J Cell Mol Med. 2006;10:650–671. doi: 10.1111/j.1582-4934.2006.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock WW, Buelow R, Sayegh MH, Turka LA. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med. 1998;4:1392–1396. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 24.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poss KD, Tonegawa S. Heme oxygenase-1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapturczak MH, Wasserfall C, Brusko T, Campell-Thompson M, Ellis TM, Atkinson MA, Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165:1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawashima A, Oda T, Yachie A, Koizumi S, Nakanishi I. Heme oxygenase-1 deficiency: the first autopsy case. Hum Pathol. 2002;33:125–130. doi: 10.1053/hupa.2002.30217. [DOI] [PubMed] [Google Scholar]
- 29.Madhavan M, Wattigney WA, Srinivasan SR, Berenson GS. Serum bilirubin distribution and its relation to cardiovascular risk in children and young adults. Atherosclerosis. 1997;131:107–113. doi: 10.1016/s0021-9150(97)06088-7. [DOI] [PubMed] [Google Scholar]
- 30.Caliskan M, Erdogan D, Gullu H, Tok D, Bilgi M, Muderrisoglu H. Low serum bilirubin concentrations are associated with impaired aortic elastic properties, but not impaired left ventricular diastolic function. Int J Clin Pract. 2007;61:218–224. doi: 10.1111/j.1742-1241.2006.00907.x. [DOI] [PubMed] [Google Scholar]
- 31.Graca-Souza AV, Arruda MA, de Freitas MS, Barja-Fidalgo C, Oliveira PL. Neutrophil activation by heme: implications for inflammatory processes. Blood. 2002;99:4160–4165. doi: 10.1182/blood.v99.11.4160. [DOI] [PubMed] [Google Scholar]
- 32.Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto FF, Dutra BN, Alves LS, Oliveira MF, Graca-Souza AV, Bozza MT. Characterization of heme as an activator of Toll-like receptor 4. J Biol Chem. 2007;282:20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- 33.Wagener FADTG, da Silva JL, Farley T, de Witte T, Kappas A, Abraham NG. Differential effects of heme oxygenase isoforms on heme mediated endothelial ICAM-1 expression. J Pharmacol Exp Ther. 1999;291:416–423. [PubMed] [Google Scholar]
- 34.Wagener FADTG, Eggert A, Boerman OC, Oyen WJ, Verhofstad A, Abraham NG, Adema G, van Kooyk Y, de Witte T, Figdor CG. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98:1802–1811. doi: 10.1182/blood.v98.6.1802. [DOI] [PubMed] [Google Scholar]
- 35.Alcaraz MJ, Fernandez P, Guillen MI. Anti-inflammatory actions of the heme oxygenase pathway. Curr Drug Des. 2003;9:2541–2551. doi: 10.2174/1381612033453749. [DOI] [PubMed] [Google Scholar]
- 36.Pae H-O, Lee YC, Chung H-T. Heme oxygenase-1 and carbon monoxide: emerging therapeutic targets in inflammation and allergy. Recent Patents Inflammation Allergy Drug Discovery. 2008;2:159–165. doi: 10.2174/187221308786241929. [DOI] [PubMed] [Google Scholar]
- 37.Soares MP, Marguti I, Cunha A, Larsen R. Immunoregulatory effects of HO-1: how does it work? Curr Opin Pharmacol. 2009;9:482–489. doi: 10.1016/j.coph.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Wang LJ, Lee TS, Lee FY, Pai RC, Chau LY. Expression of heme oxygenase-1 in atherosclerotic lesions. Am J Pathol. 1998;152:711–720. [PMC free article] [PubMed] [Google Scholar]
- 39.Nakayama M, Takahashi K, Komaru T, Fukuchi M, Shioiri H, Sato K, Kitaura T, Shirato K, Yamaguchi T, Suematsu M, Shibahara S. Increased expression of heme oxygenase-1 and bilirubin accumulation in foam cells of rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2001;21:1373–1377. doi: 10.1161/hq0801.093592. [DOI] [PubMed] [Google Scholar]
- 40.Cheng C, Noordeloos AM, Jeney V, Soares MP, Moll F, Pasterkamp G, Serruys PW, Duckers HJ. Heme oxygenase-1 determines atherosclerotic lesion progression into a vulnerable plaque. Circulation. 2009;119:3017–3027. doi: 10.1161/CIRCULATIONAHA.108.808618. [DOI] [PubMed] [Google Scholar]
- 41.Li YG, Wang DM, Chen SM, Tan XR, Fang XY, Wu JW, Zhang GH, Mai RQ. Haem oxygenase-1 expression and coronary heart disease-association between levels of haem oxygenase-1 expression and angiographic morphology as well as the quantity of coronary lesions. Acta Cardiol. 2006;61:295–300. doi: 10.2143/AC.61.3.2014831. [DOI] [PubMed] [Google Scholar]
- 42.Ameriso SF, Villamil AR, Zedda C, Parodi JC, Garrido S, Sarchi MI, Shultz M, Bocskowski J, Sevlever GE. Heme oxygenase-1 is expressed in carotid atherosclerotic plaque infected by Helicobacter pylori and is more prevalent in asymptomatic patients. Stroke. 2005;36:1896–1890. doi: 10.1161/01.STR.0000177494.43587.9e. [DOI] [PubMed] [Google Scholar]
- 43.Chen YH, Lin SY, Lin MW, Tsai HL, Kuo SS, Chen JW, Chang MJ, Wu TC, Chen LC, Ding YA, Pan WH, Jou YS, Chau LY. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type II diabetic patients. Hum Genet. 2002;111:1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- 44.Kaneda H, Ohno M, Taguchi J, Hashimoto H, Ogasawura T, Aizawa T, Ishizaka N, Nagai R. Heme oxygenase-1 gene promoter polymorphism is associated with coronary artery disease in Japanese patients with coronary risk factors. Arterioscler Thromb Vasc Biol. 2002;22:1680–1685. doi: 10.1161/01.atv.0000033515.96747.6f. [DOI] [PubMed] [Google Scholar]
- 45.Endler G, Exner M, Schillinger M, Marculescu R, Sunder-Plassmann R, Raith M, Jordanova N, Wojita J, Mannhalter C, Wagner OF, Huber K. A microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with increased bilirubin and HDL levels but not with coronary artery disease. Thromb Haemostasis. 2004;91:155–161. doi: 10.1160/TH03-05-0291. [DOI] [PubMed] [Google Scholar]
- 46.Ishikawa K, Sugawara D, Goto J, Watanabe K, Kawamura S, Shiomi M, Itabe H, Maruyama Y. Heme oxygenase-1 inhibits atherogenesis in Watanabe heritable hyperlipidemic rabbits. Circulation. 2001;104:1831–1836. doi: 10.1161/hc3901.095897. [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa K, Sugawara D, Wang XP, Suzuki K, Itabe H, Maruyama Y, Lusis AJ. Heme oxygenase-1 inhibits atherosclerosis lesion formation in ldl-receptor knockout mice. Circ Res. 2001;88:506–512. doi: 10.1161/01.res.88.5.506. [DOI] [PubMed] [Google Scholar]
- 48.Yet SF, Layne MD, Liu X, Chen YH, Ith B, Sibing NE, Perrella MA. Absence of heme oxygenase-1 exacerbates atherosclerosis lesion formation and vascular remodeling. FASEB J. 2003;17:1759–1761. doi: 10.1096/fj.03-0187fje. [DOI] [PubMed] [Google Scholar]
- 49.Juan SH, Lee TS, Tseng KW, Liou JY, Shyue SK, Wu KK, Chau LY. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;104:1519–1525. doi: 10.1161/hc3801.095663. [DOI] [PubMed] [Google Scholar]
- 50.Bouche D, Chauveau BD, Roussel JC, Mathieu P, Bradeau C, Tesson L, Soulillou JP, Iyer S, Buelow R, Anegon I. Inhibition of graft arteriosclerosis development in rat aortas following heme oxygenase-1 gene transfer. Transpl Immunol. 2002;9:235–238. doi: 10.1016/s0966-3274(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 51.Du D, Chang S, Chen H, Zhou B, Chen ZK. Adenovirus-mediated heme oxygenase transfer inhibits graft arteriosclerosis in rat aortic transplants. Transpl Proc. 2007;39:3446–3448. doi: 10.1016/j.transproceed.2007.03.114. [DOI] [PubMed] [Google Scholar]
- 52.Tsui TY, Wu X, Lau CK, Ho DW, Xu T, Siu YT, Fan ST. Prevention of chronic deterioration of heart allograft by recombinant adeno-associated virus-mediated heme oxygenase-1 gene transfer. Circulation. 2003;107:2623–2629. doi: 10.1161/01.CIR.0000066911.03770.8D. [DOI] [PubMed] [Google Scholar]
- 53.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 54.Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for α-tocopherol, inhibiting plasma and low density lipoprotein peroxidation. J Biol Chem. 1994;269:16712–16719. [PubMed] [Google Scholar]
- 55.Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Baranano DE, Dore S, Poss KD, Snyder SH. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 56.Piantadosi CA, Carraway MS, Suliman HB. Carbon monoxide, oxidative stress, and mitochondrial permeability pore transition. Free Radic Biol Med. 2006;40:1332–1339. doi: 10.1016/j.freeradbiomed.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 57.Taille C, El-Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem. 2005;280:25350–25360. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- 58.Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui T-Y, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial activation. J Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 59.Lin CC, Liu XM, Peyton KJ, Wang H, Wang WC, Lin SJ, Durante W. Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler Thromb Vasc Biol. 2008;28:739–745. doi: 10.1161/ATVBAHA.107.160085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pae HO, Oh GS, Lee BS, Rim JS, Kim YM, Chung HT. 3-Hydorxyanthranilic acid, one of L-tryptophan metabolites, inhibits monocyte chemoattractant protein-1 secretion and vascular cell adhesion molecule-1 expression via heme oxygenase-1 induction in human umbilical vein endothelial cells. Atherosclerosis. 2006;187:274–284. doi: 10.1016/j.atherosclerosis.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 61.Vachharajan TJ, Work J, Issekutz AC, Granger DN. Heme oxygenase modulates selectin expression in different vascular beds. Am J Physiol Heart Circ Physiol. 2000;278:H1613–H1617. doi: 10.1152/ajpheart.2000.278.5.H1613. [DOI] [PubMed] [Google Scholar]
- 62.Mazzone GL, Rigato I, Ostrow JD, Tiribelli C. Bilirubin effect on endothelial adhesion molecules expression is mediated by the NF-kappaB signaling pathway. Biosci Trends. 2009;3:151–157. [PubMed] [Google Scholar]
- 63.Morita T, Imai T, Yamaguchi T, Sugiyama T, Katayama S, Yoshino G. Induction of heme oxygenase-1 suppresses angiotensin II-elicited chemotactic activity through inhibition of CCR2: role of bilirubin and carbon monoxide generated by the enzyme. Antioxid Redox Signal. 2003;5:439–447. doi: 10.1089/152308603768295186. [DOI] [PubMed] [Google Scholar]
- 64.Orozco LD, Kapturczak MH, Barajas B, Wang X, Weinstein MM, Wong J, Deshane J, Bolisetty S, Shaposhnik Z, Shih D, Agarwal A, Lusis AJ, Araujo JA. Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circ Res. 2007;100:1703–1711. doi: 10.1161/CIRCRESAHA.107.151720. [DOI] [PubMed] [Google Scholar]
- 65.Sawle P, Foresti R, Mann BE, Johnson TR, Green CJ, Motterlini R. Carbon monoxide-releasing molecules (CO-RMs) attenuate the inflammatory response elicited by lipopolysaccharide in RAW264.7 murine macrophages. Br J Pharmacol. 2005;145:800–810. doi: 10.1038/sj.bjp.0706241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Otterbein LE, Bach FH, Alam J, Soares MP, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 67.Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AMK. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol. 2009;182:3809–3818. doi: 10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- 68.Sarady JK, Otterbein SL, Liu F, Otterbein LE, Choi AMK. Carbon monoxide modulates endotoxin-induced production of granulocyte macrophage colony-stimulating factor in macrophages. Am J Respir Cell Mol Biol. 2002;27:739–745. doi: 10.1165/rcmb.4816. [DOI] [PubMed] [Google Scholar]
- 69.Weis N, Weigert A, von Knethen A, Brune B. Heme oxygenase-1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Mol Biol Cell. 2009;20:1280–1288. doi: 10.1091/mbc.E08-10-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wegiel B, Baty CJ, Gallo D, Csizmadia E, Scott JR, Akhaven A, Chin BY, Kaczmarek E, Alam J, Bach FH, Zuckerbraun BS, Otterbein LE. Cell surface biliverdin reductase mediates biliverdin-induced anti-inflammatory effects via phosphatidylinositol-3-kinase and Akt. J Biol Chem. 2009;284:21369–21378. doi: 10.1074/jbc.M109.027433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 1989;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 72.Lee T-S, Chau L-Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 73.Chen S, Kapturczak MH, Wasserfall C, Glushakova OY, Campbell-Thompson M, DeShane JS, Joseph R, Cruz PE, Hauswirth WW, Madsen KM, Croker BP, Berns KI, Atkinson MA, Flotte TR, Tisher CC, Agarwal A. Interleukin-10 attenuates neointima proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. Proc Natl Acad Sci USA. 2005;102:7251–7256. doi: 10.1073/pnas.0502407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tricot O, Mallat Z, Heymes C, Belmin J, Leseche G, Tedgui A. Relation between endothelial cell apoptosis and blood direction in human atherosclerotic plaques. Circulation. 2000;101:2450–2453. doi: 10.1161/01.cir.101.21.2450. [DOI] [PubMed] [Google Scholar]
- 75.Morse D, Lin L, Choi AM, Ryter SW. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radic Biol Med. 2009;47:1–12. doi: 10.1016/j.freeradbiomed.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1029. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei Y, Liu XM, Peyton KJ, Wang H, Johnson FK, Johnson RA, Durante W. Hypochlorous acid-induced heme oxygenase-1 gene expression promotes human endothelial cell survival. Am J Physiol Cell Physiol. 2009;297:C907–C915. doi: 10.1152/ajpcell.00536.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc Res. 2002;55:396–405. doi: 10.1016/s0008-6363(02)00410-8. [DOI] [PubMed] [Google Scholar]
- 79.Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W. Antiapoptotic action of carbon monoxide in cultured vascular smooth muscle cells. Exp Biol Med (Maywood) 2003;228:572–575. doi: 10.1177/15353702-0322805-30. [DOI] [PubMed] [Google Scholar]
- 80.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 81.Chauveau C, Remy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert F-X, Tesson L, Brion R, Beriou G, Gregoire M, Josien R, Cuturi MC, Anegon I. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–1702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- 82.Mashreghi MF, Klemz R, Knosalla IS, Gerstmayer B, Janssen U, Buelow R, Jozkowicz A, Dulak J, Volk HD, Kotsch K. Inhibition of dendritic cell maturation and function is independent of heme oxygenase-1 but requires the activation of STAT3. J Immunol. 2008;180:7919–7930. doi: 10.4049/jimmunol.180.12.7919. [DOI] [PubMed] [Google Scholar]
- 83.Park DJ, Agarwal A, George JF. Heme oxygenase-1 expression in murine dendritic cell subpopulations: effect on CD8+ dendritic cell differentiation in vivo. Am J Pathol. 2010;176:2831–2839. doi: 10.2353/ajpath.2010.090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kotsch K, Martins PN, Klemz R, Janssen U, Gerstmeyer B, Dernier A, Reutzel-Selke A, Kuckelkorn U, Tullius SG, Volk HD. Heme oxygenase-1 ameliorates ischemia/reperfusion injury by targeting dendritic cell maturation and migration. Antioxid Redox Signal. 2007;9:2049–2063. doi: 10.1089/ars.2007.1801. [DOI] [PubMed] [Google Scholar]
- 85.Remy S, Blancou P, Tesson L, Tardif V, Brion R, Royer PJ, Motterlini R, Foresti R, Painchaut M, Pogu S, Gregoire M, Bach JM, Anegon I, Chauveau C. Carbon monoxide inhibits TLR-induced dendritic cell immunogenicity. J Immunol. 2009;182:1877–1884. doi: 10.4049/jimmunol.0802436. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y, Li P, Lu J, Xiong W, Oger J, Tetzlaff W, Cynader M. Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis. J Immunol. 2008;181:1887–1897. doi: 10.4049/jimmunol.181.3.1887. [DOI] [PubMed] [Google Scholar]
- 87.Cheng C, Noorderloos M, van Deel ED, Tempel D, den Dekker W, Wagtmans K, Duncker DJ, Soares MP, Laman JD, Duckers HJ. Dendritic cell function in transplantation arteriosclersosis is regulated by heme oxygenase-1. Circ Res. 2010;106:1656–1666. doi: 10.1161/CIRCRESAHA.110.216945. [DOI] [PubMed] [Google Scholar]
- 88.Pae HO, Ho GS, Choi BM, Chae SC, Chung HT. Differential expressions of heme oxygenase-1 gene in CD25− and CD25+ subsets of human CD4+ T cells. Biochem Biophys Res Commun. 2003;306:71–705. doi: 10.1016/s0006-291x(03)01037-4. [DOI] [PubMed] [Google Scholar]
- 89.Pae HO, Ho GS, Choi BM, Chae SC, Kim YM, Chung KR, Chung HT. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J Immunol. 2004;172:4744–4751. doi: 10.4049/jimmunol.172.8.4744. [DOI] [PubMed] [Google Scholar]
- 90.Yamashita K, McDaid J, Ollinger R, Tsui TY, Berberat PO, Usheva A, Czismadia E, Smith RN, Soares MP, Bach FH. Biliverdin, a natural product of heme catabolism, induces tolerance to cardiac grafts. FASEB J. 2004;18:765–767. doi: 10.1096/fj.03-0839fje. [DOI] [PubMed] [Google Scholar]
- 91.McDaid J, Yamashita K, Chora A, Ollinger R, Strom TB, Li XC, Bach FH, Soares MP. Heme oxygenase-1 modulates the alloimune response by promoting activation-induced cell death of T cells. FASEB J. 2005;19:458–460. doi: 10.1096/fj.04-2217fje. [DOI] [PubMed] [Google Scholar]
- 92.Song R, Zhou Z, Kim PK, Shapiro RA, Liu F, Ferran C, Choi AM, Otterbein LE. Carbon monoxide promotes Fas/CD95-induced apoptosis in Jurkat cells. J Biol Chem. 2004;279:44327–44334. doi: 10.1074/jbc.M406105200. [DOI] [PubMed] [Google Scholar]
- 93.Stenzel KH, Rubin AL, Novogrodsky A. Mitogenic and co-mitogenic properties of hemin. J Immunol. 1981;127:2469–2473. [PubMed] [Google Scholar]
- 94.Ke B, Buelow R, Shen XD, Milinek J, Gao F, Ritter T, Volk HD, Busuttil RW, Kupiec-Weglinski JW. Heme oxgenase-1 gene therapy: a novel immunological approach in liver allograft recipients. Transplant Proc. 2001;33:581–582. doi: 10.1016/s0041-1345(00)02151-5. [DOI] [PubMed] [Google Scholar]
- 95.Ke B, Buelow R, Shen XD, Melinik J, Amersi F, Gao F, Ritter T, Volk HD, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase 1 gene transfer prevents CD95/Fas lignd-mediated apoptosis and improves liver allograft survival via carbon monoxide signaling pathway. Hum Gene Ther. 2002;13:1189–1199. doi: 10.1089/104303402320138970. [DOI] [PubMed] [Google Scholar]
- 96.Choi BM, Pae HO, Jeong YR, Kim YM, Chung HT. Critical role of heme oxygenase-1 in Foxp3-mediated immune suppression. Biochem Biophys Res Commun. 2005;327:1066–1071. doi: 10.1016/j.bbrc.2004.12.106. [DOI] [PubMed] [Google Scholar]
- 97.Zelenay S, Chora A, Soares MP, Demengeot J. Heme oxygenase-1 is not required for mouse regulatory T-cell development and function. Int Immunol. 2007;19:11–18. doi: 10.1093/intimm/dxl116. [DOI] [PubMed] [Google Scholar]
- 98.George JF, Braun A, Brusko TM, Joseph R, Bolisetty S, Wasserfall CH, Atkinson MA, Agarwal A, Kapturczak MH. Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am J Pathol. 2008;173:154–160. doi: 10.2353/ajpath.2008.070963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, MacFarlane LA, Mallen-St Clair J, Shi GP. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 100.Bot I, de Jager SC, Zernecke A, Lindstedt KA, van Berkel TJ, Weber C, Biessen EA. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation. 2007;115:2516–2525. doi: 10.1161/CIRCULATIONAHA.106.660472. [DOI] [PubMed] [Google Scholar]
- 101.Takamiya R, Murakami M, Kajimura M, Goda N, Makino N, Takamiya Y, Yamaguchi T, Ishimura Y, Hozumi N, Suematsu M. Stabilization of mast cells by heme oxygenase: an anti-inflammatory role. Am J Physiol Heart Circ Physiol. 2002;283:H861–H870. doi: 10.1152/ajpheart.00740.2001. [DOI] [PubMed] [Google Scholar]
- 102.Yasui Y, Nakamura M, Onda T, Uehara T, Murata S, Matsui N, Fukuishi N, Akagi R, Suematsu M, Akagi M. Heme oxygenase-1 inhibits cytokine production by activated mast cells. Biochem Biophys Res Commun. 2007;354:485–490. doi: 10.1016/j.bbrc.2006.12.228. [DOI] [PubMed] [Google Scholar]
- 103.Semple JW, Freedman J. Platelets and innate immunity. Cell Mol Life Sci. 2010;67:499–511. doi: 10.1007/s00018-009-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mansouri A, Perry CA. Inhibition of platelet ADP and serotonin release by carbon monoxide. Experentia. 1984;40:515–517. doi: 10.1007/BF01952415. [DOI] [PubMed] [Google Scholar]
- 105.Brune B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987;32:497–504. [PubMed] [Google Scholar]
- 106.Wagner CT, Durante W, Christodoulides N, Hellums JD, Schafer AI. Hemodynamic forces induce the expression of heme oxygenase in cultured vascular smooth muscle cells. J Clin Invest. 1997;100:589–596. doi: 10.1172/JCI119569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chlopicki S, Olszanecki R, Marcinkiewicz E, Lomnicka M, Motterlini R. Carbon monoxide release by CORM-3 inhibits human platelets by a mechanism independent of soluble guanylate cyclase. Cardiovas Res. 2006;71:393–401. doi: 10.1016/j.cardiores.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 108.Morisaki H, Katayama T, Kotake Y, Ito M, Handa M, Ikeda Y, Takeda J, Suematsu M. Carbon monoxide modulates endotoxin-induced microvascular leukocyte adhesion through platelet-dependent mechanisms. Anesthesiology. 2002;97:701–709. doi: 10.1097/00000542-200209000-00025. [DOI] [PubMed] [Google Scholar]
- 109.Peng L, Mundada JM, Stomel JJ, Liu L, Sun YS, Yet J, Fay WP. Induction of heme oxygenase-1 expression inhibits platelet-dependent thrombosis. Antioxid Redox Signal. 2004;6:729–735. doi: 10.1089/1523086041361677. [DOI] [PubMed] [Google Scholar]
- 110.Lindenblatt N, Bordel R, Schareck W, Menger MD, Vollmar B. Vascular heme oxygenase-1 induction suppresses microvascular thrombus formation in vivo. Arterioscler Thromb Vasc Biol. 2004;24:1–6. doi: 10.1161/01.ATV.0000118279.74056.8a. [DOI] [PubMed] [Google Scholar]
- 111.True AL, Olive M, Boehm M, San H, Westrick RJ, Raghavachari N, Xu X, Lynn EG, Sack MN, Munson MT, Gladwin PJ, Nabel EG. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res. 2007;101:893–901. doi: 10.1161/CIRCRESAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- 112.Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman ZS, Katusic AW, Nath KA. Induction of heme oxygenase-1 is a beneficial response in a murine model of venous thrombosis. Am J Pathol. 2008;173:1882–1890. doi: 10.2353/ajpath.2008.080556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen B, Guo L, Fan C, Bolisetty S, Joseph R, Wright MW, Agarwal A, George JF. Carbon monoxide rescues heme oxygenase-1-deficient mice from arterial thrombosis in allogeneic aortic transplantation. Am J Pathol. 2009;175:422–429. doi: 10.2353/ajpath.2009.081033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morimoto Y, Durante W, Lancaster DG, Klattenhoff J, Tittel FK. Real-time measurements of endogenous CO production from vascular cells using an ultrasensitive laser sensor. Am J Physiol Heart Circ Physiol. 2001;280:H483–H488. doi: 10.1152/ajpheart.2001.280.1.H483. [DOI] [PubMed] [Google Scholar]
- 115.Rank JM, Straka JG, Weimer MK, Boissenmaier I, Taddeini BL, Bloomer JR. Hematin therapy in late onset congenital erythropoietic porphyria. Br J Haematol. 1990;75:617–618. doi: 10.1111/j.1365-2141.1990.tb07809.x. [DOI] [PubMed] [Google Scholar]
- 116.Dellon ES, Szczepiorkowski ZM, Dzik WH, Graeme-Cook F, Ades A, Bloomer JR, Cosimi AB, Chung RT. Treatment of recurrent allograft dysfunction with intravenous hematin after liver transplantation for erythropoietic protoporphyria. Transplantation. 2002;73:911–915. doi: 10.1097/00007890-200203270-00014. [DOI] [PubMed] [Google Scholar]
- 117.Bharucha AE, Kulkarni A, Choi KM, Camilleri M, Lempke M, Brunn GJ, Gibbons SJ, Zinsmeister AR, Farrugia G. First-in-human study demonstrating pharmacological activation of heme oxygenase-1 in humans. Clin Pharmacol Ther. 2010;87:187–190. doi: 10.1038/clpt.2009.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ogborne RM, Rushworth SA, Charalambos CA, O’Connell MA. Haem oxygenase-1: a target for dietary antioxidants. Biochem Soc Trans. 2004;32:1003–1005. doi: 10.1042/BST0321003. [DOI] [PubMed] [Google Scholar]
- 119.Erdmann K, Grosser N, Schroder H. L-methionine reduces oxidant stress in endothelial cells: role of heme oxygenase-1, ferritin, and nitric oxide. AAPS J. 2005;7:E195–E200. doi: 10.1208/aapsj070118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grosser N, Oberle S, Berndt G, Erdmann K, Hemmerle A, Schroder H. Antioxidant action of L-alanine: heme oxygenase-1 and ferritin as possible mediators. Biochem Biophys Res Commun. 2004;314:351–355. doi: 10.1016/j.bbrc.2003.12.089. [DOI] [PubMed] [Google Scholar]
- 121.Uehara K, Takahashi T, Fujii H, Shimizu H, Omori E, Matsumi M, Yokoyama M, Morita K, Akagi T, Sassa S. The lower intestinal tract-specific induction of heme oxygenase-1 by glutamine protects against endotoxemic intestinal injury. Crit Care Med. 2005;33:381–390. doi: 10.1097/01.ccm.0000153407.14237.7f. [DOI] [PubMed] [Google Scholar]
- 122.Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 123.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators: C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 124.Grosser N, Hemmerle A, Berndt G, Erdmann K, Hinkelmann U, Schurger S, Wijayanti N, Immenschuh S, Schroder H. The antioxidant defense protein heme oxygenase-1 is a novel target for statins in endothelial cells. Free Radic Biol Med. 2004;37:2061–2071. doi: 10.1016/j.freeradbiomed.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 125.Grosser N, Erdmann K, Hemmerle A, Berndt G, Hinkelmann U, Smith G, Schroder H. Rosuvastatin upregulates the antioxidant defense protein heme oxygenase-1. Biochem Biophys Res Commun. 2004;325:871–876. doi: 10.1016/j.bbrc.2004.10.123. [DOI] [PubMed] [Google Scholar]
- 126.Lee TS, Chang CC, Zhu Y, Shyy JY. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 2004;110:1296–1302. doi: 10.1161/01.CIR.0000140694.67251.9C. [DOI] [PubMed] [Google Scholar]
- 127.Hsu M, Muchova L, Moriaka I, Wong JR, Schroder H, Stevenson DK. Tissue-specific effects of statins on the expression of heme oxygenase-1 in vivo. Biochem Biophys Res Commun. 2006;343:738–744. doi: 10.1016/j.bbrc.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 128.Ali F, Zakkar M, Karu K, Lidington EA, Hamdulay SS, Boyle JJ, Zloh M, Bauer A, Haskard DO, Evans PC, Mason JC. Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow. J Biol Chem. 2009;284:18882–18892. doi: 10.1074/jbc.M109.009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Loboda A, Jazwa A, Jozkowicz A, Dorosz J, Balla J, Molema G, Dulak J. Atorvastatin prevents hypoxia-induced inhibition of endothelial nitric oxide synthase expression but does not affect heme oxygenase-1 in human microvascular endothelial cells. Atherosclerosis. 2006;187:26–30. doi: 10.1016/j.atherosclerosis.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sawayama Y, Shimizu C, Maeda N, Tatsukawa M, Kinukawa N, Koyanagi S, Kashiwagi S, Hayashi J. Effect of probucol and pravastin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia: Fukuoka Atherosclerosis Trial (FAST) J Am Coll Cardiol. 2002;39:610–616. doi: 10.1016/s0735-1097(01)01783-1. [DOI] [PubMed] [Google Scholar]
- 131.Witting PK, Pettersson K, Letters J, Stocker R. Site-specific anti-atherogenic effect of probucol in apolipoprotein E deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:e26–e33. doi: 10.1161/01.atv.20.8.e26. [DOI] [PubMed] [Google Scholar]
- 132.Deng YM, Wu BJ, Witting PK, Stocker R. Probucol protects against smooth muscle cell proliferation by upregulating heme oxygenase-1. Circulation. 2004;110:1855–1860. doi: 10.1161/01.CIR.0000142610.10530.25. [DOI] [PubMed] [Google Scholar]
- 133.Wu BJ, Kathir K, Witting PK, Beck K, Choy K, Li C, Croft KD, Mori TA, Tanous D, Adams MR, Lau AK, Stocker R. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med. 2006;203:1117–1127. doi: 10.1084/jem.20052321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grosser N, Abate A, Oberle S, Vreman HJ, Dennery PA, Becker JC, Pohle T, Seidman DS, Schroder H. Heme oxygenase-1 induction may explain the antioxidant profile of aspirin. Biochem Biophys Res Commun. 2003;308:956–960. doi: 10.1016/s0006-291x(03)01504-3. [DOI] [PubMed] [Google Scholar]
- 135.Visner GA, Lu F, Zhou H, Liu J, Kazemfar K, Agarwal A. Rapamycin induces heme oxygenase-1 in human pulmonary vascular cells: implications in the antiproliferative response to rapamycin. Circulation. 2003;107:911–916. doi: 10.1161/01.cir.0000048191.75585.60. [DOI] [PubMed] [Google Scholar]
- 136.Zhou H, Liu H, Porvasnik SL, Terada N, Agarwal A, Cheng Y, Visner GA. Heme oxygenase-1 mediates the protective effects of rapamycin in monocrotaline-induced pulmonary hypertension. Lab Invest. 2006;86:62–71. doi: 10.1038/labinvest.3700361. [DOI] [PubMed] [Google Scholar]
- 137.Choi BM, Kim YM, Jeong YR, Pae HO, Song CE, Park JE, Ahn YK, Chung HT. Induction of heme oxygenase-1 is involved in the antiproliferative effects of paclitaxel in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 2004;25:39–47. doi: 10.1016/j.bbrc.2004.06.120. [DOI] [PubMed] [Google Scholar]
- 138.Liu XM, Chapman GB, Wang H, Durante W. Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells. Circulation. 2002;105:79–84. doi: 10.1161/hc0102.101369. [DOI] [PubMed] [Google Scholar]
- 139.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression due to reactive iron. FASEB J. 1999;13:1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
- 140.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 141.Duckers HJ, Boehm M, True AL, Yet S-F, Park JL, Webb RC, Lee M-E, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;7:693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 142.Tulis DA, Durante W, Liu X, Evan AJ, Peyton KJ, Schafer AI. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation. 2001;104:2710–2715. doi: 10.1161/hc4701.099585. [DOI] [PubMed] [Google Scholar]
- 143.Abraham NG, Asija A, Drummond G, Peterson S. Heme oxygenase-1 gene therapy: recent advances and therapeutic applications. Curr Gene Ther. 2007;7:89–108. doi: 10.2174/156652307780363134. [DOI] [PubMed] [Google Scholar]
- 144.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Neil Smith R, Czismadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach FH, Choi AMK, Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 145.Moore BA, Overhaus M, Whitcomb J, Ifedigbo E, Choi AM, Otterbein LE, Bauer AJ. Brief inhalation of low-dose carbon monoxide prevents graft-induced intimal hyperplasia in swine. J Surg Res. 2007;138:121–127. doi: 10.1016/j.jss.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 146.Zuckerbraun BS, Chin BY, Wegiel B, Billiar TR, Czsimadia E, Rao J, Shimoda L, Ifedigbo E, Kanno S, Otterbein LE. Carbon monoxide reverses established pulmonary hypertension. J Exp Med. 2006;203:2109–2119. doi: 10.1084/jem.20052267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ott MC, Scott JR, Bihari A, Badhwar A, Otterbein LE, Gray DK, Harris KA, Potter RF. Inhalation of carbon monoxide prevents liver injury and inflammation following hindlimb ischemia/reperfusion. FASEB J. 2005;19:106–108. doi: 10.1096/fj.04-2514fje. [DOI] [PubMed] [Google Scholar]
- 148.Carbon monoxide now orphan drug for transplantation. Inpharma. 2008;1:21. [Google Scholar]
- 149.Mayr FB, Spiel S, Leitner J, Marsik C, Germann P, Ullrich R, Wagne O, Jilma B. Effect of carbon monoxide inhalation during experimental endotoxemia in humans. Am J Respir Crit Care Med. 2005;171:354–360. doi: 10.1164/rccm.200404-446OC. [DOI] [PubMed] [Google Scholar]
- 150.Bathoorn E, Slebos D-J, Postma DS, Koeter GH, van Oosterhout AJM, van der Toorn M, Boezen HM, Kerstjens HAM. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur Respir J. 2007;30:1131–1137. doi: 10.1183/09031936.00163206. [DOI] [PubMed] [Google Scholar]
- 151.Tulis DA, Keswani AN, Peyton KJ, Wang H, Schafer AI, Durante W. Local administration of carbon monoxide inhibits neointima formation in balloon injured rat carotid arteries. Cell Mol Biol (Noisy-le-grand) 2005;51:441–446. [PMC free article] [PubMed] [Google Scholar]
- 152.Vera T, Henegar JR, Drummond HA, Rimoldi JM, Stec DE. Protective effect of carbon monoxide-releasing compounds in ischemia-induced acute renal failure. J Am Soc Nephrol. 2005;16:950–958. doi: 10.1681/ASN.2004090736. [DOI] [PubMed] [Google Scholar]
- 153.Foresti R, Shurey C, Ansari T, Sibbons P, Mann BE, Johnson TR, Green CJ, Motterlini R. Reviewing the use of carbon monoxide-releasing molecules (CO-RMs) in biology: implications in endotoxin-mediated vascular dysfunction. Cell Mol Biol (Noisy-le-grand) 2005;51:409–423. [PubMed] [Google Scholar]
- 154.Hyvelin JM, Maurel B, Uzbekov R, Motterlini R, Lermusiaux P. Hemin prevents in-stent stenosis in rat and rabbit models by inducing heme oxygenase-1. J Vasc Res. 2010;51:417–428. doi: 10.1016/j.jvs.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 155.Ollinger R, Bilban M, Erat A, Froio A, McDaid J, Tyagi S, Csizmadia E, Graca-Souza AV, Liloia A, Soares MP, Otterbein LE, Usheva A, Yamashita K, Bach FH. Bilirubin: a natural inhibitor of vascular smooth muscle cell proliferation. Circulation. 2005;112:1030–1039. doi: 10.1161/CIRCULATIONAHA.104.528802. [DOI] [PubMed] [Google Scholar]
- 156.Nakao A, Murase N, Ho C, Toyokawa B, Billiar TR, Kanno S. Biliverdin administration prevents the formation of intimal hyperplasia induced by vascular injury. Circulation. 2005;112:587–591. doi: 10.1161/CIRCULATIONAHA.104.509778. [DOI] [PubMed] [Google Scholar]
- 157.Schwertner HA, Jackson WG, Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem. 1994;40:18–23. [PubMed] [Google Scholar]
- 158.Perlstein TS, Pande RL, Beckman JA, Creager MA. Serum total bilirubin level and prevalent lower-extremity peripheral artery disease. Arterioscler Thromb Vasc Biol. 2008;28:166–172. doi: 10.1161/ATVBAHA.107.153262. [DOI] [PubMed] [Google Scholar]