Abstract
Hunger, mostly initiated by a deficiency in energy, induces food seeking and intake. However, the drive toward food is not only regulated by physiological needs, but is motivated by the pleasure derived from ingestion of food, in particular palatable foods. Therefore, feeding is viewed as an adaptive motivated behavior that involves integrated communication between homeostatic feeding circuits and reward circuits. The initiation and termination of a feeding episode are instructed by a variety of neuronal signals, and maladaptive plasticity in almost any component of the network may lead to the development of pathological eating disorders. In this review we will summarize the latest understanding of how the feeding circuits and reward circuits in the brain interact. We will emphasize communication between the hypothalamus and the mesolimbic dopamine system and highlight complexities, discrepancies, open questions and future directions for the field.
Keywords: feeding, neural circuitry, hedonic, reward, mesolimbic system, dopamine, hypothalamus
Introduction
In mammals, feeding is coordinated by peripheral and central neuronal signaling to help maintain energy homeostasis. Feeding behavior is under complex control and is significantly influenced by emotions (i.e. pleasure, joy, stress, anxiety, depression and fear), environmental factors (i.e. temperature and time of day) and genetic traits (i.e. epigenetic modifications and genetic mutations).
Regulatory mechanisms that control feeding may be divided into two categories — homeostatic, and reward-initiated (i.e. hedonic). Homeostatic mechanisms are defined as an increased drive to eat after energy depletion, followed by termination of feeding upon replenishment. Homeostasis is regulated by the metabolic state of the body, activity levels, and the distribution of essential nutrients (carbohydrates, fats, proteins and minerals) (Blouet and Schwartz, 2010; Volkow et al., 2011). Hedonic mechanisms are defined as the motivation to consume palatable foods even when the homeostatic “set-point” has been reached (Volkow et al., 2011). The interplay between the homeostatic and hedonic systems is intimate and sculpts feeding behavior.
The hypothalamus is traditionally recognized as the main brain region regulating food intake. It regulates feeding as a function of caloric and nutritional requirements, by sensing macronutrients and through the action of circulating regulatory hormones, neuropeptides and neuromodulators such as leptin, cholecystokinin (CCK), ghrelin, orexin/hypocretin, insulin, neuropeptide Y (NPY) and endocannabinoids (Coll et al., 2007; Dietrich and Horvath, 2009; Blouet and Schwartz, 2010; Volkow et al., 2011). In particular, homeostatic food intake is tightly regulated by communication among hypothalamic nuclei including the arcuate nucleus (ARC), the paraventricular nucleus of the hypothalamus (PVH), the ventromedial and dorsomedial hypothalamus, as well as the lateral hypothalamic area (LHA). Misregulated hypothalamic function can lead to eating disorders such as anorexia, causing drastic body weight reduction, or hyperphagia, leading to body mass increase and obesity (Goldstone, 2006; Belgardt et al., 2009). Many limbic brain areas including the ventral tegmental area (VTA), nucleus accumbens (NAc), amygdala and hippocampus, as well as cortical brain regions including the medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), cingulate gyrus (ACC) and insula, have been implicated in hedonic aspects of feeding (Petrovich et al., 2005; Volkow et al., 2008; Land et al., 2014). With a growing appreciation for the role played by the reward circuit in defining hedonic aspects of feeding, binge eating disorders are increasingly viewed as sharing common mechanisms with addiction to drug of abuse (Lutter and Nestler, 2009; Kenny, 2011a; Volkow et al., 2013). Both are compulsive behavioral disorders that can be viewed as stemming from maladaptive plasticity in the mesolimbic dopaminergic (DA) system, which is essential for the development of adaptive motivated behaviors (Volkow et al., 2013). In this review, we will describe how regulation of food intake by the hypothalamus can be modulated by the neurocircuitry for reward and motivation (Baicy et al., 2007; Farooqi et al., 2007) (Fig. 1).
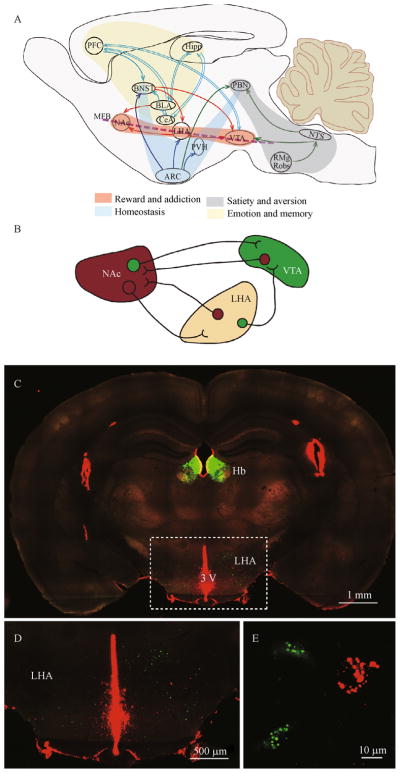
Figure 1.
Neural circuits regulate feeding behaviors. (A) Homeostatic feeding is mainly regulated by hypothalamic regions receiving input from the arcuate nucleus (ARC) including paraventricular nucleus of the hypothalamus (PVH). The neural circuit for reward/addiction involves the mesolimbic DA system, including the ventral tegmental area (VTA) and the nucleus accumbens (NAc). The lateral hypothalamic area (LHA) relates homeostatic feeding to reward by directly synapsing on neurons in the VTA and NAc. Brain regions in charge of emotion and memory also modulate feeding behavior, such as the prefrontal cortex (PFC), the hippocampus (Hipp) and the amygdala nuclei including the basal lateral amygdala (BLA), the central amygdala (CeA) and the bed nucleus of stria terminalis (BNST). Nucleus tractus solitarius (NTS) in the hindbrain directly receives inputs from gastrointestinal structures, and communicates the peripheral signals to the parabrachial nucleus (PBN) to regulate satiety and aversion. The NTS also receives anorexigenic signals from the serotonergic neurons in the dorsal raphe. (B) A simplified diagram of LHA-mesolimbic interactions. (C) Retrograde tracing showing LHA neurons project to the NAc (Red) and VTA (Green). Red and green fluorescent beads were injected to the NAc and VTA, respectively. Hb: Habenula.
The neural circuit of homeostatic regulation of feeding has been discussed in many excellent reviews (Morton et al., 2006; Morton and Salovitz, 2006; Sternson, 2013; Morton et al., 2014). In this review, we focus on the interactions between the neural circuitry regulating feeding and the neural circuitry of reward. We hope to convince the reader that the interaction between the lateral hypothalamic area (LHA) and the mesolimic dopamine (DA) system is a key element in the regulation of feeding, and a major target for maladaptive plasticity underlying the development of overeating. Specifically, we focus on the regulation of hedonic aspects of feeding by ventral tegmental (VTA) DA neurons and nucleus accumbens (NAc) medium spiny neurons (MSNs) and the integration of the hedonic input from the reward circuitry with homeostatic regulation of feeding by neurons in the LHA.
The lateral hypothalamus (LHA)— the linker between the hypothalamic feeding circuitry and the reward circuitry
The LHA is a large and heterogeneous area with several distinct subregions. As one of the most extensively interconnected areas of the hypothalamus, with a vast array of interoceptive and exteroceptive afferent inputs and an equally rich efferent connectivity, the LHA is positioned to integrate homeostatic information and orchestrate adaptive responses by modulating cognitive, motor, autonomic, and endocrine functions. The LHA receives metabolic state information through both neural and humoral routes and can affect energy assimilation and expenditure through direct access to behavioral, autonomic, and endocrine effector pathways.
The LHA was classically identified as a “hunger center” about 60 years ago, with observations that electrical stimulation of the LHA is reinforcing and induces feeding, while lesions to the LHA depress feeding (Anand and Brobeck, 1951; Teitelbaum and Stellar, 1954; Morrison and Mayer, 1957; Miller, 1960). Diverse subtypes of neurons are loosely distributed around the fornix to form the LHA within the lateral part of the tuberal region of the hypothalamus. However, this region and the multiple cell types within it have been strongly implicated in the regulation of energy expenditure and feeding (Willie et al., 2001; Saper et al., 2005). Major neurochemically defined subtypes of LHA neurons include Melanin Concentrating Hormone (MCH) secreting cells (Skofitsch et al., 1985; Nahon et al., 1989), which likely co-release GABA (Harthoorn et al., 2005; Meister, 2007); orexin/hypocretin-secreting neurons, which are likely glutamatergic (Rosin et al., 2003); thyrotropin-releasing hormone (TRH)-secreting neurons (Horjales-Araujo et al., 2014); as well as non-MCH/Orexin expressing GABAergic neurons (Karnani et al., 2013), a subset of which expresses leptin receptors (Leinninger et al., 2009) and neurotensin (Leinninger et al., 2011; Kempadoo et al., 2013; Goforth et al., 2014). All of these cellular subtypes are strongly implicated in regulating feeding (Qu et al., 1996; Lu et al., 2000; Kokkotou et al., 2001; Ludwig et al., 2001).
A complex wiring pattern of the LHA has emerged from tracing experiments, whereby subregions of the LHA are connected with almost every major division of the brain (Hahn and Swanson, 2010; Hahn and Swanson, 2012). Even so, it is clear that the LHA receives significant inputs from the NAc, the Bed Nucleus of Stria Terminalis (BNST), and multiple hypothalamic nuclei, including the preoptic nucleus, the dorsomedial nucleus (DMH), the ventromedial nucleus (VMH) and the ARC. LHA neurons give rise to many fibers in the medial forebrain bundle, which connects forebrain structures including the NAc to the tegmentum (Ciriello et al., 2003; Hahn and Swanson, 2010; Hahn and Swanson, 2012). These wiring patterns not only enable fast communication between the LHA and the mesolimbic reward system, but also position the LHA as a linker engaging the circuits of homeostatic feeding and of reward/emotion.
Classic studies of intracranial self-stimulation have been highly influential in defining the role of the LHA as an integrator of rewarding aspects of feeding. Intracranial stimulation of the LHA induces voracious feeding and moreover, following removal of food, the lever-pressing rate for LHA self-stimulation gradually increases with time, resembling a learned behavior (Olds and Milner, 1954; Atrens et al., 1982). Interestingly, when food and the opportunity to self-stimulate the LHA are simultaneously available, rats develop a preference for electrical stimulation of the LHA over eating regular chow. However, access to palatable food dampens the increasing rate of level pressing for stimuli (Routtenberg and Lindy, 1965; Spies, 1965). These classical experiments suggest that 1) Activation of the LHA initiates food intake; 2) LHA stimulation is reinforcing and ‘rewarding’ (Hoebel and Teitelbaum, 1962; Frank et al., 1982); and 3) Pleasure derived from eating palatable foods is more intense than direct activation of the LHA, potentially due to involvement of additional elements of the reward circuitry (Routtenberg and Lindy, 1965; Spies, 1965; Coons and Cruce, 1968; Wise, 1974). Thus, it is likely that the initiation of food intake and the rewarding aspects of hedonic feeding (as well as direct LHA stimulation) are mediated by distinct pathways that converge in the LHA (Frank et al., 1982).
The rewarding character of feeding evoked by activation of the LHA is mediated by the DA system, since feeding evoked by stimulation of the LHA is prevented by blocking striatal DA signaling (Saper et al., 2002). Furthermore, the LHA closely interacts with the mesolimic DA system: LHA neurons project to the VTA (Leinninger et al., 2009) and the NAc, while the NAc provides reciprocal input to the LHA (Bittencourt et al., 1992; Sano and Yokoi, 2007) (Fig. 1B–E). However, the NAc–LHA and LHA–VTA pathways may mediate different aspects of eating behaviors. The stimulatory role of the LHA in driving food consumption appears to be constrained by tonic inhibition from striatal pathways (for review see Kelley et al., 2005b) since inhibition of neurons in the NAc medial shell dramatically promotes voracious eating in well fed animals and correlates with increased Fos expression in LHA and ARC neurons (for review see Kelley et al., 2005b). It is likely that the inhibitory function of NAc neurons on the LHA is indirect, directed through the ventral pallidum (Stratford and Kelley, 1999). More so, the different roles of the VTA and NAc in the regulation of LHA function are far from being resolved, and we venture that this will be a hotbed of discovery in coming years.
An even more complex picture of the functional organization and regulation of the LHA is emerging, as recent observations demonstrate that food intake is also initiated by optogenetic enhancement of inhibitory inputs into the LHA (Atasoy et al., 2008; Atasoy et al., 2012; Betley et al., 2013; Jennings et al., 2013). Specifically, activation of inhibitory inputs from the BNST to the LHA, which suppresses the firing of glutamatergic LHA neurons, induces voracious feeding (Jennings et al., 2013). Furthermore, activation of AgRP axons within the LHA, which presumably also release GABA (Atasoy et al., 2008; Atasoy et al., 2012) and evoke robust feeding, comparable to that driven by direct activation of AgRP cell bodies in the arcuate nucleus (Betley et al., 2013). Resolving the discrepancy between induction of feeding by electric stimulation of the LHA (Miller, 1960; Coons and Cruce, 1968; Wise, 1974), or by optogenetic enhancement of inhibitory input into the LHA (Jennings et al., 2013) requires further investigation. While one explanation is that the electrical stimulation is dominated by activation of local inhibitory circuits, an altenative explanation is that the inhibitory inputs from the BNST and ARC may target specific groups of neurons whose activity may be overridden by electric stimulation of the LHA.
Thus, the LHA may be envisioned as a major component of the “core-forebrain feeding circuitry” (which also includes the PVT, BNST and PVH) (Betley et al., 2013), that comprises of both pro-feeding and pro-satiety “decision-making” neurons. Potentially, the pro-feeding neurons could be regulated by reward, while the pro-satiety neurons are inhibited by inputs from the BNST and ARC. Future investigation is essential in order to confirm this hypothesis and identify these potential neuronal populations.
The nucleus accumbens (NAc) – an integrator of hedonic feeding
The mesolimbic VTA—NAc reward circuitry is a relatively well studied neural system, and is responsible for action selection and the development of adaptive motivated behaviors (Kauer and Malenka, 2007). Experiences are evaluated by the organism and favored actions are reinforced by the neural circutry of reward. The NAc, located in the ventral striatum, is widely regarded as a central node of reward, and integrates glutamatergic inputs from multiple brain regions, including the prefrontal cortex (PFC), the ventral subiculum (vSub), the basolateral amygdala (BLA) (Cardinal et al., 2002), and DA input from the VTA (Kauer and Malenka, 2007; Citri and Malenka, 2008). Based on the different afferent and efferent projections (Zahm and Brog, 1992) and neurochemical characters, two subregions of the NAc are generally recognized as the NAc core (surrounding the anterior commissure) and shell (surrounding the core). Medium spiny neurons (MSN) within the NAc receive DAergic input from the VTA, and project back to the VTA either directly, or indirectly via the ventral pallidum. The direct pathway comprises D1 receptor-expressing MSNs, and encodes a “Go” signal, while D2 receptor-expressing MSNs project indirectly to the VTA and encode a “No Go” signal (Kauer and Malenka, 2007). Modulation of this circuit is thought to underlie the development of addiction, as well as hedonic feeding. For example, a decrease in the levels of D2 DA receptors is observed in the striatum of cocaine addicts (Trifilieff and Martinez, 2014), as well as obese and overweight individuals (Wang et al., 2001; Volkow and Wise, 2005; Haltia et al., 2007). A negative correlation has also been observed between the body mass index (BMI) of overweight individuals and the expression level of D2 receptors in the striatum (Wang et al., 2001; Haltia et al., 2007).
The NAc shell evaluates information coding homeostatic status by integrating afferents from the LHA and POMC neurons in the ARC (Stratford and Kelley, 1999; Kenny, 2011b). The specific activation of melanocortin 4 receptors (MC4Rs) on D1R MSNs from POMC (Lim et al., 2012) also mediates stress-induced anorexigenic responses and weight loss (Millington, 2007; Chung et al., 2009). Meanwhile, the efferents of the NAc shell control feeding through both the cortical motor output systems and the descending signals to the hypothalamus (Kelley et al., 1996; Kelley, 2004). Lesion or local depression caused by enhancing GABA tone in the NAc shell, results in heightened appetite, hyperphagia and weight gain (Stanley et al., 1993; Peciña and Berridge, 1995; Peciña and Berridge, 2000; Wang et al., 2014), along with increased Fos expression within NPY/AgRP cells in the ARC and decreased expression of Fos in POMC neurons (Zheng et al., 2003). These effects could be counteracted by increasing the GABAergic tone within the ARC (Zheng et al., 2003), suggesting a direct inhibitory control of the NAc over NPY/AgRP neurons to limit feeding. Moreover, the NAc shell further controls feeding by depressing other hypothalamic substrates such as the LHA. The inhibition of the LHA is mediated by the ventral pallidum, adding another level of NAc control of the hypothalamus via the indirect pathway (Stratford and Kelley, 1999). The NAc shell also receives reciprocal direct inputs from the LHA, likely from Orexin or MCH neurons, which might serve as feedback loops, regulating the NAc-to-LHA circuit.
In light of these observations, the NAc is identified to exert multidimensional control over food seeking and intake. On one hand, DA in the NAc critically modulates goal-seeking instrumental strategies and augments hedonic experience, particularly with regard to palatable food (Kelley et al., 2005b; Morton et al., 2006; Murray et al., 2014); on the other hand, GABAergic transmission from the NAc exerts a sentinel-like control function over motor outputs. This integrative function of the NAc shows its fundamental importance in the anticipation of rewards and preparing actions toward gratification (Sterling and Eyer, 1988).
Neuropeptides in the LHA—regulation of food intake and mesolimbic function
Two major orexigenic neuropeptides are exclusively produced in LHA neurons and project widely throughout the brain: orexins/hypocretins (Fadel and Deutch, 2002) and MCH (Shimada et al., 1998; Chung et al., 2009). Both of these peptides regulate the activity of VTA DA neurons and NAc medium spiny neurons, impacting both the development of drug addiction, as well as the regulation of feeding. Additionally, neurotensin, most likely an anorexigenic neuropeptide (Boules et al., 2000; Feifel et al., 2010), is also expressed by a subpopulation of LHA neurons, and interestingly, this group of neurons mediate leptin function (Sahu et al., 2001) on orexin neurons in the LHA as well as neurons in the VTA (Leinninger et al., 2011).
Orexin levels in the hypothalamus regulate feeding (Horvath, 2005) and injection of either orexin or MCH into hypothalamic nuclei, including the PVN, DMN, and LHA, elicits feeding in well-fed animals, (Qu et al., 1996; Sakurai et al., 1998; Dube et al., 1999). Orexin neurons have been reported to be directly inhibited by glucose or the anorexigenic peptide leptin, while excited by the orexigenic peptide ghrelin (Yamanaka et al., 2003). LHA orexin neurons send direct projections to the VTA, PFC and striatum, including the NAc shell. In the VTA, orexin-positive afferents intermingle with TH-positive neurons, while in the NAc, orexin-positive terminals intertwine with DA fibers (Fadel and Deutch, 2002). Therefore, orexin neurons in the LHA potentially influence forebrain DA transmission, by acting either directly in the VTA, or in the NAc on inputs from the VTA. In support of this notion, orexin has been found to impact excitatory transmission onto VTA DA neurons, both by producing a LTP–like increase in the AMPA/NMDA ratio and by modulating NMDA receptor trafficking and subunit composition (Borgland et al., 2010). These orexin-induced synaptic modifications are required for sensitization to cocaine, and are blocked by VTA-specific antagonism of orexin receptors (Borgland et al., 2010).
MCH-expressing neurons in the LHA also project widely throughout the brain and MCH receptors are found co-expressed with DA receptors on NAc neurons. Infusion of MCH into the NAc shell increases feeding, while blocking MCH signaling with antagonists decreases feeding (Georgescu et al., 2005). Similar effects of MCH were observed on the regulation of cocaine-elicited behaviors in addiction models: blocking MCH signaling in the NAc decreases cocaine-induced conditioned place preference, as well as behavioral sensitization to cocaine (Chung et al., 2009). Consistent with this observation, MCH receptor 1 knockout mice are hypophagic, lean and hyperactive (Marsh et al., 2002; Lalonde and Qian, 2007). While MCH has been reported to reduce neuronal firing in the NAc shell via a combination of reducing presynaptic excitatory inputs and activation of post-synaptic K+ channel (Sears et al., 2010), MCH receptor activation has also been reported to potentiate the action of DA in the NAc, synergistically increasing firing of NAc MSNs (Chung et al., 2009). Obviously, future investigation is required in order to resolve the role of MCH in regulation of NAc activity.
Receptors for the anorexigenic neuropeptide leptin have also been identified on a subpopulation of LHA GABAergic neurons (Hakansson et al., 1999; Yamanaka et al., 2003; Fulton et al., 2006). However, not all the leptin receptor-expressing neurons in the LHA respond to leptin in the same fashion: one third of leptin receptor-expressing LHA neurons are depolarized by leptin; another third are hyperpolarized by leptin and the remaining do not respond. The reason for these differential effects of leptin on LHA GABAergic neurons is unknown (Leinninger et al., 2009). Nevertheless, ablation of leptin receptors from LHA GABAergic neurons that project to the VTA specifically decreases mesolimbic DA release and contributes to the development of obesity (Fulton et al., 2006; Leinninger et al., 2009).
Feeding peptides can also directly modulate the activity of mesolimbic DA neurons. For example, DA neurons in the VTA express receptors to leptin (Hommel et al., 2006), ghrelin, insulin, orexin, melanocortin and GLP-1 (Narayanan et al., 2010; Dossat et al., 2011; Dossat et al., 2013). Furthermore, orexigenic peptides like ghrelin increase the activity of VTA DA cells and increase DA release in the NAc when exposed to food stimuli (Jerlhag et al., 2012; Skibicka et al., 2013), potentiating an increase in food intake. In contrast, anorexigenic peptides like leptin inhibit DA firing, decrease DA release (Rada et al., 2005; Johnson and Kenny, 2010) and reduce food intake, while decreasing VTA leptin signaling was found to increase sensitivity to palatable foods (Ishiwari et al., 2004; Hommel et al., 2006; Domingos et al., 2011; Murray et al., 2014). As deletion of leptin receptors in GABAergic neurons, but not in AgRP, POMC or glutamatergic neurons, leads to massive increase of body weight, the effect of leptin in the VTA may be indirect and mediated by GABAergic interneurons, rather than through a direct effect on VTA DA neurons (Vong et al., 2011).
In summary, the LHA is positioned as a crucial component, linking the homeostatic feeding circuitry (the ARC) and the reward circuitry (the VTA and NAc) (Fig. 2), and likely governs the transition from homeostatic to hedonic eating. The major neuropeptides (MCH and orexin) secreted by the LHA regulate the activity of neurons of the mesolimbic system to promote feeding, potentially by modulating the hedonic aspects of feeding. Meanwhile, these orexigenic functions are balanced by glutamatergic neurons in the LHA whose action leads to reduction in food intake. Moreover, circulating leptin regulates leptin receptor expressing neurons in the LHA that directly synapse on VTA DA neurons. This communication between the LHA and the mesolimbic system is likely crucial for defining the outcome of an interaction with palatable food, and in formation of maladaptive feeding habits that lead to obesity. It should also be noted that LHA neurons participate in the regulation of additional motivated processes such as arousal, sleep and sexual behavior (Hansen et al., 1981; Krilowicz et al., 1994; Kelley et al., 2005a; Gutierrez et al., 2011).
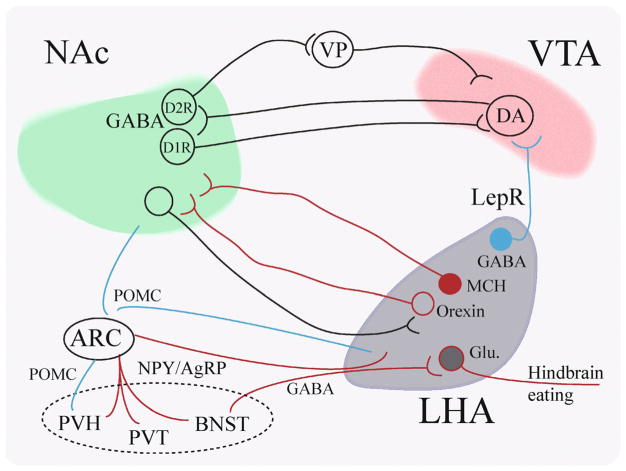
Figure 2.
Interactions of LHA-NAc-VTA neurocircuitry and the regulation of food intake. The NAc shell contains DA receptor 1 (D1R)-expressing neurons, as well as D2R-expressiing neurons, which project to the VTA either directly (D1R-MSNs) or indirectly (D2R-MSNs), through the ventral pallidum (VP). Neurons in the NAc are regulated by both MCH and orexin input from the LHA to promote eating. There are roughly four major subtypes of LHA neurons: MCH, orexin, GABAergic and Glutamatergic (Glu) neurons. A subgroup of the GABAergic neurons expresses Leptin receptors and projects to the VTA. A subgroup of glutamatergic neurons receives inhibitory input from the BNST and its inhibition promotes eating. ARC NPY/AgRP is the major “receiver” to mediate body hormones and energy status of the body to “forebrain feeding circuit” composed of paraventricular hypothalamus (PVH), paraventricular hypothalamic nucleus (PVT), the Bed Nucleus of Stria Terminalis (BNST) as well as LHA.
Mesolimbic DA signaling — a shared pathway for drug addiction and feeding
DA has long been implicated in modulation of the neural circuitry of reward. More recently, accumulated evidence suggests that DA is associated with hedonic aspects of feeding (Volkow et al., 2008; Kenny, 2011b). Human fMRI studies in cocaine addicts show overlapping patterns of brain activation to cocaine and palatable foods (Tomasi et al., 2014). Also, in a human PET study, DA was found to be released in the dorsal striatum in proportion to the self-reported pleasure derived from the ingestion of energy-dense food (Small et al., 2003). In accordance, VTA activation by electrical stimulation promotes feeding in well-fed rats (Trojniar et al., 2007). Interestingly, DA depletion reduces the effort an animal is willing to exert in order to obtain food without affecting intake when food is freely available (Salamone et al., 1994); while voracious eating induced by inhibition of the NAc does not contain a reinforcing character because the animals will not exert an effort in order to obtain food.
Numerous animal studies suggest that striatal D2R pathways are modified in obesity. Reduced D2R expression is observed in leptin-resistant obese rats (Pfaffly et al., 2010), as well as diet-induced binge-eating animals (Avena et al., 2008). The reduction of D2Rs further develops as the rats gaining weight (Thanos et al., 2008). Lentivirus-mediated knockdown of striatal D2Rs showed acceleration in the development of compulsive food seeking for palatable food (Johnson and Kenny, 2010). Therefore, reduced DA input onto D2R MSNs may undermine the animal’s ability to resist the temptation to feed, and reinforcing D2R function might dampen the drive toward compulsive feeding. However, these speculations require further experimental interrogation.
Collectively, DA functions to modulate motivation and reinforce reward (Volkow and Wise, 2005; Wise, 2006; Berridge, 2009). Interestingly, DA release has been found to increase in the NAc of control rats upon exposure to normal chow, while in obese rats, with a history of extended access to palatable food, only energy-dense food could trigger an increase in DA levels in the NAc (Geiger et al., 2009). In this sense, both addiction and over-eating can be viewed as the acquisition of alternative homeostatic states that develop following prolonged exposure to the addictive substance (drugs of abuse or comforting foods). The newly established pathological state may be actively defended by the reward circuits in the brain (Morton et al., 2006). This view necessitates a new experimental perspective to understand the circuit modifications underlying the development of addictions and compulsive consumption.
In summary, it is clear that DA released from the VTA is a crucial modulator of feeding, specifically in terms of the rewarding aspects of food. The evidence primarily points to a function for hypothalamic peptides in regulating DA release through direct action in the VTA, as well as action on DA terminals in the striatum, particularly the NAc. Much remains to be discovered about the specific modes of action of DA in the NAc, and how it modulates the activity of D1R and D2R-expressing MSNs to regulate feeding.
Integration of hedonic and homeostatic feeding
The hedonic reward system and the homeostatic feeding neurocircuitry maintain a strong interaction. The homeostatic state of the organism strongly modifies the perceived rewarding value. For example, food deprivation strongly augments the rewarding value of drugs of abuse, including heroin, amphetamine and cocaine (Carroll et al., 1979; Stuber et al., 2002); drives for electrical stimulations in brain ‘pleasure areas’ are augmented by starvation (Fulton et al., 2000), and dampened by feeding of palatable food (Routtenberg and Lindy, 1965; Spies, 1965). As described above, the intimate connections between the LHA, a key component of the forebrain feeding neural circuit, and the mesolimbic system, including the VTA and the NAc (Fig. 1), provide strong evidence for a direct anatomical and functional relationship between the hedonic and homeostatic feeding behaviors. Hormones such as leptin and insulin regulate the central nodes of this reciprocal system through their fluctuations according to energy status. Consistent with this, central administration of insulin or leptin diminishes food reward (sucrose preference). The mechanism may involve increased DA transporter expression on VTA DA neurons, reducing DA tone in the NAc (Figlewicz, 2003). Conversely, fasting intensifies the rewarding values of substances. These observations suggest that by decreasing neuronal input from anorexigenic hormones, energy restriction increases responses to rewarding stimuli as an adaptive mechanism, motivating animals that are threatened by caloric insufficiency to seek and obtain energy dense foods.
In vulnerable individuals, the consumption of high quantities of palatable food (similar to drugs of abuse) likely upsets the balanced interaction between these circuits, resulting in an enhanced reinforcing value of food and in a weakened control over impulses. This perturbation is a result of conditioned learning and the resetting of reward thresholds in at-risk individuals. The undermining of the cortical top-down regulatory networks over the subcortical regions results in impulsive and compulsive food intake. Recently, experimental evidence has been obtained supporting this model in the context of exposure to drugs of abuse (Stuber et al., 2011; Pascoli et al., 2014). These papers describe synaptic mechanisms hijacked by drugs of abuse (Volkow and Wise, 2005; Volkow et al., 2008; Volkow et al., 2011; Volkow et al., 2013). One study revealed that following prolonged cocaine self-administration, a reduction in the strength of synapses from the PFC onto D1R-expressing MSNs in the NAc shell is observed, in parallel to an increase in the strength of synaptic inputs from the ventral hippocampus (Pascoli et al., 2014).
A separate study observed that mice would perform optical self-stimulation of inputs from the basolateral amygdala (BLA) into the NAc, while optogenetic inhibition of the input from the BLA to the NAc reduced cue-evoked sucrose intake (Stuber et al., 2011). The BLA and hippocampus are crucial components of the emotional and contextual circuits of the brain and indeed, similar to addictive substances (Cota et al., 2006), palatable foods enhance mood in humans (Davis et al., 2004). Palatable foods are believed able to carry out powerful reinforcing effects by fast sensory inputs and by slower post-ingestive effects (Volkow and Wise, 2005). Conversly, emotional states strongly modify reinstatement of addictive drugs and may produce similar unsuppressable cravings leading to repeated binging of comforting/mood-enhancing foods. Supporting this notion, high co-morbidity is seen of anxiety, depression, drug addiction, and obesity or other eating disorders (Everson et al., 2002; Musselman et al., 2003; McCarty et al., 2009), and overeating in obese or bulimic individuals show a compulsive anxiety-triggered element (Menatti et al., 2013).
Perspective
We are currently in the midst of an exciting phase of discovery in the study of neural circuitry function in regulating behavior, including food intake. The combination of different genetically encoded tools (optogenetic and pharmacogenetic) with the increasing availability of animal models provides opportunities to delineate neural circuits and define their function with high resolution. Until recently, the majority of research in investigation of the neural circuitry of feeding was based on global manipulations: ablation of brain regions, pharmacological excitation or inhibition of brain tissue, and electrical stimulation of brain nuclei. In this review we focused on describing the interaction of LHA—VTA—NAc. Taking a page from the recent advances in identifying the synaptic and circuit mechanisms underlying the development of addiction to drug of abuse, we believe the next developments in understanding the regulation of feeding will come from identifying loci of synaptic plasticity affected by feeding, and the regulation of the plasticity by peptides and hormones for feeding and satiety. More importantly, as is being demonstrated by the new pioneers of this field we now have much improved genetic access to many elements of the neurocircuitry, and through implementing pharmacogenetic and optogenetic tools, we can expect to obtain modifications and corrections to the circuit diagrams associating the reward circuitry with the feeding circuitry. Thus, we can expect the next few years to lead to significant insight into the neural circuit mechanisms underlying the extremely important topic of regulation of feeding, which is of fundamental relevance to human health and well-being.
Acknowledgments
The authors want to thank the generous support from the US-Israel Binational Science Foundation (BSF; grant #2011266).
Footnotes
Compliance with ethics guidelines
Jing-Jing Liu, Diptendu Mukherjee, Doron Haritan, Bogna Ignatowska-Jankowska, Ji Liu, Ami Citri and Zhiping P. Pang declare that they have no conflict of interests. This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Anand BK, Brobeck JR. Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951;77(2):323–324. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488(7410):172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atrens DM, Williams MP, Brady CJ, Hunt GE. Energy balance and hypothalamic self-stimulation. Behav Brain Res. 1982;5(2):131–142. doi: 10.1016/0166-4328(82)90048-1. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32(1):20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, Licinio J. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci USA. 2007;104(46):18276–18279. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgardt BF, Okamura T, Brüning JC. Hormone and glucose signalling in POMC and AgRP neurons. J Physiol. 2009;587(Pt 22):5305–5314. doi: 10.1113/jphysiol.2009.179192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97(5):537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155(6):1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319(2):218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res. 2010;209(1):1–12. doi: 10.1016/j.bbr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Ungless MA, Bonci A. Convergent actions of orexin/hypocretin and CRF on dopamine neurons: Emerging players in addiction. Brain Res. 2010;1314:139–144. doi: 10.1016/j.brainres.2009.10.068. [DOI] [PubMed] [Google Scholar]
- Boules M, Cusack B, Zhao L, Fauq A, McCormick DJ, Richelson E. A novel neurotensin peptide analog given extracranially decreases food intake and weight in rodents. Brain Res. 2000;865(1):35–44. doi: 10.1016/s0006-8993(00)02187-9. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205(4403):319–321. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A, Civelli O. The melanin-concentrating hormone system modulates cocaine reward. Proc Natl Acad Sci USA. 2009;106(16):6772–6777. doi: 10.1073/pnas.0811331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriello J, McMurray JC, Babic T, de Oliveira CV. Collateral axonal projections from hypothalamic hypocretin neurons to cardiovascular sites in nucleus ambiguus and nucleus tractus solitarius. Brain Res. 2003;991(1–2):133–141. doi: 10.1016/j.brainres.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33(1):18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Coll AP, Farooqi IS, O’Rahilly S. The hormonal control of food intake. Cell. 2007;129(2):251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coons EE, Cruce JA. Lateral hypothalamus: food current intensity in maintaining self-stimulation of hunger. Science. 1968;159(3819):1117–1119. doi: 10.1126/science.159.3819.1117. [DOI] [PubMed] [Google Scholar]
- Cota D, Barrera JG, Seeley RJ. Leptin in energy balance and reward: two faces of the same coin? Neuron. 2006;51(6):678–680. doi: 10.1016/j.neuron.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite. 2004;42(2):131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Horvath TL. Feeding signals and brain circuitry. Eur J Neurosci. 2009;30(9):1688–1696. doi: 10.1111/j.1460-9568.2009.06963.x. [DOI] [PubMed] [Google Scholar]
- Domingos AI, Vaynshteyn J, Voss HU, Ren X, Gradinaru V, Zang F, Deisseroth K, de Araujo IE, Friedman J. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14(12):1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat AM, Diaz R, Gallo L, Panagos A, Kay K, Williams DL. Nucleus accumbens GLP-1 receptors influence meal size and palatability. Am J Physiol Endocrinol Metab. 2013;304(12):E1314–E1320. doi: 10.1152/ajpendo.00137.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31(41):14453–14457. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube MG, Kalra SP, Kalra PS. Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res. 1999;842(2):473–477. doi: 10.1016/s0006-8993(99)01824-7. [DOI] [PubMed] [Google Scholar]
- Everson SA, Maty SC, Lynch JW, Kaplan GA. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. J Psychosom Res. 2002;53(4):891–895. doi: 10.1016/s0022-3999(02)00303-3. [DOI] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317(5843):1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Goldenberg J, Melendez G, Shilling PD. The acute and subchronic effects of a brain-penetrating, neurotensin-1 receptor agonist on feeding, body weight and temperature. Neuropharmacology. 2010;58(1):195–198. doi: 10.1016/j.neuropharm.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP. Insulin, food intake, and reward. Semin Clin Neuropsychiatry. 2003;8(2):82–93. doi: 10.1053/scnp.2003.50012. [DOI] [PubMed] [Google Scholar]
- Frank RA, Preshaw RL, Stutz RM, Valenstein ES. Lateral hypothalamic stimulation: stimulus-bound eating and self-deprivation. Physiol Behav. 1982;29(1):17–21. doi: 10.1016/0031-9384(82)90359-6. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51(6):811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287(5450):125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159(4):1193–1199. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goforth PB, Leinninger GM, Patterson CM, Satin LS, Myers MG., Jr Leptin acts via lateral hypothalamic area neurotensin neurons to inhibit orexin neurons by multiple GABA-independent mechanisms. J Neurosci. 2014;34:11405–11415. doi: 10.1523/JNEUROSCI.5167-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone AP. The hypothalamus, hormones, and hunger: alterations in human obesity and illness. Prog Brain Res. 2006;153:57–73. doi: 10.1016/S0079-6123(06)53003-1. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Lobo MK, Zhang F, de Lecea L. Neural integration of reward, arousal, and feeding: recruitment of VTA, lateral hypothalamus, and ventral striatal neurons. IUBMB Life. 2011;63(10):824–830. doi: 10.1002/iub.539. [DOI] [PubMed] [Google Scholar]
- Hahn JD, Swanson LW. Distinct patterns of neuronal inputs and outputs of the juxtaparaventricular and suprafornical regions of the lateral hypothalamic area in the male rat. Brain Res Brain Res Rev. 2010;64(1):14–103. doi: 10.1016/j.brainresrev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JD, Swanson LW. Connections of the lateral hypothalamic area juxtadorsomedial region in the male rat. J Comp Neurol. 2012;520(9):1831–1890. doi: 10.1002/cne.23064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson M, de Lecea L, Sutcliffe JG, Yanagisawa M, Meister B. Leptin receptor- and STAT3-immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus. J Neuroendocrinol. 1999;11(8):653–663. doi: 10.1046/j.1365-2826.1999.00378.x. [DOI] [PubMed] [Google Scholar]
- Haltia LT, Rinne JO, Merisaari H, Maguire RP, Savontaus E, Helin S, Någren K, Kaasinen V. Effects of intravenous glucose on dopaminergic function in the human brain in vivo. Synapse. 2007;61(9):748–756. doi: 10.1002/syn.20418. [DOI] [PubMed] [Google Scholar]
- Hansen S, Stanfield EJ, Everitt BJ. The effects of lesions of lateral tegmental noradrenergic neurons on components of sexual behavior and pseudopregnancy in female rats. Neuroscience. 1981;6(6):1105–1117. doi: 10.1016/0306-4522(81)90075-0. [DOI] [PubMed] [Google Scholar]
- Harthoorn LF, Sañé A, Nethe M, Van Heerikhuize JJ. Multi-transcriptional profiling of melanin-concentrating hormone and orexin-containing neurons. Cell Mol Neurobiol. 2005;25(8):1209–1223. doi: 10.1007/s10571-005-8184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science. 1962;135(3501):375–377. doi: 10.1126/science.135.3501.375. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51(6):801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Horjales-Araujo E, Hellysaz A, Broberger C. Lateral hypothalamic thyrotropin-releasing hormone neurons: distribution and relationship to histochemically defined cell populations in the rat. Neuroscience. 2014;277:87–102. doi: 10.1016/j.neuroscience.2014.06.043. [DOI] [PubMed] [Google Scholar]
- Horvath TL. The hardship of obesity: a soft-wired hypothalamus. Nat Neurosci. 2005;8(5):561–565. doi: 10.1038/nn1453. [DOI] [PubMed] [Google Scholar]
- Ishiwari K, Weber SM, Mingote S, Correa M, Salamone JD. Accumbens dopamine and the regulation of effort in food-seeking behavior: modulation of work output by different ratio or force requirements. Behav Brain Res. 2004;151(1–2):83–91. doi: 10.1016/j.bbr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341(6153):1517–1521. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Janson AC, Waters S, Engel JA. Concomitant release of ventral tegmental acetylcholine and accumbal dopamine by ghrelin in rats. PLoS ONE. 2012;7(11):e49557. doi: 10.1371/journal.pone.0049557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats (vol 13, pg 635, 2010) Nat Neurosci. 2010;13:1033–1033. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnani MM, Szabó G, Erdélyi F, Burdakov D. Lateral hypothalamic GAD65 neurons are spontaneously firing and distinct from orexin- and melanin-concentrating hormone neurons. J Physiol. 2013;591(Pt 4):933–953. doi: 10.1113/jphysiol.2012.243493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8(11):844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27(8):765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005a;493(1):72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005b;86(5):773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bless EP, Swanson CJ. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther. 1996;278(3):1499–1507. [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, Bonci A. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci. 2013;33:7618–7626. doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011a;12(11):638–651. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011b;69(4):664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkotou EG, Tritos NA, Mastaitis JW, Slieker L, Maratos-Flier E. Melanin-concentrating hormone receptor is a target of leptin action in the mouse brain. Endocrinology. 2001;142(2):680–686. doi: 10.1210/endo.142.2.7981. [DOI] [PubMed] [Google Scholar]
- Krilowicz BL, Szymusiak R, McGinty D. Regulation of posterior lateral hypothalamic arousal related neuronal discharge by preoptic anterior hypothalamic warming. Brain Res. 1994;668(1–2):30–38. doi: 10.1016/0006-8993(94)90507-x. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Qian S. Exploratory activity, motor coordination, and spatial learning in Mchr1 knockout mice. Behav Brain Res. 2007;178(2):293–304. doi: 10.1016/j.bbr.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, Sarhan M, Guarnieri DJ, Deisseroth K, Aghajanian GK, DiLeone RJ. Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci. 2014;17(2):248–253. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, Jones JC, Rhodes CJ, Chua S, Jr, Diano S, Horvath TL, Seeley RJ, Becker JB, Münzberg H, Myers MG., Jr Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10(2):89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, Seasholtz AF, Thompson RC, Myers MG., Jr Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14(3):313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487(7406):183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav. 2000;37(4):335–344. doi: 10.1006/hbeh.2000.1584. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107(3):379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr. 2009;139(3):629–632. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, Guan XM, Jiang MM, Feng Y, Camacho RE, Shen Z, Frazier EG, Yu H, Metzger JM, Kuca SJ, Shearman LP, Gopal-Truter S, MacNeil DJ, Strack AM, MacIntyre DE, Van der Ploeg LH, Qian S. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci USA. 2002;99(5):3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty CA, Kosterman R, Mason WA, McCauley E, Hawkins JD, Herrenkohl TI, Lengua LJ. Longitudinal associations among depression, obesity and alcohol use disorders in young adulthood. Gen Hosp Psychiatry. 2009;31(5):442–450. doi: 10.1016/j.genhosppsych.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav. 2007;92(1–2):263–271. doi: 10.1016/j.physbeh.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Menatti AR, Weeks JW, Levinson CA, McGowan MM. Exploring the relationship between social anxiety and bulimic symptoms: mediational effects of perfectionism among females. Cognit Ther Res. 2013;37(5):914–922. doi: 10.1007/s10608-013-9521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NE. Motivational effects of brain stimulation and drugs. Fed Proc. 1960;19:846–854. [PubMed] [Google Scholar]
- Millington GW. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr Metab (Lond) 2007;4(1):18. doi: 10.1186/1743-7075-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SD, Mayer J. Adipsia and aphagia in rats after lateral subthalamic lesions. Am J Physiol. 1957;191(2):248–254. doi: 10.1152/ajplegacy.1957.191.2.248. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15(6):367–378. doi: 10.1038/nrn3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton TD, Salovitz B. Evolving a theoretical model of child safety in maltreating families. Child Abuse Negl. 2006;30(12):1317–1327. doi: 10.1016/j.chiabu.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Murray S, Tulloch A, Gold MS, Avena NM. Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nat Rev Endocrinol. 2014;10(9):540–552. doi: 10.1038/nrendo.2014.91. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Betan E, Larsen H, Phillips LS. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54(3):317–329. doi: 10.1016/s0006-3223(03)00569-9. [DOI] [PubMed] [Google Scholar]
- Nahon JL, Presse F, Bittencourt JC, Sawchenko PE, Vale W. The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology. 1989;125(4):2056–2065. doi: 10.1210/endo-125-4-2056. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolic hormones, dopamine circuits, and feeding. Front Neuroendocrinol. 2010;31(1):104–112. doi: 10.1016/j.yfrne.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47(6):419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Lüscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509(7501):459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Central enhancement of taste pleasure by intraventricular morphine. Neurobiology (Bp) 1995;3(3–4):269–280. [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res. 2000;863(1–2):71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25:8295–8302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffly J, Michaelides M, Wang GJ, Pessin JE, Volkow ND, Thanos PK. Leptin increases striatal dopamine D2 receptor binding in leptin-deficient obese (ob/ob) mice. Synapse. 2010;64(7):503–510. doi: 10.1002/syn.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380(6571):243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134(3):737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465(4):593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Lindy J. Effects of the availability of rewarding septal and hypothalamic stimulation on bar pressing for food under conditions of deprivation. J Comp Physiol Psychol. 1965;60(2):158–161. doi: 10.1037/h0022365. [DOI] [PubMed] [Google Scholar]
- Sahu A, Carraway RE, Wang YP. Evidence that neurotensin mediates the central effect of leptin on food intake in rat. Brain Res. 2001;888(2):343–347. doi: 10.1016/s0006-8993(00)03107-3. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65(2):221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Sano H, Yokoi M. Striatal medium spiny neurons terminate in a distinct region in the lateral hypothalamic area and do not directly innervate orexin/hypocretin- or melanin-concentrating hormone-containing neurons. J Neurosci. 2007;27:6948–6955. doi: 10.1523/JNEUROSCI.0514-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36(2):199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28(3):152–157. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Sears RM, Liu RJ, Narayanan NS, Sharf R, Yeckel MF, Laubach M, Aghajanian GK, DiLeone RJ. Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin-concentrating hormone. J Neurosci. 2010;30:8263–8273. doi: 10.1523/JNEUROSCI.5858-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396(6712):670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Shirazi RH, Rabasa-Papio C, Alvarez-Crespo M, Neuber C, Vogel H, Dickson SL. Divergent circuitry underlying food reward and intake effects of ghrelin: dopaminergic VTA-accumbens projection mediates ghrelin’s effect on food reward but not food intake. Neuropharmacology. 2013;73:274–283. doi: 10.1016/j.neuropharm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM, Zamir N. Immunohistochemical localization of a melanin concentrating hormone-like peptide in the rat brain. Brain Res Bull. 1985;15(6):635–649. doi: 10.1016/0361-9230(85)90213-8. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19(4):1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Spies G. Food versus intracranial self-stimulation reinforcement in food-deprived rats. J Comp Physiol Psychol. 1965;60(2):153–157. doi: 10.1037/h0022367. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Willett VL, 3rd, Donias HW, Ha LH, Spears LC. The lateral hypothalamus: a primary site mediating excitatory amino acid-elicited eating. Brain Res. 1993;630(1–2):41–49. doi: 10.1016/0006-8993(93)90640-9. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: a New Paradigm to Explain Arousal Pathology. John Wiley & Sons; 1988. [Google Scholar]
- Sternson SM. Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron. 2013;77(5):810–824. doi: 10.1016/j.neuron.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19(24):11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Evans SB, Higgins MS, Pu Y, Figlewicz DP. Food restriction modulates amphetamine-conditioned place preference and nucleus accumbens dopamine release in the rat. Synapse. 2002;46(2):83–90. doi: 10.1002/syn.10120. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475(7356):377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum P, Stellar E. Recovery from the failure to eat produced by hypothalamic lesions. Science. 1954;120(3126):894–895. doi: 10.1126/science.120.3126.894. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62(1):50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Wang R, Caparelli EC, Logan J, Volkow ND. Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: Association to striatal D2/D3 receptors. Hum Brain Mapp. 2014 doi: 10.1002/hbm.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Martinez D. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology. 2014;76(Pt B):498–509. doi: 10.1016/j.neuropharm.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojniar W, Plucińska K, Ignatowska-Jankowska B, Jankowski M. Damage to the nucleus accumbens shell but not core impairs ventral tegmental area stimulation-induced feeding. J Physiol Pharmacol. 2007;58(Suppl 3):63–71. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15(1):37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14(1):2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8(5):555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71(1):142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357(9253):354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wang L, Shen M, Yu Y, Tao Y, Zheng P, Wang F, Ma L. Optogenetic activation of GABAergic neurons in the nucleus accumbens decreases the activity of the ventral pallidum and the expression of cocaine-context-associated memory. Int J Neuropsychopharmacol. 2014;17(5):753–763. doi: 10.1017/S1461145713001570. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24(1):429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Wise RA. Lateral hypothalamic electrical stimulation: does it make animals ‘hungry’? Brain Res. 1974;67(2):187–209. doi: 10.1016/0006-8993(74)90272-8. [DOI] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38(5):701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50(4):751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284(6):R1436–R1444. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]