Abstract
Study Objectives:
Parkinson disease (PD) non-motor symptoms are associated with sleep disorders and impair quality of life. Our objective was to assess the effect of obstructive sleep apnea (OSA) treatment using continuous positive airway pressure (CPAP) on PD non-motor symptoms.
Methods:
In this prospective observational study, 67 patients with idiopathic PD underwent polysomnography. Those with moderate-severe OSA were offered CPAP therapy. Subjects were divided into those without OSA (OSA−), and those with OSA (OSA+). Analyses were conducted for 6 and 12 months' follow-up data. At 6 months, those who had used CPAP at home for at least 1 month were considered CPAP users (OSA+CPAP+), whereas those who did not try it, or declined further treatment following a short trial were considered non-users (OSA+CPAP−). For the 12-month analysis, only those still actively using CPAP at 12 months were included in the OSA+CPAP+ group. Non-motor symptom measurements were: Epworth Sleepiness Scale, Montreal Cognitive Assessment (MoCA), Unified Parkinson's Disease Rating Scale part 1 (UPDRS1), Parkinson's Disease Sleep Scale (PDSS), Fatigue Severity Scale, Apathy Scale, Beck Depression Inventory, and Hospital Anxiety and Depression Scale (HADS).
Results:
Sixty-five participants were re-assessed at least once. At 6 months, 30 participants were categorized as OSA+CPAP+, 11 OSA+CPAP−, and 18 OSA−. At 12 months, 21 were categorized as OSA+CPAP+, 21 OSA+CPAP−, and 17 OSA−. The UPDRS1 and PDSS improved from baseline in OSA+CPAP+ at 6 months (−2.7, standard deviation [SD] 4.0, P = .001, and 7.9, SD 19.0, P = .03, respectively) and 12 months (−4.1, SD 5.4, P = .002, and 11.4, SD 24.4, P = .04, respectively), but not in other groups. The MoCA and HADS-A improved in OSA+CPAP+ at 12 months (1.7, SD 3.5, P = .04, and −2.1, SD 3.8, P = .02, respectively). The MoCA improved in those with low baseline MoCA and those with REM sleep behavior disorder. Mean CPAP use in users at 12 months was 3 hours 36 minutes per night.
Conclusions:
CPAP treatment of OSA in PD is associated with improved overall non-motor symptoms, sleep quality, anxiety, and global cognitive function over a 12-month period.
Citation:
Kaminska M, Mery VP, Lafontaine AL, Robinson A, Benedetti A, Gros P, Kimoff RJ. Change in cognition and other non-motor symptoms with obstructive sleep apnea treatment in Parkinson disease. J Clin Sleep Med. 2018;14(5):819–828.
Keywords: cognitive function, CPAP, non-motor symptoms, obstructive sleep apnea, Parkinson disease, RBD
BRIEF SUMMARY
Current Knowledge/Study Rationale: Non-motor symptoms of Parkinson disease (PD) include sleep disorders, mood disturbances, progressive cognitive dysfunction frequently leading to dementia, and others. They constitute a prominent source of disability in PD. In the general population, similar symptoms have been linked to obstructive sleep apnea, but cognition has not consistently been found to improve with continuous positive airway pressure (CPAP) treatment.
Study Impact: Individuals with PD and moderate-severe obstructive sleep apnea who used CPAP over a 12-month period showed improved non-motor symptoms, including an overall non-motor symptom score, sleep, anxiety and notably, global cognitive function. The improvement in cognition was associated with baseline reduced cognition and REM sleep behavior disorder. Hence, treating obstructive sleep apnea with CPAP in neurodegenerative disorders such as PD may be beneficial for highly relevant patient outcomes.
INTRODUCTION
Non-motor symptoms are increasingly recognized as having a major detrimental impact on function and quality of life in Parkinson disease (PD).1–3 Important symptoms include sleep disturbances, daytime sleepiness, cognitive dysfunction, and mood disturbances.4 Cognitive impairment is of particular importance as it is frequent and may progress to dementia. Dementia constitutes a major burden on the patient and society as it precludes ability to sustain employment and determines need for increased assistance and institutionalization.5 The prevalence of dementia in PD has been variable in studies to date but increases to up to 80% with increasing age and disease duration.6 In almost 50% of patients with PD with normal baseline cognition, cognitive impairment developed after 6 years of follow-up, and dementia developed within 5 years in all those with incident mild cognitive impairment.7
Sleep disturbances are also common in PD, including sleep fragmentation, insomnia, restless legs syndrome (RLS), and REM sleep behavior disorder (RBD).8 Recently, obstructive sleep apnea (OSA) has gained attention as a potentially important comorbidity in PD. Reported prevalence is highly variable, and it remains unclear whether it is higher than in the general population. There are, however, data suggesting that upper airway motor dysfunction may be implicated.9 More importantly, OSA, when concomitant with PD, might aggravate non-motor symptoms.
In the general population, in addition to sleepiness, OSA has been associated with impaired cognitive and psychomotor performance.10 Response of neurocognitive dysfunction to OSA treatment using the standard positive airway pressure (CPAP) therapy has been variable and incomplete.11,12
We have recently reported an association, in PD, between OSA and increased sleepiness as well as reduced global cognitive function.13 The aim of this study was to assess the effect of CPAP treatment of OSA on non-motor symptoms, including a global measure of cognitive function, in a cohort of patients with PD followed over a period of 12 months.
METHODS
This was a prospective observational cohort study following a cross-sectional study of non-motor symptoms in relation to OSA in PD.13
Study Subjects
Subjects with idiopathic PD were recruited from November 2011 to July 2014 from a movement disorder clinic in an academic tertiary care center.13 The inclusion criteria were: diagnosis of idiopathic PD14; ability to transfer with minimal assistance (required for laboratory polysomnography [PSG)]; and adequate knowledge of English or French for completion of study assessment. Exclusion criteria were: other major neurological disorder; unstable cardiac disease or uncontrolled hypertension; active cancer or other disorder with an expected survival of fewer than 6 months; active treatment of OSA; inability to provide informed consent, or to comply with study procedures or requirements, including as a result of dementia. Our institutional Research Ethics Board approved the study and all participants provided written consent.
Procedures
Participants underwent a baseline evaluation followed by a laboratory overnight PSG. Assessment included the Movement Disorder Society Unified Parkinson's Disease Rating Scale (UPDRS)15 and the Hoehn & Yahr scale (H&Y), a PD motor severity scale with stages 1 to 5 (stages 1, 2, and 3 are considered milder).16 Restless legs syndrome (RLS) was assessed using the International Restless Legs Syndrome Study Group Rating Scale,17 and confirmed by clinical interview. In this study, the diagnosis of RBD was based on clinical criteria using an in-house questionnaire including questions on movements/vocalization during sleep, dream enactment, and self- or bed-partner injury together with a validated single-item questionnaire,18 followed by confirmation during a clinical interview with the study sleep physician and/or neurologist.
L-Dopa equivalent daily dosage was calculated according to a standard formula.19
Individuals found to have moderate-severe OSA were offered CPAP therapy. Auto-PAP (PR REMstar Auto with A-Flex, Philips Respironics, Murrysville, Pennsylvania, United States) was initiated in those agreeing via a standard commercial provider but free of charge to participants. Auto or fixed CPAP was adjusted as deemed optimal by the sleep specialist study physician (MK) for therapy clinically. Usage and efficacy data were obtained from the devices' microprocessor.
Outcomes are reported for follow-up at 6 and 12 months. Participants were initially reassessed at 3, 6, and 12 months. However, the 3-month visit was missed by several participants due to difficulties in scheduling the multiple visits especially in those starting CPAP, together with delays in CPAP initiation and adaptation in these patients. Results are shown for 6 and 12 months. When a participant missed their 6-month follow-up but 3-month data were available, it was used to maximize the sample at 6 months. This occurred for three patients with OSA (two treated and one untreated).
Outcomes
Assessment were performed at baseline and follow-up as follows:
Daytime sleepiness: Epworth Sleepiness Scale (ESS). An ESS score ≥ 11 is considered abnormal daytime sleepiness.20
Cognition: Montreal Cognitive Assessment (MoCA); a 30-point scale assessing different cognitive domains used as a screening test for mild cognitive impairment. A score < 26 is considered as impairment.21
Global PD nonmotor symptom assessment: UPDRS part 1; a higher score implies greater symptoms.
Sleep quality: Parkinson's Disease Sleep Scale (PDSS); visual analog scale addressing several sleep symptoms, with lower scores considered poorer sleep quality.22
Fatigue: Fatigue Severity Scale; a 9-item Likert scale where an average score ≥ 4 is considered as abnormal fatigue in PD.23
Apathy: Apathy Scale; a score ≥ 14 is considered as apathetic.24
Anxiety: Hospital Anxiety and Depression Scale (HADS-A); a score ≥ 8 in the anxiety item is indicative of abnormal anxiety.25
Depressive symptoms: Beck Depression Inventory scale; a score ≥ 14 is considered as depression.26 Hospital Anxiety and Depression Scale (HADS-D); a score ≥ 8 in the depression item is indicative of depression.
The MoCA and the UPDRS (except the “Patient Questionnaire” part) were administered in a standardized fashion by a neurologist (VM) or an experienced, trained research nurse (AR). Other questionnaires were filled out by the study participants directly.
Polysomnography
Diagnostic PSG was performed using the Harmonie System (Stellate, Montreal, Quebec, Canada), with recording from standard electroencephalographic leads (C4/C3/F3/F4/O1/O2/ M1/M2); bilateral electrooculogram; chin, bilateral anterior tibialis and extensor digitorum electromyograms (EMG); airflow via nasal pressure cannula; thoracoabdominal movements via inductive plethysmography (Respitrace Systems, Ardsley, New York, United States); single-lead electrocardiogram; pulse oximetry; snoring; digital video recording and body position. All PSG tests were scored manually by a single Registered Polysomnographic Technologist (RPSGT) with 30 years of experience, who was blinded to questionnaire data and clinical status. The scoring of sleep stages, arousal, and periodic limb movements was according to current American Academy of Sleep Medicine (AASM) criteria.27 The respiratory analyses were done using AASM research (Chicago) criteria,28 including a 4% oxygen desaturation index. If the total sleep time was < 2 hours, subjects were asked to repeat the PSG (n = 3). OSA was defined as an apnea-hypopnea index (AHI) ≥ 15 events/h.
Sample Size
Using the MoCA as the outcome of interest, we assumed no significant change without intervention based on published data where MoCA remained stable for 3 years in patients with PD with SD 1.7.29 The minimal clinically important difference (MCID) is unknown but improvement of 1.5 was considered of interest.30 Using a paired t test with an estimated standard deviation (SD) = 2, α = .05 and power 80%, 16 participants with OSA treated with CPAP therapy were needed. Estimating a 50% prevalence of OSA in the referred patients, 50% of those with OSA using CPAP for 12 months, and adjusting for 10% loss to follow-up, we estimated needing 71 participants screened for OSA.
Statistical Analysis
Subjects were divided into those without OSA (OSA−), and those with OSA (OSA+). Analyses were conducted for 6 and 12 months' follow-up data. At 6 months, those who had used CPAP at home for at least 1 month were considered CPAP users (OSA+CPAP+), whereas those who did not try it, or declined further treatment following a short trial were considered nonusers (OSA+CPAP−). For the 12-month analysis, only those still actively using CPAP at 12 months were included in the OSA+CPAP+ group.
Baseline characteristics between OSA+ versus OSA− groups, and OSA+CPAP+ versus OSA+CPAP− at 12 months, were compared using t test, chi square, or Fisher exact tests. Within-group comparison of outcome variables from baseline to follow-up was done using paired t tests. Regarding missing data, if an entire visit was missing, the data were considered missing. If the visit took place but parts of questionnaires were missing, the last available value was carried forward. The level of significance was .05. Analyses were done using R.31
RESULTS
Study Population
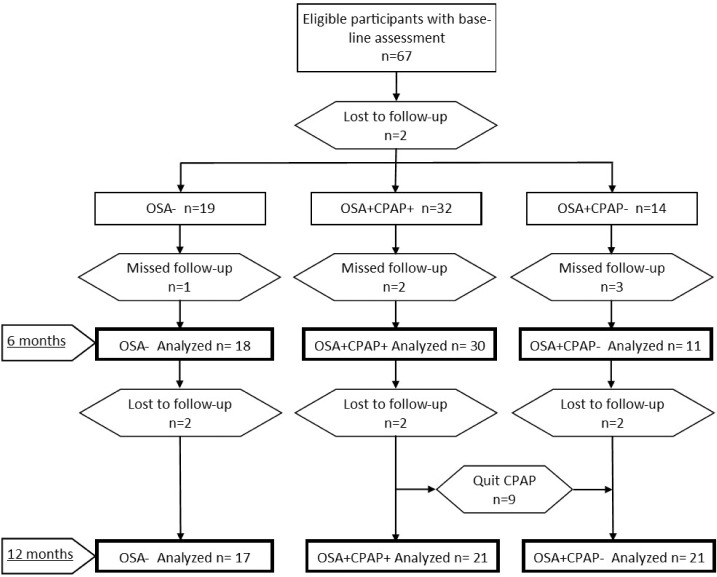
Of the 67 eligible participants with baseline assessment,13 2 were lost to follow-up before the 6-month assessment, and 6 more afterward. Six participants missed the 6-month assessment but were seen at 12 months. Of the 32 individuals considered OSA+CPAP+ at 6 months, 9 discontinued CPAP therapy and were included as OSA+CPAP− at 12 months. Data were available for 18 OSA−, 30 OSA+CPAP+ and 11 OSA+CPAP− at 6 months, and 17 OSA−, 21 OSA+CPAP+ and 21 OSA+CPAP− at 12 months (Figure 1). The mean MoCA in the 8 individuals who were lost to follow-up was 26.6, SD 3.3 (P = .28 compared with remaining participants).
Figure 1. Study participant flowchart.
CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea.
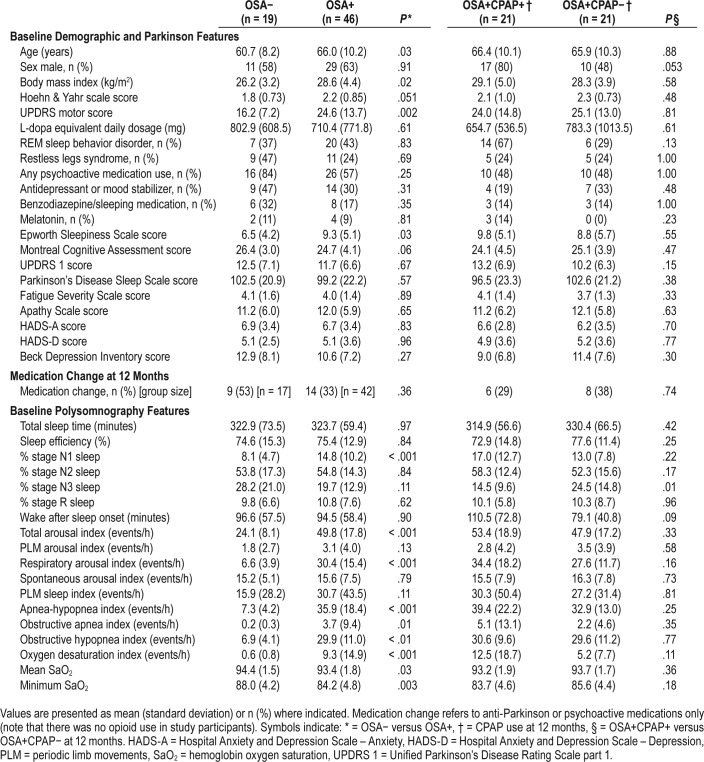
Patients with PD and OSA were older, had a higher body mass index (BMI) and greater motor dysfunction (Table 1). They also had a greater proportion of stage N1 sleep on PSG, and more total and respiratory arousals suggesting poorer sleep quality. Their sleep efficiency was reduced similarly to those without OSA. The mean AHI for those with OSA was 35.9 events/h (SD 18.4 events/h). Participants using CPAP at 12 months had less stage N3 sleep on baseline PSG than OSA+CPAP−, but no other significantly different baseline clinical characteristics, except for a trend to higher mean BMI and greater proportion with RBD.
Table 1.
Participant characteristics.
Outcomes at Follow-Up
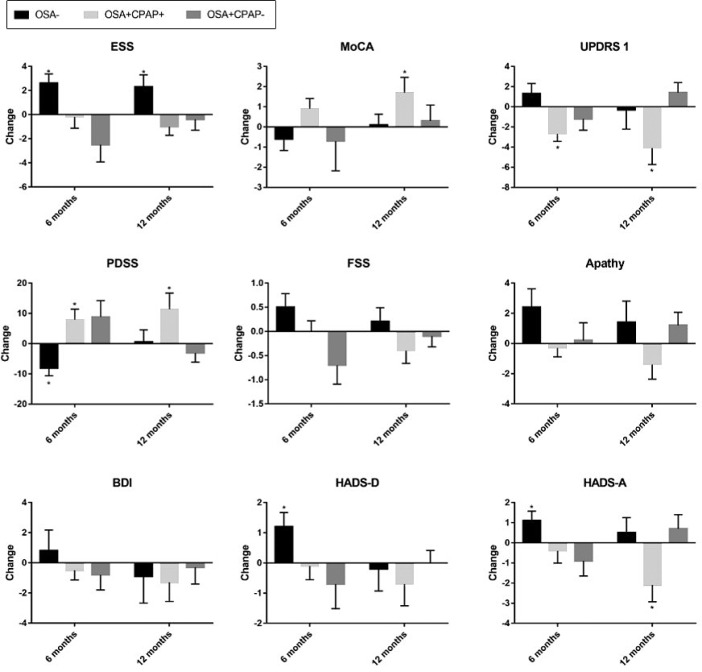
At 6 months, in the OSA− group, ESS, PDSS, HADS-D, and HADS-A scores deteriorated (Figure 2, Table S1 in the supplemental material) but this was no longer apparent at 12 months, except for ESS. In the OSA+CPAP+ group, but not others, there was significant improvement in UPDRS1 and in PDSS at both 6 and 12 months (Figure 2, Table S1). The MoCA and HADS-A were significantly improved at 12 months (Figure 2, Table S1).
Figure 2. Change in outcome measures at 6 and 12 months.
Change in outcomes compared with baseline. * = P < .05 for change from baseline. OSA+: n = 18 at 6 months, n = 17 at 12 months; OSA+CPAP+: n = 30 at 6 months, n = 21 at 12 months; OSA+CPAP−: n = 11 at 6 months, n = 21 at 12 months. Apathy score = a higher score reflects greater apathy; BDI = Beck Depression Inventory, a higher score reflects more depressive tendency; ESS = Epworth Sleepiness Scale, a higher score reflects greater sleepiness; FSS = Fatigue Severity Scale, a higher score reflects greater fatigue; HADS-A = Hospital Anxiety and Depression Scale – Anxiety, a higher score reflect greater anxiety; HADS-D = Hospital Anxiety and Depression Scale – Depression, a higher score reflects more depressive tendency; MoCA = Montreal Cognitive Assessment, a lower score reflects worse cognition; PDSS = Parkinson's Disease Sleep Scale, a lower score reflects poorer sleep quality; UPDRS 1 = Unified Parkinson's Disease Rating Scale part 1, a higher score reflects greater non-motor symptoms.
Individual UPDRS1 items were assessed (Table S2 in the supplemental material). Items showing a statistically signifi-cant improvement in OSA+CPAP+ at 6 months were Anxious Mood, Daytime Sleepiness, and Light Headedness on Standing. Items showing a trend to improvement were Depressed Mood and Fatigue. At 12 months, significant improvements were found in Depressed Mood, Anxious Mood, Light Headedness on Standing, and Fatigue, with a trend for Daytime Sleepiness.
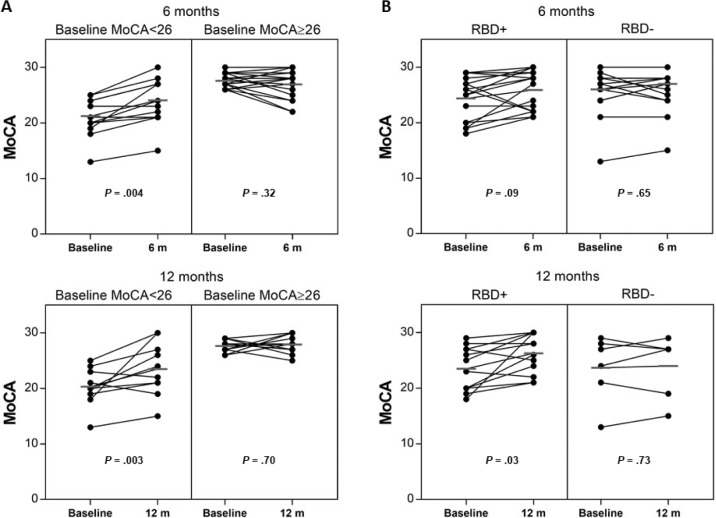
To further evaluate the effect of CPAP therapy on cognition, change in MoCA was assessed in participants with baseline cognitive impairment, i.e. MoCA < 26. At 6 months, there was a mean change of −0.8 (SD 3.9, P = .67, n = 5) in OSA−, +2.8 (SD 2.1, P = .004, n = 13) in OSA+CPAP+, and −1.2 (SD 6.4, P = .68, n = 6) in OSA+CPAP−. At 12 months, mean change was −0.8 (SD 3.2, P = .61, n = 5) in OSA−, +3.2 (SD 4.0, P = .03, n = 10) in OSA+CPAP+, and +1.1 (SD 4.7, P = .48, n = 10) in OSA+CPAP−. Hence, significant improvement was seen in OSA+CPAP+ with baseline MoCA < 26 at both time points, but not in those with MoCA in the normal range at baseline (Figure 3A).
Figure 3. MoCA change at 6 and 12 months in OSA+CPAP+ by subgroups.
(A) Change in OSA+CPAP+ with low versus high baseline MoCA score. (B) Change in OSA+CPAP+ subgroups based on presence or absence of REM sleep behavior disorder.
In that RBD has previously been associated with cognitive dysfunction in PD, we assessed the change in MoCA in participants with and without RBD. At baseline, there was no difference in MoCA between those with versus without RBD (25.1, SD 3.6, n = 27 versus 25.2, SD 4.1, n = 38, respectively; P = .87). In those with RBD, the MoCA improved in OSA+CPAP+ at 6 months by +1.4, SD 3.2 (P = .09, n = 16, including 8 with baseline MoCA ≥ 26) and at 12 months by +2.8, SD 3.7 (P = .03, n = 12, including 5 with baseline MoCA ≥ 26) (Figure 3B). There was no significant change in MoCA scores in OSA− or OSA+CPAP− groups with RBD. In those without RBD, CPAP therapy did not yield any significant improvement in MoCA at 6 months (+0.3, SD 2.3, n = 14, P = .65) or 12 months (+0.3, SD 3.5, n = 6, P = .73). For other outcomes, there were no consistent differences between those with and without RBD.
CPAP Use
Of the 46 OSA+ participants who were followed (Figure 1), 7 were uninterested and never tried CPAP and 7 tried it but did not continue beyond a few nights. These were considered OSA+CPAP− at 6 months. Among initial CPAP users, 22 were regular users, 3 were sporadic users with mean nightly use ≤ 1 hour, 3 abandoned use at 1 months (no perceived benefit: n = 2; too impaired to manage CPAP: n = 1) and 4 abandoned use at 3 months (poor tolerance: n = 3; eye infection: n = 1 who later restarted CPAP). Additionally, 9 abandoned use after 6 months (Figure 1).
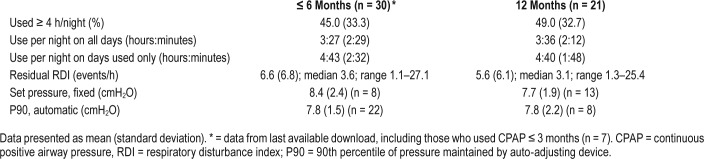
Details of CPAP use and efficacy for users are shown in Table 2. The “ ≤ 6-months” data come from the last available device report while the participant was using CPAP. From these reports, mean nightly use was 3 hours 27 minutes, and use ≥ 4 h/night was recorded on 45% of nights. At 12 months, only data from ongoing users are presented. Mean use was 3 hours 36 minutes with 49% of nights use ≥ 4 h/night. Overall OSA was well controlled on CPAP, with residual events (from device report) ≥ 10 events/h in 3 and 2 participants at 6 and 12 months, respectively.
Table 2.
CPAP data for OSA+CPAP+ group.
Among OSA+CPAP+, CPAP use was not significantly different between subjects with versus without baseline cognitive impairment. For subjects with baseline MoCA < 26, mean CPAP use at 6 months was 3 hours 29 minutes (SD 2 hours 33 minutes) with 45% of nights use > 4 h/night (n = 13), and at 12 months mean use was 4 hours 8 minutes (SD 2 hours 0 minutes) with 53% use > 4 h/night (n = 10). There was also no difference in nightly use in OSA+CPAP+ with or without RBD.
DISCUSSION
In this 12-month prospective observational cohort study, we have found that CPAP treatment of OSA in patients with PD is associated with improvement in non-motor symptoms globally, subjective sleep quality, cognitive function, and anxiety. Our results point to OSA as a previously overlooked but potentially important comorbidity in PD. We demonstrate that OSA treatment is feasible long term in a substantial proportion of patients with PD, and may result in clinically meaningful improvement in important patient-related outcomes.
It remains unclear whether the prevalence of OSA in PD is different than in the general population. However, once present, OSA may have detrimental consequences. Sleep disorders in PD have been associated with non-motor symptoms,32 which are a major impediment to quality of life in these patients.33–35 OSA in PD has specifically been associated with poorer cognition in some cross-sectional studies,13,32 but not others.36 Methodological differences in patient selection, OSA diagnosis, and cognitive testing may account for these inconsistencies. The MoCA has been shown to be sensitive for mild executive dys-function often found in PD.37 It was developed as a screening tool, but is sensitive to change in Alzheimer disease,38 and stroke.30 The MCID is unknown, but we considered a change of 1.5 to be relevant, and used this for our sample size calculation. Of note, a difference of 0.4 points was associated with improvement in other cognitive measures, suggesting that even this small difference may be relevant.39 We have found a clearly greater absolute improvement of 1.7 points at 12 months in CPAP-treated patients. The improvement was even greater at 3.2 in those with reduced MoCA at baseline. Therefore, CPAP treatment of OSA appears to hold great promise in patients with PD and OSA who have reduced cognitive function. This also suggests utility in targeting patients with PD with reduced cognition for sleep testing.
Interestingly, the improvement in cognitive function was primarily found in patients treated for OSA with low-baseline MoCA, with or without RBD, as well as in those with RBD at baseline. This was despite no association between MoCA scores and RBD at baseline. Our findings are in contrast with those of others who have found RBD to be associated with reduced cognitive function in idiopathic and PD-related RBD.40 RBD is a neurodegenerative process that is frequent in PD, often a precursor and a marker of more severe disease.41 It is possible that RBD and OSA may have a synergistic effect on cognitive dysfunction in PD, such that treating OSA in this context may allow greatest benefits on cognition. Mechanistically, neuroinflammation may be involved. RBD has been associated with microglial activation, a marker of neuroinflammation, in the substantia nigra, putamen, and caudate.42 OSA promotes systemic inflammation,43 including in PD,44 and neuroinflammation,45 and may thus exacerbate RBD-related neuroinflammation and clinical manifestations, including cognitive and potentially other symptoms as well as overall disease progression. Another mechanism whereby OSA can exacerbate cognitive dysfunction could be through sleep fragmentation, which has been associated with reduced clearance of metabolic waste.46 This could explain the need for a longer duration of treatment to see an effect and the progressive improvement in MoCA (including in the subset of those still using CPAP at 12 months). Further work will be required to determine whether CPAP can prevent or delay onset of dementia, and whether it could be considered neuroprotective treatment in PD.
In our study, the diagnosis of RBD was based on clinical diagnosis without systematic PSG confirmation. PSG for RBD confirmation not infrequently needs to be repeated as RBD features are not uniformly present on each night and REM sleep may be absent.47,48 It is possible that some of our patients had RBD-like manifestations of OSA49 rather than RBD proper. However, in a typical clinical context, these patients with PD would likely not have undergone PSG or received a diagnosis of OSA,50 and hence would have carried a clinical diagnosis of RBD. This clinical syndrome remains relevant, as identification and treatment of OSA in patients with PD with reduced cognition and those with RBD symptoms may lead to meaningful cognitive improvement over a sustained time period.
In this study, cognition improved without a significant improvement in subjective daytime sleepiness. This suggests dissociation of those symptoms, and is consistent with non-PD literature proposing that independent pathways may mediate these effects in OSA.51
A short-term randomized trial of therapeutic vs sham CPAP has found improvement in sleep quality and sleepiness in patients with PD with OSA.52 The same group has evaluated cognitive function and found no improvement with CPAP treatment of OSA at 3 or 6 weeks, or at follow-up in those with continued use.53 The discrepancy compared with our study may relate to a smaller sample size and lower severity of OSA in their subjects, as well as the shorter study duration, in that we observed a greater improvement at 12 than 6 months in MoCA score. Further research will be needed to identify patient subsets more likely to benefit from CPAP therapy with respect to cognition, and to gain understanding regarding duration of treatment required to show benefit.
The UPDRS part 1 (Non-Motor Aspects of Experiences of Daily Living) is a well-validated measure of non-motor symptoms in PD. Its MCID is unknown, but the rate of decline is approximately 0.42 per year.54 It was deemed responsive to deep brain stimulation, with a change of 3.1 points,55 a smaller improvement than that observed in our study in the CPAP− treated group. Our results therefore point to a clinically meaningful improvement in non-motor symptoms. We also found an improvement in PDSS, which is a well-validated PD-specific scale assessing nocturnal sleep disturbances.56 HADS-A also improved at 12 months, corresponding to improvement in Mood items on the UPDRS1, though depression scales did not show change. There was also no significant improvement in fatigue or apathy.
In the OSA− group, several outcomes appeared to deteriorate at 6 months but moved closer to baseline by 12 months, suggesting a regression to the mean phenomenon. This contrasts with our findings of improvements in outcomes in the OSA+CPAP+ group at both time points. Our findings also highlight the importance of performing longer follow-up in studies assessing patient-related outcomes, as this is more relevant to clinical care, and may demonstrate benefits that are not evident in a shorter study setting. Results from this “real life” study are generalizable to patients with PD of comparable severity.
It is interesting that improvement in symptoms and cognition occurred with relatively modest nightly CPAP use. In non-PD OSA, it has been shown that improvement in different outcomes is maximized with longer CPAP use, but that the optimal duration varies for different outcomes.57 In general, however, at least 4 hours per night of use are recommended. In this PD cohort, mean use of roughly 3.5 hours led to marked improvement in outcomes, though mean use on nights used was over 4.5 hours. This may be explained in part by the generally short and fragmented sleep in PD.8 Hence, as a proportion of these patients' usual sleep time, this may in fact be non-trivial use. The brain in PD may be more responsive to treatment perhaps due to greater ongoing insult as a result of the combination of the PD disease process, intrinsic sleep fragmentation, and OSA. In practical terms, our results suggest that participants should be allowed and encouraged to continue CPAP use even if it is less than 4 h/night.
CPAP use is challenging in patients with PD. A recent study of CPAP in PD had an attrition rate of 75% due to CPAP intolerance.58 In our study, of the 39 participants who were initially willing to try CPAP, 21 continued to use it at 12 months. In addition to usual support to enhance CPAP adherence, in patients with PD, facilitation of treatment by enlisting caregivers' help, using masks that are easy to put on and take off, and close supervision from sleep professionals can help foster more successful CPAP therapy and perhaps greater symptomatic benefit. However, further research should continue to develop treatment modalities that are easier to use for physically and cognitively impaired patients, and more easily tolerable.
Limitations of our study include potential selection bias, as it is likely that recruited participants had more sleep complaints than the general PD population. They may then have been more motivated to pursue treatment and respond. Those using CPAP may also represent a generally more motivated group using other interventions to improve their health status. The improvement in outcomes at 12 months is clinically interesting, but it applies only to those who were able to pursue CPAP therapy for that duration of time. A randomized trial will be needed to better define the effect. Nevertheless, our findings remain highly suggestive of the efficacy, and effectiveness, of CPAP treatment of OSA in PD.
Some participants crossed over from CPAP+ to CPAP− during the course of the study, and the OSA+CPAP+ group at 6 months included patients who no longer were actively using CPAP at the time of the assessment. We believe the participant grouping used for analyses to be the most pragmatic for this study. At 12 months, only ongoing users were included. It is unknown how any benefits of CPAP develop or accrue over time, in particular regarding cognitive function.
Another limitation is that we have not assessed efficacy of CPAP treatment by PSG. We used CPAP machine data, which have been shown to be reliable in OSA in the general population, using the same device.59 Furthermore, we did not reassess OSA status at 6 or 12 months. The deteriorating sleepiness in the OSA− group is interesting in that regard. Though it is likely that it resulted primarily from progression of PD and medication effects, we cannot exclude the possibility that OSA may have developed in some in the OSA− group over the course of follow-up. Additionally, medication changes in the OSA+ group may also have led to changes in severity of the OSA.9
In this study, patients with PD who were treated for moderate-severe OSA over 12 months had significant improvements in non-motor symptoms, including cognition that improved primarily in those with low-baseline MoCA or concomitant RBD symptoms. These promising results support the need for further research on OSA in PD, including medium- to long-term randomized trials of CPAP therapy or other OSA treatments to determine more precisely their effect on non-motor symptoms including cognitive function, and its specific domains. Our results bring to light OSA as a potentially important but to date underappreciated contributor to non-motor symptoms in PD, and suggest it may be warranted to more systematically test patients with PD with prominent non-motor symptoms for OSA, as treatment for OSA is feasible and may be a potential disease modifier.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Study funding: Department of Medicine of the McGill University Health Centre; Research Institute of the McGill University Health Centre; American Thoracic Society Foundation; Philips Respironics (Investigator-initiated study; in-kind support only); VitalAire Inc. (Investigator-initiated study; personnel time only).
Dr. Marta Kaminska holds research operating grants from the Canadian Institutes of Health Research and is supported by the Fonds de Recherche du Quebec – Sante. She has received research support from Philips-Respironics Inc., ResMed Inc. and VitalAire Inc. She has received consultant fees from and serves as advisory board member for Biron Soins du Sommeil. Dr. Anne-Louise Lafontaine serves as advisory board member for Abbvie and Merz. Dr. Andrea Benedetti holds operating grants from the Canadian Institutes of Health Research and is supported by the Fonds de Recherche du Quebec – Sante. Dr. R. John Kimoff holds research operating grants from the Canadian Institutes of Health Research, Fonds de Recherche du Quebec – Sante, and The Multiple Sclerosis Society of Canada. He has received research operating support from Philips-Respironics Inc., ResMed Inc., and VitalAire Inc. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to the movement disorders specialists E. Fon, R. Postuma, A. Dagher, M. Sidel, D. Rabinovitch, and all the staff at the McGill Movement Disorders clinic for their help with participant recruitment. We also thank Allen Olha who diligently scored the polysomnography tests.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- EMG
electromyography
- ESS
Epworth Sleepiness Scale
- HADS-A
Hospital Anxiety and Depression Scale, Anxiety component
- HADS-D
Hospital Anxiety and Depression Scale, Depression component
- MCID
minimal clinically important difference
- MoCA
Cognition, Montreal Cognitive Assessment
- OSA
obstructive sleep apnea
- PD
Parkinson disease
- PDSS
Parkinson's Disease Sleep Scale
- PLM
periodic limb movements
- PSG
polysomnography
- RBD
REM sleep behavior disorder
- RLS
restless legs syndrome
- SD
standard deviation
- UPDRS
Unified Parkinson's Disease Rating Scale (Movement Disorder Society Revision)
REFERENCES
- 1.Sjodahl HC, Hagell P, Nilsson MH. Motor and non-motor predictors of illness-related distress in Parkinson's disease. Parkinsonism Relat Disord. 2012;18(3):299–302. doi: 10.1016/j.parkreldis.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Gomez-Esteban JC, Tijero B, Somme J, et al. Impact of psychiatric symptoms and sleep disorders on the quality of life of patients with Parkinson's disease. J Neurol. 2011;258(3):494–499. doi: 10.1007/s00415-010-5786-y. [DOI] [PubMed] [Google Scholar]
- 3.Quelhas R, Costa M. Anxiety, depression, and quality of life in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2009;21(4):413–419. doi: 10.1176/jnp.2009.21.4.413. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher DA, Goetz CG, Stebbins G, Lees AJ, Schrag A. Validation of the MDS-UPDRS Part I for nonmotor symptoms in Parkinson's disease. Mov Disord. 2012;27(1):79–83. doi: 10.1002/mds.23939. [DOI] [PubMed] [Google Scholar]
- 5.Poewe W. The natural history of Parkinson's disease. J Neurol. 2006;253(Suppl 7):VII2–VII6. doi: 10.1007/s00415-006-7002-7. [DOI] [PubMed] [Google Scholar]
- 6.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 7.Pigott K, Rick J, Xie SX, et al. Longitudinal study of normal cognition in Parkinson disease. Neurology. 2015;85(15):1276–1282. doi: 10.1212/WNL.0000000000002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diederich NJ, McIntyre DJ. Sleep disorders in Parkinson's disease: many causes, few therapeutic options. J Neurol Sci. 2012;314(1-2):12–19. doi: 10.1016/j.jns.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Gros P, Mery VP, Lafontaine AL, et al. Obstructive sleep apnea in Parkinson's disease patients: effect of Sinemet CR taken at bedtime. Sleep Breath. 2016;20(1):205–212. doi: 10.1007/s11325-015-1208-9. [DOI] [PubMed] [Google Scholar]
- 10.Davies CR, Harrington JJ. Impact of obstructive sleep apnea on neurocognitive function and impact of continuous positive air pressure. Sleep Med Clin. 2016;11(3):287–298. doi: 10.1016/j.jsmc.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Ferini-Strambi L, Baietto C, Di Gioia MR, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP) Brain Res Bull. 2003;61(1):87–92. doi: 10.1016/s0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 12.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35(12):1593–1602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mery VP, Gros P, Lafontaine AL, et al. Reduced cognitive function in patients with Parkinson disease and obstructive sleep apnea. Neurology. 2017;88(12):1120–1128. doi: 10.1212/WNL.0000000000003738. [DOI] [PubMed] [Google Scholar]
- 14.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 16.Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19(9):1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 17.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 18.Postuma RB, Arnulf I, Hogl B, et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord. 2012;27(7):913–916. doi: 10.1002/mds.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25(15):2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhuri KR, Pal S, DiMarco A, et al. The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73(6):629–635. doi: 10.1136/jnnp.73.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 24.Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 1992;4(2):134–139. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- 25.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 26.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 27.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 28.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 29.Lessig S, Nie D, Xu R, Corey-Bloom J. Changes on brief cognitive instruments over time in Parkinson's disease. Mov Disord. 2012;27(9):1125–1128. doi: 10.1002/mds.25070. [DOI] [PubMed] [Google Scholar]
- 30.Marzolini S, Oh P, McIlroy W, Brooks D. The effects of an aerobic and resistance exercise training program on cognition following stroke. Neurorehabil Neural Repair. 2013;27(5):392–402. doi: 10.1177/1545968312465192. [DOI] [PubMed] [Google Scholar]
- 31.Vienna, Austria: R foundation for Statistical Computing; 2008. R: A language and environment for statistical computing. [Google Scholar]
- 32.Neikrug AB, Maglione JE, Liu L, et al. Effects of sleep disorders on the non-motor symptoms of Parkinson disease. J Clin Sleep Med. 2013;9(11):1119–1129. doi: 10.5664/jcsm.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsaa EB, Larsen JP, Wentzel-Larsen T, Herlofson K, Alves G. Predictors and course of health-related quality of life in Parkinson's disease. Mov Disord. 2008;23(10):1420–1427. doi: 10.1002/mds.22121. [DOI] [PubMed] [Google Scholar]
- 34.Havlikova E, Rosenberger J, Nagyova I, et al. Impact of fatigue on quality of life in patients with Parkinson's disease. Eur J Neurol. 2008;15(5):475–480. doi: 10.1111/j.1468-1331.2008.02103.x. [DOI] [PubMed] [Google Scholar]
- 35.Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson's disease: the relative importance of the symptoms. Mov Disord. 2008;23(10):1428–1434. doi: 10.1002/mds.21667. [DOI] [PubMed] [Google Scholar]
- 36.Beland SG, Postuma RB, Latreille V, et al. Observational study of the relation between parkinson's disease and sleep apnea. J Parkinsons Dis. 2015;5(4):805–811. doi: 10.3233/JPD-150602. [DOI] [PubMed] [Google Scholar]
- 37.Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costa AS, Reich A, Fimm B, Ketteler ST, Schulz JB, Reetz K. Evidence of the sensitivity of the MoCA alternate forms in monitoring cognitive change in early Alzheimer's disease. Dement Geriatr Cogn Disord. 2013;37(1-2):95–103. doi: 10.1159/000351864. [DOI] [PubMed] [Google Scholar]
- 39.Cheng Y, Wang YJ, Yan JC, Zhou R, Zhou HD. Effects of carotid artery stenting on cognitive function in patients with mild cognitive impairment and carotid stenosis. Exp Ther Med. 2013;5(4):1019–1024. doi: 10.3892/etm.2013.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gagnon JF, Vendette M, Postuma RB, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson's disease. Ann Neurol. 2009;66(1):39–47. doi: 10.1002/ana.21680. [DOI] [PubMed] [Google Scholar]
- 41.Bugalho P, Viana-Baptista M. REM sleep behavior disorder and motor dysfunction in Parkinson's disease--a longitudinal study. Parkinsonism Relat Disord. 2013;19(12):1084–1087. doi: 10.1016/j.parkreldis.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Stokholm MG, Iranzo A, Ostergaard K, et al. Assessment of neuroinflammation in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol. 2017;16(10):789–796. doi: 10.1016/S1474-4422(17)30173-4. [DOI] [PubMed] [Google Scholar]
- 43.Htoo AK, Greenberg H, Tongia S, et al. Activation of nuclear factor kappaB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep Breath. 2006;10(1):43–50. doi: 10.1007/s11325-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 44.Ho-Wo-Cheong D, O'Sullivan M, Mery VP, et al. Inflammatory markers are associated with obstructive sleep apnea (OSA) in Parkinson's disease (PD) [abstract] Am J Resp Crit Care Med. 2017;195:A4540. [Google Scholar]
- 45.Zhan G, Serrano F, Fenik P, et al. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respi Crit Care Med. 2005;172(7):921–929. doi: 10.1164/rccm.200504-581OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: a beginner's guide. Neurochem Res. 2015;40(12):2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandez-Arcos A, Iranzo A, Serradell M, Gaig C, Santamaria J. The clinical phenotype of idiopathic rapid eye movement sleep behavior disorder at presentation: a study in 203 consecutive patients. Sleep. 2016;39(1):121–132. doi: 10.5665/sleep.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferri R, Marelli S, Cosentino FI, Rundo F, Ferini-Strambi L, Zucconi M. Night-to-night variability of automatic quantitative parameters of the chin EMG amplitude (Atonia Index) in REM sleep behavior disorder. J Clin Sleep Med. 2013;9(3):253–258. doi: 10.5664/jcsm.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iranzo A, Santamaria J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28(2):203–206. doi: 10.1093/sleep/28.2.203. [DOI] [PubMed] [Google Scholar]
- 50.Cochen De Cock V, Abouda M, Leu S, et al. Is obstructive sleep apnea a problem in Parkinson's disease? Sleep Med. 2010;11(3):247–252. doi: 10.1016/j.sleep.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Gagnon K, Baril AA, Gagnon JF, et al. Cognitive impairment in obstructive sleep apnea. Pathol Biol (Paris) 2014;62(5):233–240. doi: 10.1016/j.patbio.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 52.Neikrug AB, Liu L, Avanzino JA, et al. Continuous positive airway pressure improves sleep and daytime sleepiness in patients with Parkinson disease and sleep apnea. Sleep. 2014;37(1):177–185. doi: 10.5665/sleep.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harmell AL, Neikrug AB, Palmer BW, et al. Obstructive sleep apnea and cognition in Parkinson's disease. Sleep Med. 2016;21:28–34. doi: 10.1016/j.sleep.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lang AE, Eberly S, Goetz CG, et al. Movement disorder society unified Parkinson disease rating scale experiences in daily living: longitudinal changes and correlation with other assessments. Mov Disord. 2013;28(14):1980–1986. doi: 10.1002/mds.25671. [DOI] [PubMed] [Google Scholar]
- 55.Chou KL, Taylor JL, Patil PG. The MDS-UPDRS tracks motor and non-motor improvement due to subthalamic nucleus deep brain stimulation in Parkinson disease. Parkinsonism Relat Disord. 2013;19(11):966–969. doi: 10.1016/j.parkreldis.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez-Martin P, Visser M, Rodriguez-Blazquez C, Marinus J, Chaudhuri KR, van Hilten JJ. SCOPA-sleep and PDSS: two scales for assessment of sleep disorder in Parkinson's disease. Mov Disord. 2008;23(12):1681–1688. doi: 10.1002/mds.22110. [DOI] [PubMed] [Google Scholar]
- 57.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terzaghi M, Spelta L, Minafra B, et al. Treating sleep apnea in Parkinson's disease with C-PAP: feasibility concerns and effects on cognition and alertness. Sleep Med. 2017;33:114–118. doi: 10.1016/j.sleep.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 59.Berry RB, Kushida CA, Kryger MH, Soto-Calderon H, Staley B, Kuna ST. Respiratory event detection by a positive airway pressure device. Sleep. 2012;35(3):361–367. doi: 10.5665/sleep.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.