Abstract
A series of amides of ethacrynic acid was prepared and evaluated for their ability to inhibit Wnt signaling and decrease the survival of CLL cells. Several of the most potent derivatives were active in the low micromolar range. Reduction of the α,β-unsaturated carbon–carbon double bond of EA abrogated both the inhibition of Wnt signaling as well as the decrease in CLL survival. Preliminary mechanism of action studies suggest that these derivatives covalently modify sulfhydryl groups present on transcription factors important for Wnt/β-catenin signaling.
Keywords: Chronic lymphocytic leukemia, Wnt/β-catenin signaling, Ethacrynic acid amides, Lef-1
Chronic lymphocytic leukemia (CLL), the most common adult leukemia in the United States, is characterized by the accumulation of mature-appearing, but functionally incompetent small lymphocytes. There is as yet no cure for this disease, nor has conventional chemotherapy been definitively shown to prolong patient survival. Late in the disease course, patients typically develop pronounced bone marrow dysfunction due to chemotherapy-induced toxicity and disease progression, making them intolerant to further treatment with cytotoxic agents. Thus, it is necessary to develop new treatments that target the molecular defects in CLL with minimal bone marrow toxicities. The clonal expansion of B-lymphocytes in CLL is caused by an abnormal balance between the signaling for survival and cell death.1
Wnt signaling pathways play a number of key roles in embryonic development and maintenance of homeostasis in mature tissues. Wnt proteins are a large family of secreted glycoproteins that activate signal transduction pathways to control a wide variety of cellular processes such as determination of cell fate, proliferation, migration, and polarity. Wnts are capable of signaling through several pathways, the best-characterized being the canonical β-catenin/Tcf-LEF mediated pathway. Canonical Wnts stabilize β-catenin protein, which has implications in the genesis of many human cancers. Indeed, growing evidence suggests that deregulation of the Wnt/β-catenin pathway is directly linked to tumorigenesis. 2,3 Recently, it has been demonstrated that the Wnt signaling pathway is activated in CLL cells, and that uncontrolled Wnt/β-catenin signaling may contribute to the defect in apoptosis that characterizes this malignancy.4,5 Therefore, the Wnt/β-catenin signaling molecules are attractive candidates for developing targeted therapies for CLL.
Cell-based screens of libraries of natural products and synthetic small molecules have provided useful tools for the study of complex cellular processes. Indeed, a number of small molecules have been identified that modulate Wnt/β-catenin signaling, including NSAIDs, 6 quercetin,7 ICG-001,8 and others.9 To identify additional molecules that regulate the canonical Wnt/β-catenin signaling pathway, we employed a β-catenin dependent cell reporter system to screen for specific antagonists of the Wnt/β-catenin signaling pathway from a library of 960 known FDA-approved drugs (MicroSource Gen-Plus library). One such molecule, ethacrynic acid (1, EA) was identified among the few inhibitors. Ethacrynic acid, a once commonly used loop diuretic drug, was previously shown to be uniquely cytotoxic toward primary CLL cells.10 However, EA is not ideal as a chemotherapeutic agent for CLL treatment due to its diuretic properties and relative lack of potency. Therefore, as initial efforts to optimize this lead, we report here the synthesis and in vitro evaluation of some amide derivatives of EA for inhibition of Wnt signaling and for decreasing the survival of cells from CLL patients.
The preparation of the amide derivatives of EA was accomplished by refluxing EA in benzene with thionyl chloride to form the EA acyl chloride intermediate followed by reaction in pyridine with desired amines (Scheme 1).
Scheme 1.
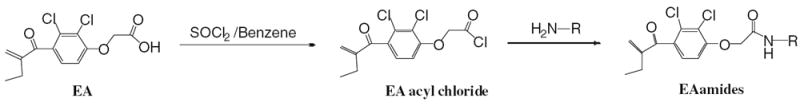
Thus, by this procedure over 40 compounds were prepared and evaluated. Furthermore, to explore the contribution of the C–C double bond of the α–β-unsaturated carbonyl function to the bioactivity, we reduced the double bond of EA by catalytic hydrogenation11 to afford EA-R (42). In addition, a few simple alkyl esters of EA and a “truncated” decarboxylated version of EA (compound 43) were prepared. Although EA esters were reported to kill CLL cells at low micromolar concentrations,12 those esters along with 43 were also highly toxic to peripheral blood mononuclear cells (PBMC) in our assays and were not studied further for Wnt and CLL activity (data not shown).
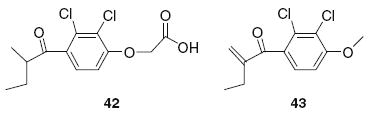
The mechanism of ethacrynic acid cytotoxicity has been attributed to the drug’s known capacity to inhibit glutathione S-transferase (GST), causing increased cellular oxidative stress. However, a recent study13 showed that the antioxidant N-acetyl-l-cysteine (NAC) protected ethacrynic acid-induced cell death with no effect on cellular glutathione levels, whereas the free radical scavenger 3(2) tert-butyl-4-hydroxyanisole (BHA) did not repress ethacrynic acid-induced cell death, suggesting the existence of additional or alternative pathways that are altered by the drug. Since EA is classified as an α,β-unsaturated ketone, its Wnt inhibition activities are most likely due to the alkylation effects on Wnt proteins which are comprised of cysteine-rich glycoproteins.14 Indeed, we found that inhibition of Wnt signaling by EA can be blocked by adding N-acetyl-l-cysteine or 2-aminoethanethiol to the media prior to testing (data not shown). Moreover, decreased survival of CLL cells and inhibition of Wnt signaling by EA were completely abrogated after reduction of the α–β-double bond by hydrogenation (Fig. 1, EA-R and Table 1, compound 42), suggesting that this Michael acceptor function is essential for its activity.
Figure 1.
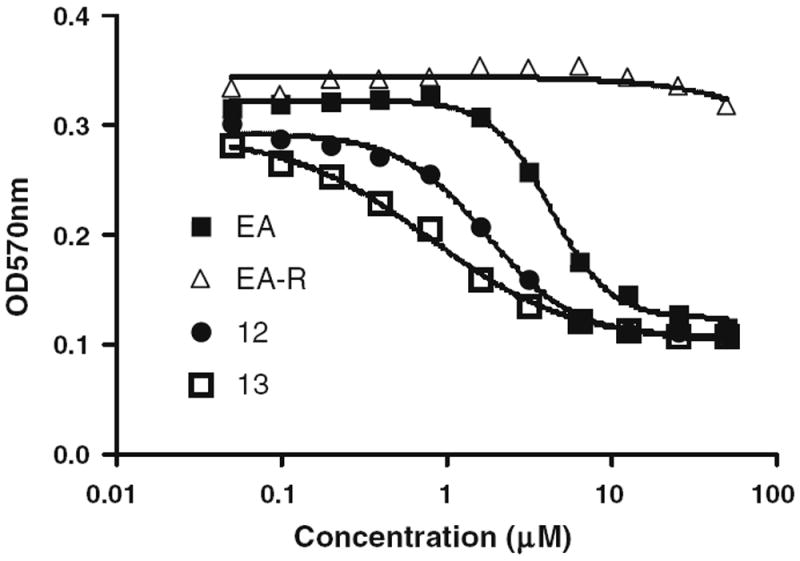
Comparison of EA and EA-R on CLL viability.18 This experiment was performed as described in Ref. 18.
Table 1.
Inhibition of Wnt signaling and CLL survival by ethacrynic amides
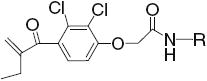
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | CLL inhibition EC50 (μM) | Wnt inhibition IC50 (μM) | Entry | R | CLL inhibition EC50 (μM) | Wnt inhibition IC50 (μM) |
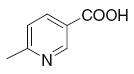
| 1 | Ethacrynic acid | 9.9 | 32.7 | 21 |
|
14.9 | >50 |
| 2 |
|
4.1 | >50 | 22 |
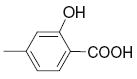
|
>25 | >50 |
| 3 |
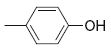
|
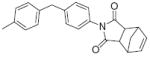
6.4 | >50 | 23 |
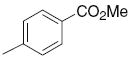
|
4.1 | 11.38 |
| 4 |
|
3.7 | 4.76 | 24 |
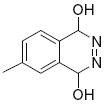
|
8.5 | 6.58 |
| 5 | −OH | 4.5 | 9.89 | 25 |
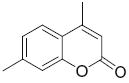
|
2.8 | 2.63 |
| 6 |
|
5.0 | 5.81 | 26 |
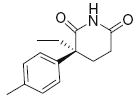
|
2.8 | 4.42 |
| 7 |
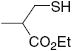
|
4.8 | >50 | 27 |
|
1.8 | 2.93 |
| 8 |
|
7.8 | 6.84 | 28 |
|
3.9 | 5.06 |
| 9 |
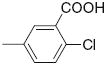
|
15.9 | >50 | 29 |
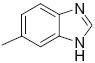
|
3.2 | 3.62 |
| 10 |
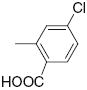
|
13.8 | 9.62 | 30 |
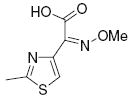
|
>25 | >50 |
| 11 |
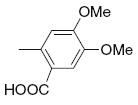
|
21.9 | >50 | 31 |
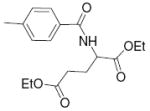
|
2.1 | 2.61 |
| 12 |
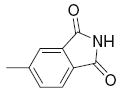
|
2.5 | 4.88 | 32 |
|
2.1 | 2.97 |
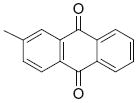
| 13 |
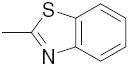
|
1.5 | 4.86 | 33 |
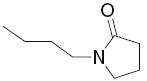
|
13.8 | >50 |
| 14 |
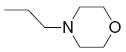
|
6.4 | >50 | 34 |
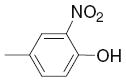
|
7.4 | 4.23 |
| 15 |
|
3.2 | >50 | 35 |
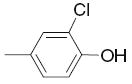
|
5.5 | 3.80 |
| 16 |
|
>25 | >50 | 36 |
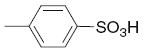
|
>25 | >50 |
| 17 |
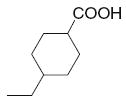
|
8.0 | >50 | 37 |
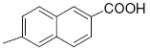
|
3.7 | 2.93 |
| 18 |
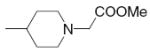
|
10.6 | >50 | 38 |
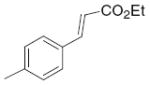
|
1.7 | 3.79 |
| 19 |
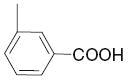
|
20.6 | 5.85 | 39 |
|
3.0 | 2.51 |
| 20 |
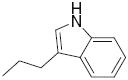
|
5.9 | 10.7 | 40 |
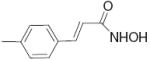
|
2.6 | 1.81 |
| 41 |
|
>25 | >50 | 42 | EA-R | >25 | >50 |
Several compounds were found to effectively decrease CLL survival and antagonize Wnt signaling at low micromolar concentrations (25, 29, 31, 37, 39 and 40). These results correlate with our earlier findings that Wnt signaling genes are over-expressed and active in CLL.5 It is possible that EA derivatives might inhibit Wnt signaling by covalent modification of sulfhydryl groups of Wnt-dependent genes such as Lef-1 (which is highly expressed in CLL) and this possibility is the subject of ongoing studies in our laboratories.
Structure–activity trends among the amides in terms of Wnt signaling inhibition revealed that aromatic-containing amides were generally more active than aliphatic amides. Moreover, the larger aromatic substitutions (benzothiazole, phthalimide, naphthyl carboxylicacid, etc.) showed good activity in both systems. It is noteworthy that the IC50s for inhibition of Wnt signaling are consistently lower than the EC50s for inhibition of CLL survival, except for most of the aromatic carboxylic acids. This suggests that the active EA derivatives may have some other target receptor in the cell, a target that may impact CLL survival in addition to the Wnt signaling alone. An example of a possible off-target receptor for the EA derivatives might be inhibition of NF-κB activity through direct inhibition of IKK-β, wherein the cysteine 179 in the activation loop of IKK-β can be covalently modified by Michael acceptors.15 Two well known examples of this are prostaglandin J2 and prostaglandin A1, both of which contain the α,β-unsaturated carbonyl function, and thus the EA derivatives may be acting in a similar manner.
In summary, we have synthesized amides of EA19-23 with enhanced potency, relative to EA, toward the inhibition of Wnt signaling and of CLL cell survival (Table 1 and Fig. 2 see Supplement). Differences in the potency among the various derivatives may be simply due to relative efficiency of compound delivery to cells and their ability to access the nuclear compartment and make contact with transcription factors important in Wnt signaling.
Further in vivo studies of these amide compounds are underway.
Supplementary Material
Acknowledgments
We thank Michael Rosenbach, Haowen Zhou, Michael Chan, for the technical assistance, and Nancy Noon for secretarial support. This work was supported in part by the Leukemia and Lymphoma Society SCOR grant, and Grants CA81534 and CA113318, both from the National Institutes of Health.
Footnotes
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bmcl.2008.12.067.
References and notes
- 1.Caligaris-Cappio F, Hamblin TJ. J Clin Oncol. 1999;17:399. doi: 10.1200/JCO.1999.17.1.399. [DOI] [PubMed] [Google Scholar]
- 2.Peifer M, Polakis P. Science. 2000;287:1606. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 3.Polakis P. Genes Dev. 2000;14:1837. [PubMed] [Google Scholar]
- 4.Rosenwald A, Alizadeh AA, Widhopf G, Simon R, Davis RE, Yu X, Yang L, Pickeral OK, Rassenti LZ, Powell J, Botstein D, Byrd JC, Grever MR, Cheson BD, Chiorazzi N, Wilson WH, Kipps TJ, Brown PO, Staudt LM. J Exp Med. 2001;194:1639. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu D, Zhao Y, Tawatao R, Cottam HB, Sen M, Leoni LM, Kipps TJ, Corr M, Carson DA. Proc Natl Acad Sci U S A. 2004;101:3118. doi: 10.1073/pnas.0308648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu D, Cottam HB, Corr M, Carson DA. Proc Natl Acad Sci U S A. 2005;102:18567. doi: 10.1073/pnas.0509316102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park CH, Hahm ER, Lee JH, Jung KC, Yang CH. Biochem Biophys Res Commun. 2005;331:1222. doi: 10.1016/j.bbrc.2005.03.242. [DOI] [PubMed] [Google Scholar]
- 8.Teo J-L, Ma H, Nguyen C, Lam C, Kahn M. Proc Natl Acad Sci U S A. 2005;102:12171. doi: 10.1073/pnas.0504600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker N, Clevers H. Nat Rev Drug Discov. 2006;5:997. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 10.Twentyman PR, Lambert E, Muller M, Rees JK. Leukemia. 1992;6:726. [PubMed] [Google Scholar]
- 11.Woltersdorf OW, Jr, DeSolms SJ, Schultz EM, Cragoe EJ., Jr J Med Chem. 1977;20:1400. doi: 10.1021/jm00221a010. [DOI] [PubMed] [Google Scholar]
- 12.Zhao G, Liu C, Wang R, Song D, Wang X, Lou H, Jing Y. Bioorg Med Chem. 2007;15:2701. doi: 10.1016/j.bmc.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 13.Aizawa S, Ookawa K, Kudo T, Asano J, Hayakari M, Tsuchida S. Cancer Sci. 2003;94:886. doi: 10.1111/j.1349-7006.2003.tb01371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi-Yanaga F, Sasaguri T. J Pharmacol Sci. 2007;104:293. doi: 10.1254/jphs.cr0070024. [DOI] [PubMed] [Google Scholar]
- 15.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Nature. 2000;403:103. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 16.Wnt signaling inhibition method I: The human embryonic kidney cell line HEK293 was grown and transfected with various expression plasmids encoding proteins in the Wnt and β-catenin pathway, exactly as described previously.6 They included the β-catenin regulated TOPFlash reporter gene and expression plasmids for Wnt1, Wnt3, β-catenin, DSH, LRP6, as described.
- 17.Wnt signaling inhibition method II: To determine the specificity of compounds on Wnt/β-catenin pathway inhibition, CellSensor LEF/TCF-bla SW480 cell-based assay (Invitrogen, Carlsbad, CA) was used according to the supplier’s instructions, but modified for 96 well format. Cells were plated at 25,000 cells/well in assay medium in 96-well black plates with clear bottom (Corning) the day prior to compound treatment. Compounds were added to cells at a final concentration ranging from 33.3 to 0.5 μM, incubated for 20 h and then combined with LiveBLAzer™-FRET B/G Substrate (CCF4-AM) for 2 h at room temperature. Fluorescence emission values at 465 and 535 nm were obtained using a standard fluorescence plate reader and the 465/535 ratios were calculated for each treatment (n = 2 for each data point). Results were normalized to untreated control cells (set at 100%, n = 4), plotted as % of control, and EC50 determined using Prism 4.0a software (GraphPad).
- 18.Fresh CLL or peripheral blood mononuclear cells (PBMC) were plated at 2.5 × 105 per well and treated with compounds for 48 h. Then 1/10 V of 5 mg/mL MTT was added, and cells were incubated at 37 °C overnight. Finally, ½ V of Lysis buffer was added to dissolve the insoluble purple formazan product, incubated at 37 °C overnight, and OD at 570 nm was read and recorded.
- 19.General procedure for synthesis: To a mixture of 1 mmol of ethacrynic acid in 10 mL of benzene, 1 mL of thionyl chloride was added. The mixture was heated at reflux for 1.5 h, solvent was removed in vacuo. Another 10 mL of benzene was added and distilled off again. The residue was dissolved in a small volume of benzene for the next step. The resulting ethacrynic chloride solution was added dropwise to a solution of 1 mmol of amine in pyridine (10 mL) at 0 °C with stirring. The reaction was stirred at ambient temperature for 3 h, the solvent was distilled off in vacuo, the residue was dissolved in ethyl acetate, and washed with water and brine. The organic layer was dried over anhydrous MgSO4, and the residue was purified by silica gel column chromatography (dichloromethane:methanol from 100:0 to 100:5) to obtain the pure EA amides shown in Table 1.
- 20.Selected data for compound 4: 1H NMR (400 MHz, CDCl3) δ 9.23 (br, 1H), 7.89 (d, J = 8 Hz, 1H), 7.70 (d, J = 8 Hz, 1H), 7.21 (d, J = 8 Hz, 2H), 6.98 (d, J = 8 Hz, 2H), 6.20 (br, 1H), 5.98 (d, J = 8 Hz, 1H), 5.62 (d, J = 12 Hz, 1H), 4.79 (d, J = 12 Hz, 1H), 2.77 (s, 2H), 2.44 (q, J = 8 Hz, 2H), 1.17 (t, J = 8 Hz, 3H). MS (ESI) m/z: 422, [M+H]+.
- 21.Selected data for compound 6: 1H NMR (400 MHz, CDCl3) δ 9.47 (br, 1H), 8.03 (d, J = 8 Hz, 2H), 7.73 (d, J = 8 Hz, 2H), 7.21 (d, J = 8 Hz, 1H), 6.99 (d, J = 8 Hz, 1H), 5.98 (d, J = 8 Hz, 1H), 5.62 (d, J = 8 Hz, 1H), 4.81 (s, 2H), 2.90 (br, 1H), 2.45 (q, J = 8 Hz, 2H), 1.17 (t, J = 8 Hz, 3H). MS (ESI) m/z: 423, [M+H]+.
- 22.Selected data for compound 37: 1H NMR (400 MHz, CDCl3) δ 9.30 (br, 1H), 8.57 (s, 1H), 8.40 (s, 1H), 7.95(d, J = 8 Hz, 1H), 7.93 (d, J = 8 Hz, 1H), 7.86 (d, J = 8 Hz, 1H), 7.62 (d, J = 8 Hz, 1H), 7.22 (d, J = 8 Hz, 1H), 7.04 (d, J = 8Hz, 1H), 5.99 (s, 1H), 5.63 (s, 1H), 4.83 (s, 2H), 2.60 (br, 1H), 2.48 (q, J = 8 Hz, 2H), 1.17 (t, J = 8 HZ, 3H). MS (ESI) m/z: 473, [M+H]+.
- 23.Selected data for compound 40: 1H NMR (400 MHz, CDCl3) δ 10.70 (br, 1H), 10.38 (br, 1H), 9.00 (br, 1H), 7.64 (d, J = 1.6 Hz, 2H), 7.50 (d, J = 1.6 Hz, 2H), 7.32 (d, J = 8.4 Hz, 1H), 7.15 (d, J = 8.4 Hz, 1H), 6.37 (s, 1H), 6.33 (s, 1H), 6.06 (s, 1H), 5.56 (s, 1H), 4.97 (s, 2H), 2.36 (q, J = 6.8 Hz, 2H), 1.07 (t, J = 7.6 Hz, 3H). MS (ESI) m/z: 463, [M+H]+.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


