Abstract
Exposure to an environmental endocrine disruptor (e.g., vinclozolin) during embryonic gonadal sex determination appears to alter the male germ line epigenome and subsequently promotes transgenerational adult onset disease. The epigenetic mechanism involves the induction of new imprinted-like genes/DNA sequences in the germ line that appear to transmit disease phenotypes. The disease phenotypes include testis abnormalities, prostate disease, kidney disease, immune abnormalities, and tumor development. This epigenetic transgenerational disease mechanism provides a unique perspective from which to view inheritable adult onset disease states, such as cancer, and ultimately offers new insights into novel diagnostic and therapeutic strategies.
Keywords: epigenetics, transgenerational, adult onset disease, gonadal development, DNA methylation
I. REVIEW
Epigenetics refers to the regulation of genome activity that does not directly involve changes in the DNA sequence. The stability of the DNA sequence is extensive and is not readily mutated or modified. The epigenetic regulation of the genome involves factors such as histone modifications and DNA methylation that directs chromatin structure and gene transcription. Much of the research on environmental factors and toxicants has not demonstrated direct modification of the genomic DNA sequence; however, these factors can alter epigenetic factors. A consideration of environment-genome interactions requires that epigenetic regulation be considered as a component of the molecular basis on which the environmental factors influence the genome.
Environmental exposures have been found to promote several transgenerational disease states or phenotypes.1 In general, an embryonic or early postnatal exposure is required for these transgenerational phenotypes to develop. For example, embryonic exposure to diethylstilbesterol (DES) promotes reproductive tract defects in both male and female F2 generation offspring.2 Another example is the ability of embryonic nutritional defects (i.e., caloric restriction) to promote an F2 generation diabetes phenotype.3 The reproducibility and frequency of these disease phenotypes suggests that they are more likely the result of epigenetic alterations rather than due to DNA sequence mutations, since DNA sequences are highly stable. The potential that these phenotypes are induced by an epigenetic mechanism has not yet been directly identified, but studies suggest such a phenomenon may exist.
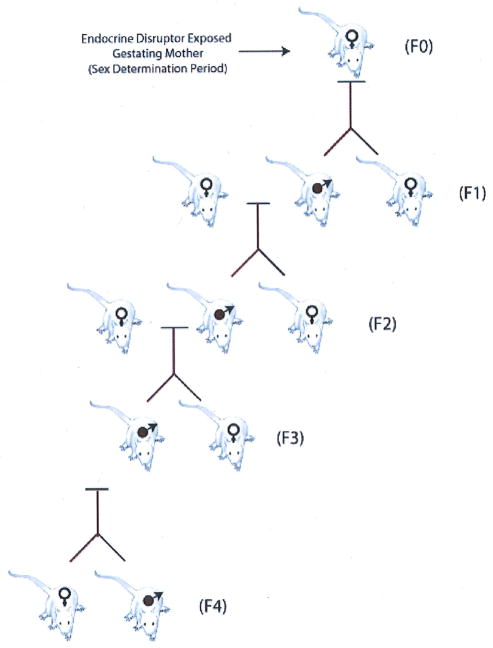
A transgenerational phenomenon can be defined as the ability of an acquired physiological phenotype (i.e., disease state) to be transmitted to subsequent generations through the male germ line, such that the subsequent generation is not directly exposed to the environmental factor or toxicant. For example, the exposure of a gestating mother exposes the F0 generation mother, the F1 generation embryo, and the germ line that will generate the F2 generation. Since multiple generations are exposed, the phenotypes in the F0, F1, and F2 generations could be due to toxicology of the direct exposure and not necessarily transmitted transgenerationally through an alternate mechanism. Therefore, in the above case the F3 generation would be the first unequivocal transgenerational generation not directly exposed. This does not rule out that the F2 generation is not a transgenerational phenotype, but simply points out the limitation in concluding this as a result of the direct exposure of the germ line in the F2 generation. A multigenerational exposure, such as that of an exposed gestating mother, can transmit a phenotype due to the toxicology of the direct exposure, but a transgenerational phenotype involves the transmission of a phenotype independent of the direct exposure (Fig. 1).
FIGURE 1.
Transgenerational transmission of adult onset disease through the male germ line following endocrine disruptor exposure of a gestating female.
Recently, the observation was made that the transient exposure of an F0 generation gestating rat at the time of embryonic sex determination to an endocrine disruptor promotes an adult onset disease of spermatogenic defects and male sub-fertility.4 Observations demonstrate that 90% of all male progeny for four generations (F1–F4) developed spermatogenic defects following the direct exposure of the F0 gestating rat.4 This transgenerational phenotype was only transmitted through the male germ line (i.e., sperm) and was not passed through the female germ line (i.e., oocyte). Prior to 120 days of age, the primary disease phenotype was a spermatogenic cell defect in the male testis.4,5 Animals that were allowed to age up to 14 months were found to have additional transgenerational disease phenotypes beyond spermatogenic cell defects. These aged animals had the following disease state frequencies: 17% tumor development, 51% prostate disease, 38% kidney disease, 29%) immune abnormalities, and 27% severe spermatogenic defects in males from F1 to F4 generations.6 Female animals also developed transgenerational disease states including tumor development and kidney disease.
Exposure of gestating female rats to endocrine disruptors induced a transgenerational phenotype of increased tumor frequencies. These tumors included skin merkel cell tumor, lung sarcoma, knee synovial cell tumor, and mammary adenomas, and fibroadenomas in the male rats. Mammary gland tumors were the most frequent tumors observed and they occurred at similar frequencies in the aged male exposed F1–F4 generations. Along with tumors, the aged males developed regions of hyperplasia in the ventral prostate. However, in the female vinclozolin F1–F3 generations, the tumors were limited to mammary adenomas, fibroadenomas, and carcinomas, as well as pituitary tumors and a brain astrocytoma. Interestingly, these female tumors were specific to the F2 and F3 generations. Aged match controls developed very few tumors in the females (i.e., less than 2%) and none of the males.6
The transgenerational phenotype was induced by exposing the F0 gestating rat to the endocrine disruptor vinclozolin. Vinclozolin is an antiandrogenic compound used as a fungicide in the fruit industry (e.g., wineries).7 The spermatogenic cell defect can also be induced by the pesticide methoxychlor, which has a mixture of estrogenic, antiestrogenic, and antiandrogenic metabolites.4,8 The ability of endocrine disruptors to promote adult onset disease has been previously reviewed.9 Endocrine disruptors are a large class of environmental toxicants ranging from plastics to pesticides.10 These environmental toxicants generally do not promote DNA sequence mutations, which generally occur at a frequency less than 0.01%.11 The frequency of the transgenerational phenotype described above (occurring in 30–90% of the sample population) could not be attributed to DNA sequence mutations. Therefore, the hypothesis was developed that the transgenerational phenotype observed is an epigenetic transgenerational phenotype resulting from changes in gene function that are not related to a DNA sequence mutation.1,4
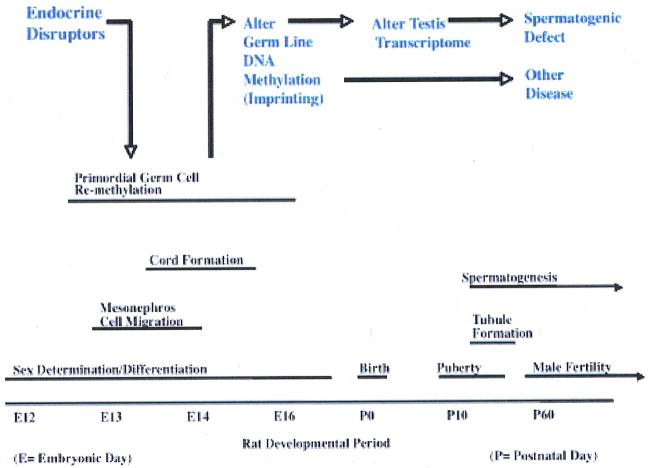
Sex determination has been shown to involve a demethylation and remethylation of the germ line in a sex-specific manner.12 Potential permanent reprogramming of DNA methylation of the male germ line is speculated to be involved in the transgenerational phenotype. The epigenetic transgenerational phenotype appears to involve an endocrine disruptor (i.e., vinclozolin) exposure during embryonic sex determination resulting in an epigenetically reprogramming (i.e., DNA methylation) of the male germ line that induces new imprinted-like DNA sequences (Fig. 2). This altered epigenome is then transmitted through the male germ line to all subsequent progeny (F1–F4) causing a dysregulation of the genome altering transcriptomes in a variety of organs and promoting transgenerational disease.4,6,13
FIGURE 2.
Proposed mechanism involved in the transgenerational epigenetic phenomena.
A large number of studies have demonstrated that embryonic or postnatal exposures can induce adult onset disease.9,10 The mechanism for this fetal basis of adult onset disease, however, is largely unknown, but likely involves in part epigenetic alterations in the genome. Many adult onset disease phenotypes are not transgenerational, but manifest in the exposed individuals. These individual disease exposures and phenotypes may also involve epigenetic mechanisms, but currently cannot be separated from the results of the direct exposure. A recent study demonstrated a pubertal exposure to bisphenol A (BPA) promoted an alteration in DNA methylation of a number of genes and in the adult promoted a high frequency of prostate disease.14 Therefore, an embryonic, postnatal, or adult exposure could cause an epigenetic event that then alters the physiology of a tissue and promotes disease. It is likely that rapidly developing tissues will be more sensitive to environmental exposures and epigenetic modifications. The ability of an environmental exposure to induce an abnormal phenotype or physiology is likely to involve epigenetic mechanisms in the toxicology of the agent or compound. Epigenetics will be an important process to consider in the investigation of environmental exposures, environment-genome interactions, and the toxicology of specific compounds.
Tumor formation in the Sprague-Dawley rat model is not uncommon. Male and female rats often develop a variety of tumors as they age (i.e., 24 months old) at frequencies of 0.01% to 7.0%. In the male rat, the common tumor is the adenoma of the pituitary, which occurs at an average frequency of 46% in 24-month-old animals. Whereas most mammary, lung, skin, brain, and prostate tumors occur at less than 1–2% in 24-month-old rats. In females, the most common types of tumors are the pituitary adenomas at 70% and mammary gland fibroadenomas at 32% frequencies. Tumors found in the skin, brain, lung, and uterus occur at < 1–2%) in 24-month-old rats (Charles River Web site: www.criver.com). The tumor frequency of > 10%) at an age of < 14 months old in the endocrine disruptor F1–F4 generations has not been previously observed.
Many human cancers to date have epigenetic components to their disease etiology including prostate,15–17 colon,18–20 lung,20 intestinal,21 and breast.20 These epigenetic alterations are usually the result of DNA methylation changes; however, alterations in chromatin remodeling and histone modifications 17,22–24 are also associated with cancer development and progression. Speculations have been made that these epigenetic alterations in cancer could be the result of environmental exposure.25–28 The fetal basis of cancer susceptibility and progression has also been postulated to involve epigenetics.19,25,27,29 These previous links between epigenetics and cancer suggest the epigenetic transgenerational phenotype observed may be a factor to consider in cancer biology.
The epigenetic transgenerational disease phenotype described was due to an embryonic exposure promoting an adult onset disease. The speculation is drawn that an altered germ line epigenome is the causal mechanism for the adult onset disease observed. The identification of novel epigenetic diagnostics and therapeutic strategies based on this unique disease etiology is anticipated to provide a significant advance in our understanding of disease phenotype transmission and development. An elucidation of the role of epigenetics in environment-genome interactions will provide critical insights for environmental health and disease.
Acknowledgments
We acknowledge the assistance of Ms. Jill Griffin and Ms. Rochelle Pedersen in the preparation of the manuscript. This research was supported in part by grants from the USA National Institutes of Health, NIH/NIEHS to MKS.
References
- 1.Anway MD, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006;147(6 Suppl):S43–9. doi: 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- 2.Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Increased tumors but uncompromised fertility in the female descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 1998;19(9):1655–63. doi: 10.1093/carcin/19.9.1655. [DOI] [PubMed] [Google Scholar]
- 3.Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005;566(Pt 1):225–36. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disrupters and male fertility. Science. 2005;308(5727):1466–9. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anway MD, Memon MA, Uzumcu M, Skinner MK. Transgenerational effect of the endocrine disrupter vinclozolin on male spermatogenesis. J Androl. 2006;27(6):868–79. doi: 10.2164/jandrol.106.000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anway MD, Leathers C, Skinner MK. Endocrine disrupter vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology. 2006;147(12):5515–23. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE., Jr Environmental hormone disrupters: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol. 1994;126(2):276–85. doi: 10.1006/taap.1994.1117. [DOI] [PubMed] [Google Scholar]
- 8.Cupp AS, Uzumcu M, Suzuki H, Dirks K, Phillips B, Skinner MK. Effect of transient embryonic in vivo exposure to the endocrine disruptor methoxychlor on embryonic and postnatal testis development. J Androl. 2003;24(5):736–45. doi: 10.1002/j.1939-4640.2003.tb02736.x. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004;56(3):311–7. doi: 10.1203/01.PDR.0000135998.08025.FB. [DOI] [PubMed] [Google Scholar]
- 10.Heindel JJ. The fetal basis of adult disease: Role of environmental exposures--introduction. Birth Defects Res A Clin Mol Teratol. 2005;73(3):131–2. doi: 10.1002/bdra.20119. [DOI] [PubMed] [Google Scholar]
- 11.Barber R, Plumb MA, Boulton E, Roux I, Dubrova YE. Elevated mutation rates in the germ line of first- and second-generation offspring of irradiated male mice. Proc Natl Acad Sci U S A. 2002;99(10):6877–82. doi: 10.1073/pnas.102015399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamazaki Y, Mann MR, Lee SS, Marh J, McCarrey JR, Yanagimachi R, Bartolomei MS. Reprogramming of primordial germ cells begins before migration into the genital ridge, making these cells inadequate donors for reproductive cloning. Proc Natl Acad Sci U S A. 2003;100(21):12207–12. doi: 10.1073/pnas.2035119100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang HS, Anway MD, Rekow SS, Skinner MK. Transgenerational epigenetic imprinting of the male germline by endocrine disruptor exposure during gonadal sex determination. Endocrinology. 2006;147(12):5524–41. doi: 10.1210/en.2006-0987. [DOI] [PubMed] [Google Scholar]
- 14.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66(11):5624–32. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobosy JR, Roberts JL, Fu VX, Jarrard DF. The expanding role of epigenetics in the development, diagnosis and treatment of prostate cancer and benign prostatic hyperplasia. J Urol. 2007;177(3):822–31. doi: 10.1016/j.juro.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 16.Manoharan M, Ramachandran K, Soloway MS, Singal R. Epigenetic targets in the diagnosis and treatment of prostate cancer. Int Braz J Urol. 2007;33(1):11–8. doi: 10.1590/s1677-55382007000100003. [DOI] [PubMed] [Google Scholar]
- 17.Schulz WA, Hatina J. Epigenetics of prostate cancer: beyond DNA methylation. J Cell Mol Med. 2006;10(1):100–25. doi: 10.1111/j.1582-4934.2006.tb00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feinberg AP. The epigenetics of cancer etiology. Semin Cancer Biol. 2004;14(6):427–32. doi: 10.1016/j.semcancer.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Jass JR. Heredity and DNA methylation in colorectal cancer. Gut. 2007;56(1):154–5. [PMC free article] [PubMed] [Google Scholar]
- 20.Shames DS, Girard L, Gao B, Sato M, Lewis CM, Shivapurkar N, Jiang A, Perou CM, Kim YH, Pollack JR, Fong KM, Lam CL, Wong M, Shyr, Nanda R, Olopade OI, Gerald W, Euhus DM, Shay JW, Gazdar AF, Minna JD. A genome-wide screen for promoter methylation in lung cancer identifies novel methylation markers for multiple malignancies. PLoS Med. 2006;3(12):e486. doi: 10.1371/journal.pmed.0030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuchiya T, Tamura G, Sato K, Endoh Y, Sakata K, Jin Z, Motoyama T, Usuba O, Kimura W, Nishizuka S, Wilson KT, James SP, Yin J, Fleisher AS, Zou T, Silverberg SG, Kong D, Meltzer SJ. Distinct methylation patterns of two APC gene promoters in normal and cancerous gastric epithelia. Oncogene. 2000;19(32):3642–6. doi: 10.1038/sj.onc.1203704. [DOI] [PubMed] [Google Scholar]
- 22.Herranz M, Esteller M. DNA methylation and histone modifications in patients with cancer: potential prognostic and therapeutic targets. Methods Mol Biol. 2007;361:25–62. doi: 10.1385/1-59745-208-4:25. [DOI] [PubMed] [Google Scholar]
- 23.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6(8):597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 24.Ting AH, McGarvey KM, Baylin SB. The cancer epigenome--components and functional correlates. Genes Dev. 2006;20(23):3215–31. doi: 10.1101/gad.1464906. [DOI] [PubMed] [Google Scholar]
- 25.Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: Linking early developmental environment to adult disease. Reprod Toxicol. 2007;23(3):297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Herceg Z. Epigenetics and cancer: towards an evaluation of the impact of environmental and dietary factors. Mutagenesis. 2007;22(2):91–103. doi: 10.1093/mutage/gel068. [DOI] [PubMed] [Google Scholar]
- 27.van Vliet J, Oates NA, Whitelaw E. Epigenetic mechanisms in the context of complex diseases. Cell Mol Life Sci. 2007;64:1531–8. doi: 10.1007/s00018-007-6526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weidman JR, Dolinoy DC, Murphy SK, Jirtle RL. Cancer susceptibility: epigenetic manifestation of environmental exposures. Cancer J. 2007;13(1):9–16. doi: 10.1097/PPO.0b013e31803c71f2. [DOI] [PubMed] [Google Scholar]
- 29.Dolinoy DC, Das R, Weidman JR, Jirtle RL. Metastable epialleles, imprinting, and the fetal origins of adult diseases. Pediatr Res. 2007;61(5 Pt 2):30R–7R. doi: 10.1203/pdr.0b013e31804575f7. [DOI] [PubMed] [Google Scholar]