Abstract
Study Objectives:
A relationship between obstructive sleep apnea (OSA) and exposure to the World Trade Center (WTC) dust and fumes has been suggested in responders but little is known about a possible relationship in community members. We characterized sleep studies performed in community members with WTC dust exposure to improve our understanding of the relationship between the diagnosis and severity of OSA and WTC dust exposure in this population.
Methods:
Single-center, retrospective study of patients enrolled in a clinical treatment program for community members with WTC dust exposure. Patients were included if they had undergone sleep studies for evaluation of possible OSA through September 2016 and provided written informed consent.
Results:
The total number of patients included in the analysis was 143. Patients were predominantly male (61%), never smokers (59%) and had a median body mass index of 31 kg/m2. Most reported upper and lower respiratory symptoms. An apnea-hypopnea index (AHI) ≥ 5 events/h was measured in 66% of the patients, and respiratory disturbance index was ≥ 5 events/h in 97%. The proportion of patients with moderate-severe OSA (defined by the AHI 4% criteria) was 50%. Multivariate logistic regression revealed that acute WTC dust cloud exposure was associated with severity but not diagnosis of OSA.
Conclusions:
We identified a high rate of OSA in the WTC community cohort who were referred for sleep studies. Exposure to the massive WTC dust cloud caused by the WTC collapse was independently associated with the severity of OSA in this population. This finding highlights the role that environmental exposures may play in the development of OSA.
Citation:
Ahuja S, Zhu Z, Shao Y, Berger KI, Reibman J, Ahmed O. Obstructive sleep apnea in community members exposed to World Trade Center dust and fumes. J Clin Sleep Med. 2018;14(5):735–743.
Keywords: environment, inflammation, obstructive sleep apnea, particulate matter, rhinitis, upper airway, World Trade Center
BRIEF SUMMARY
Current Knowledge/Study Rationale: Adverse health effects associated with World Trade Center (WTC) dust exposure in the WTC responder and local community population are well described. An association between aerodigestive disorders and obstructive sleep apnea (OSA) has been reported in responders involved in the rescue and response to the WTC disaster; the relationship to the type of exposure remains unclear. There is even less information available about OSA in the community members who lived or worked in the area surrounding the WTC disaster.
Study Impact: Our study reveals a dose-response relationship between WTC exposure and severity of OSA in community members and highlights the potential role environmental exposures may play in the development of OSA.
INTRODUCTION
The destruction and collapse of the World Trade Center (WTC) towers in New York City on September 11, 2001 resulted in significant environmental and occupational exposures to the rescue and recovery workers (responders), as well as to the larger group of local community members (local residents, local workers, tower evacuees, or those involved in the cleanup of the surrounding area). The collapse of the WTC towers released 1.2 million tons of material, producing massive dust clouds as the buildings collapsed.1 The dust consisted of a combination of building debris and combustion products and was extremely caustic with a pH of 9 to 11. Many evacuees, local workers, and local residents were caught in these dust clouds as they attempted to flee the area with potential for massive and acute WTC dust exposure. This dust settled over southern Manhattan and areas of Brooklyn in New York City. It had a high propensity to aerosolize, allowing for easy resuspension and recontamination of sites that were inadequately cleaned.1–4 Community members had potential for chronic exposure to re-suspended dust, as well as fumes from the fires that burned for months.2,3,5,6 Acute and chronic WTC dust exposure has been associated with adverse health effects in responders and community members, including increased incidence of persistent upper and lower respiratory symptoms and a higher burden of posttraumatic stress disorder (PTSD) compared to the general population.7–12
Symptoms of snoring and sleep disruption have been described in those who participated in rescue and recovery activities during the WTC disaster (responder population). A link between dust cloud exposure and obstructive sleep apnea (OSA) has been suggested in some studies.13–15 Interestingly, typical risk factors for OSA such as elevated body mass index (BMI) may not be associated with the OSA reported in the WTC responder population, suggesting an alternative mechanism.14 Responses to a screening questionnaire suggest an association between OSA and gastroesophageal reflux and chronic rhinosinusitis symptoms with increased odds ratios (ORs) for OSA of 2.7 and 2.21 respectively.16 These data resulted in the United States government including OSA as a WTC-associated condition in the WTC-exposed population with aerodigestive disorders.
The World Trade Center Environmental Health Center (WTC EHC) provides medical and mental health care to affected community members.2,5 The program started as a pilot program in 2005, and obtained federal grant funding in 2008. It was included as a Center of Excellence under the James Zadroga Health and Compensation Act of 2010, with federal rules and regulations for patient inclusion and treatment of medical conditions.2 The WTC dust/fume exposure of community members differs from that of the responders, with many having both acute exposure to the dust cloud and subsequent chronic exposures. The population in the WTC EHC includes more women, and those with varied socioeconomic characteristics when compared to the responders.5 Despite this, there are many similarities between the health outcomes in the WTC responders and WTC community members. Indeed, anecdotal sleep complaints are common in community members as well as in responders. Symptoms of gastroesophageal reflux disease (GERD), rhinosinusitis, lower respiratory symptoms, and PTSD are also well documented in the community.2,5,7,8,10,17 As such, there is an unmet need to investigate sleep disorders in the community cohort affected by the WTC collapse. The aim of this study was to evaluate the association between WTC exposures with diagnosis and severity of OSA in the WTC exposed community population referred for sleep studies in the WTC EHC.
METHODS
Patient Selection
Individuals were self-referred to the WTC EHC with medical and/or mental health symptoms related to the WTC towers collapse as previously described.5 The Institutional Review Board of New York University School of Medicine approved the research database and only patients who provided signed consent to have their records included in a database and retrospectively reviewed were included for analysis. Patients were included for analysis if they completed an initial visit at Bellevue Hospital between 2005 and September 2016 and underwent polysomnography (PSG) ordered by the WTC EHC clinic.
Procedures and Definitions
Clinical Protocol and Definitions
At the time of enrollment in the WTC EHC, patients underwent a series of interviewer-administered questionnaires to obtain information regarding demographics and degree of WTC exposure.5 Upon enrollment, patients were asked but not required to sign consent for review of clinical information. Questions about WTC exposure included those that obtain information about presence in the WTC dust cloud exposure on September 11, 2001, and individuals were classified as having WTC dust cloud exposure if they reported having been enveloped in the dust on September 11 as the WTC towers collapsed.5 Individuals were also classified for potential exposure as local residents, cleanup workers, or local workers.5 Questionnaires also included detailed assessment of symptoms, including the onset of the individual symptom, severity, and temporal relationship to the WTC collapse. Standardized medical evaluation included spirometry. Mental health screening was performed using the PTSD checklist (PCL-17), with a score ≥ 44 considered consistent with “probable PTSD.”17 Symptoms of depression and anxiety were assessed using the Hopkins Symptom checklist (HSCL-25), with a score of ≥ 1.75 considered positive for either condition.18 Information about tobacco use was obtained and for this study; patients with pack-year smoking history of 5 or more were considered smokers.
Sleep disorders were not anticipated as an adverse health event; thus, questions interrogating sleep disruption were not included. However, patients in the WTC EHC were referred for PSG to evaluate clinical suspicion of OSA based on symptoms. Under federal regulations for the WTC EHC, patients were eligible for certification of OSA as a WTC-associated condition if they had OSA exacerbated by or related to another WTC-related aerodigestive disorder. Thus, most patients were referred for evaluation if they had a concurrent aerodigestive symptom.
Sleep Assessment
Captured data included the type of sleep study performed, total sleep time, sleep and REM latency, sleep efficiency and time in each sleep stage, total apnea-hypopnea index (AHI) with an associated oxygen desaturation of 4% (AHI 4%), respiratory disturbance index (RDI) and mean and nadir oxygen saturation during sleep.
A diagnosis of OSA was defined as an AHI 4% ≥ 5 events/h. Severity of OSA was characterized using standardized definitions: AHI of 5–14.9, 15–29.9, ≥ 30 events/h were defined as mild, moderate, and severe OSA respectively.19 In patients with split-night sleep studies, the diagnostic portion of the study was used to determine AHI.
Statistical Methods
Information on characteristics and mental health scores were maintained in the WTC Data Center Oracle database. PSG data were collected and managed using REDCap electronic data capture tools hosted at New York University.20 Categorical variables were summarized using counts and percentages and the significance of between-group difference was assessed by chi-square test. Continuous variables were summarized using median and interquartile range and difference between groups was assessed by Wilcoxon rank-sum test. Log transformation was conducted on AHI 4% and RDI. Patients with incomplete covariate information were excluded from regression analysis. Linear regression was used to quantify the association between log-transformed AHI 4%, log-transformed RDI, and covariates of interest. Logistic regression was used to quantify the association between diagnosis of OSA and covariates of interest. Multivariate models were constructed using covariates that were significant in univariate analyses. A value of P = .05 was used to test for two-sided statistical significance. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, North Carolina, United States).
RESULTS
Baseline Characteristics
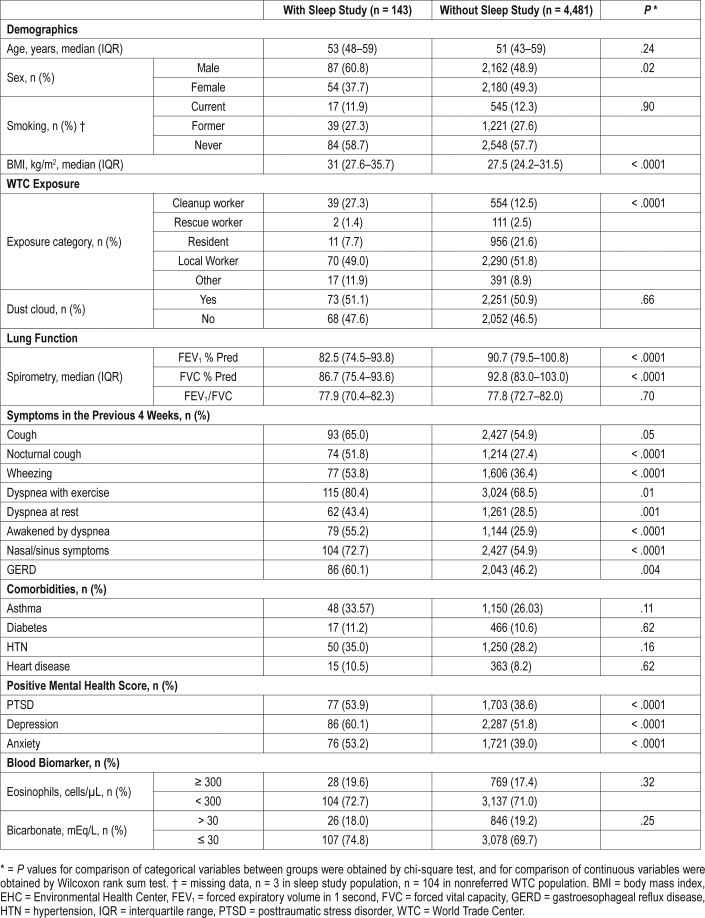
Two hundred nineteen patients with informed consent in the WTC EHC underwent sleep studies between 2008 and September 2016. Of these, 143 studies were available for review (final sleep study population). The characteristics of the patients who underwent sleep studies and those that did not are shown (Table 1). Patients with sleep studies were predominantly male (61%), never smokers (59%), and had a median BMI of 31. Many were local workers (49%) and 50% of the patients were in the initial WTC dust cloud. Many reported cough, wheezing, dyspnea, rhinosinusitis, GERD, and headaches that occurred more than twice a week in the month preceding their initial interview. Many individuals scored positive for probable PTSD (54%), depression (60%), or anxiety (53%).
Table 1.
WTC EHC patients with sleep studies versus WTC EHC patients without studies.
Compared to the overall WTC EHC population, patients who underwent sleep studies were more frequently male and had a higher BMI. There was no significant difference in WTC dust cloud exposure. More were cleanup workers. As expected due to referral requirements, the rate of upper and lower respiratory symptoms was increased in the sleep study population. Lung function was reduced in the sleep study population as measured by the forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). More patients in the sleep study group scored positive for PTSD, depression, and anxiety compared to the overall group.
Polysomnography Data
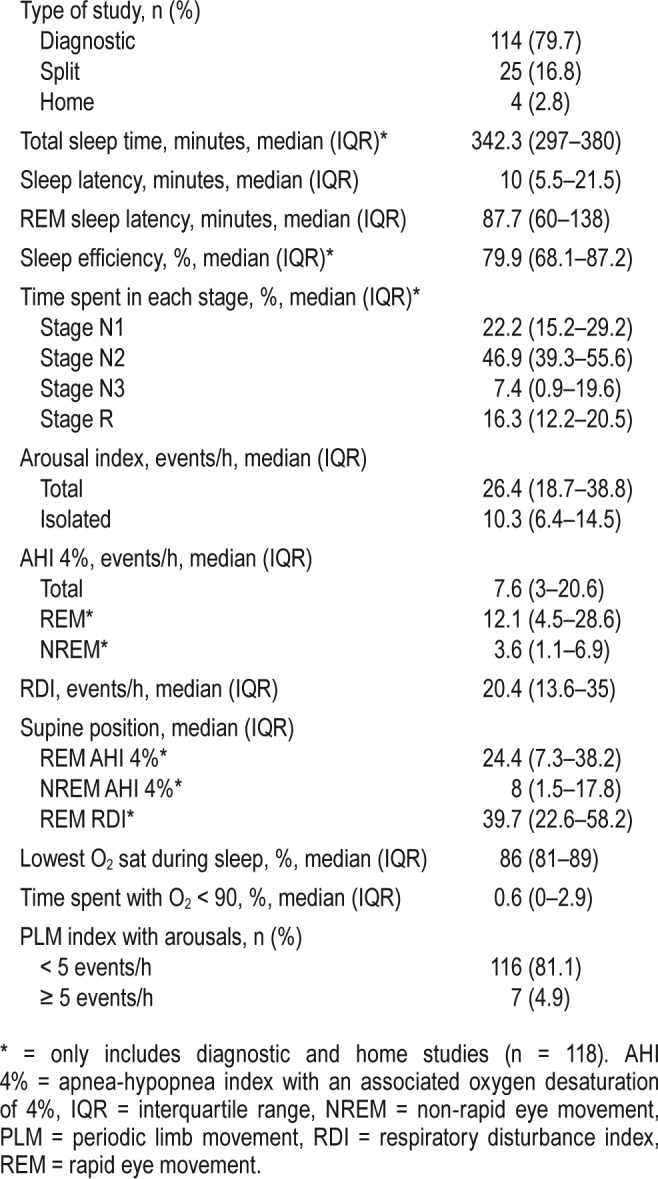
The data collected by PSG are shown in Table 2. Approximately 80% of the patients had full-night in-laboratory PSG, whereas a smaller amount underwent split-night PSG or home studies. Sufficient amounts of sleep time were recorded for adequate evaluation of sleep structure. Sleep efficiency was mildly reduced, but both sleep latency and REM latency were within the expected range. Time spent in stage 3 and REM sleep was reduced compared to normal reference rates.21 The median total AHI 4% and RDI were 7.6 and 20.4 events/h, respectively. Supine REM AHI was greater than total AHI, with a median supine REM AHI 4% of 24.4 events/h. The nadir O2 saturation during sleep was markedly reduced at 86% with a median time spent with O2 < 90% of less than 1% of the total sleep time. The nadir O2 saturation was associated with higher AHI, as well as lower FEV1 and FVC. No association was seen between nadir O2 saturation and FEV1/ FVC (Table S1 in the supplemental material).
Table 2.
Polysomnography data in sleep study population (n = 143).
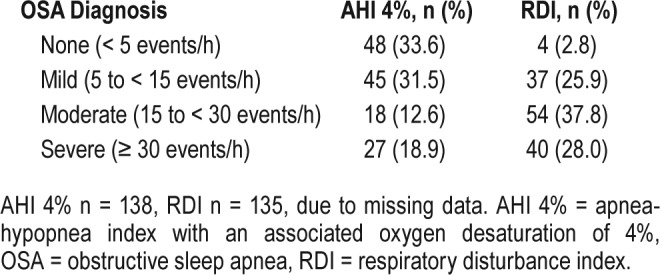
A diagnosis of OSA was confirmed in 67% of the sleep study population (Table 3). Of those with OSA, severity was mild in many (50%), with fewer patients meeting criteria for moderate (20%) or severe (30%) OSA. Most patients who underwent PSG had evidence of respiratory effort-related arousals reflected in the elevated RDI.
Table 3.
Sleep apnea severity.
Association of Diagnosis of OSA With Baseline Characteristics and WTC Exposure
Using AHI, approximately 66% of the sleep study population met criteria for a diagnosis of OSA. We therefore first investigated whether there were characteristics associated with the absence or presence of an AHI-defined diagnosis of OSA. Older age, elevated BMI, hypertension (HTN), asthma, and reduced FVC were associated with the diagnosis of OSA (Table 4). Neither WTC exposure category nor WTC dust cloud exposure was associated with the diagnosis of OSA. In multivariate analysis BMI and HTN remained significantly associated with the diagnosis of OSA (Table S2 in the supplemental material).
Table 4.
Characteristics and exposures associated with AHI 4%.
Association of Severity of OSA With Baseline Characteristics and WTC Exposure
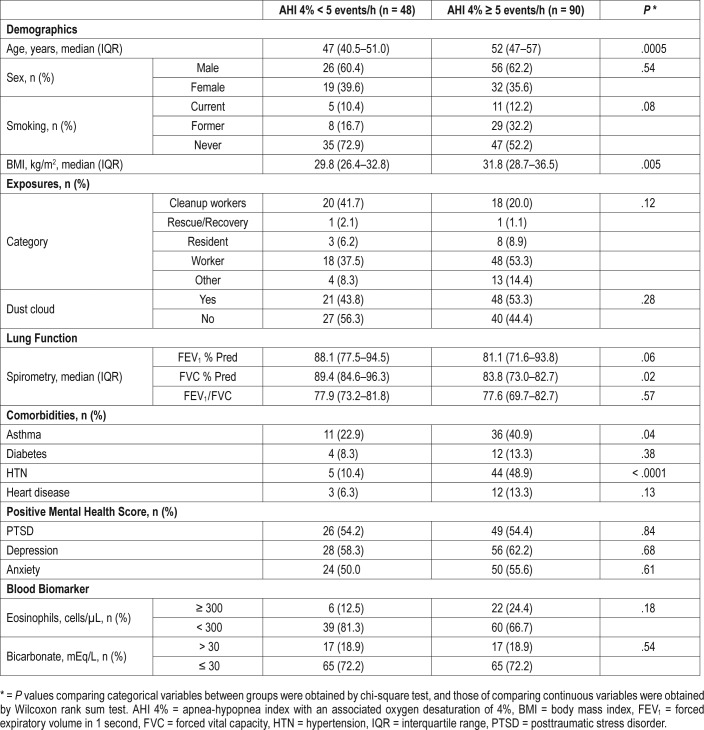
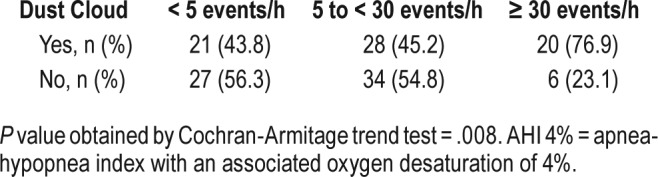
Using AHI criteria, we next investigated whether the severity of OSA was associated with baseline characteristics and WTC exposures (Table 5). Consistent with data from other populations,22–24 male sex, elevated BMI, and reported heart disease were associated with severity of OSA. However, in contrast with the presence of OSA, more severe OSA was associated with exposure to the WTC dust cloud and younger age. In addition, reported asthma was associated with severity of OSA. The associations between AHI and WTC dust cloud exposure, as well as age, sex, and BMI remained significant in multivariate analysis (Table 6). A trend test also revealed an association between WTC dust cloud exposure and OSA severity (Table 7).
Table 5.
Univariate analysis of basic characteristics and log AHI 4% (n = 138).
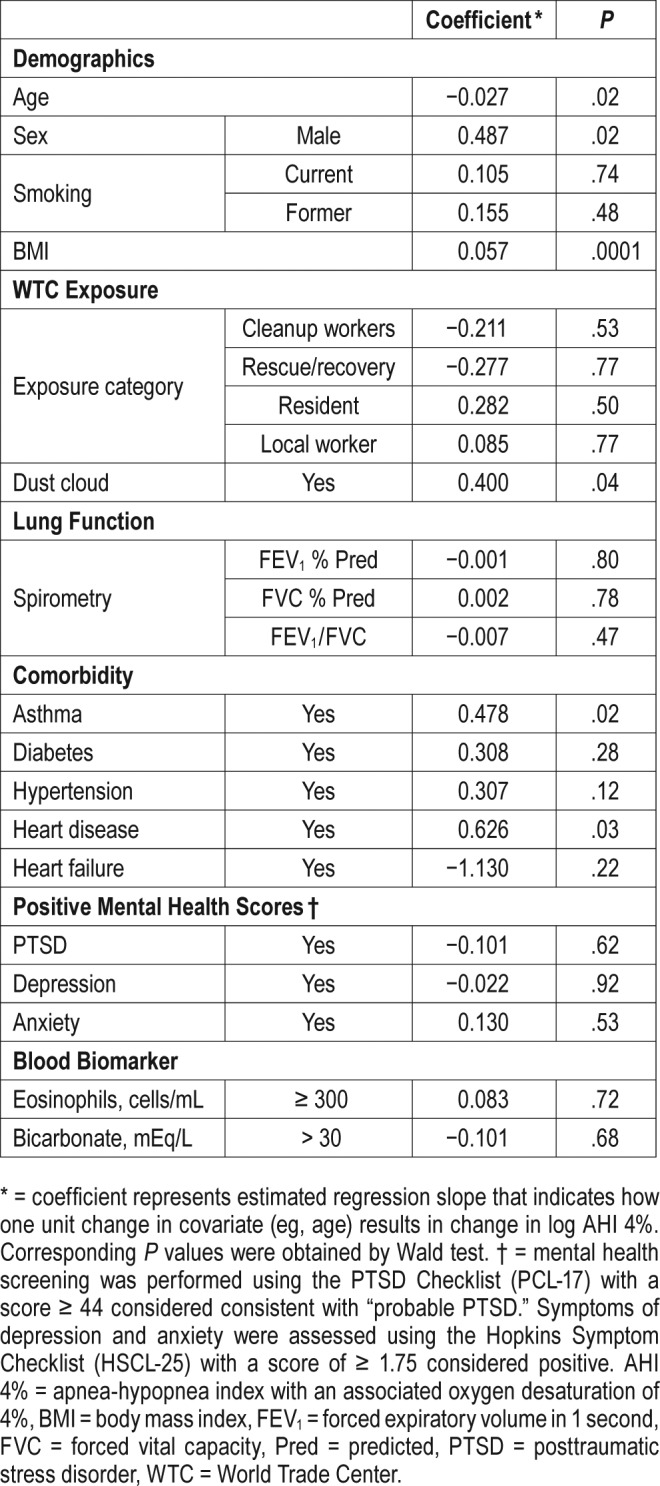
Table 6.
Multivariate analysis of basic characteristics and log AHI 4%.
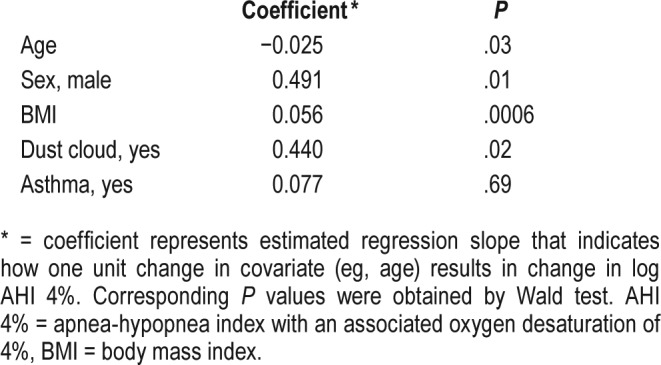
Table 7.
Trend test for AHI 4% versus dust cloud exposure.
DISCUSSION
We described patients with sleep disturbances in the community population with environmental exposures to dust and fumes from the destruction of the WTC towers. We strove to better understand the interplay between these environmental exposures and sleep disorders. PSG confirmed that almost all the patients who were referred for PSG had evidence of sleep disruption, reflected in their elevated RDI, and most had OSA by AHI criteria. Although many patients had mild disease, 50% of the patients with OSA had moderate-severe disease.
We characterized acute exposure to WTC dust and fumes using exposure to the initial dust clouds created by collapse of the WTC buildings on September 11, 2001. Community members who were local workers and residents had potential for both acute exposure to the WTC dust cloud, as well as more chronic exposure from re-suspended dust and persistent fumes. Patients who were cleanup workers were more likely to have had chronic exposure. Contrary to our initial hypothesis, we did not detect a significant relationship between WTC exposure as defined by WTC dust cloud exposure or exposure category, and the presence of OSA as defined by an elevated AHI score. We did identify an association between the diagnosis of OSA in the WTC community population and known risk factors of OSA in the general population including older age and elevated BMI,19,25 in contrast to the lack of association between BMI and OSA described in WTC responders.14 The limited sample size is likely a key factor in our failure to establish statistical significance between the diagnosis of OSA and WTC exposures in the community population. The high rate of sleep disturbance in the sleep study population suggests underreferral for sleep studies and therefore potential underdiagnosis of OSA in WTC community members, which may further have affected our ability to identify a relationship. The requirement for aerodigestive symptoms for certification may also have biased our population and reduced our ability to identify a relationship between OSA and WTC dust exposure.
Rates of elevated PTSD scores in the WTC population referred for sleep studies were significantly higher than in the nonreferred WTC population. An association between OSA and PTSD has been well described in the literature.26 Although the temporal relationship between PTSD and OSA has not been definitively established, there are several proposed mechanisms to explain the association. It is possible that the high rates of elevated PTSD scores in our population may provide a potential explanation for the high prevalence of OSA. It has been suggested that sleep fragmentation and deprivation in PTSD with resultant decreased REM may decrease airway tone and critical pressure, thereby facilitating upper airway collapse and OSA.19,25 Further, sleep disturbance prior to trauma may be a risk factor for the development of psychiatric disorders.27
Our study showed an association between WTC dust cloud exposure and the severity of OSA. The association between WTC dust cloud exposure and the severity of OSA using a multivariate analysis and a trend test suggested a dose-response relationship between exposure and the severity of OSA. The mechanisms by which WTC dust exposure and OSA are related remain unclear and warrant further investigation. WTC dust cloud exposure has been associated with other adverse health effects including severe lower respiratory symptoms,28 paresthesias,29 and PTSD.11 Dust cloud exposure in our study may be a marker for the presence of more severe respiratory symptoms, driving the high prevalence and severity of OSA in the WTC population. This would support a link between OSA and upper airway inflammation in the WTC exposed cohort.14 It has been postulated that exposure to the massive dust cloud resulted in irritation of the nose, throat, and airways with subsequent respiratory symptoms and abnormal lung function.1 Chronic rhinosinusitis is highly prevalent in the WTC exposed population and previous studies have shown associations between rhinosinusitis, nasal obstruction, and OSA.30,31 The proposed mechanism for this combination has been suggested to involve mucosal congestion and edema causing increased nasal and posterior pharyngeal resistance, thereby resulting in flow limitation.14 In a cohort of WTC dust-exposed rescue and recovery workers, Webber et al. demonstrated that chronic rhinosinusitis symptoms are associated with OSA (OR of 2.21).16
Interestingly, rates of asthma are higher in the WTC exposed population than the general population32 and the link between asthma and OSA has also been described. Literature suggests that the increased negative pressures during inspiration and active contraction of respiratory muscles during expiration during asthma exacerbations may lead to increased upper airway collapsibility.33 In addition, a recent study has identified preexistent asthma as a risk factor for the development of clinically relevant OSA.34 In our study, however, asthma was not significantly associated with diagnosis or severity of OSA.
Prior studies have suggested ongoing inflammation following WTC exposure and the possibility exists that this inflammation may provide a mechanism by which WTC dust exposures induce sleep disruption. Weiden et al. showed that inflammatory blood biomarkers expressed within 6 months of WTC exposure predicted the decline in lung function over many years.35 Kazeros et al. showed an association of blood eosinophils and C-reactive protein with discrete lung function limitations in the WTC EHC.6 Using in vitro human lung cell cultures, Payne et al. showed that WTC particulate matter up-regulates oxidative stress and the release of inflammatory mediators, providing a plausible explanation for the development of respiratory symptoms.36 Although yet to be proven, a similar localized inflammatory pathway affecting the upper airway, or even systemic pathway, may mediate the association of WTC exposures with OSA.
An additional mechanism linking OSA and particulate matter exposures may be via neuronal mechanisms. Increases in RDI and time with O2 desaturation have been linked to particulate matter (PM10) in a cohort of patients from the Sleep Heart Health Study.37 The authors postulated that translocation of inhaled particulate matter along the olfactory nerve allowed for deposition in the striatum and cerebellum, areas known to play a role in regulation of the sleep-wake cycle, and position and muscle tone during sleep.38,39 Moreover, many patients with upper and lower respiratory symptoms from WTC exposures have increased responsiveness to challenge with methacholine.8,40 The possibility exists that aberrant sensory neural pathways may contribute to the sleep abnormalities noted in these patients. This exposure may have triggered a proinflammatory response and altered neurotransmitter levels.41,42 Recent studies suggest a relationship between exposure to diesel exhaust particles and sensory neural responses in the lung.43
There are several potential limitations of our study. Current American Academy of Sleep Medicine guidelines suggest the use of AHI 3% instead of AHI 4% in the diagnosis of sleep apnea.44 Because these data were not reported at the time our sleep studies were performed, our data may underestimate the incidence and severity of OSA in our cohort. Further, referral bias due to federal regulations for certification of WTC-related sleep apnea may have affected our cohort and limited generalizability of our study. In addition, we were unable to assess the baseline, pre-WTC exposure, prevalence of OSA. These findings strongly support improved detection of potential sleep disturbance in the whole cohort with screening questionnaires in the future. Finally, we did not have specific medication data available to include in the analysis of our population.
In summary, there was a high rate of sleep disruption and OSA in WTC community members referred for sleep studies in the WTC EHC. OSA diagnosis was associated with known risk factors, such as BMI and older age; however, our data suggest that WTC dust cloud exposure was independently associated with the severity of OSA in this population. This finding highlights the role that environmental and occupational exposures may play in the development of OSA. Further studies need to be performed to confirm these findings and elucidate the mechanism of this relationship.
DISCLOSURE STATEMENT
Work was performed at NYU School of Medicine, Bellevue Hospital. All authors have seen and approved the manuscript. The authors report no conflicts of interest. Funding was provided by CDC-NIOSH 200-2017-93427, CDC-NIOSH 200-2017-93327, and NYU NIEHS Center Grant P30 ES00260.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- EHC
Environmental Health Center
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- GERD
gastroesophageal reflux disease
- HSCL-25
Hopkins Symptom Checklist
- HTN
hypertension
- OR
odds ratio
- OSA
obstructive sleep apnea
- PCL-17
Posttraumatic Stress Disorder Checklist
- PSG
polysomnography
- PTSD
posttraumatic stress disorder
- RDI
respiratory disturbance index
- WTC
World Trade Center
REFERENCES
- 1.Rom WN, Reibman J, Rogers L, et al. Emerging exposures and respiratory health: World Trade Center dust. Proc Am Thorac Soc. 2010;7(2):142–145. doi: 10.1513/pats.200908-092RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reibman J, Levy-Carrick N, Miles T, et al. Destruction of the World Trade Center towers. Lessons learned from an environmental health disaster. Ann Am Thorac Soc. 2016;13(5):577–583. doi: 10.1513/AnnalsATS.201509-572PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lioy PJ, Weisel CP, Millette JR, et al. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect. 2002;110(7):703–714. doi: 10.1289/ehp.02110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippmann M, Cohen MD, Chen L-C. Health effects of World Trade Center (WTC) dust: an unprecedented disaster with inadequate risk management. Crit Rev Toxicol. 2015;45(6):492–530. doi: 10.3109/10408444.2015.1044601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reibman J, Liu M, Cheng Q, et al. Characteristics of a residential and working community with diverse exposure to World Trade Center dust, gas, and fumes. J Occup Environ Med. 2009;51(5):534–541. doi: 10.1097/JOM.0b013e3181a0365b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazeros A, Zhang E, Cheng X, et al. Systemic inflammation associated with World Trade Center dust exposures and airway abnormalities in the local community. J Occup Environ Med. 2015;57(6):610–616. doi: 10.1097/JOM.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 7.Lin S, Reibman J, Bowers JA, et al. Upper respiratory symptoms and other health effects among residents living near the World Trade Center site after September 11, 2001. Am J Epidemiol. 2005;162(6):499–507. doi: 10.1093/aje/kwi233. [DOI] [PubMed] [Google Scholar]
- 8.Reibman J, Lin S, Hwang SA, et al. The World Trade Center residents' respiratory health study: new-onset respiratory symptoms and pulmonary function. Environ Health Perspect. 2005;113(4):406–411. doi: 10.1289/ehp.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbert R, Moline J, Skloot G, et al. The World Trade Center disaster and the health of workers: five-year assessment of a unique medical screening program. Environ Heatlh Perspect. 2006;114(12):1853–1858. doi: 10.1289/ehp.9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neria Y, DiGrande L, Adams BG. Posttraumatic stress disorder following the September 11, 2001, terrorist attacks: a review of the literature among highly exposed populations. Am Psychol. 2011;66(6):429–446. doi: 10.1037/a0024791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen RL, Levy-Carrick N, Reibman J, et al. Elevated C-reactive protein and posttraumatic stress pathology among survivors of the 9/11 World Trade Center attacks. J Psychiatr Res. 2017;89:14–21. doi: 10.1016/j.jpsychires.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Brackbill RM, Hadler JL, DiGrande L, et al. Asthma and posttraumatic stress symptoms 5 to 6 years following exposure to the World Trade Center terrorist attack. JAMA. 2009;302(5):502–516. doi: 10.1001/jama.2009.1121. [DOI] [PubMed] [Google Scholar]
- 13.Glaser MS, Shah N, Webber MP, et al. Obstructive sleep apnea and World Trade Center exposure. J Occup Environ Med. 2014;56(Suppl 10):S30–S34. doi: 10.1097/JOM.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 14.Sunderram J, Udasin I, Kelly-McNeil K, et al. Unique features of obstructive sleep apnea in World Trade Center responders with aerodigestive disorders. J Occup Environ Med. 2011;53(9):975–980. doi: 10.1097/JOM.0b013e3182305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de la Hoz RE, Aurora RN, Landsbergis P, Bienenfeld LA, Afilaka AA, Herbert R. Snoring and obstructive sleep apnea among former World Trade Center rescue workers and volunteers. J Occup Environ Med. 2010;52(1):29–32. doi: 10.1097/JOM.0b013e3181c2bb18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webber MP, Lee R, Soo J, et al. Prevalence and incidence of high risk for obstructive sleep apnea in World Trade Center-exposed rescue/recovery workers. Sleep Breath. 2011;15(3):283–294. doi: 10.1007/s11325-010-0379-7. [DOI] [PubMed] [Google Scholar]
- 17.Farfel M, DiGrande L, Brackbill R, et al. An overview of 9/11 experiences and respiratory and mental health conditions among World Trade Center Health Registry enrollees. J Urban Health. 2008;85(6):880–909. doi: 10.1007/s11524-008-9317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav Sci. 1974;19(1):1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- 19.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 22.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 24.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289(17):2230–2237. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 25.de la Hoz RE, Mallea JM, Kramer SJ, Bienenfeld LA, Wisnivesky JP, Aurora RN. Polysomnographic diagnoses among former World Trade Center rescue workers and volunteers. Arch Environ Occup Health. 2012;67(4):239–242. doi: 10.1080/19338244.2012.725230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405–1411. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 27.Bryant RA, Creamer M, O'Donnell M, Silove D, McFarlane AC. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. 2010;33(1):69–74. doi: 10.1093/sleep/33.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caplan-Shaw C, Kazeros A, Pradhan D, et al. Improvement in severe lower respiratory symptoms and small airway function in World Trade Center dust exposed community members. Am J Ind Med. 2016;59(9):777–787. doi: 10.1002/ajim.22642. [DOI] [PubMed] [Google Scholar]
- 29.Marmor M, Shao Y, Bhatt DH, et al. Paresthesias among community members exposed to the World Trade Center disaster. J Occup Environ Med. 2017;59(4):389–396. doi: 10.1097/JOM.0000000000000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young T, Finn L, Kim H. Nasal obstruction as a risk factor for sleep-disordered breathing. The University of Wisconsin Sleep and Respiratory Research Group. J Allergy Clin Immunol. 1997;99(2):S757–S762. doi: 10.1016/s0091-6749(97)70124-6. [DOI] [PubMed] [Google Scholar]
- 31.Friedman M, Tanyeri H, Lim JW, Landsberg ROY, Vaidyanathan K, Caldarelli D. Effect of improved nasal breathing on obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;122(1):71–74. doi: 10.1016/S0194-5998(00)70147-1. [DOI] [PubMed] [Google Scholar]
- 32.Kim H, Herbert R, Landrigan P, et al. Increased rates of asthma among World Trade Center disaster responders. Am J Ind Med. 2012;55(1):44–53. doi: 10.1002/ajim.21025. [DOI] [PubMed] [Google Scholar]
- 33.Heil M, Jensen OE. Flows in Deformable Tubes and Channels. In: Carpenter PW, Pedley TJ, editors. Flow Past Highly Compliant Boundaries and in Collapsible Tubes: Proceedings of the IUTAM Symposium held at the University of Warwick; 26-30 March 2001; United Kingdom. Dordrecht, Netherlands: Springer Science+Business Media; 2003. [Google Scholar]
- 34.Teodorescu M, Barnet JH, Hagen EW, Palta M, Young TB, Peppard PE. Association between asthma and risk of developing obstructive sleep apnea. JAMA. 2015;313(2):156–164. doi: 10.1001/jama.2014.17822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiden MD, Kwon S, Caraher E, et al. Biomarkers of World Trade Center particulate matter exposure: physiology of distal airway and blood biomarkers that predict FEV(1) decline. Semin Respir Crit Care Med. 2015;36(3):323–333. doi: 10.1055/s-0035-1547349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Payne JP, Kemp SJ, Dewar A, et al. Effects of airborne World Trade Center dust on cytokine release by primary human lung cells in vitro. J Occup Environ Med. 2004;46(5):420–427. doi: 10.1097/01.jom.0000126021.25149.64. [DOI] [PubMed] [Google Scholar]
- 37.Zanobetti A, Redline S, Schwartz J, et al. Associations of PM10 with sleep and sleep-disordered breathing in adults from seven U.S. urban areas. Am J Respir Crit Care Med. 2010;182(6):819–825. doi: 10.1164/rccm.200912-1797OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elder A, Gelein R, Silva V, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114(8):1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunchillos JD, De Andres I. Participation of the cerebellum in the regulation of the sleep-wakefulness cycle. Results in cerebellectomized cats. Electroencephalogr Clin Neurophysiol. 1982;53(5):549–558. doi: 10.1016/0013-4694(82)90067-0. [DOI] [PubMed] [Google Scholar]
- 40.Prezant DJ, Weiden M, Banauch GI, et al. Cough and bronchial responsiveness in firefighters at the World Trade Center site. N Engl J Med. 2002;347(11):806–815. doi: 10.1056/NEJMoa021300. [DOI] [PubMed] [Google Scholar]
- 41.Campbell A, Oldham M, Becaria A, et al. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005;26(1):133–140. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Kleinman MT, Araujo JA, Nel A, et al. Inhaled ultrafine particulate matter affects CNS inflammatory processes and may act via MAP kinase signaling pathways. Toxicol Lett. 2008;178(2):127–130. doi: 10.1016/j.toxlet.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson RK, Birrell MA, Adcock JJ, et al. Mechanistic link between diesel exhaust particles and respiratory reflexes. J Allergy Clin Immunol. 2018;141(3):1074–1084. doi: 10.1016/j.jaci.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.