Abstract
Our work is concerned with the origins and therapy of human cancers. Members of the epidermal growth factor receptor (EGFR) family of tyrosine kinases, also known as erbB or HER receptors, are over expressed and/or activated in many types of human tumors and represent important therapeutic targets in cancer therapy. Studies from our laboratory identified targeted therapy as a way to treat cancer. Rational therapeutics targeting and disabling erbB receptors have been developed to reverse the malignant properties of tumors. Reversal of the malignant phenotype, best seen with disabling the HER2 receptors using monoclonal antibodies is a distinct process from that seen with blocking of ligand binding to cognate receptors as has been done for EGFr receptors. Here we review the mechanisms of action deduced from a number of approaches developed in our laboratory and elsewhere, including monoclonal antibodies, peptide mimetics, recombinant proteins and small molecules. The biochemical and biological principles which have been uncovered during these studies of disabling HER2 homomeric or HER2-EGFr heteromeric receptors will help the development of novel and more efficient therapeutics targeting erbB family receptors.
Keywords: erbB receptors, Targeted therapy, Monoclonal antibody, Peptide mimetic, Recombinant protein
1. Introduction
The epidermal growth factor receptor (EGFR) family of tyrosine kinases, also known as erbB or HER receptors, are over expressed and/or activated in many types of human tumors. There are four members in the erbB family: EGFR, HER2 (also known as p185her2/neu and Neu), HER3 (erbB3) and HER4 (erbB4). Activation of erbB receptors leads not only to increased cell proliferation, but also to resistance to growth-inhibitory cytokines. Moreover the activated erbB receptors lead to a transformed phenotype which is associated with the expression of selective immune suppressive and proangiogenic cytokines and chemokines. This tumor-host interaction creates an environment that favors tumor progression [1,2]. All members of the erbB family share structural similarities, which include an extracellular ligand binding domain, a transmembrane domain, and an intracellular kinase domain.
The erbB family of tyrosine kinases have emerged as important therapeutic targets in oncology. Receptor-directed therapy represents a powerful cancer management strategy. Numerous antibodies, recombinant proteins, peptide mimetics and small molecules have been developed for targeting EGF receptors. Some of the antibodies (cetuximab, panitumumab, and trastuzumab) and small molecules (gefitinib, erlotinib, and lapatinib) have been approved clinically and are already benefiting patients.
Although the results are encouraging, there is still a need for the development of novel and more efficient therapies, because many patients are either not sensitive to current drugs/antibodies or develop resistance after a few months of treatment. The mechanisms by which therapeutics exert their activity on malignant properties are incompletely defined and the multiple pathways by which resistance to targeted therapy arises are also poorly understood.
Our laboratory initiated the rational therapeutic targeting and disabling of erbB receptors to reverse the malignant properties of tumors. Reversal of phenotype is very distinct from blocking of ligand binding to cognate receptors as has been done for EGFr receptors. We have developed a number of approaches including monoclonal antibodies, peptide mimetics, recombinant proteins and small molecules. Here we review the results obtained in our laboratory and elsewhere for erbB targeted therapy mediated by various therapeutic modalities.
2. Antibodies
For many years, monoclonal antibodies were considered primarily useful for molecular diagnostics. However, monoclonal antibodies have been developed for anti-cancer treatment, and they have emerged as a class of biologic cancer therapeutics. We generated a panel of monoclonal antibodies against the rat p185neu receptor, including 7.16.4, 7.21.2 and A11 [3]. These antibodies have high affinities for the oncogenic rodent p185neu receptor. From staining of human malignant tissues, we observed that 7.16.4 also interacts with p185her2/neu on human breast adenocarcinoma cells [4] and other human tissues (Paco Real, Mark Greene, 1986, unpublished data). The 7.16.4 can effectively inhibit anchorage dependent cell growth under most serum conditions although this is clearly dependent on the characteristics of the fetal calf serum used. Many of these monoclonals including the prototypic 7.16.4 can also limit anchorage independent growth and reverse the phenotype of cells transformed by either mutant p185neu or by over expressed p185her2/neu [3].
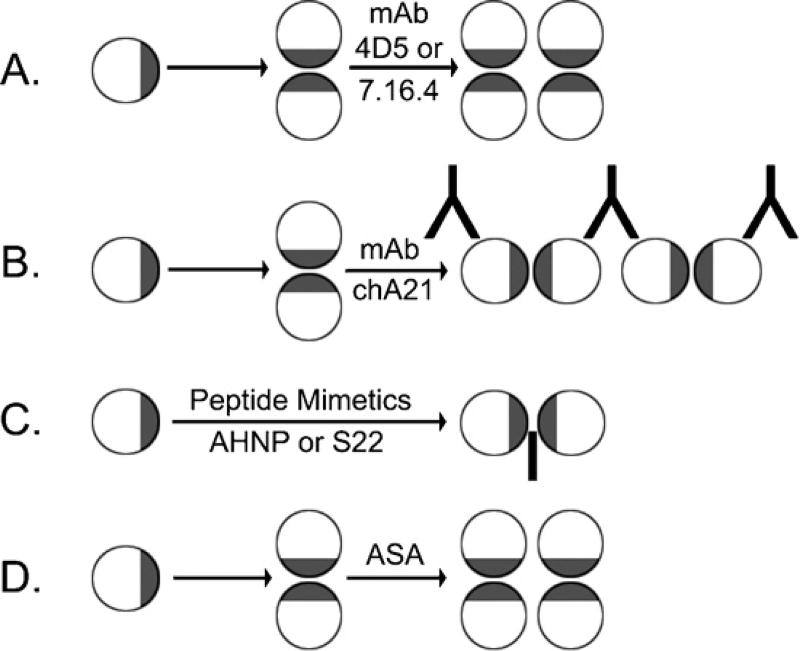
mAb 7.16.4 is able to bind to human tumor tissues in a manner comparable to the binding and staining of rhuMAb4D5. 7.16.4 can readily displace rhuMAb4D5 (a humanized anti-p185her2/neu antibody developed several years later and independently by scientists at Genentech) from its interaction with p185her2/neu in p185her2/neu expressing T6–17 cells [4]. More importantly, rhuMAb4D5 and 7.16.4 bind to the same or an overlapping epitope on the ectodomain of p185her2/neu. RhuMAb4D5 was only slightly more effective (IC50, 1 µg/ml) at reversing phenotype than the 7.16.4 monoclonal antibody (IC50, 10 µg/ml). These studies indicated that both 7.16.4 and 4D5 interact with the same epitope and have similar biological activities and mediate their activities by the same mechanisms. Mechanisms of phenotype reversal include creation of ineffective tetramers which lack kinase activity (Fig. 1).
Fig. 1.
Mechanisms of function of therapeutic agents targeting ectodomains of receptors. Receptor ectodomains were represented by circular disks, in which the dimerization interfaces were colored by gray. (A) Monoclonal antibodies 4D5 and 7.16.4 can promote the formation of functionally defective receptor tetramers; (B) monoclonal antibody chA21 appears to cross-link dimers of the receptors to form large defective receptor complexes which are internalized; (C) peptide mimetics AHNP (antibody mimetic) and S22 (dimerization-blocking peptide) can interfere with receptor dimers; (D) like the parental antibody, ASA may also promote the formation of defective receptor tetramers.
A chimeric anti-p185her2/neu antibody chA21 that specifically inhibits the growth of p185her2/neu-over expressing cancer cells in vitro and in vivo has also been developed ([5] and Huihao Zhou et al., unpublished data). As suggested by the crystal structure of the single-chain Fv (ScFv) fragment of chA21 in complex with an N-terminal fragment of the p185her2/neu ectodomain, chA21 binds to an epitope located diametrically opposite to the putative p185her2/neu dimerization interface, indicating that chA21 does not directly block the dimerization of p185her2/neu species.
The chA21 epitope is distinct from those of the other anti-p185her2/neu therapeutic antibodies including Trastuzumab(4D5) and Pertuzumab(2C4). Binding of the bivalent monoclonal antibody chA21 to its distinct epitope also leads to internalization and downregulation of p185her2/neu in SK-BR-3 cells (Huihao Zhou et al., unpublished data).
We previously demonstrated that therapeutic monoclonal antibodies can promote EGFR or p185her2/neu-EGFR to form tetramers. EGF-induced phosphorylation in the tetramers was significantly lower than that of the dimers [6]. The impaired receptor tetramers were observed after even a brief 15-min treatment with a mixture of mAbs, and these tetramers had significantly diminished kinase activity. Our study also demonstrated that formation of the impaired terameric receptor forms was a physiologically relevant process largely independent of the receptor density. We also showed that antibodies can rapidly shift the equilibrium from active dimeric to an impaired tetrameric receptor complex state. This mechanism may present an important component for the antibodies’ antitumor activity (Fig. 1A) and indicates a rapid time frame for reversal of the malignant phenotype.
We also sought to understand the mechanism by which chA21 functions because of its unique interaction epitope. We found that monovalent ScFv of chA21 was not able to induce p185her2/neu down-regulation, whereas bivalent chA21 could produce that effect. Furthermore, the chA21-mediated p185her2/neu down-regulation is dose-dependent in a bell-shaped curve reflecting the stoichiometry of the process. These data lead to a cross-linking model, in which chA21 interacts with two p185her2/neu molecules on separate homo- or hetero dimers, resulting in cross-linking of these dimers to form a large complex in the cell membrane for internalization and degradation (Fig. 1B).
This mechanism is also reminiscent of our previous work documenting that internalization of receptors can be enhanced by antibodies that promote active receptor dimers to associate into inactive tetramers.
3. Peptide mimetics
We developed two additional ectodomain targeted approaches for disabling erbB receptor signaling using mimetic peptides. The first approach was focused on mimicking the effects of anti-erbB receptor MAbs, whereas the second approach targets receptor dimerization interfaces. Both strategies have proven efficient in the inhibition of erbB receptor functions in both in vitro and in vivo studies.
3.1. Antibody mimetics, novel antibody surrogates created by using the deconstructed heavy chain CDR3, typified by AHNP
There are appreciated limitations associated with the systemic use of intact antibody molecules as therapeutic or diagnostic entities. They may engender a host immune response, possess little ability to penetrate into tumors and practically are associated with a high production cost [7,8]. Attempts to reduce the size of antibodies resulted in the development of smaller versions of antibodies that retain specificity [single-chain Fvs, Fabs, Fab(2)s, minibodies, domain-deleted antibodies] and have increased penetration into solid tumors [9]. A promising alternative approach to overcome the limitations of high-molecular-weight therapeutics was to design mimetic peptides derived from the antigen-binding site of antibodies.
Our approach was to deconstruct the antibody binding site. Based on our crystallographic studies of protein antigen complexes we recognized that the CDR3 of the heavy chain surrounded by aromatics created a highly energetic surface for antigen binding [10]. Through studies of the deconstructed antigen binding surface of a monoclonal antibody we established a general principle that a single CDR3 loop from the heavy chain of the antibody is sufficient to mimic its parental monoclonal antibody function [11–16]. The CDR peptide is much smaller than the antibody but retains binding affinity to the antigen as well as the biological activity of the antibody. Our studies of the CDR mimetics demonstrated that the constrained CDRs could mediate binding in a context independent manner and formed the theoretical basis for the technique of CDR grafting [11,12,17]. We also extended this notion to create a synthetic CDR peptidomimetic species that was biologically active [15].
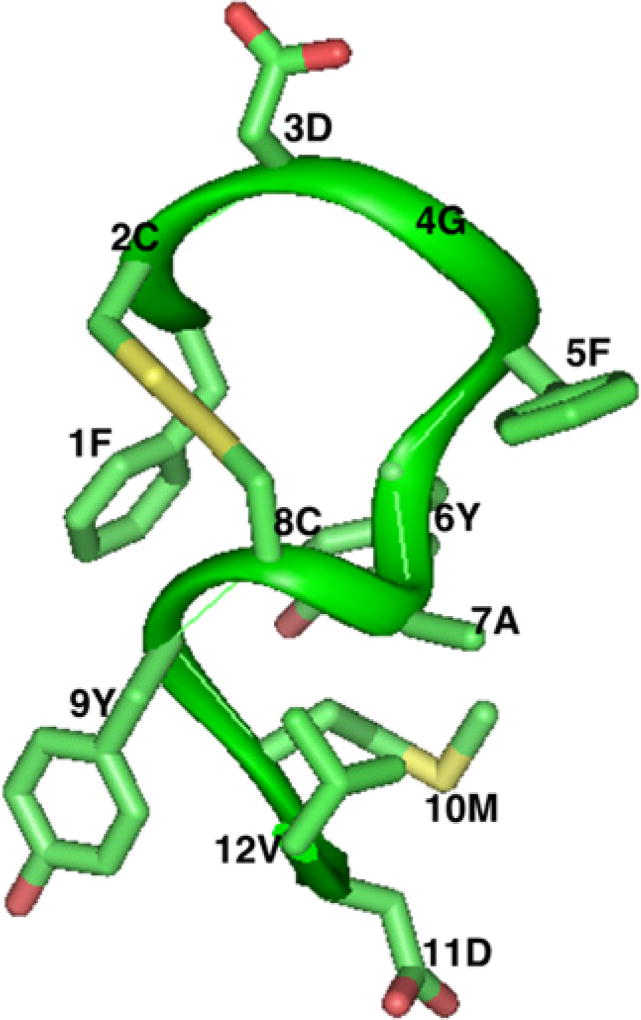
Based on the shared structural properties of the third CDR of the heavy chain of the 7.16.4 and rhuMAb4D5 antibodies, we developed a 1.5 kDa anti-HER2 peptidomimetic named AHNP (anti her2/neu peptide). The structure of AHNP was determined by solution NMR (Fig. 2) and demonstrated a single conformation. It also revealed structural features distinct from molecular modeling. These features were then incorporated into newly designed peptidomimetics. As shown by both in vitro and in vivo studies, AHNP exhibits the activity to disable p185her2/neu tyrosine kinases to the extent comparable to that of the monoclonal antibodies [17].
Fig. 2.
Three-dimensional structure of AHNP as determined by NMR.
AHNP has been shown to bind to p185her2/neu with submicromolar affinity. It inhibited proliferation of p185her2/neu-expressing tumor cells. The peptide mimetic also showed good activity in both anchorage-independent colony growth assays and most importantly in the growth of p185her2/neu-expressing tumors in athymic mice. In addition, AHNP sensitized the tumor cells to apoptosis when it was used in conjunction with ionizing radiation or chemotherapeutic agents and was as effective as Herceptin in reducing tumor size and more effective than Herceptin in its ability to inhibit proliferation.
However while the intact monoclonal antibodies actively down regulate p185her2/neu from the cell surface, AHNP has no effect on receptor down regulation, indicating that the small molecule disables the p185her2/neu complex principally by promoting misaligned monomers and limiting the monomers ability to create a stable dimer with an activated kinase. Our data showed that AHNP indicating a shared binding site for the peptide mimetic and the parental antibody.
To further develop AHNP as an antitumor agent useful for preclinical trials and as a radiopharmaceutical to be used for tumor imaging, a number of derivatives of AHNP have been designed. These second generation species have important advantages over AHNP in terms of their binding properties, specificity, and solubility [18]. Structure-function relationships have been studied using surface plasmon resonance technology. Some of the AHNP analogues have improved binding properties, solubility, and cytotoxic activity relative to AHNP. Residues in the exocyclic region of AHNP are essential for high-affinity binding. Kinetic and equilibrium analysis of peptide receptor binding for various AHNP analogues revealed a strong correlation between peptide binding characteristics and their biological activity. We also showed that the dissociation rate constant is a better indicator than receptor binding affinities for predicting the biological activity of AHNP analogues.
The antitumor effects of AHNP can be enhanced when combined with chemotherapeutic drugs. For example, a dimeric AHNP peptide (AHNP bivalent) was designed to conjugate with Taxol to deliver the drug into tumor cells through p185Her2/neu internalization [19]. The Taxol conjugated AHNP bivalent prodrug binds to p185Her2/neu, and induces receptor internalization and down regulation. Free Taxol is subsequently released inside the targeted cell and results in additional cytotoxicity. This strategy can be generalized to selectively target cancer cells by directing cytotoxic drugs into the tumor-marker expressing cells, through receptor-mediated internalization.
Our efforts revealed the possibility of mimicking the well-documented antibody effects and its applications in tumor therapy using much smaller antibody-based cyclic peptides with significant therapeutic advantages.
To improve AHNP efficacy, we engineered a fusion protein containing AHNP and a nonimmunoglobulin protein, streptavidin [20]. The recombinant protein, AHNP-SA (ASA) bound to p185her2/neu with a high affinity (Biacore: 8.8 nM), inhibited the proliferation of p185her2/neu-overexpressing cells, and reduced tumor growth induced by p185her2/neu transformed cells. Compared with AHNP, ASA showed less activity in an anchorage-independent assay using polyHEMA but more activity in an in vivo tumor growth assay.
We expected ASA to possess a serum half-life close to that of SA (12 h) [21] since the newly developed ASA species has a similar core structure and molecular weight to that of SA. The AHNP molecule’s serum half-life is about 2–3 h. Our studies revealed that the bacterially produced tetrameric ASA can be used as an antibody-surrogate molecule. ASA can be also used as a diagnostic tool to detect p185her2/neu in early breast cancer developments using FACTT, an ultra-sensitive detection technology [22]. We can extend these scaffolds to other polymerizing molecules so that AHNP fusions with dimeric, trimeric or tetrameric scaffolds may lead to new fully humanized recombinant species suitable for the diagnosis and treatment of p185her2/neu-related tumors.
ASA functions in the same way as the parental antibody which is to promote signaling-defective receptor ensembles [6]. Of note signaling-defective receptor ensembles (inactive tetrameric complexes) appear to operationally behave in a manner similar to defective misaligned dimers induced by AHNP (Fig. 1C and D). Therefore a unifying principle of disabled receptor kinases caused by antibody or peptide induced ectodomain alterations is one of the bases for the targeted therapeutic process.
Our experiments serve as a defining principle to create new forms of antibody-surrogate molecules in a modular fashion. Biologically active peptides can be linked to a suitable scaffold upon which multiple copies of relevant CDRs are disposed. A small biologically active synthetic peptide can be engineered to enhance functional aspects by structural oligomerization and can be produced recombinantly using bacterial expression.
3.2. Targeting erbB receptor dimerization interface using peptidomimetics
Early studies from our laboratory established that dimer formation was important for p185Her2/neu activation and that mutations in the transmembrane region facilitated dimers and kinase activation [23]. Studies primarily from our laboratory and David Stern’s showed that heteromeric kinases could also be induced [24–26]. The EGF-like growth factors bind to the ectodomain of EGFR and other receptors of the same family, leading to the formation of homo- and heterodimers [27,28].
Dimerization consequently stimulates the intrinsic tyrosine kinase activity of the receptors, triggering tyrosine autophosphorylation in the cytoplasmic domain. Phosphorylated tyrosine residues then serve as docking sites for intracellular signaling molecules involved in the regulation of signaling cascades [29–34].
We observed that co-expression of p185her2/neu with EGFR in NIH 3T3 fibroblasts augmented the effects of EGF on the transformed phenotype [25]. Our laboratory also showed that heterodimers were far more active than homodimers of either p185c-neu or EGFR [35]. Numerous studies have confirmed the enhanced transforming abilities of erbB heterodimers [36–42] and found that erbB heteromers are a dominant mediator of malignant properties and also are relevant to the emergence of resistance to agents which target only one member of the erbB family.
Targeting protein-protein interaction (dimerization) surfaces is a promising approach for rational drug design against heteromeric receptors ([43,44], Synthetic peptides and peptidomimetics that disrupt protein-protein interactions act as inhibitors of HIV-1 protease [45], HIV-1 reverse transcriptase [46], herpes simplex virus ribonucleotide reductase [47], and thymidilate synthase.
We identified distinct loops within the C-terminal part of the subdomain IV as a receptor–receptor interaction site for the erbB receptors. Next we created a peptide mimetic of this loop and demonstrated that this rationally designed loop mimetic was able to target homomeric and heteromeric dimerization interfaces.
Dose-dependent inhibition of erbB receptor dimerization was observed using this loop mimetic in cell lines transfected with different combinations of erbB receptors [48].
Our structural models for dimeric erbB receptors were used to create rationally designed loop mimetic constrained peptides. These species mimicked the potential dimerization site in subdomain IV of p185her2/neu. In surface plasmon resonance studies, our designed peptide mimetics have been shown to selectively bind to the p185her2/neu subdomain IV with submicromolar affinities [48].
4. Trans inhibitory mutants of p185her2/neu
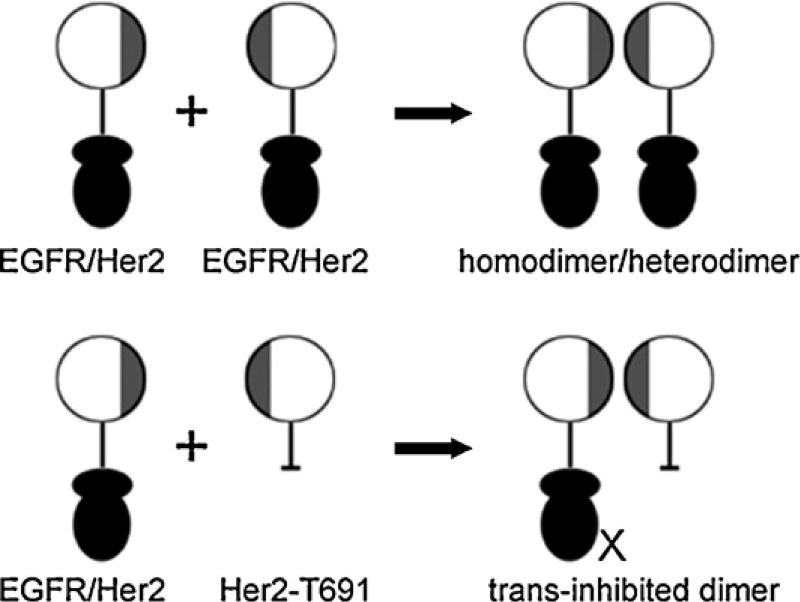
Receptor dimerization is a dominant event for signaling and transformation mediated by the erbB family tyrosine kinases. Because p185her2/neu is the preferred heterodimerization partner of the other erbB family members, we selectively targeted erbB receptors using a kinase deletion mutant consisting of the ectodomain of the p185her2/neu and the transmembrane region but without an endodomain. Species lacking a functional endodomain are still able to lead to the formation of heterodimers while ectodomain lacking forms cannot form dimers. Therefore it is the ectodomain which is most important for dimer formation. We have shown that the endodomain deleted form of p185her2/neu, called T691, can form kinase inactive heterodimers with EGFR or other erbB receptors and thus acts to “trans inhibit” erbB-dependent phenotypes (Fig. 3) [35,49–51].
Fig. 3.
Representations of T691, a trans inhibitory mutant of p185her2/neu. Receptor ectodomains were represented by circular disks, in which the dimerization interfaces were colored by gray. Intracellular kinase domains of the receptors were represented by two filled ellipses, and transmembrane domain was represented by lines.
For example, in EGFR positive U87MG human glioblastoma cells, expression of the p185her2/neu ectodomain inhibits EGF-, but not platelet-derived growth factor-induced DNA synthesis; inhibits cell proliferation in the presence of EGF, but not platelet-derived growth factor; inhibits the ability of U87MG to form colonies in soft agar; and inhibits tumor growth in athymic mice [51].
Our studies established that trans receptor inhibition can abrogate abnormal growth of these tumors. This leads to a general approach for erbB receptor-specific trans inhibition of human neoplasia. These receptor-based inhibitory strategies exploit the thermodynamic preference for erbB ectodomains to heterodimerize, thereby creating erbB receptor assemblies which are defective in signaling and do not internalize. Pharmaceuticals that mimic the p185her2/neu ectodomain may therefore have important therapeutic applications in advanced human malignancies expressing erbB receptors. Our studies also define a rationale for the application of the p185her2/neu ectodomain in gene therapy approaches to human malignant glioma and to other systemic epithelial malignancies expressing erbB family receptors.
5. Small molecules
The intracellular domain of the erbB family receptors includes the tyrosine kinase domain (TKD). TKD is essential for the activation of the receptor and the consequent induction of the downstream pathways that regulate the cell proliferation and other critical functions. The EGFR kinase domain crystal structure was solved by Stamos et al. [52]. The structure shows a bilobate arrangement including an N-lobe, an activation loop and a C-lobe. ATP and substrate bind to the active site of kinase domain and the substrate can be phosphorylated in concomitance with the hydrolysis of ATP. The C-terminal tail of TKD contains several important tyrosine residues whose state of phosphorylation is critical for downstream signal cascades.
Several small molecule tyrosine kinase inhibitors (TKIs) have recently been developed and many of them have already been approved by FDA and used in clinics. Lapatinib (specific to both EGFR and p185her2/neu), Gefitinib (specific to EGFR) and Erlotinib (specific to EGFR) are examples of the most successful reversible kinase inhibitors of the erbB family receptors. The clinical response to these small molecule inhibitors is highly dependent upon certain mutations in the kinase domain [53–55]. Despite significant initial effects observed in patients with mutant EGFR following TKI treatment, most patients eventually develop resistant secondary mutations after a few months of treatment [56]. In addition, for irreversible kinase inhibitors that form a covalent disulfide bond with a cysteine residue (Cys773) in the kinase domain [57], limited success is observed because of the toxicity due to concurrent inhibition of wild type EGFR [58].
Recently we have developed small organic compounds targeting the intracellular kinase domain of EGFR. The compounds can bind to the intracellular domain and mimic the effects produced by the drug sensitizing mutations (Greene MI, Berezov A, unpublished data). This new species represents a novel type of noncompetitive anti-EGFR kinase small molecule inhibitor. We believe that this type of compounds can provide new insights into kinase targeting molecules for cancer and other diseases.
6. Summary
We discovered the activity of anti neu monoclonal antibody targeted therapies in the early 1980s. Now anti-erbB receptor therapy plays an important role in cancer therapy. For decades, we and others have been focused on developing novel therapeutics targeting erbB family tyrosine kinases. Although mAbs and small molecule inhibitors have shown early clinical promises, the mechanisms of action of these therapeutics remain complex and not fully understood. There is still a need to develop more effective receptor-targeting entitles and to improve the understanding of their mechanisms of action.
We have tried to define the first order principles to explain ectodomain mediated disabling of erbB receptors. Our laboratory described several operational mechanisms effected by mAbs. These include receptor downregulation, cell cycle arrest, angiogenesis inhibition and facilitation of antibody-dependent cell-mediated cytotoxicity (ADCC).
Endodomain targeted therapy is different and its principles are distinct. mAb alone cause p185her2/neu ubiquitination and degradation of the receptor. However the small molecule TKI lapatinib inhibited p185her2/neu phosporylation, prevents receptor ubiquitination and degradation, which in turn result in accumulation of stabilized receptor monomer and inactive receptor dimers at cell surface [59]. Kinase activation may be a required step for receptor ubiquitination and degradation, and small molecule TKIs prevent receptor down regulation as a consequence. These findings support combinations of anti-receptor mAb and a receptor-stabilizing TKI in receptor targeted therapy since their modes of action may improve disabling of erbB receptor functions.
Moreover, the treatment regimen that combines erbB targeted therapy with conventional chemotherapy will need to be further explored and optimized. Finding the optimal combinations of receptor targeting biologics and low molecular weight compounds is necessary. In addition identification of predictive biomarkers for drug response, will be useful in monitoring the effectiveness of targeted therapeutics in converting cancer from a lethal process into a chronic disease treatable with less toxic therapies than chemotherapy and high dose irradiation.
References
- 1.Wen XF, Yang G, Mao W, Thornton A, Liu J, Bast RC, Jr, et al. HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25(52):6986–96. doi: 10.1038/sj.onc.1209685. [DOI] [PubMed] [Google Scholar]
- 2.De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, et al. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214(3):559–67. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 3.Drebin JA, Link VC, Greene MI. Monoclonal antibodies reactive with distinct domains of the neu oncogene-encoded p185 molecule exert synergistic antitumor effects in vivo. Oncogene. 1988;2(3):273–7. [PubMed] [Google Scholar]
- 4.Zhang H, Wang Q, Montone KT, Peavey JE, Drebin JA, Greene MI, et al. Shared antigenic epitopes and pathobiological functions of anti-p185(her2/neu) monoclonal antibodies. Oncogene. 1999;67(1):15–25. doi: 10.1006/exmp.1999.2266. [DOI] [PubMed] [Google Scholar]
- 5.Hu S, Zhu Z, Li L, Chang L, Li W, Cheng L, et al. Epitope mapping and structural analysis of an anti-ErbB2 antibody A21: molecular basis for tumor inhibitory mechanism. Proteins. 2008;70(3):938–49. doi: 10.1002/prot.21551. [DOI] [PubMed] [Google Scholar]
- 6.Furuuchi K, Berezov A, Kumagai T, Greene MI. Targeted antireceptor therapy with monoclonal antibodies leads to the formation of inactivated tetrameric forms of ErbB receptors. J Immunol. 2007;178(2):1021–9. doi: 10.4049/jimmunol.178.2.1021. [DOI] [PubMed] [Google Scholar]
- 7.Maynard J, Georgiou G. Antibody engineering. Annu Rev Biomed Eng. 2000:2339–76. doi: 10.1146/annurev.bioeng.2.1.339. [DOI] [PubMed] [Google Scholar]
- 8.Hudson PJ, Souriau C. Engineered antibodies. Nat Med. 2003;9(1):129–34. doi: 10.1038/nm0103-129. [DOI] [PubMed] [Google Scholar]
- 9.Reff ME, Heard C. A review of modifications to recombinant antibodies: attempt to increase efficacy in oncology applications. Crit Rev Oncol Hematol. 2001;40(1):25–35. doi: 10.1016/s1040-8428(01)00132-9. [DOI] [PubMed] [Google Scholar]
- 10.Bhat TN, Bentley GA, Boulot G, Greene MI, Tello D, Dall’Acqua W, et al. Bound water molecules and conformational stabilization help mediate an antigen-antibody association. Proc Natl Acad Sci U S A. 1994;91(3):1089–93. doi: 10.1073/pnas.91.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruck C, Co MS, Slaoui M, Gaulton GN, Smith T, Fields BN, et al. Nucleic acid sequence of an internal image-bearing monoclonal anti-idiotype and its comparison to the sequence of the external antigen. Proc Natl Acad Sci U S A. 1986;83(17):6578–82. doi: 10.1073/pnas.83.17.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams WV, Moss DA, Kieber-Emmons T, Cohen JA, Myers JN, Weiner DB, et al. Development of biologically active peptides based on antibody structure. Proc Natl Acad Sci U S A. 1989;86(14):5537–41. doi: 10.1073/pnas.86.14.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams WV, Kieber-Emmons T, VonFeldt J, Greene MI, Weiner DB. Design of bioactive peptides based on antibody hypervariable region structures. Development of conformationally constrained and dimeric peptides with enhanced affinity. J Biol Chem. 1991;266(8):5182–90. [PubMed] [Google Scholar]
- 14.Dougall WC, Peterson NC, Greene MI. Antibody-structure-based design of pharmacological agents. Trends Biotechnol. 1994;12(9):372–9. doi: 10.1016/0167-7799(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 15.Saragovi HU, Fitzpatrick D, Raktabutr A, Nakanishi H, Kahn M, Greene MI. Design and synthesis of a mimetic from an antibody complementarity-determining region. Science. 1991;253(5021):792–5. doi: 10.1126/science.1876837. [DOI] [PubMed] [Google Scholar]
- 16.Murali R, Greene MI. Structure-based design of immunologically active therapeutic peptides. Immunol Res. 1998;17(1–2):163–9. doi: 10.1007/BF02786441. [DOI] [PubMed] [Google Scholar]
- 17.Park BW, Zhang HT, Wu C, Berezov A, Zhang X, Dua R, et al. Rationally designed anti-HER2/neu peptide mimetic disables P185HER2/neu tyrosine kinases in vitro and in vivo. Nat Biotechnol. 2000;18(2):194–8. doi: 10.1038/72651. [DOI] [PubMed] [Google Scholar]
- 18.Berezov A, Zhang HT, Greene MI, Murali R. Disabling erbB receptors with rationally designed exocyclic mimetics of antibodies: structure-function analysis. J Med Chem. 2001;44(16):2565–74. doi: 10.1021/jm000527m. [DOI] [PubMed] [Google Scholar]
- 19.Guillemard V, Nedev HN, Berezov A, Murali R, Saragovi HU. HER2-mediated internalization of a targeted prodrug cytotoxic conjugate is dependent on the valency of the targeting ligand. DNA Cell Biol. 2005;24(6):350–8. doi: 10.1089/dna.2005.24.351. [DOI] [PubMed] [Google Scholar]
- 20.Masuda K, Richter M, Song X, Berezov A, Masuda K, Murali R, et al. AHNP-streptavidin: a tetrameric bacterially produced antibody surrogate fusion protein against p185her2/neu. Oncogene. 2006;25(59):7740–6. doi: 10.1038/sj.onc.1209745. [DOI] [PubMed] [Google Scholar]
- 21.Rosebrough SF. Pharmacokinetics and biodistribution of radiolabeled avidin, streptavidin and biotin. Nucl Med Biol. 1993;20(5):663–8. doi: 10.1016/0969-8051(93)90037-u. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Cheng X, Richter M, Greene MI. A sensitive and high-throughput assay to detect low-abundance proteins in serum. Nat Med. 2006;12(4):473–7. doi: 10.1038/nm1378. [DOI] [PubMed] [Google Scholar]
- 23.Weiner DB, Kokai Y, Wada T, Cohen JA, Williams WV, Greene MI. Linkage of tyrosine kinase activity with transforming ability of the p185neu oncoprotein. Oncogene. 1989;4(10):1175–83. [PubMed] [Google Scholar]
- 24.Wada T, Qian XL, Greene MI. Intermolecular association of the p185neu protein and EGF receptor modulates EGF receptor function. Cell. 1990;61(7):1339–47. doi: 10.1016/0092-8674(90)90697-d. [DOI] [PubMed] [Google Scholar]
- 25.Kokai Y, Myers JN, Wada T, Brown VI, LeVea CM, Davis JG, et al. Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell. 1989;58(2):287–92. doi: 10.1016/0092-8674(89)90843-x. [DOI] [PubMed] [Google Scholar]
- 26.Stern DF, Kamps MP. EGF-stimulated tyrosine phosphorylation of p185neu: a potential model for receptor interactions. EMBO J. 1988;7(4):995–1001. doi: 10.1002/j.1460-2075.1988.tb02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackel PO, Zwick E, Prenzel N, Ullrich A. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol. 1999;11(2):184–9. doi: 10.1016/s0955-0674(99)80024-6. [DOI] [PubMed] [Google Scholar]
- 28.Zwick E, Hackel PO, Prenzel N, Ullrich A. The EGF receptor as central transducer of heterologous signalling systems. Trends Pharmacol Sci. 1999;20(10):408–12. doi: 10.1016/s0165-6147(99)01373-5. [DOI] [PubMed] [Google Scholar]
- 29.Cooper JA, Howell B. The when and how of Src regulation. Cell. 1993;73(6):1051–4. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- 30.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274(12):8335–43. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 31.Emlet DR, Moscatello DK, Ludlow LB, Wong AJ. Subsets of epidermal growth factor receptors during activation and endocytosis. J Biol Chem. 1997;272(7):4079–86. doi: 10.1074/jbc.272.7.4079. [DOI] [PubMed] [Google Scholar]
- 32.Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, et al. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4(6):1029–40. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- 33.Ettenberg SA, Keane MM, Nau MM, Frankel M, Wang LM, Pierce JH, et al. cbl-b inhibits epidermal growth factor receptor signaling. Oncogene. 1999;18(10):1855–66. doi: 10.1038/sj.onc.1202499. [DOI] [PubMed] [Google Scholar]
- 34.Rojas M, Yao S, Lin YZ. Controlling epidermal growth factor (EGF)-stimulated Ras activation in intact cells by a cell-permeable peptide mimicking phosphorylated EGF receptor. J Biol Chem. 1996;271(44):27456–61. doi: 10.1074/jbc.271.44.27456. [DOI] [PubMed] [Google Scholar]
- 35.Qian X, LeVea CM, Freeman JK, Dougall WC, Greene MI. Heterodimerization of epidermal growth factor receptor and wild-type or kinase-deficient Neu: a mechanism of interreceptor kinase activation and transphosphorylation. Proc Natl Acad Sci U S A. 1994;91(4):1500–4. doi: 10.1073/pnas.91.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alimandi M, Romano A, Curia MC, Muraro R, Fedi P, Aaronson SA, et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10(9):1813–21. [PubMed] [Google Scholar]
- 37.Cohen BD, Kiener PA, Green JM, Foy L, Fell HP, Zhang K. The relationship between human epidermal growth-like factor receptor expression and cellular transformation in NIH3T3 cells. J Biol Chem. 1996;271(48):30897–903. doi: 10.1074/jbc.271.48.30897. [DOI] [PubMed] [Google Scholar]
- 38.Cohen BD, Green JM, Foy L, Fell HP. HER4-mediated biological and biochemical properties in NIH 3T3 cells. Evidence for HER1–HER4 heterodimers. J Biol Chem. 1996;271(9):4813–8. doi: 10.1074/jbc.271.9.4813. [DOI] [PubMed] [Google Scholar]
- 39.Wallasch C, Weiss FU, Niederfellner G, Jallal B, Issing W, Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J. 1995;14(17):4267–75. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang K, Sun J, Liu N, Wen D, Chang D, Thomason A, et al. Transformation of NIH 3T3 cells by HER3 or HER4 receptors requires the presence of HER1 or HER2. J Biol Chem. 1996;271(7):3884–90. [PubMed] [Google Scholar]
- 41.Klapper LN, Glathe S, Vaisman N, Hynes NE, Andrews GC, Sela M, et al. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci U S A. 1999;96(9):4995–5000. doi: 10.1073/pnas.96.9.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carraway KL, 3rd, Cantley LC. A neu acquaintance for erbB3 and erbB4: a role for receptor heterodimerization in growth signaling. Cell. 1994;78(1):5–8. doi: 10.1016/0092-8674(94)90564-9. [DOI] [PubMed] [Google Scholar]
- 43.Peczuh MW, Hamilton AD. Peptide and protein recognition by designed molecules. Chem Rev. 2000;100(7):2479–94. doi: 10.1021/cr9900026. [DOI] [PubMed] [Google Scholar]
- 44.Zutshi R, Brickner M, Chmielewski J. Inhibiting the assembly of protein–protein interfaces. Curr Opin Chem Biol. 1998;2(1):62–6. doi: 10.1016/s1367-5931(98)80036-7. [DOI] [PubMed] [Google Scholar]
- 45.Schramm HJ, Boetzel J, Büttner J, Fritsche E, Göhring W, Jaeger E, et al. The inhibition of human immunodeficiency virus proteases by ‘interface peptides’. Antiviral Res. 1996;30(2–3):155–70. doi: 10.1016/0166-3542(96)00940-0. [DOI] [PubMed] [Google Scholar]
- 46.Divita G, Restle T, Goody RS, Chermann JC, Baillon JG. Inhibition of human immunodeficiency virus type 1 reverse transcriptase dimerization using synthetic peptides derived from the connection domain. J Biol Chem. 1994;269(18):13080–3. [PubMed] [Google Scholar]
- 47.Dutia BM, Frame MC, Subak-Sharpe JH, Clark WN, Marsden HS. Specific inhibition of herpesvirus ribonucleotide reductase by synthetic peptides. Nature. 1986;321(6068):439–41. doi: 10.1038/321439a0. [DOI] [PubMed] [Google Scholar]
- 48.Berezov A, Chen J, Liu Q, Zhang HT, Greene MI, Murali R. Disabling receptor ensembles with rationally designed interface peptidomimetics. J Biol Chem. 2002;277(31):28330–9. doi: 10.1074/jbc.M202880200. [DOI] [PubMed] [Google Scholar]
- 49.Qian X, Dougall WC, Fei Z, Greene MI. Intermolecular association and trans-phosphorylation of different neu-kinase forms permit SH2-dependent signaling and oncogenic transformation. Oncogene. 1995;10(1):211–9. [PubMed] [Google Scholar]
- 50.Qian X, O’Rourke DM, Zhao H, Greene MI. Inhibition of p185neu kinase activity and cellular transformation by co-expression of a truncated neu protein. Oncogene. 1996;13(10):2149–57. [PubMed] [Google Scholar]
- 51.O’Rourke DM, Qian X, Zhang HT, Davis JG, Nute E, Meinkoth J, et al. Trans receptor inhibition of human glioblastoma cells by erbB family ectodomains. Proc Natl Acad Sci U S A. 1997;94(7):3250–5. doi: 10.1073/pnas.94.7.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stamos J, Sliwkowski MX, Eigenbrot C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol Chem. 2002;277(48):46265–72. doi: 10.1074/jbc.M207135200. [DOI] [PubMed] [Google Scholar]
- 53.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 55.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 56.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wissner A, Mansour TS. The development of HKI-272 and related compounds for the treatment of cancer. Arch Pharm (Weinheim) 2008;341(8):465–77. doi: 10.1002/ardp.200800009. [DOI] [PubMed] [Google Scholar]
- 58.Li D, Shimamura T, Ji H, Chen L, Haringsma HJ, McNamara K, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007;12(1):81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28(6):803–14. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]