Abstract
Study Objectives:
The goals of this study were to (1) evaluate the degree of decisional conflict (DC) experienced by caregivers of children with obstructive sleep apnea (OSA) without tonsillar hypertrophy; and (2) describe the association between DC, quality of life (QOL), and OSA severity.
Methods:
This study comprised children evaluated in the multidisciplinary upper airway center at the Cincinnati Children's Hospital Medical Center from December 2014 to May 2016. Caregivers were asked to complete surveys (Pediatric Quality of Life Inventory 4.0 [PedsQL], OSA-18, Epworth Sleepiness Scale, Family Impact Questionnaire, Decisional Conflict Scale, CollaboRATE scale, and SURE questionnaire) during a clinic visit. Polysomnography data were collected. Analysis included Kruskal-Wallis, Wilcoxon rank-sum, and regression testing.
Results:
Caregivers of 76 children participated; 16 (21.1%) had high DC. There were no significant differences in demographics between those with low and high DC; the low DC group had a higher obstructive apnea-hypopnea index (13.2 versus 12.3 events/h; P = .013). Overall and disease-specific QOL, sleepiness, family impact scores, and DC did not differ by OSA severity except for the PedsQL physical subcategory (P = .02). DC was associated with the total PedsQL (P = .043) on univariate regression; however, this did not persist (P = .61) after controlling for demographic variables. DC scores correlated well with CollaboRATE and SURE throughout the analysis (P < .001).
Conclusions:
The proportion of caregivers of children with OSA without tonsillar hypertrophy who experienced a high level of DC regarding their child's treatment was 21.1%. Neither DC nor OSA severity was related to QOL in children with OSA. The briefer SURE or CollaboRATE scales were adequate tools to measure DC in these children.
Citation:
Manning AM, Duggins AL, Tiemeyer KA, Mullen LA, Crisalli JA, Cohen AP, Ishman SL. Characterizing decisional conflict for caregivers of children with obstructive sleep apnea without tonsillar hypertrophy. J Clin Sleep Med. 2018;14(5):849–855.
Keywords: decision-making, decisional conflict, pediatric obstructive sleep apnea, persistent obstructive sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: Management of children with obstructive sleep apnea (OSA) without tonsillar hypertrophy typically involves an array of possible medical and surgical treatments from which caregivers must choose. Our intent was to examine the degree of decisional conflict (DC) engendered by this process and the relationship between DC, quality of life, and OSA severity.
Study Impact: This is the first study to examine the effect of DC on caregivers of children with OSA without tonsillar hypertrophy. We found that more than 20% of these caregivers experienced high levels of DC regarding their child's treatment for OSA.
INTRODUCTION
Decisional conflict (DC) is a state of personal uncertainty regarding the best course of action to take when there are multiple options, all of which involve some degree of risk, incongruity with personal values, or potential for regret. Multiple studies have characterized situations in which DC is most likely to occur, reporting that it is maximal when there are no clear benefits of one treatment over another, when patient values relating to the risks and benefits affect treatment preference, and when patients consider their decision to be a high-stakes choice.1–3 Research indicates that parents who are faced with decisions regarding elective surgery for their children experience DC.4 To date, four studies5–8 have examined DC in parents of children who are scheduled for consultation regarding elective otolaryngologic procedures; these studies report significant DC in 7% to 33% of caregivers.5–8
In view of the number of treatment options available for children with obstructive sleep apnea (OSA) without tonsillar hypertrophy and the lack of research regarding DC in parents of these children, we thought that it was important to explore this topic. The relevance of DC in this clinical scenario is reflected in past studies showing a sleep surgery success rate of approximately 60%9 and adherence to medical options such as continuous positive airway pressure rarely above 50%.10
With these data in mind, the primary objective of the current study was to examine the degree of DC experienced by caregivers of children with OSA and without tonsillar hypertrophy and to ascertain if there is an association between DC, quality of life (QOL), and OSA severity. Our secondary aim was to examine the correlation among the Decisional Conflict Scale (DCS) and two simplified measures of DC—the CollaboRATE and SURE scales—to determine if either of these briefer assessments could be effectively used to evaluate DC in this cohort.
METHODS
Participants
Our study included consecutive children from 0 to 18 years of age with polysomnography (PSG)-confirmed OSA but without tonsillar hypertrophy, who were evaluated by the multidisciplinary team at the upper airway center (UAC) at Cincinnati Children's Hospital Medical Center from December 2014 to May 2016. The team includes pediatric specialists in otolaryngology, pulmonary medicine, sleep medicine, radiology, genetics, and plastic surgery. The patient population served by this clinic includes children and young adults with persistent or recurrent OSA following tonsillectomy and those with primary OSA without large tonsils at presentation. Patients older than 18 years were excluded from the analysis, as were those with an obstructive apnea-hypopnea index (oAHI) < 1 event/h and those who had not completed the DC outcome measures or did not complete all surveys. Approval for the study was obtained from the Cincinnati Children's Hospital Institutional Review Board.
PSG tests were scored according to the 2007 scoring rules from the American Academy of Sleep Medicine.11 An obstructive apnea was defined as cessation of airflow with continued respiratory effort for at least two respiratory cycles. An obstructive hypopnea was defined as a reduction in airflow ≥ 30% for at least two respiratory cycles with continued respiratory effort associated with a ≥ 3% desaturation or an arousal.11 The oAHI was calculated as the number of obstructive apneas and obstructive hypopneas per hour of sleep time. Mild OSA was defined as an oAHI ≥ 1 to < 5 events/h, moderate OSA was defined as an oAHI ≥ 5 to < 10 events/h, and severe OSA was defined as an oAHI ≥ 10 events/h.
Survey Evaluation
Caregivers of all clinic patients were asked to complete the following four surveys designed to assess QOL at each clinic visit: the Pediatric Quality of Life Inventory 4.0 (PedsQL),12 OSA-18,13 Epworth Sleepiness Scale (ESS),14 and Family Impact Questionnaire (FIQ).15 The original version of the ESS was validated in adult patients; this study used a modified version for children used by prior studies16 that removed mention of alcohol from question #7. Participants in this study were also asked to complete three surveys designed to assess DC at the end of the clinic visit; these surveys were the DCS,1 Collabo-RATE,17–19 and SURE20,21 questionnaires.
The FIQ15 is a 50-item survey in which caregivers are asked to compare the effect of their child's medical disorder on several dimensions of family functioning with the effect on most children of the same age. Items are scaled using a four-point ordinal Likert scale. Items are asked in six different categories, five of which measure the child's negative effect on parental feelings (negative feelings toward parenting, effect on social relationships, financial effect, effect on marriage (if applicable), and effect on siblings (if applicable) and one item measuring the child's positive effect on parental feelings. Scores in each subcategory are summed. Previous research has reported good internal consistencies for the positive impact score (α = 0.81) and combined negative impact score (α = 0.92).22
The DCS1 is a 16-item survey that asks parents to respond to statements related to their medical decision making on a five-point ordinal Likert scale. Items are divided into five subscales: informed, values clarity; support; uncertainty; effective decision. Scores are summed, divided by 16, and multiplied by 25. Scores range from 0 (feels completely certain about the best choice) to 100 (feels extremely uncertain about the best choice). The validation study indicates that a score lower than 25 is associated with effectively doing what is decided, and a score higher than 37.5 is associated with decisional delay and uncertainty regarding the implementation of the decision. We used a cutoff of 25 or higher on the DCS to designate a high level of DC.
The CollaboRATE Scale17–19 is a three-item validated measure of shared decision making with questions pertaining to how well a healthcare provider explains a health issue, elicits patient preferences, and integrates patient preferences. Items are asked on a 10-point Likert scale, with 0 indicating that no effort was made on the part of the provider and 9 indicating that every effort was made. A score on a 0–100 scale is obtained by summing the item scores and multiplying by 3.704. A top score is also recorded when participants answer the highest response (9) on all three questions.17
Last, the SURE scale20,21 is a four-item checklist consisting of dichotomous (yes/no) questions designed to screen for DC. Scores range from 0, which signifies extreme DC, to 4, which signifies no DC; a score of 3 or lower indicates DC and is correlated with a DCS higher than 37.5. Questions are designed to incorporate four of the five subscales of the DCS (informed, values clarity, support, and uncertainty).
Treatment Options
For each visit, the treatment ultimately selected by the family was recorded. Possible management options included a variety of medical and surgical treatments, either alone or in combination. Medical options included positive airway pressure, supplemental O2, nasal steroids, leukotriene inhibitors, systemic antihistamines, weight loss, and a dental appliance. Surgical options included pharyngoplasty, supraglottoplasty, tonsillectomy, adenoidectomy (primary or revision), lingual tonsillectomy, genioglossal advancement, posterior midline glossectomy, hyoid suspension, uvulopalatopharyngoplasty, mandibular distraction, two-jaw advancement surgery, inferior nasal turbinate reduction, septoplasty, and tracheostomy.
Treatment Decisions
Prior to recommending specific management options, the multidisciplinary UAC team of providers discussed each patient at a weekly care conference and agreed on a unified treatment plan. Providers used a number of clinical factors to select these options, including the patient's age, OSA severity, comorbidi-ties, and site(s) of obstruction. Site(s) of obstruction were determined based on findings from dynamic airway cine magnetic resonance imaging and/or drug-induced sleep endoscopy. The final recommendation decided on by the team was then discussed with each family by both the pulmonologist and the otolaryngologist during the clinic visit. A shared decision-making tool was in development during this timeframe and was used for most of these discussions.23
Statistical Analysis
For demographic and baseline PSG variables, the Wilcoxon rank-sum test was used for all continuous variables and the Kruskal-Wallis test was used for categorical variables (race, comorbidities). For the comparison of families with low and high DC, significance results were obtained with the two-sample Wilcoxon rank-sum (Mann-Whitney U) test. For the evaluation of DC versus OSA severity, patients were grouped as those with mild OSA or those with moderate to severe OSA. Spearman correlation was used to evaluate the relationship between DCS, oAHI, and QOL measures. Univariable regression analysis was used to assess the relationship between DCS scores and demographic variables, PSG results, and outcomes questionnaire scores. Backward and forward model selection found none of these factors to be significant enough to be included in a multivariable model (the CollaboRATE and SURE scales were excluded, as they also measure DC and were col-linear with the DCS scores). Values of P < .05 were considered significant. Statistical analysis was carried out using Stata 12.0 (StataCorp LP, College Station, Texas, United States).
RESULTS
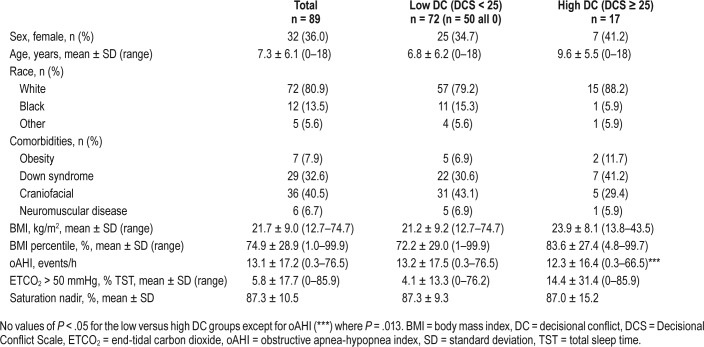
The study population comprised 250 patients who were evaluated in the UAC clinic during the study period. Of these, 42 were excluded based on age older than 18 years or oAHI < 1 event/h, leaving 208 children. An additional 132 were excluded for missing or incomplete survey data. There were no differences in age, sex, race, body mass index (BMI) percentile, apnea-hypopnea index or obstructive index between those patients included and excluded from the study (P = .17–.97). DC scales and QOL surveys were completed for 76 patients during the study period. The mean age of the study participants was 7.9 ± 6.2 years; 34% were female and 80% were classified as white race. Down syndrome was diagnosed in 34.2% of these children and 36.8% had craniofacial syndromes. The mean BMI in this cohort was 22.1 ± 9.1 kg/m2 whereas the mean BMI percentile for age was 75.4 ± 29.0.
Patients were analyzed based on their reported levels of DC. Sixteen (21.1%) had a high level of DC whereas 60 (78.9%) had a low level of DC. The two groups did not differ significantly in age, race, comorbid conditions, BMI, BMI percentile for age, or treatment (surgical versus nonsurgical) option selected (Table 1). Similarly, there was no significant difference in the pretreatment oAHI, peak PSG CO2 level, or oxygen saturation nadir between the low and high DC groups. Children with high DC had a higher percentage of total sleep time with end-tidal CO2 > 50 mmHg than those with low DC (15.7% versus 2.5%, P = .013). Separate subgroup analyses of DC did not reveal any differences in the likelihood of high DC for families of children with Down syndrome (P = .19) or craniofacial syndromes (P = .70) when compared to children without these comorbidities.
Table 1.
Demographic and polysomnography data by level of decisional conflict for children without tonsillar hypertrophy seen in an upper airway clinic.
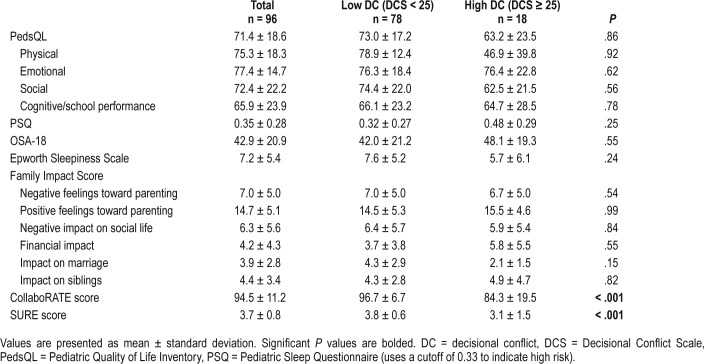
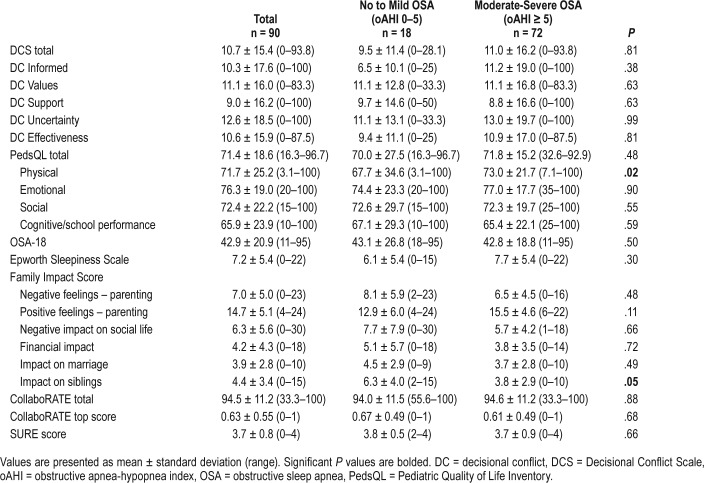
QOL, sleepiness, and family impact were examined in low versus high DC groups (Table 2). No significant difference was found in QOL between these two groups. When QOL, sleepiness, family impact, and DC were examined in low versus moderate to high disease severity groups (oAHI 1–5 versus oAHI ≥ 5 events/h), the only significant relationship found was in the physical well-being dimension of the PedsQL (Table 3). Patients with moderate to severe OSA scored lower in physical QOL than did patients with mild OSA (94.4 ± 6.4 versus 73.9 ± 9.7, P = .02). We also assessed to see if there are any differences in QOL, sleepiness, and family impact between the families of children who had previous tonsillectomy versus those without tonsillar hypertrophy and there were no differences in any of the measures assessed (P = .11–.98) (Table S1 in the supplemental material).
Table 2.
Survey results regarding quality of life, sleepiness, family impact, and decisional conflict for children without tonsillar hypertrophy in an upper airway clinic.
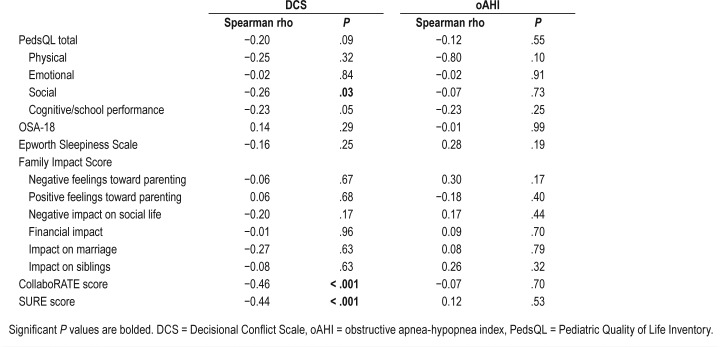
Table 3.
Correlation between quality of life surveys and DCS and oAHI.
The correlations between DC and OSA severity compared with global QOL, sleep-related QOL, sleepiness, and family impact were examined. The social functioning dimension of the PedsQL questionnaire was found to correlate negatively with DC (Spearman rho −0.26, P = .04) (Table 4). None of the survey results was correlated with disease severity.
Table 4.
Survey results regarding quality of life, sleepiness, family impact, and decisional conflict for children without tonsillar hypertrophy by OSA severity.
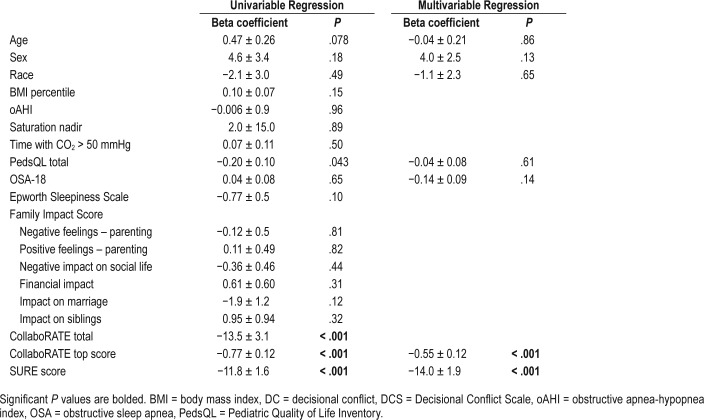
On univariable regression, the PedsQL physical well-being dimension was the only score that was associated with DC (P = .039); however, this difference did not persist when controlling for demographic data in multivariate analysis (Table 5). DCS scores correlated well with CollaboRATE and SURE scores (P < .001) throughout our analysis.
Table 5.
Regression associations between decisional conflict scores and demographic, sleep, and outcomes questionnaire scores for children with OSA.
DISCUSSION
In this cohort of family members of children with OSA without tonsillar hypertrophy, 21.1% of caregivers experienced significant DC prior to their clinical encounter. There were no baseline differences in demographic (including complex co-morbid conditions including Down syndrome or craniofacial syndromes) or PSG variables between those with and without DC except for an increased percentage of total sleep time with end-tidal CO2 > 50 mm Hg in the families with high DC. Moreover, there were no differences in patient-reported outcomes of QOL, sleepiness, or family impact among families with high and low DC. An evaluation of the correlation between DC and patient-reported outcomes revealed that only the social functioning aspect of the PedsQL had a negative correlation with DC; none of these factors correlated with OSA severity. Similarly, regression analysis revealed no significant predictors of DC. The CollaboRATE and SURE scores correlated well with the DCS throughout the analysis.
Overall, the rate of DC in our cohort of patients with OSA but without tonsillar hypertrophy was similar to that seen in prior studies. Ritchie et al.6 found that 16.9% of caregivers of children seen in a pediatric otolaryngology clinic had high DC. Families with significant DC were more likely to experience negative emotions during their clinical visit and thought that they had less autonomy in decision-making. Hong et al.24 also reported high DC in 26% of families whose children were being considered for ear tube placement or adenotonsillectomy. Carr et al.5 reported that 7.8% of parents of children undergoing tonsillectomy had high DC, but this evaluation was carried out after the decision was made to proceed with tonsillectomy. Overall, these findings suggest that caregivers would benefit from more decisional support. Current efforts at our institution are focused on the development of decisional aid tools to enhance shared decision making in this complex patient population. The data for the current study were collected as a part of the development of a shared decision-making tool.23
Although we found that there was a significant difference in total sleep time with end-tidal CO2 > 50 mm Hg between those children whose families had high and low DC, it is unlikely that this is clinically significant, as there was substantial overlap in these values between the low and high DC groups. No previous studies have evaluated the relationship between end-tidal CO2 and DC.
As it is known that children with OSA can experience reduced QOL,25,26 we hypothesized that this may be a determinate of DC in these patients. We found that children with more severe OSA had lower physical QOL scores than did those with no OSA or mild OSA. Although this suggests that caregivers of children with lower physical QOL may have more severe OSA, there was no consistent relationship between multiple QOL measures and OSA severity.
Additionally, the four-item SURE scale and the three-item CollaboRATE scale, which are both much shorter than the DCS, have been validated18,19,21 for use in adults, but not in children. The consistent correlation seen between the SURE and CollaboRATE surveys with the DCS suggests that these briefer scales could be used to evaluate DC in children with OSA but without tonsillar hypertrophy.
Our study has several limitations. First, the sample size was small; however, the proportion of families with high DC was consistent with that seen in other pediatric otolaryngology studies. In addition, we carried out multiple comparisons among families with low versus high DC, which may have led to spurious conclusions. Despite this, almost all of the associations evaluated were negative. Last, our study population included infants younger than those in whom tonsillar hyper-trophy typically develops as well as children with persistent or recurrent OSA after tonsillectomy; however, we found no significant differences in DC, regardless of patient age, suggesting that it is reasonable to pool these results.
CONCLUSIONS
Caregivers of children with OSA without tonsillar hypertrophy experience a high level of DC regarding the treatment of their child's disease. This finding is consistent with previously reported data. Neither DC nor OSA severity was related to QOL in children with OSA. The briefer SURE or CollaboRATE scales may serve as adequate tools to measure DC in these children.
DISCLOSURE STATEMENT
This work was presented at the Society for Ear, Nose & Throat Advances in Children, December 3, 2016, Orlando, FL. Work for this study was performed at Cincinnati Children's Hospital Medical Center. All authors have reviewed and approved this manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- DC
decisional conflict
- DCS
Decisional Conflict Scale
- ESS
Epworth Sleepiness Scale
- FIQ
family impact questionnaire
- oAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- QOL
quality of life
- UAC
upper airway center, a multidisciplinary sleep clinic at Cincinnati Children's Hospital Medical Center
REFERENCES
- 1.O'Connor AM. User Manual-Decisional Conflict Scale. [Accessed June 6, 2016]. http://decisionaid.ohri.ca/docs/develop/User_manuals/UM_Decisional_Conflict.pdf. Published 1993. Updated 2010.
- 2.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15(1):25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 3.Becerra Pérez MM, Menear M, Brehaut JC, Légaré F. Extent and predictors of decision regret about health care decisions: a systematic review. Med Decis Making. 2016;36(6):777–790. doi: 10.1177/0272989X16636113. [DOI] [PubMed] [Google Scholar]
- 4.Lorenzo AJ, Braga LH, Zlateska B, et al. Analysis of decisional conflict among parents who consent to hypospadias repair: single institution prospective study of 100 couples. J Urol. 2012;188(2):571–575. doi: 10.1016/j.juro.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Carr MM, Derr JB, Karikari K. Decisional conflict and regret in parents whose children undergo tonsillectomy. Otolaryngol Head Neck Surg. 2016;155(5):863–868. doi: 10.1177/0194599816655996. [DOI] [PubMed] [Google Scholar]
- 6.Ritchie KC, Chorney J, Hong P. Parents' decisional conflict, self-determination and emotional experiences in pediatric otolaryngology: a prospective descriptive-comparative study. Int J Pediatr Otorhinolaryngol. 2016;86:114–117. doi: 10.1016/j.ijporl.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Chorney J, Haworth R, Graham ME, Ritchie K, Curran JA, Hong P. Understanding shared decision making in pediatric otolaryngology. Otolaryngol Head Neck Surg. 2015;152(5):941–947. doi: 10.1177/0194599815574998. [DOI] [PubMed] [Google Scholar]
- 8.Hong P, Gorodzinsky AY, Taylor BA, Chorney JM. Parental decision making in pediatric otoplasty: the role of shared decision making in parental decisional conflict and decisional regret. Laryngoscope. 2016;126(Suppl 5):S5–S13. doi: 10.1002/lary.26071. [DOI] [PubMed] [Google Scholar]
- 9.Manickam PV, Shott SR, Boss EF, et al. Systematic review of site of obstruction identification and non-CPAP treatment options for children with persistent pediatric obstructive sleep apnea. Laryngoscope. 2016;126(2):491–500. doi: 10.1002/lary.25459. [DOI] [PubMed] [Google Scholar]
- 10.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Franco RA, Rosenfeld RM, Rao M. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;123(1 Pt 1):9–16. doi: 10.1067/mhn.2000.105254. [DOI] [PubMed] [Google Scholar]
- 14.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 15.Donenberg GL, Baker BL. The impact of young children with externalizing behaviors on their families. J Abnorm Child Psychol. 1993;21(2):179–198. doi: 10.1007/BF00911315. [DOI] [PubMed] [Google Scholar]
- 16.Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114(3):768–775. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- 17.Elwyn G, Barr PJ, Grande SW, Thompson R, Walsh T, Ozanne EM. Developing CollaboRATE: a fast and frugal patient-reported measure of shared decision making in clinical encounters. Patient Educ Couns. 2013;93(1):102–107. doi: 10.1016/j.pec.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Barr PJ, Thompson R, Walsh T, Grande SW, Ozanne EM, Elwyn G. The psychometric properties of CollaboRATE: a fast and frugal patient-reported measure of the shared decision-making process. J Med Internet Res. 2014;16(1):e2. doi: 10.2196/jmir.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barr PJ, Thompson R, Walsh T, Grande SW, Ozanne EM, Elwyn G. Correction: the psychometric properties of CollaboRATE: a fast and frugal patient-reported measure of the shared decision-making process. J Med Internet Res. 2015;17(2):e32. doi: 10.2196/jmir.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Légaré F, Kearing S, Clay K, et al. Are you SURE?: Assessing patient decisional conflict with a 4-item screening test. Can Fam Physician. 2010;56(8):e308–e314. [PMC free article] [PubMed] [Google Scholar]
- 21.Parayre AF, Labrecque M, Rousseau M, Turcotte S, Legare F. Validation of SURE, a four-item clinical checklist for detecting decisional conflict in patients. Med Decis Making. 2014;34(1):54–62. doi: 10.1177/0272989X13491463. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhower AS, Baker BL, Blacher J. Preschool children with intellectual disability: syndrome specificity, behavior problems, and maternal well-being. J Intellect Disabil Res. 2005;49(9):657–671. doi: 10.1111/j.1365-2788.2005.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergeron M, Duggins AL, Cohen AP, et al. A shared decision-making tool for obstructive sleep apnea without tonsillar hypertrophy: a randomized controlled trial. Laryngoscope. 2018;128(4):1007–1015. doi: 10.1002/lary.26967. [DOI] [PubMed] [Google Scholar]
- 24.Hong P, Maguire E, Purcell M, Ritchie KC, Chorney J. Decision-making quality in parents considering adenotonsillectomy or tympanostomy tube insertion for their children. JAMA Otolaryngol Head Neck Surg. 2017;143(3):260–266. doi: 10.1001/jamaoto.2016.3365. [DOI] [PubMed] [Google Scholar]
- 25.Garetz SL, Mitchell RB, Parker PD, et al. Quality of life and obstructive sleep apnea symptoms after pediatric adenotonsillectomy. Pediatrics. 2015;135(2):e477–e486. doi: 10.1542/peds.2014-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldassari CM, Mitchell RB, Schubert C, Rudnick EF. Pediatric obstructive sleep apnea and quality of life: a meta-analysis. Otolaryngol Head Neck Surg. 2008;138(3):265–273. doi: 10.1016/j.otohns.2007.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.