Abstract
[Purpose] This study evaluated the effects of repetitive peripheral magnetic stimulation of the soleus muscle on spinal cord and peripheral motor nerve excitability. [Subjects and Methods] Twelve healthy adults (mean age 22 years) who provided written informed consent were administered repetitive peripheral magnetic stimulation for 10 min. Pre-and post-stimulation latencies and amplitudes of H- and M-waves of the soleus muscle were measured using electromyography and compared using paired t-tests. [Results] Pre- and post-stimulation latencies (28.3 ± 3.3 vs. 29.1 ± 1.3 ms, respectively) and amplitudes (35.8 ± 1.3 vs. 35.8 ± 1.1 mV, respectively) of H-waves were similar. Pre-stimulation latencies of M-waves were significantly higher than post-stimulation latencies (6.1 ± 2.2 vs. 5.0 ± 0.9 ms, respectively), although pre- and post-stimulation amplitudes were similar (12.2 ± 1.4 vs. 12.2 ± 1.3 mV, respectively). Motor neuron excitability, based on the excitability of motor nerves and peripheral nerve action, was increased by M-waves following magnetic stimulation. [Conclusion] The lack of effect of magnetic stimulation on the amplitude and latency of the H-reflex suggests that magnetic stimulation did not activate sensory nerve synapses of α motor neurons in the spinal cord. However, because motor nerves were stimulated together with sensory nerves, the increased H-wave amplitude may have reflected changes in peripheral rather than in α motor nerves.
Keywords: Repetitive peripheral magnetic stimulation, H wave, M wave
INTRODUCTION
Functional electrical stimulation (FES) of paralyzed muscles shortly after stroke has been found to promote muscle contraction and improve the function of paralyzed muscles1). Application of an integrated volitional control electrical stimulator (IVES) during activities of daily living (ADL) and gait is a new method of electrical stimulation therapy. The stimulation intensity of IVES is proportional to voluntary muscle contractions, with no stimulation during rest, making possible its therapeutic use during ADL. Electromyogram (EMG)-triggered electrical stimulation is one of the rehabilitation techniques employed to facilitate motor restoration in chronic stroke survivors. Electrical stimulation of target muscles during voluntary movements can therefore promote appropriate contraction of these muscles2).
Many studies using IVES have targeted muscles of the upper extremities1, 2). Administration of IVES for 8 hours/day for 8 days was shown to improve separate movements of the fingers, increased the utility of the upper extremities, and promote stable wrist orthosis3). The combination of IVES targeting muscles of the lower extremities and Botox injections in patients with hemiplegic stroke showed a locomotor effect. IVES was found to stimulate the tibialis anterior muscle and improve ankle movement during gait rehabilitation. Moreover, ambulation activity was improved by stimulating muscles of both the lower and upper extremities. Because many studies have shown that electrical stimulation improves paralysis4−6), electrical stimulation therapy is used to train muscles of the upper and/or lower extremities in hemiplegic patients. Electrical stimulation therapy has been reported to improve the functions of paralyzed limbs while suppressing spasticity.
Pain electrically stimulates algesthesia nerves through nociceptors around their outer layers. Thus, increased intensity of exogenous electrical stimulation can increase pain, and long-time application of electrical stimulation may become unpleasant or difficult7, 8). Repetitive peripheral magnetic stimulation (rPMS) is less painful, increasing its clinical applications9). rPMS acts on intramuscular motor axons, which evoke muscle contraction10). Moreover, rPMS has a greater depth of penetration than electrical muscle stimulation and does not include current flowing through the skin or locally high current densities11). Because both magnetic and electrical stimulation act peripherally, both promote extreme muscle contraction, with the combination of rPMS and occupational therapy improving arm function in stroke patients. This study was designed to clarify the effects of rPMS of the soleus muscle on the excitability of the spinal cord and peripheral motor nerves.
SUBJECTS AND METHODS
Twelve healthy adults (mean age 22.0 years), with no history of neurological disorders or injuries, participated in this study. All subjects provided written informed consent, and the study was conducted according to the Declaration of Helsinki.
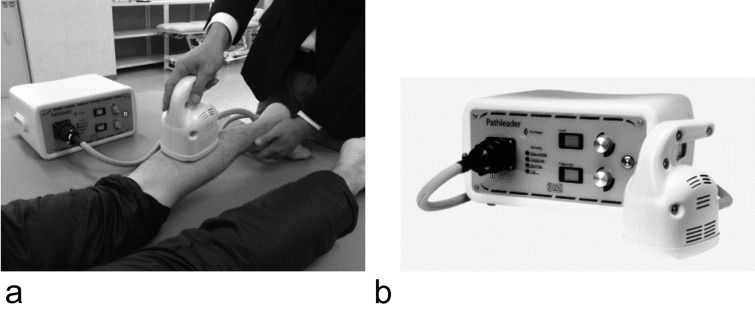
All subjects were examined before and after rPMS. rPMS was delivered to the soleus muscle of the right leg using a Pathleader stimulator (IFG, Japan, Fig. 1) and coil, with the subject in the supine position. The stimulation frequency was 40 Hz and the stimulation intensity was 70. Each stimulus was delivered for 3 sec, with a pause of 7 sec between stimuli. Stimuli were therefore applied 6 times/min for 10 min.
Fig. 1.
Stimulation. a: stimulation situation. b: rPMS (IFG Japan).
The percutaneous electrical stimulus was a square-wave pulse of 500 µs duration delivered with a stimulator (Nihon-koden, Japan). The H-reflex was elicited by placing the cathode over the posterior tibial nerve proximal to the anode in the popliteal fossa to avoid anodal block while in the prone position. Pre- and post-stimulation latencies and amplitudes of the H-wave and M-wave of the soleus muscle were each measured 64 times, and their means were calculated. The stimulation level was set to obtain H-wave amplitudes approximately equal to M-wave amplitudes.
Differences between the values before and after rPMS were tested for significance using paired t tests. SPSS ver 21 was used for all statistical analyses. Differences with p<0.05 were considered statistically significant.
RESULTS
Pre- and post-stimulation latencies (28.3 ± 3.3 vs. 29.1 ± 1.3 ms) and amplitudes (35.8 ± 1.3 vs. 35.8 ± 1.1 mV) of H-wave reflexes were similar. Pre-stimulation latencies of M-wave reflexes were significantly higher than post-stimulation latencies (6.1 ± 2.2 vs. 5.0 ± 0.9 ms, p<0.05; Table 1), although pre- and post-stimulation amplitudes were similar (12.2 ± 1.4 vs. 12.2 ± 1.3 mV). Motor neuron excitability, as shown by the excitability of motor nerves and peripheral nerve action, was increased by M-waves following magnetic stimulation.
Table 1. The latencies and amplitudes of H wave and M wave during rPMS and in the absence of stimulation.
| H wave | M wave | ||||
|---|---|---|---|---|---|
| Latency (msec) | Amplitude (mV) | Latency (msec) | Amplitude (mV) | ||
| rPMS | Pre | 28.3 ± 3.3 | 35.8 ± 1.3 | 6.1 ± 2.2 | 12.2 ± 1.4 |
| Post | 29.1 ± 1.3 | 35.8 ± 1.1 | 5.0 ± 0.9* | 12.2 ± 1.3 | |
All results are reported as mean ± standard deviation. *p<0.05.
DISCUSSION
This study assessed the effects of rPMS on neuromuscular and spinal reflexes by examining its effects on the soleus muscle. rPMS did not stimulate H wave reflexes. The application of rPMS to a muscle induces proprioceptive inflow to the central nervous system (CNS) via two mechanisms: First, rPMS indirectly activates mechanoreceptors via stimulation-induced rhythmic contractions and relaxations as well as via muscle vibration This includes the depolarization of fiber groups Ia, Ib and II. Second, rPMS directly activates sensorimotor nerve fibers via prodromic and antidromic conduction12). The rPMS protocol probably did not affect spinal excitability but acted on the muscle fibers, which are part of fast twitch units and are primarily responsible for the generation of maximal M waves. rPMS likely modified the integrity of neuromuscular propagation13). A similar type of consecutive magnetic stimulation was found to directly stimulate proprioceptors, a mechanism thought to promote the activation of nerve fibers13). The effectiveness of EMS of the soleus muscle for 10 minutes was assessed by comparing peak magnitudes of the H-and M-waves and the H/M ratio14). The H/M ratio increased due to a significant increase in the amplitude of the H wave and a significant decrease in the amplitude of the M wave14).
Because the amplitude and latency of the H-reflex reflect the activation of sensory nerve synapses of the α motor neurons in the spinal cord, our results suggest that magnetic stimulation had no effect. However, because motor nerves were stimulated together with sensory nerves, the decreased M-wave latencies may have reflected changes in peripheral rather than in α motor nerves. Similar results were observed during magnetic stimulation, suggesting an influence on motor nerves but not on the spinal cord15). Because shortening M-wave latency was significant and did not influence M-wave amplitude, rPMS likely influenced the peripheral muscles and nerves.
This study has some limitations. In this study, we did not have a control group for comparison because it was a repeated measures design. And, since we did not examine stimulus intensity and time, we need to consider this in a future study. In conclusion, this study assessed the effects of rPMS on patients with neuromuscular diseases by examining its effects on M waves and H waves during exercise. Future studies are needed to examine the effects of stimulation intensity and stimulation time.
Conflict of interest
None.
REFERENCES
- 1.Eraifej J, Clark W, France B, et al. : Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: a systematic review and meta-analysis. Syst Rev, 2017, 6: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamaguchi T, Tanabe S, Muraoka Y, et al. : Effects of integrated volitional control electrical stimulation (IVES) on upper extremity function in chronic stroke. Keio J Med, 2011, 60: 90–95. [DOI] [PubMed] [Google Scholar]
- 3.Kasashima Y, Fujiwara T, Muraoka Y, et al. : Newly developed therapy for paretic arm using an integrated volitional control electrical stimulator and a wrist-hand splint. The Japan Association of Rehabilitation Medicine. 2006, 43: 353–357 (In Japanese). [Google Scholar]
- 4.Yan T, Hui-Chan CW, Li LS: Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke: a randomized placebo-controlled trial. Stroke, 2005, 36: 80–85. [DOI] [PubMed] [Google Scholar]
- 5.Embrey DG, Holtz SL, Alon G, et al. : Functional electrical stimulation to dorsiflexors and plantar flexors during gait to improve walking in adults with chronic hemiplegia. Arch Phys Med Rehabil, 2010, 91: 687–696. [DOI] [PubMed] [Google Scholar]
- 6.Powell J, Pandyan AD, Granat M, et al. : Electrical stimulation of wrist extensors in poststroke hemiplegia. Stroke, 1999, 30: 1384–1389. [DOI] [PubMed] [Google Scholar]
- 7.Adriaensen H, Gybels J, Handwerker HO, et al. : Response properties of thin myelinated (A-delta) fibers in human skin nerves. J Neurophysiol, 1983, 49: 111–122. [DOI] [PubMed] [Google Scholar]
- 8.Izumi S, Yashima K: Manufacture of the peripheral nerve continuation pulse electromagnetic stimulation. BIO Clin, 2015, 30: 45–49(In Japanese). [Google Scholar]
- 9.Yashima K, Takagi T, Izumi S, et al. : Dorsiflexion movement of the wrist by magnetic stimulation. J Biomech, 2016, 40: 1–7(In Japanese). [Google Scholar]
- 10.Machetanz J, Bischoff C, Pichlmeier R, et al. : Magnetically induced muscle contraction is caused by motor nerve stimulation and not by direct muscle activation. Muscle Nerve, 1994, 17: 1170–1175. [DOI] [PubMed] [Google Scholar]
- 11.Barker AT: The history and basic principles of magnetic nerve stimulation. Electroencephalogr Clin Neurophysiol Suppl, 1999, 51: 3–21. [PubMed] [Google Scholar]
- 12.Struppler A, Angerer B, Gündisch C, et al. : Modulatory effect of repetitive peripheral magnetic stimulation on skeletal muscle tone in healthy subjects: stabilization of the elbow joint. Exp Brain Res, 2004, 157: 59–66. [DOI] [PubMed] [Google Scholar]
- 13.Behrens M, Mau-Möller A, Zschorlich V, et al. : Repetitive peripheral magnetic stimulation (15 Hz RPMS) of the human soleus muscle did not affect spinal excitability. J Sports Sci Med, 2011, 10: 39–44. [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki K, Hiraoka T, Tsubahara A, et al. : Considerations for safety of high-frequency repetitive peripheral magnetic stimulation of skeletal muscles in rats: assessment by histological analysis of muscles and biochemical blood tests. Jpn J Compr Rehabil Sci, 2015, 6: 56–63. [Google Scholar]
- 15.Tanino Y, Daikuya S, Nishimori T, et al. : M wave and H-reflex of soleus muscle before and after electrical muscle stimulation in healthy subjects. Electromyogr Clin Neurophysiol, 2003, 43: 381–384. [PubMed] [Google Scholar]