Abstract
Within this issue of Neurogastroenterology and Motility, an article by Pohl et al highlights new insights from a powerful porcine model of the link between early life adversity and relapsing functional gastrointestinal disorders. Early weaning stress closely mimics the early life psychosocial stressors that have been linked to adult onset gastrointestinal dysfunction. This early weaning model provides reproducible and highly translatable outcomes in young stress-challenged pigs. Due to the convincingly comparable neurological and gastroenterological anatomy and physiology between pigs and human beings, gastrointestinal stress and injury studies utilizing swine models will provide invaluable insights to improve our understanding and treatment of gastrointestinal disease in human beings. Future studies to examine mechanisms underlying this link between early life adversity and functional gastrointestinal disorders will explore the roles of gender and hypo-maturity in gastrointestinal responses to stress.
Keywords: Early life adversity, irritable bowel syndrome, porcine models, intestinal barrier function
Early Life Stressors and GI Dysfunction in Humans
Early life adversity (ELA) has repeatedly been shown to be associated with an increased incidence of chronic gastrointestinal (GI) disease in adulthood1. For example, there is a correlation between instances of childhood abuse and adult functional GI disorders (FGID)2,3. Traumatic childhood events such as physical or sexual abuse are thought to predispose individuals to psychological distress and exaggerated reactions to stress, manifesting as worsened symptoms of and increased disability from FGID2. It is important to note key gender differences related to childhood sexual abuse; there is a heavy bias toward increased female representation in victims reporting sexual abuse, as well as increased susceptibility of female victims to developing post-traumatic stress disorder4. Higher levels of psychological distress such as anxiety and depression increase the likelihood of developing irritable bowel syndrome (IBS) following GI infection5,6. Childhood relationship deprivation may correlate with increased adulthood FGID; 61% of IBS sufferers report having had an ‘unsatisfactory’ relationship with their parents, supporting the notion that a number of different sources of stress can lead to future development of FGID such as IBS7. In addition, restricted fetal growth also correlates with increased incidence of FGID. In particular, low birthweight significantly increases incidence of IBS and hastens the onset of illness into adolescence8. These studies suggest that even pre-natal stressors that lead to low birthweight may also contribute to FGID. Ongoing studies to understand mechanisms involved in this process are critically needed to uncover novel and more effective clinical interventions for FGID patients.
Translational Animal Models for Stress and GI Research
Studying such a complex and somewhat nebulous concept of childhood stress poses a significant challenge in developing research models. Rodent studies utilizing early maternal separation have shown promise in furthering our understanding of the complicated relationship between ELA and FGID. For instance, rat models utilizing neonatal maternal separation of early life stress reproducibly induce visceral hypersensitivity to acute stressors9. However, it is important to consider that rodent models, while informative and important to building our understanding of human disease, often fail to fully recapitulate human GI biology and disease because of fundamental differences between rodent and human GI tracts10. For example, the human and porcine ENS have interneuronal networks and plexi that rodents do not have, and neurotransmitter associations with particular neuronal subtypes is more common between humans and pigs than between humans and rodents11–13. Translating findings from rodent models of early life stress to effective clinical interventions for FGID patients has been slow and inconsistent14. On this basis, large animal models, particularly swine, have been increasingly appreciated as an important translational model for the study of human GI biology due to their remarkably similar GI anatomy and physiology15,16. Of particular psychological importance, the gyrencephalic neuroanatomy is advanced in both human beings and pigs, suggesting pigs have similar higher centers related to interpretation of social and physical stress17. Additionally, perinatal developmental stages of the porcine intestinal mucosa, including the subepithelial immune system and enteric nervous system, are more similar to human infants as compared to neonatal rodents12. In the present issue, Pohl et al utilize a powerful translational swine model of stress-induced digestive disease refined for over a decade by this research group18.
Weaning Stress as a Translational Model
Human infants and neonatal swine experience significant stress in early life associated with homeostatic changes related to birth and weaning. Adaptive changes to these early-life stressors are likely contributors to future GI disease susceptibility. While the early-life stressors, both psychological and physical, experienced by people may vary from those experienced by young pigs, they likely have a similar biological potential to challenge homeostasis and induce pathophysiological changes during the highly plastic phases of early development12. In United States swine production, it is common practice to wean piglets at approximately three weeks-of-age, at least 1-week earlier than the standard weaning age in the European Union that was developed to reduce stress in livestock production19. In the early weaning stress (EWS) model employed in the accompanying article, EWS pigs were weaned at 15 days-of-age while control animals were weaned at the 28 days-of-age18. Previous studies by this group have established that EWS in piglets, including early maternal deprivation as well as an abrupt change in environment, diet and companionship, induces significant psychological, dietary and environmental stress that closely mimics human ELA. The link between ELA and FGID is still not completely understood, but this emerging area of research along with previous work from this group highlights the effects of ELA on both short term (9 weeks-of-age, juvenile) and long term (20 weeks-of-age, adult) intestinal permeability, immune function, and overall measurable GI disease20–22. One mechanism contributing to this EWS-FGID pathogenesis is the activation of the hypothalamic-pituitary axis leading to increased release of corticotrophin releasing factor (CRF) and cortisol in juvenile pigs that have effects on intestinal function20,22. Interestingly, previous work has shown upregulation of CRF receptors that have been co-localized with markers of mast cells22, suggesting a more direct hypothalamic-intestinal axis during stress.
In a previous EWS model, both EWS (3 weeks-of-age) and late-weaned (4 weeks-of-age) pigs had marked increases in serum cortisol at the time of weaning, suggesting that the perceived weaning stress is comparable in both age groups21. Interestingly, in this previous study, increased levels of cortisol were detected in later weaned pigs, but there were higher elevations in CRF in the earlier weaned pigs. The disparity in response to EWS by the younger animals along with the apparent importance of CRF suggests there is an increased inherent susceptibility to GI dysfunction in younger pigs, likely associated with the action of CRF on enteric mast cells and possibly the hypomaturity of the enteric and central nervous systems21. The present study by Pohl et al also highlights the role of EWS in chronic FGID characterized by chronic, functional diarrhea into adulthood, associated with persistently increased mast cell numbers, particularly near enteric ganglia, and persistently increased intestinal permeability18. This is consistent with the primary underlying pathology associated with the development of IBS in people, particularly increased mast cell activation23,24, and associated with increased gut permeability25–27. Overall, the gut is thought to become hypersensitive to stimuli, with strong evidence of a direct link to the higher centers of the brain that sense pain (Fig. 1).
Figure 1.
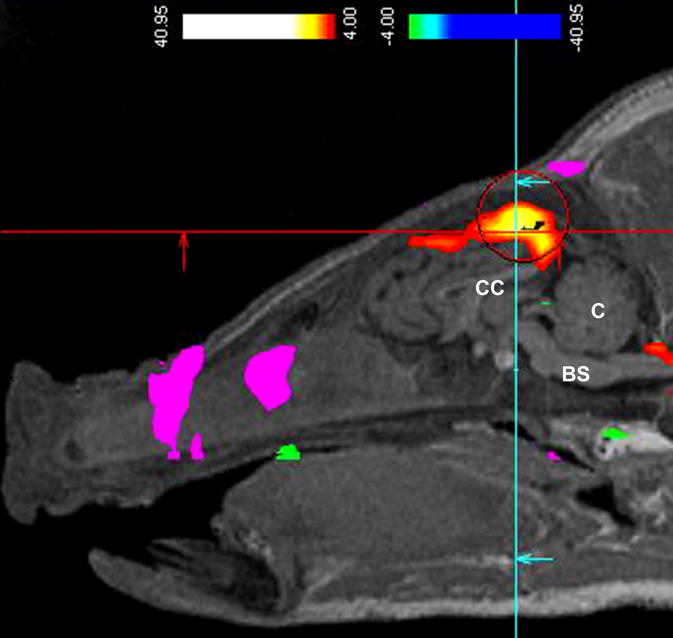
Preliminary functional magnetic resonance imaging (fMRI) data on a pig with early weaning stress using the technique of blood-oxygen-level dependent (BOLD) contrast imaging. Intestinal hypersensitivity was noted by fMRI BOLD highlighting of the cingulate cortex region of the brain (intense orange coloration within the ‘crosshairs’) in response to inflation of a balloon tip-catheter inflation within the rectum/descending colon. The pig was under general anesthesia, and positioned with the nose to the left in a horizontal plane, and with the corpus callosum (CC), cerebellum(C), and brain stem (BS) indicated for the purposes of orientation. Pigs in the absence of early weaning stress (i.e late weaned) had very little BOLD signal with the same level of rectal distension. This technique is similar to that used in people as a diagnostic method to detect visceral hypersensitivity and by inference, IBS. The increased detection of oxygenated blood within specific regions of the cortex can be quantified, and has been used as an indicator of increased visceral input to regions of the brain involved in interpreting visceral pain. This data was collected in a preliminary trial performed by Pease A, Moeser AJ, and Blikslager AT, to evaluate the feasibility of fMRI BOLD in pigs under NC State Institutional Animal Care and Use Committee approval.
Intestinal Repair Defect in Neonatal Swine
Intestinal barrier defects are a hallmark of both IBS and the EWS model developed by Pohl et al. The role of the intestinal barrier in numerous GI pathologies has been well documented, and there is new evidence that aberrations in barrier function may be of greater consequence in the neonate. Recent work in our lab, which focuses on intestinal barrier repair mechanisms using the pig as a model28, has uncovered a defect in the ability of neonatal pigs to restitute the intestinal barrier following brief ischemic injury to the jejunum. This appears to be linked to immature development of the enteric nervous system, and may contribute to the pathogenesis of FGID both in early life as well as adulthood. We know that the enteric nervous system (ENS) serves as the link between brain-gut axis and is intricately involved in visceral sensation, motility, absorption and secretion, and intestinal barrier function29. The ENS is hypo-mature at birth in both pigs and humans, and it undergoes significant postnatal changes30,31. There is remarkable plasticity in the ENS during the development and adaptive phases in early growth periods and alterations in these processes have the potential to lead to life-long alterations in GI physiology.32 A growing body of evidence indicates that enteric glial cells, a previously under-appreciated community of cells critical to the ENS, plays a pivotal role in promoting intestinal epithelial repair and barrier function. This is achieved by enteric glial cell release of paracrine factors such as glial-derived neurotrophic factor, pro-epidermal growth factor, 11β prostaglandin F2α and S-nitrosoglutathione33–36. Enteric glial cells form a dense network in the lamina propria in close proximity to intestinal epithelial cells, and we know that this network continues development into the postnatal period37–39. Preliminary work from our lab has shown that the the number of glial cells and the relative extent of the glial cells network appears to be reduced in neonatal pigs as compared to older animals.
Conclusion
There is a clear connection between ELA and FGID. The present study, together with a growing body of evidence, has revealed that early life stress induces changes in intestinal permeability, enteric nervous system development, CRF release, and mast cell activation that are critical components of FGID development that is conserved across higher order mammals12. Pohl et al have also identified an important sex-related variation in response to EWS in that female pigs demonstrated enhanced susceptibility to FGID outcomes18. Further studies utilizing increasingly important swine models discussed in this review may well provide a better understanding of the role of sex hormones and hypomaturity in the development of GI disease, and will likely uncover novel approaches to treating FGID patients.
Acknowledgments
Funding: Large Animal Models Core, Center for Gastrointestinal Biology and Disease, P30 DK34987
ABBREVIATIONS
- ELA
Early life adversity
- GI
gastrointestinal
- FGID
functional GI disorders
- IBS
irritable bowel syndrome
- EWS
early weaning stress
- ENS
enteric nervous system
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Talley NJ, Fett SL, Zinsmeister AR. Self-reported abuse and gastrointestinal disease in outpatients: association with irritable bowel-type symptoms. Am J Gastroenterol. 1995;90:366–371. [PubMed] [Google Scholar]
- 2.Chitkara DK, van Tilburg MA, Blois-Martin N, et al. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker EA, Katon WJ, Roy-Byrne PP, et al. Histories of sexual victimization in patients with irritable bowel syndrome or inflammatory bowel disease. Am J Psychiatry. 1993;150:1502–1506. doi: 10.1176/ajp.150.10.1502. [DOI] [PubMed] [Google Scholar]
- 4.Walker JL, Carey PD, Mohr N, et al. Gender differences in the prevalence of childhood sexual abuse and in the development of pediatric PTSD. Arch Womens Ment Health. 2004;7:111–121. doi: 10.1007/s00737-003-0039-z. [DOI] [PubMed] [Google Scholar]
- 5.Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 6.Coutinho SV, Plotsky PM, Sablad M, et al. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 7.Hislop IG. Childhood deprivation: an antecedent of the irritable bowel syndrome. Med J Aust. 1979;1:372–374. doi: 10.5694/j.1326-5377.1979.tb126963.x. [DOI] [PubMed] [Google Scholar]
- 8.Bengtson MB, Ronning T, Vatn MH, et al. Irritable bowel syndrome in twins: genes and environment. Gut. 2006;55:1754–1759. doi: 10.1136/gut.2006.097287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Wijngaard RM, Klooker TK, Welting O, et al. Essential role for TRPV1 in stress-induced (mast cell-dependent) colonic hypersensitivity in maternally separated rats. Neurogastroenterol Motil. 2009;21:1107–e1194. doi: 10.1111/j.1365-2982.2009.01339.x. [DOI] [PubMed] [Google Scholar]
- 10.Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 11.Brown DR, Timmermans JP. Lessons from the porcine enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl 1):50–54. doi: 10.1111/j.1743-3150.2004.00475.x. [DOI] [PubMed] [Google Scholar]
- 12.Pohl CS, Medland JE, Moeser AJ. Early-life stress origins of gastrointestinal disease: animal models, intestinal pathophysiology, and translational implications. Am J Physiol Gastrointest Liver Physiol. 2015;309:G927–941. doi: 10.1152/ajpgi.00206.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timmermans JP, Hens J, Adriaensen D. Outer submucous plexus: an intrinsic nerve network involved in both secretory and motility processes in the intestine of large mammals and humans. Anat Rec. 2001;262:71–78. doi: 10.1002/1097-0185(20010101)262:1<71::AID-AR1012>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Holschneider DP, Bradesi S, Mayer EA. The role of experimental models in developing new treatments for irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2011;5:43–57. doi: 10.1586/egh.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler A, Gonzalez L, Blikslager A. Large Animal Models: The Key to Translational Discovery in Digestive Disease Research. Cell Mol Gastroenterol Hepatol. 2016;2:716–724. doi: 10.1016/j.jcmgh.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez LM, Moeser AJ, Blikslager AT. Porcine models of digestive disease: the future of large animal translational research. Transl Res. 2015;166:12–27. doi: 10.1016/j.trsl.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gieling ET, Schuurman T, Nordquist RE, et al. The pig as a model animal for studying cognition and neurobehavioral disorders. Curr Top Behav Neurosci. 2011;7:359–383. doi: 10.1007/7854_2010_112. [DOI] [PubMed] [Google Scholar]
- 18.Pohl CS, Medland JE, Mackey E, et al. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. Neurogastroenterol Motil. 2017 doi: 10.1111/nmo.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Council Directive 2008/120/EC of 18 December 2008 laying down minimum standards for the protection of pigs (Codified version) Official Journal of the European Union. 2009;L 47(5) [Google Scholar]
- 20.Smith F, Clark JE, Overman BL, et al. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. 2010;298:G352–363. doi: 10.1152/ajpgi.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moeser AJ, Ryan KA, Nighot PK, et al. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am J Physiol Gastrointest Liver Physiol. 2007;293:G413–421. doi: 10.1152/ajpgi.00304.2006. [DOI] [PubMed] [Google Scholar]
- 22.Moeser AJ, Klok CV, Ryan KA, et al. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol Gastrointest Liver Physiol. 2007;292:G173–181. doi: 10.1152/ajpgi.00197.2006. [DOI] [PubMed] [Google Scholar]
- 23.Matricon J, Meleine M, Gelot A, et al. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;36:1009–1031. doi: 10.1111/apt.12080. [DOI] [PubMed] [Google Scholar]
- 24.Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213–1221. doi: 10.1136/gut.2010.213108. [DOI] [PubMed] [Google Scholar]
- 25.Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288–1294. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 26.Shulman RJ, Eakin MN, Czyzewski DI, et al. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr. 2008;153:646–650. doi: 10.1016/j.jpeds.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mujagic Z, Ludidi S, Keszthelyi D, et al. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment Pharmacol Ther. 2014;40:288–297. doi: 10.1111/apt.12829. [DOI] [PubMed] [Google Scholar]
- 28.Blikslager AT, Moeser AJ, Gookin JL, et al. Restoration of barrier function in injured intestinal mucosa. Physiological Reviews. 2007;87:545–564. doi: 10.1152/physrev.00012.2006. [DOI] [PubMed] [Google Scholar]
- 29.Furness JB, Callaghan BP, Rivera LR, et al. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 30.Wester T, O’Briain DS, Puri P. Notable postnatal alterations in the myenteric plexus of normal human bowel. Gut. 1999;44:666–674. doi: 10.1136/gut.44.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paran TS, Rolle U, Puri P. Postnatal development of the mucosal plexus in the porcine small and large intestine. Pediatr Surg Int. 2006;22:997–1001. doi: 10.1007/s00383-006-1786-5. [DOI] [PubMed] [Google Scholar]
- 32.Kabouridis PS, Pachnis V. Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. J Clin Invest. 2015;125:956–964. doi: 10.1172/JCI76308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Landeghem L, Chevalier J, Mahe MM, et al. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am J Physiol Gastrointest Liver Physiol. 2011;300:G976–987. doi: 10.1152/ajpgi.00427.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savidge TC, Newman P, Pothoulakis C, et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–1358. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 35.Coquenlorge S, Van Landeghem L, Jaulin J, et al. The arachidonic acid metabolite 11beta-ProstaglandinF2alpha controls intestinal epithelial healing: deficiency in patients with Crohn–s disease. Sci Rep. 2016;6:25203. doi: 10.1038/srep25203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao W, Wang W, Chen W, et al. GDNF is involved in the barrier-inducing effect of enteric glial cells on intestinal epithelial cells under acute ischemia reperfusion stimulation. Mol Neurobiol. 2014;50:274–289. doi: 10.1007/s12035-014-8730-9. [DOI] [PubMed] [Google Scholar]
- 37.Cossais F, Durand T, Chevalier J, et al. Postnatal development of the myenteric glial network and its modulation by butyrate. Am J Physiol Gastrointest Liver Physiol. 2016;310:G941–951. doi: 10.1152/ajpgi.00232.2015. [DOI] [PubMed] [Google Scholar]
- 38.Neunlist M, Aubert P, Bonnaud S, et al. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. Am J Physiol Gastrointest Liver Physiol. 2007;292:G231–241. doi: 10.1152/ajpgi.00276.2005. [DOI] [PubMed] [Google Scholar]
- 39.Kabouridis PS, Lasrado R, McCallum S, et al. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron. 2015;85:289–295. doi: 10.1016/j.neuron.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]


