Abstract
Study Objectives:
To estimate the prevalence of obstructive sleep apnea (OSA) in children with Down syndrome.
Methods:
Two authors independently searched databases, namely PubMed, MEDLINE, EMBASE, and the Cochrane Review database. The keywords used were “Down syndrome,” “Trisomy 21,” “OSA,” “sleep apnea syndromes,” “polysomnography” and “polygraphy.” The prevalence of OSA based on apnea-hypopnea index (AHI) greater than 1, 1.5, 2, 5, and 10 event/h was estimated using a random-effects model. Subgroup analyses were conducted for children in different countries, sample size, study year, and risk of bias. Finally, the prevalence of OSA was compared between two types of sleep studies (polysomnography versus polygraphy).
Results:
A total of 18 studies (1,200 children) were included (mean age: 7.7 years; 56% boys; mean sample size: 67 patients). Five studies had low risk of bias, and nine and four studies had moderate and high risk of bias, respectively. The OSA was evaluated through polygraphy in 2 studies, and polysomnography in 16 studies. For children who underwent polysomnography, the prevalences of OSA based on AHI > 1, 1.5, 2, 5, and 10 events/h were 69%, 76%, 75%, 50%, and 34%, respectively. Subgroup analyses revealed no significant difference among all subgroups. Meta-regression showed that AHI > 5 events/h was inversely correlated with age (P < .001). Moreover, the prevalence of OSA based on AHI > 1.5 events/h was lower in polygraphy compared with polysomnography (59% versus 76%, P = .037).
Conclusions:
OSA is highly prevalent in children with Down syndrome. Prevalence of moderate to severe OSA is higher in younger age.
Citation:
Lee CF, Lee CH, Hsueh WY, Lin MT, Kang KT. Prevalence of obstructive sleep apnea in children with Down syndrome: a meta-analysis. J Clin Sleep Med. 2018;14(5):867–875.
Keywords: child, Down syndrome, polysomnography, prevalence, sleep apnea syndromes
INTRODUCTION
Down syndrome (DS) was first described by John Langdon Down in 1866.1 DS is caused by an extra copy of chromosome 212 and is the most common genetic disorder, with a prevalence of approximately 1 in 732 infants in the United States.3 Children with DS generally have intellectual disability of varying degrees.4,5 Individuals with DS are at risk of heart defects,6 celiac disease,7 hypothyroidism,8 and otolaryngologic diseases.9,10
Correlations between DS and sleep problems have received increasing attention.11–13 In particular, obstructive sleep apnea (OSA) is frequently diagnosed in children with DS13 and these children also have numerous predisposing factors for OSA, such as macroglossia,14,15 midfacial hypoplasia,16 over-weight,17 and poor muscle tone.18 Studies have demonstrated that OSA is highly prevalent in children with DS.11–13 However, a meta-analysis on the prevalence of OSA in children with DS has not been conducted. Because untreated pediatric OSA is associated with long-term adverse consequences,19 epidemiological studies on children with DS and OSA should be a priority.
This study aimed to clarify the epidemiology of OSA in children with DS. First, the prevalence and severity of OSA in children with DS were estimated, and the correlation between pediatric OSA and age in patients with DS was explored. Second, the prevalence of OSA was compared among patients from different countries, and in studies with different sample sizes, study years, risks of bias, and using two types of sleep studies—polysomnography (PSG) and polygraphy.
METHODS
Search Strategy
A meta-analysis was performed based on the PRISMA statement and the recommendations of the Meta-Analysis of Observational Studies in Epidemiology group.20 The study protocol was registered in PROSPERO (CRD42017071063).21 Two authors independently searched numerous databases, including PubMed, MEDLINE, EMBASE, and the Cochrane Review database for articles published until February 2017. The reference sections of the identified articles were searched to yield additional articles. The keywords searched for were “sleep apnea,” “OSA,” “sleep apnea syndromes,” “sleep-disordered breathing,” “sleep disorders,” “sleep apnea syndromes,” “dyssomnias,” “polysomnography (PSG),” “apnea-hypopnea index (AHI),” “polygraphy,” “Down syndrome,” “Down's syndrome,” “Trisomy 21,” “Mongolism,” and “Mongoloid.” Table 1 and Table S1 in the supplemental material describe the literature search process and list the keywords used.
Table 1.
MeSH terms and keywords used in the searching process.
The inclusion criteria were as follows: patients with DS younger than 18 years who underwent a sleep study (ie, PSG or polygraphy) for OSA diagnosis. Pediatric OSA diagnosis was based on AHI in the sleep studies.22 Therefore, the publications included in this meta-analysis contained information on AHI, and publications that failed to report AHI were excluded.
The exclusion criteria were based primarily on the absence of one of the inclusion criteria. Adults with DS were excluded.23 Children who underwent pulse oximetry for OSA diagnosis were excluded.24 Case reports, abstracts, letters to editors, and unpublished studies were excluded. The initial search was conducted by the two key reviewers (Lee CF and Lee CH) independently and was verified by the other two researchers (Lin MT and Kang KT).
Risk-of-Bias Assessment
To assess the external and internal validity of the meta-analysis, a risk-of-bias tool was used for a systematic review of the prevalence studies.25 The tool comprised 10 items: (1) national representativeness, (2) target population representativeness, (3) random selection or census undertaken, (4) minimal nonresponse bias, (5) data collected from subjects, (6) acceptable case definition used, (7) valid and reliable study instrument used, (8) same mode of data collection for all subjects, (9) length of the shortest prevalence period, and (10) appropriateness of numerator(s) and denominator(s) for the parameter. Items 1–4 are used to assess the external validity (selection and nonresponse bias) and items 5–10 are used to assess the internal validity of the study (measurement and analysis bias). All of these items are rated as high or low. Item 11, the summary assessment, is used to evaluate the overall risk of the study bias and is based on the author's subjective judgement of the preceding 10 items rated as low, moderate, or high risk. The toolset was adapted to each article separately by two authors (Lin MT and Kang KT), and disagreements were resolved through consensus.
Statistical Analysis
Data were extracted and analyzed using Comprehensive Meta-analysis Version 2 (Biostat Inc., Englewood, NJ, USA, 2005). Using polygraphy instead of PSG may have resulted in underestimating pediatric OSA diagnosis. Therefore, primary analysis for OSA prevalence was conducted for children with DS assessed using PSG. For children who underwent PSG, the prevalence of OSA based on an AHI of > 1, 1.5, 2, 5, and 10 events/h in children with DS was calculated using a random-effect model. Statistical heterogeneity among the studies was assessed using I2 statistics that measured the proportion of overall variation attributable to between-study heterogeneity.26 The I2 statistics > 50% indicated moderate heterogeneity, whereas that > 75% indicated high heterogeneity. Subgroup analyses were conducted to compare the prevalence of OSA among children from different countries, and in studies with different sample sizes, study years, and risks of bias. A mixed-effect meta-regression model was used to explore the correlation between age and prevalence of OSA. Finally, the prevalence of OSA was compared among studies using two types of sleep studies (ie, PSG versus polygraphy). A value of P < .05 was considered statistically significant.
RESULTS
Literature Search
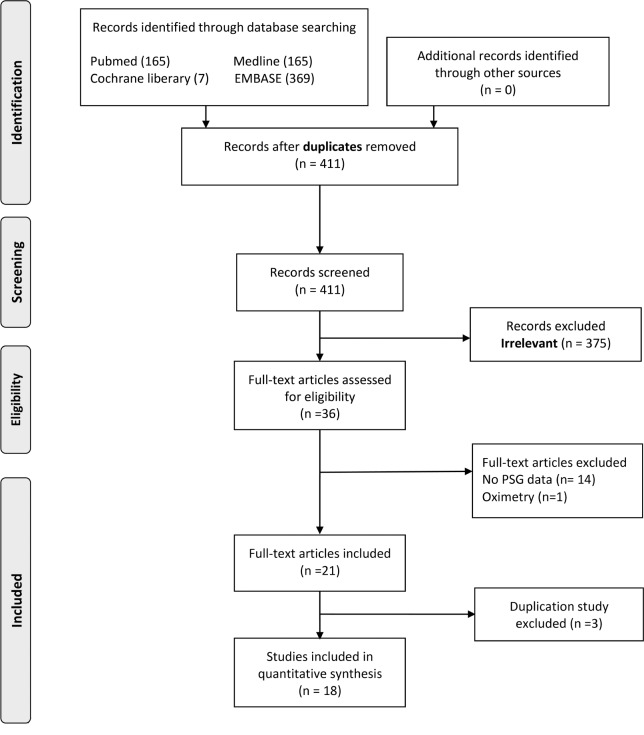
Figure 1 illustrates the literature search process. The initial web-based search yielded 411 studies and abstracts. Studies that were unpublished, contained no AHI data, or did not assess a pediatric population were excluded. Ultimately, 36 potentially pertinent studies were identified. Finally, 18 studies were included in qualitative analyses (Table 2).27–44
Figure 1. Flow diagram of literature search.
Table 2.
Basic demographics of included studies.
Basic Demographics
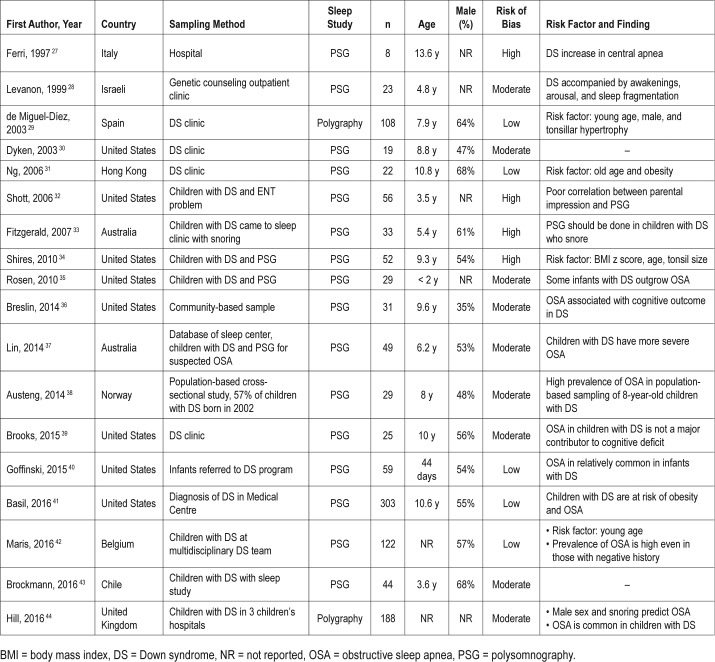
Table 2 lists the basic demographics of the patients in all the included studies. The meta-analysis included 18 studies and 1,200 children with DS. The mean age was 7.7 years, boys comprised 56% of all the children, and the mean sample size was 67. Seven studies were performed before 2008, and 11 studies were performed after 2008. Eight studies were conducted in the United States, whereas the others were conducted in Italy,27 Israel,28 Spain,29 Hong Kong,31 Australia,33,37 Norway,38 Belgium,42 Chile,43 and the United Kingdom.44 Most studies recruited their participants from a DS clinic in a hospital-based setting. The OSA was diagnosed through polygraphy in 2 studies and PSG in 16 studies. Table 2 summarizes the risk factors for OSA and the main findings of the included studies.
The studies enrolled in this meta-analysis used either PSG or polygraphy to evaluate OSA in children with DS.27–44 The polygraphy tended to use the same equipment and software as the PSG. The major difference between these two types of examination methods is that electroencephalography signals are standard equipment in the PSG but are not included in polygraphy montages.29,44 Table S2 in the supplemental material lists the details (eg, place, scoring criteria, and channels) of the sleep studies (ie, polygraphy and PSG) of the included studies.
Table S3 in the supplemental material lists the risk of bias results in detail. Five studies had a low risk of bias, nine studies had a moderate risk of bias, and four studies had a high risk of bias.
OSA Prevalence in Children With DS
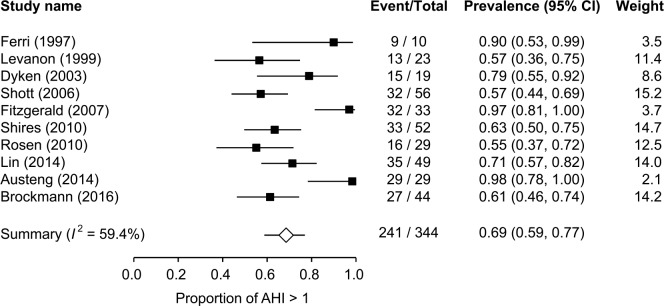
For children with DS who underwent PSG, OSA prevalence based on AHI > 1 event/h was 69% (95% confidence interval [CI], 59% to 77%, I2 = 59.4%) (Figure 2). OSA prevalence based on AHI > 1.5 events/h was 76% (95% CI, 61% to 86%, I2 = 65.1%) (Figure S1A in the supplemental material). OSA prevalence based on AHI > 2 events/h was 75% (95% CI, 64% to 84%, I2 = 77.3%) (Figure S1B in the supplemental material). The 95% CI of the prevalence of AHI > 1 event/h (95% CI, 59% to 77%), 1.5 events/h (95% CI, 61% to 86%), and 2 events/h (95% CI, 64% to 84%) are overlapping, implying that the differences between the prevalence of AHI > 1, 1.5, 2 events/h are statistically insignificant. Moreover, OSA prevalence based on AHI > 5 events/h was 50% (95% CI, 38% to 62%, I2 = 81.8%) (Figure S2 in the supplemental material), and the prevalence of OSA based on AHI > 10 events/h was 34% (95% CI, 20% to 51%, I2 = 87.8%) (Figure S3 in the supplemental material).
Figure 2. Prevalence of OSA based on AHI > 1 event/h in children with Down syndrome.
AHI = apnea-hypopnea index, OSA = obstructive sleep apnea.
Subgroup Analysis
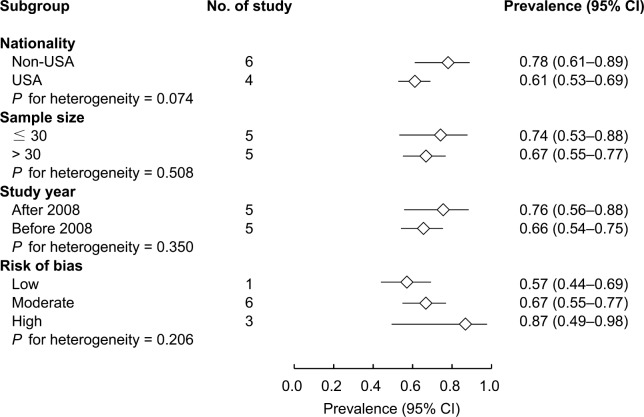
For children with DS who underwent PSG, subgroup analysis was performed to compare OSA prevalence (ie, AHI > 1 event/h) among different nationalities, and in studies with different sample sizes, study years, and risks of bias (Figure 3). The results showed that OSA prevalence did not significantly differ by nationality (non-American versus American; 78% versus 61%, P = .074), or for different sample sizes (< 30 versus > 30 patients; 74% versus 67%, P = .508), study years (after 2008 versus before 2008; 76% versus 66%, P = .350), or risks of bias (low versus moderate versus high; 57% versus 67% versus 87%, P = .206).
Figure 3. Subgroups analysis for prevalence of OSA based on AHI > 1 event/h.
AHI = apnea-hypopnea index, OSA = obstructive sleep apnea.
Meta-Regression for Age Versus AHI
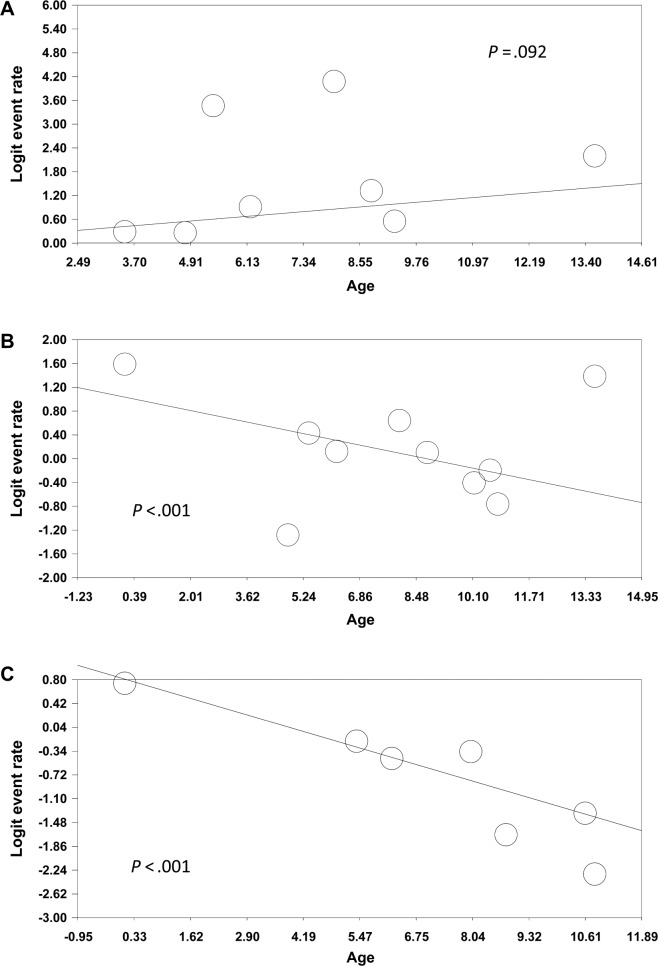
A meta-regression model was used to analyze the correlation between age and the prevalence of OSA. Age was not significantly correlated with the prevalence of AHI > 1 event/h in children with DS (P = .092) (Figure 4A). However, age was inversely correlated with the prevalence of AHI > 5 events/h in children with DS (P < .001) (Figure 4B). Age was also inversely correlated with the prevalence of AHI > 10 events/h in children with DS (P < .001) (Figure 4C), indicating that some children with DS exhibited moderate-to-severe OSA in their early childhood.
Figure 4. Meta-regression for association between the prevalence of OSA based on (A) AHI > 1, (B) AHI > 5, (C) AHI > 10 events/h and ages.
AHI = apnea-hypopnea index, OSA = obstructive sleep apnea.
PSG Versus Polygraphy
Finally, this study compared the prevalence of OSA based on AHI > 1, 1.5, 2, 5, and 10 events/h among children who underwent PSG and polygraphy (Figure S4 in the supplemental material). The prevalence of OSA based on an AHI > 1.5 events/h was significantly lower for children who underwent polygraphy than for those who underwent PSG (59% versus 76%, P = .037). However, the prevalence of OSA based on AHI > 1, 2, 5, and 10 events/h did not significantly differ among the children who underwent PSG and polygraphy (all P > .05).
DISCUSSION
This study was the first to our knowledge to perform a meta-analysis determining the prevalence and severity of OSA in children with DS. The results revealed that OSA (ie, AHI > 1, 1.5 or 2 events/h) was highly prevalent, affecting 69% to 76% of children with DS. Furthermore, approximately half of these children (50%) had moderate-to-severe OSA (ie, AHI > 5 events/h). In particular, this meta-analysis found that some children with DS had moderate-to-severe OSA in their young ages. A weak correlation was identified between parent report and PSG results.45–48 The American Academy of Pediatrics guidelines for the care of children with DS recommend that all children with DS be referred to a pediatric sleep laboratory for a sleep study or polysomnography by age 4 years.49 From a clinical perspective, this study provides substantial information on children with DS. Because OSA is typically diagnosed in early childhood, proper diagnosis and management are required to promote the well-being of children with DS.
In this meta-analysis, children with DS were mostly recruited from a hospital-based setting, that is, a DS clinic or genetic counseling department. Parents of children with DS sought medical advice because their children had clinical symptoms during sleep.45,46 Clinical symptoms such as snoring or breathing pauses are unreliable for predicting pediatric OSA,45,46 and children with DS and OSA do not necessarily snore.47 Maris et al. reported that the prevalence of OSA in children with DS without clinical symptoms was as high as 53.8%.42 These findings suggest a weak correlation between parental perception of sleep problems and PSG results.48 Therefore, a clinical physician should either test all children with DS for OSA32 or construct a predictive model to identify those at high risk of the disease.50 The only population-based study included in this meta-analysis was conducted by Austeng et al., who reported cross-sectional data in a limited age group and geographical area.38 However, the sample size in the study by Austeng et al. was relatively small, with only 29 children at age 8 years. Therefore, a population-based OSA survey on the DS population is strongly desired.
When evaluating sleep in children with DS, it is important to consider the variety of research methodologies, including different sampling methods, basic demographic sleep laboratory settings, and evolving sleep scoring criteria. In 2007, the American Academy of Sleep Medicine published rules for scoring respiratory events.51 However, different applications of the rules have resulted in disparities in OSA interpretation. This meta-analysis subclassified the participants according to study year before and after 2008 to verify the effect of different sleep scoring criteria on OSA prevalence in children with DS. Subgroup analysis was also conducted for different nationalities, sample sizes, and risks of bias. This meta-analysis revealed that OSA is highly prevalent in all subgroups and differences in OSA prevalence among the subgroups are nonsignificant. Despite disparities in sleep scoring criteria and baseline characteristics, OSA is widely observed in children with DS, warranting the need for OSA diagnosis and management strategies.
The evidence correlating age with pediatric OSA is controversial.52–54 A systematic review by Lumeng and Chervin revealed that less convincing data exist to prove differences in prevalence by age.52 In the DS population, the correlation between age and pediatric OSA is complicated. Dyken et al.30, Ng et al.31, and Shires et al.34 observed that OSA is associated with older age in children with DS. By contrast, de Miguel-Díez et al. reported that age less than 8 years is associated with OSA.29 Maris et al. reported a significant inverse correlation between age and OSA in children with DS.42 Rosen35 and Goffinski et al.40 discovered that OSA is relatively common in infants with DS. This meta-analysis revealed that moderate-to-severe OSA was inversely correlated with age in children with DS. Because of the lack of longitudinal studies reported in the literature,55 this result suggests that OSA in children with DS may start from a young age, warranting early diagnosis and management of childhood OSA in the DS population.
Several predisposing factors for OSA in children have been proposed, including obesity56 and adenotonsillar hypertrophy.54 In the DS population, these risk factors have been examined but the results are inconsistent. Basil et al. asserted that OSA risk was elevated in obese children with DS.41 By contrast, Hill et al. reported that tonsillar hypertrophy and obesity do not predict OSA in children with DS.44 Furthermore, enlargement of the lingual tonsils is relatively common in children with DS and is widely considered a risk factor for OSA.57 Recently, Maris et al. further identified that children with DS and OSA had multilevel collapse in sleep endoscopy.58 Such anatomy-based studies pertaining to the risk factors of OSA in children with DS have methodological problems because of a lack of normal controls.57–59 Future studies should clarify the risk factors of OSA in the DS population to facilitate identification of high-risk subjects.
Overnight PSG is the “gold standard” for diagnosing pediatric OSA.51 Previous studies have suggested that the use of polygraphy compared with overnight PSG may underestimate pediatric OSA.22 Similarly, the current meta-analysis revealed that the prevalence of pediatric OSA is slightly lower in studies using polygraphy compared with those using PSG. On the basis of these findings, we recommend that children with DS be monitored using overnight PSG to thoroughly understand their sleep profile.
This study had some limitations. First, the studies included in this meta-analysis were mostly hospital-based studies. Compared with hospital-based data, population-based studies avoid referral bias and provide more information on clinical practices in the general population.60,61 Population-based studies are, therefore, highly desirable. Second, insufficient longitudinal data are available that track the OSA from childhood to adulthood in the DS population.55 Understanding the natural history of OSA in children with DS is therefore vital to the establishment of treatment strategies.62–64 Third, some studies included in this review included central respiratory events in their AHI, which have inflated their estimated prevalence of OSA. Fourth, limited evidence exists comparing quality of life65 and neurocognitive66 and cardiometabolic changes67 in children with DS with and without OSA. Future studies should link adverse consequences with pediatric OSA in the DS population.68
Terminology
The authors acknowledge that the terms “mongolism” and “mongoloid” are now considered unacceptable and are no longer in common use.69 They were used as search terms in the current review only to capture all relevant literature. The authors imply no endorsement of the use of these terms.
CONCLUSIONS
Children with DS have a higher incidence of OSA. The prevalence of OSA based on an AHI > 1, 1.5, 2, 5, and 10 events/h is 69%, 76%, 75%, 50%, and 34%, respectively. The prevalence of moderate-to-severe OSA is higher at a younger age. The use of PSG is recommended for diagnosing OSA in children with DS. Population-based studies, longitudinal data, and more information regarding the effect on quality of life and neuro-cognitive outcomes are required in children with DS with and without OSA.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank all contributors for their great work in children with Down syndrome and OSA. The authors thank the anonymous reviewers and the editors for their comments. Author contributions: Chia-Fan Lee and Chia-Hsuan Lee contributed equally as the first author to this article. Kun-Tai Kang and Ming-Tzer Lin both equally participated in this study's design and coordination and are acknowledged as co-corresponding authors.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CI
confidence interval
- DS
Down syndrome
- OSA
obstructive sleep apnea
- PSG
polysomnography
REFERENCES
- 1.Down JL. Observations on an ethnic classification of idiots. 1866. Ment Retard. 1995;33(1):54–56. [PubMed] [Google Scholar]
- 2.Antonarakis SE, Lyle R, Dermitzakis ET, Reymond A, Deutsch S. Chromosome 21 and down syndrome: from genomics to pathophysiology. Nat Rev Genet. 2004;5(10):725–738. doi: 10.1038/nrg1448. [DOI] [PubMed] [Google Scholar]
- 3.Sherman SL, Allen EG, Bean LH, Freeman SB. Epidemiology of Down syndrome. Ment Retard Dev Disabil Res Rev. 2007;13(3):221–227. doi: 10.1002/mrdd.20157. [DOI] [PubMed] [Google Scholar]
- 4.Eisenhower AS, Baker BL, Blacher J. Preschool children with intellectual disability: syndrome specificity, behaviour problems, and maternal well-being. J Intellect Disabil Res. 2005;49(Pt 9):657–671. doi: 10.1111/j.1365-2788.2005.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman RS, Hesketh LJ. Behavioral phenotype of individuals with Down syndrome. Ment Retard Dev Disabil Res Rev. 2000;6(2):84–95. doi: 10.1002/1098-2779(2000)6:2<84::AID-MRDD2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 6.Freeman SB, Taft LF, Dooley KJ, et al. Population-based study of congenital heart defects in Down syndrome. Am J Med Genet. 1998;80(3):213–217. [PubMed] [Google Scholar]
- 7.George EK, Mearin ML, Bouquet J, et al. High frequency of celiac disease in Down syndrome. J Pediatr. 1996;128(4):555–557. doi: 10.1016/s0022-3476(96)70369-4. [DOI] [PubMed] [Google Scholar]
- 8.Fort P, Lifshitz F, Bellisario R, et al. Abnormalities of thyroid function in infants with Down syndrome. J Pediatr. 1984;104(4):545–549. doi: 10.1016/s0022-3476(84)80544-2. [DOI] [PubMed] [Google Scholar]
- 9.Shott SR. Down syndrome: common otolaryngologic manifestations. Am J Med Genet C Semin Med Genet. 2006;142C(3):131–140. doi: 10.1002/ajmg.c.30095. [DOI] [PubMed] [Google Scholar]
- 10.Ramia M, Musharrafieh U, Khaddage W, Sabri A. Revisiting Down syndrome from the ENT perspective: review of literature and recommendations. Eur Arch Otorhinolaryngol. 2014;271(5):863–869. doi: 10.1007/s00405-013-2563-4. [DOI] [PubMed] [Google Scholar]
- 11.Churchill SS, Kieckhefer GM, Landis CA, Ward TM. Sleep measurement and monitoring in children with Down syndrome: a review of the literature, 1960-2010. Sleep Med Rev. 2012;16(5):477–488. doi: 10.1016/j.smrv.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lal C, White DR, Joseph JE, van Bakergem K, LaRosa A. Sleep-disordered breathing in Down syndrome. Chest. 2015;147(2):570–579. doi: 10.1378/chest.14-0266. [DOI] [PubMed] [Google Scholar]
- 13.Marcus CL, Keens TG, Bautista DB, von Pechmann WS, Ward SL. Obstructive sleep apnea in children with Down syndrome. Pediatrics. 1991;88(1):132–139. [PubMed] [Google Scholar]
- 14.Donnelly LF, Shott SR, LaRose CR, Chini BA, Amin RS. Causes of persistent obstructive sleep apnea despite previous tonsillectomy and adenoidectomy in children with down syndrome as depicted on static and dynamic cine MRI. AJR Am J Roentgenol. 2004;183(1):175–181. doi: 10.2214/ajr.183.1.1830175. [DOI] [PubMed] [Google Scholar]
- 15.Guimaraes CV, Donnelly LF, Shott SR, Amin RS, Kalra M. Relative rather than absolute macroglossia in patients with Down syndrome: implications for treatment of obstructive sleep apnea. Pediatr Radiol. 2008;38(10):1062–1067. doi: 10.1007/s00247-008-0941-7. [DOI] [PubMed] [Google Scholar]
- 16.Suri S, Tompson BD, Cornfoot L. Cranial base, maxillary and mandibular morphology in Down syndrome. Angle Orthod. 2010;80(5):861–869. doi: 10.2319/111709-650.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cronk C, Crocker AC, Pueschel SM, et al. Growth charts for children with Down syndrome: 1 month to 18 years of age. Pediatrics. 1988;81(1):102–110. [PubMed] [Google Scholar]
- 18.Palisano RJ, Walter SD, Russell DJ, et al. Gross motor function of children with down syndrome: creation of motor growth curves. Arch Phys Med Rehabil. 2001;82(4):494–500. doi: 10.1053/apmr.2001.21956. [DOI] [PubMed] [Google Scholar]
- 19.Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc. 2008;5(2):274–282. doi: 10.1513/pats.200708-138MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 21.PROSPERO International Prospective Register of Systematic Reviews. Obstructive sleep apnea and Down syndrome: a systematic review and meta-analysis. PROSPERO 2017:CRD42017071063. [Accessed August 2, 2017]. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42017071063.
- 22.Tan HL, Gozal D, Ramirez HM, Bandla HP, Kheirandish-Gozal L. Overnight polysomnography versus respiratory polygraphy in the diagnosis of pediatric obstructive sleep apnea. Sleep. 2014;37(2):255–260. doi: 10.5665/sleep.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trois MS, Capone GT, Lutz JA, et al. Obstructive sleep apnea in adults with Down syndrome. J Clin Sleep Med. 2009;5(4):317–323. [PMC free article] [PubMed] [Google Scholar]
- 24.Coverstone AM, Bird M, Sicard M, et al. Overnight pulse oximetry for evaluation of sleep apnea among children with trisomy 21. J Clin Sleep Med. 2014;10(12):1309–1315. doi: 10.5664/jcsm.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferri R, Curzi-Dascalova L, Del Gracco S, et al. Respiratory patterns during sleep in Down's syndrome: importance of central apnoeas. J Sleep Res. 1997;6(2):134–141. doi: 10.1046/j.1365-2869.1997.00030.x. [DOI] [PubMed] [Google Scholar]
- 28.Levanon A, Tarasiuk A, Tal A. Sleep characteristics in children with Down syndrome. J Pediatr. 1999;134(6):755–760. doi: 10.1016/s0022-3476(99)70293-3. [DOI] [PubMed] [Google Scholar]
- 29.de Miguel-Díez J, Villa-Asensi JR, Alvarez-Sala JL. Prevalence of sleep-disordered breathing in children with Down syndrome: polygraphic findings in 108 children. Sleep. 2003;26(8):1006–1009. doi: 10.1093/sleep/26.8.1006. [DOI] [PubMed] [Google Scholar]
- 30.Dyken ME, Lin-Dyken DC, Poulton S, Zimmerman MB, Sedars E. Prospective polysomnographic analysis of obstructive sleep apnea in down syndrome. Arch Pediatr Adolesc Med. 2003;157(7):655–660. doi: 10.1001/archpedi.157.7.655. [DOI] [PubMed] [Google Scholar]
- 31.Ng DK, Hui HN, Chan CH, et al. Obstructive sleep apnoea in children with Down syndrome. Singapore Med J. 2006;47(9):774–779. [PubMed] [Google Scholar]
- 32.Shott SR, Amin R, Chini B, Heubi C, Hotze S, Akers R. Obstructive sleep apnea: Should all children with Down syndrome be tested? Arch Otolaryngol Head Neck Surg. 2006;132(4):432–436. doi: 10.1001/archotol.132.4.432. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald DA, Paul A, Richmond C. Severity of obstructive apnoea in children with Down syndrome who snore. Arch Dis Child. 2007;92(5):423–425. doi: 10.1136/adc.2006.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shires CB, Anold SL, Schoumacher RA, Dehoff GW, Donepudi SK, Stocks RM. Body mass index as an indicator of obstructive sleep apnea in pediatric Down syndrome. Int J Pediatr Otorhinolaryngol. 2010;74(7):768–772. doi: 10.1016/j.ijporl.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 35.Rosen D. Some infants with Down syndrome spontaneously outgrow their obstructive sleep apnea. Clin Pediatr (Phila) 2010;49(11):1068–1071. doi: 10.1177/0009922810378037. [DOI] [PubMed] [Google Scholar]
- 36.Breslin J, Spanò G, Bootzin R, Anand P, Nadel L, Edgin J. Obstructive sleep apnea syndrome and cognition in Down syndrome. Dev Med Child Neurol. 2014;56(7):657–664. doi: 10.1111/dmcn.12376. [DOI] [PubMed] [Google Scholar]
- 37.Lin SC, Davey MJ, Horne RS, Nixon GM. Screening for obstructive sleep apnea in children with Down syndrome. J Pediatr. 2014;165(1):117–122. doi: 10.1016/j.jpeds.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 38.Austeng ME, Øverland B, Kværner KJ, et al. Obstructive sleep apnea in younger school children with Down syndrome. Int J Pediatr Otorhinolaryngol. 2014;78(7):1026–1029. doi: 10.1016/j.ijporl.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 39.Brooks LJ, Olsen MN, Bacevice AM, Beebe A, Konstantinopoulou S, Taylor HG. Relationship between sleep, sleep apnea, and neuropsychological function in children with Down syndrome. Sleep Breath. 2015;19(1):197–204. doi: 10.1007/s11325-014-0992-y. [DOI] [PubMed] [Google Scholar]
- 40.Goffinski A, Stanley MA, Shepherd N, et al. Obstructive sleep apnea in young infants with Down syndrome evaluated in a Down syndrome specialty clinic. Am J Med Genet A. 2015;167A(2):324–330. doi: 10.1002/ajmg.a.36903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basil JS, Santoro SL, Martin LJ, Healy KW, Chini BA, Saal HM. Retrospective study of obesity in children with Down syndrome. J Pediatr. 2016;173:143–148. doi: 10.1016/j.jpeds.2016.02.046. [DOI] [PubMed] [Google Scholar]
- 42.Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Prevalence of obstructive sleep apnea in children with Down syndrome. Sleep. 2016;39(3):699–704. doi: 10.5665/sleep.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brockmann PE, Damiani F, Nuñez F, et al. Sleep-disordered breathing in children with Down syndrome: Usefulness of home polysomnography. Int J Pediatr Otorhinolaryngol. 2016;83:47–50. doi: 10.1016/j.ijporl.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 44.Hill CM, Evans HJ, Elphick H, et al. Prevalence and predictors of obstructive sleep apnoea in young children with Down syndrome. Sleep Med. 2016;27-28:99–106. doi: 10.1016/j.sleep.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Kang KT, Weng WC, Lee CH, et al. Detection of pediatric obstructive sleep apnea syndrome: history or anatomical findings? Sleep Med. 2015;16(5):617–624. doi: 10.1016/j.sleep.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 46.Kang KT, Weng WC, Lee CH, Hsiao TY, Lee PL, Hsu WC. Clinical risk assessment model for pediatric obstructive sleep apnea. Laryngoscope. 2016;126(10):2403–2409. doi: 10.1002/lary.25912. [DOI] [PubMed] [Google Scholar]
- 47.Ng DK, Chan CH, Cheung JM. Children with Down syndrome and OSA do not necessarily snore. Arch Dis Child. 2007;92(11):1047–1048. [PMC free article] [PubMed] [Google Scholar]
- 48.Rosen D, Lombardo A, Skotko B, Davidson EJ. Parental perceptions of sleep disturbances and sleep-disordered breathing in children with Down syndrome. Clin Pediatr (Phila) 2011;50(2):121–125. doi: 10.1177/0009922810384260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bull MJ. Health supervision for children with Down syndrome. Pediatrics. 2011;128(2):393–406. doi: 10.1542/peds.2011-1605. [DOI] [PubMed] [Google Scholar]
- 50.Skotko BG, Macklin EA, Muselli M, et al. A predictive model for obstructive sleep apnea and Down syndrome. Am J Med Genet A. 2017;173(4):889–896. doi: 10.1002/ajmg.a.38137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 52.Don DM, Geller KA, Koempel JA, Ward SD. Age specific differences in pediatric obstructive sleep apnea. Int J Pediatr Otorhinolaryngol. 2009;73(7):1025–1028. doi: 10.1016/j.ijporl.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang KT, Chou CH, Weng WC, Lee PL, Hsu WC. Associations between adenotonsillar hypertrophy, age, and obesity in children with obstructive sleep apnea. PLoS One. 2013;8(10):e78666. doi: 10.1371/journal.pone.0078666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bixler EO, Fernandez-Mendoza J, Liao D, et al. Natural history of sleep disordered breathing in prepubertal children transitioning to adolescence. Eur Respir J. 2016;47(5):1402–1409. doi: 10.1183/13993003.01771-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang KT, Lee PL, Weng WC, Hsu WC. Body weight status and obstructive sleep apnea in children. Int J Obes (Lond) 2012;36:920–924. doi: 10.1038/ijo.2012.5. [DOI] [PubMed] [Google Scholar]
- 57.Donnelly LF, Shott SR, LaRose CR, Chini BA, Amin RS. Causes of persistent obstructive sleep apnea despite previous tonsillectomy and adenoidectomy in children with down syndrome as depicted on static and dynamic cine MRI. AJR Am J Roentgenol. 2004;183(1):175–181. doi: 10.2214/ajr.183.1.1830175. [DOI] [PubMed] [Google Scholar]
- 58.Maris M, Verhulst S, Saldien V, et al. Drug-induced sedation endoscopy in surgically naive children with Down syndrome and obstructive sleep apnea. Sleep Med. 2016;24:63–70. doi: 10.1016/j.sleep.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 59.Jayaratne YSN, Elsharkawi I, Macklin EA, et al. The facial morphology in Down syndrome: a 3D comparison of patients with and without obstructive sleep apnea. Am J Med Genet A. 2017;173(11):3013–3021. doi: 10.1002/ajmg.a.38399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee CH, Chang WH, Ko JY, Yeh TH, Hsu WC, Kang KT. Revision adenoidectomy in children: a population-based cohort study in Taiwan. Eur Arch Otorhinolaryngol. 2017;274(10):3627–3635. doi: 10.1007/s00405-017-4655-z. [DOI] [PubMed] [Google Scholar]
- 61.Lee CH, Hsu WC, Ko JY, Yeh TH, Chang WH, Kang KT. Epidemiology and trend of pediatric adenoidectomy: a population-based study in Taiwan from 1997 to 2012. Acta Otolaryngol. 2017;137(12):1265–1270. doi: 10.1080/00016489.2017.1357191. [DOI] [PubMed] [Google Scholar]
- 62.Farhood Z, Isley JW, Ong AA, et al. Adenotonsillectomy outcomes in patients with Down syndrome and obstructive sleep apnea. Laryngoscope. 2017;127(6):1465–1470. doi: 10.1002/lary.26398. [DOI] [PubMed] [Google Scholar]
- 63.Nation J, Brigger M. The efficacy of adenotonsillectomy for obstructive sleep apnea in children with Down syndrome: a systematic review. Otolaryngol Head Neck Surg. 2017;157(3):401–408. doi: 10.1177/0194599817703921. [DOI] [PubMed] [Google Scholar]
- 64.Kang KT, Koltai PJ, Lee CH, Lin MT, Hsu WC. Lingual tonsillectomy for treatment of pediatric obstructive sleep apnea: a meta-analysis. JAMA Otolaryngol Head Neck Surg. 2017;143(6):561–568. doi: 10.1001/jamaoto.2016.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang KT, Weng WC, Yeh TH, Lee PL, Hsu WC. Validation of the Chinese version OSA-18 quality of life questionnaire in Taiwanese children with obstructive sleep apnea. J Formos Med Assoc. 2014;113(7):454–462. doi: 10.1016/j.jfma.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Gozal D, Kheirandish-Gozal L. Neurocognitive and behavioral morbidity in children with sleep disorders. Curr Opin Pulm Med. 2007;13(6):505–509. doi: 10.1097/MCP.0b013e3282ef6880. [DOI] [PubMed] [Google Scholar]
- 67.Kang KT, Chiu SN, Weng WC, Lee PL, Hsu WC. Comparisons of office and 24-hour ambulatory blood pressure monitoring in children with obstructive sleep apnea. J Pediatr. 2017;182:177.e2–183.e2. doi: 10.1016/j.jpeds.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 68.Mallah ME, Bailey E, Trivedi M, Kremer T, Rhein LM. Pediatric Obstructive Sleep Apnea in High-Risk Populations: Clinical Implications. Pediatr Ann. 2017;46(9):e336–e339. doi: 10.3928/19382359-20170815-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodríguez-Hernández ML, Montoya E. Fifty years of evolution of the term Down's syndrome. Lancet. 2011;378(9789):402. doi: 10.1016/S0140-6736(11)61212-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.