Abstract
Human female reproductive tract development rests mostly upon hematoxilyn and eosin stained sections despite recent advances on molecular mechanisms in mouse studies. We report application of immunohistochemical methods to explore the ontogeny of epithelial and mesenchymal differentiation markers (keratins, homobox proteins, steroid receptors), transcription factors and signaling molecules (TP63 and RUNX1) during human female reproductive tract development. Keratins 6, 7, 8, 10, 14 and 19 (KRT6, KRT7, KRT8, KRT10, KRT14, KRT19) were expressed in a temporally and spatially dynamic fashion. The undifferentiated Müllerian duct and uterovaginal canal, lined by simple columnar epithelia, expressed KRT7, KRT8 and KRT19. Glandular derivatives of the Müllerian duct (uterine tube, uterine corpus and endocervix) maintained expression of these keratins, while tissues that undergo stratified squamous differentiation (exocervix and vagina) expressed KRT6, KRT14 and KRT10 during development in an age-dependent fashion. TP63 and RUNX1 were expressed prior to KRT14, as these two transcription factors are known to be upstream from KRT14 in developing Müllerian epithelium. In the vagina, KRT10, a marker of terminal differentiation, appeared after endogenous estrogens transformed the epithelium to a thick glycogenated squamous epithelium. Uroplakin, a protein unique to urothelium, was expressed only in the bladder, urethra and vaginal introitus, but not in the female reproductive tract itself. Mesenchymal differentiation was examined through immunostaining for HOXA11 (expressed in uterine mesenchyme) and ISL1 (expressed in vaginal mesenchyme). A detailed ontogeny of estrogen receptor alpha (ESR1), progesterone receptor (PGR) and the androgen receptor (AR) provides the mechanistic underpinning for the teratogenicity of estrogens, progestins and androgens on female reproductive tract development. Immunohistochemical analysis of differentiation markers and signaling molecules advance our understanding of normal development of the human female reproductive tract. These observations demonstrate remarkable similarities in mouse and human female reproductive tract development, but also highlight some key differences.
Keywords: Müllerian duct, Uterovaginal canal, Uterus, Cervix, Vagina, Keratins, Estrogen receptor
1. Introduction
Parenchyma of the female reproductive tract develops from epithelia of the Müllerian duct and urogenital sinus (Orvis and Behringer, 2007; Guioli et al., 2007; Koff, 1933; Kurita and Nakamura, 2008; Bulmer, 1957; Robboy et al., 2017; Robboy and Mutter, 2014; Mutter and Robboy, 2014). The bilateral Müllerian ducts develop as invaginations of coelomic epithelium forming ductal structures that grow caudally down the urogenital ridges to join the endodermal urogenital sinus (UGS) (Kobayashi and Behringer, 2003; Jaubert et al., 2009; Robboy et al., 2017). As the right and left Müllerian ducts approach the UGS, they fuse in the midline to form the uterovaginal canal. The degree of midline fusion of the Müllerian ducts varies with the species. In rats and mice only the caudal portions of the Müllerian ducts fuse in the midline to form the cervix and the Müllerian vagina. Unfused Müllerian ducts in rats and mice form bilateral oviducts, bilateral uterine horns and cervical canals. In humans, the Müllerian ducts undergo extensive fusion to form the midline uterovaginal canal destined to form the uterine fundus, uterine corpus, uterine cervix and vagina (Kobayashi and Behringer, 2003; Koff, 1933; Kurita and Nakamura, 2008; Robboy et al., 2017; Moore and Persaud, 2003). Relatively short bilateral uterine tubes are all that remain of the individual right and left human Müllerian ducts.
The process of vaginal development in mice differs somewhat from that in humans. At birth the so-called Müllerian vagina (fused Müllerian ducts) is in contact with a dorsal projection of the UGS, the called the sinus vagina (Kurita, 2011). The sinus vagina is a transient epithelial structure that maintains a connection with the Müllerian vaginal epithelium throughout development. Cell lineage tracing studies carried out in mice (Kurita, 2010) have shown that as organogenesis progresses, the sinus vagina is gradually reduced in size relative to the Müllerian vagina. Indeed, at puberty when the vagina opens to the exterior, urogenital sinus epithelium (UGE) was detected only in the vulva, and not in the vagina. Thus, in mice adult vaginal epithelium is derived solely from Müllerian duct epithelium (MDE) (Kurita, 2010).
The contribution of Müllerian versus urogenital sinus epithelium (UGE) to human vaginal epithelium has been debated for over half a century. The two most popular theories of derivation of human vaginal epithelium are based upon the analysis of H& E-stained histological sections (Bulmer, 1957; Koff, 1933). Koff concluded that epithelium of the upper 4/5ths of the vagina is lined with Müllerian-derived epithelium, while the lower 1/5th of the vagina is derived from UGE (Koff, 1933). Bulmer asserts that UGE replaces Müllerian epithelium (MDE) of the lower uterovaginal canal, and thus concluded that human vaginal epithelium is exclusively derived from UGE (Bulmer, 1957). Using immunohistochemical markers of Müllerian epithelium (PAX2) and urogenital sinus epithelium (FOXA1), we have shown that during the course of human vaginal development the PAX2/FOXA1 boundary within the vaginal rudiment progressively extends cranially so that at 21 weeks of gestation epithelial FOXA1 staining was observed from the vaginal introitus to the cervix. Thus, our immunohistochemical studies reported in a companion article (Robboy et al., 2017) support Bulmer’s proposal (1957) that human vaginal epithelium derives solely from UGE.
The cellular and molecular mechanisms of epithelial differentiation in Müllerian duct-derived organs have been extensively studied utilizing animal models. In mouse, the epithelium of the midline fused Müllerian ducts (the anlagen of the Müllerian vagina) is undifferentiated and simple columnar at 16.5 days of gestation, whereas the sinus vagina consists of a solid dorsal epithelial cord expressing TRP63 (specifically the ΔN isoform) and keratin 14 (KRT14), markers of squamous epithelium (Kurita and Cunha, 2001; Kurita et al., 2005, 2004). In the course of development the epithelium of the Müllerian vagina expresses ΔNp63 followed temporally by keratin 14 during conversion of the simple columnar epithelium of the Müllerian vagina into stratified squamous epithelium. This process progresses from caudal to cranial within the Müllerian vagina, and is completed around postnatal day 7 (Kurita and Cunha, 2001; Kurita et al., 2005, 2004). The potential importance of ΔNp63 in human vaginal development comes from immunohistochemical studies demonstrating expression of TP63 during human vaginal development (Kurita et al., 2005; Fritsch et al., 2012, 2013).
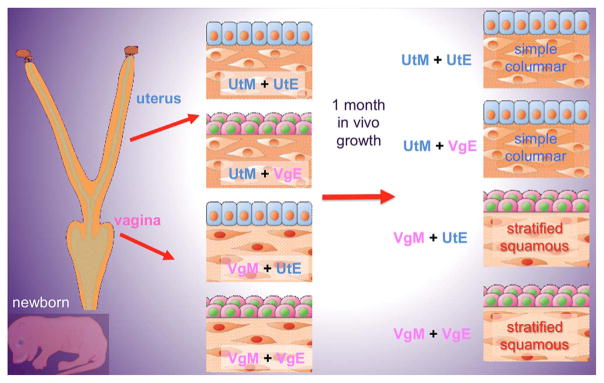
Epithelial differentiation in the cervix/vagina versus that of the uterus is induced and specified by the associated mesenchyme. This conclusion is based upon heterotypic tissue recombinants of epithelium and mesenchyme derived from the neonatal mouse uterus and vagina (Cunha, 1976). Homotypic vaginal (VgM + VgE) and uterine (UtM + UtE) tissue recombinants express vaginal or uterine epithelial differentiation as expected (Fig. 1). In heterotypic tissue recombinants vaginal mesenchyme induces uterine epithelium to undergo vaginal epithelial differentiation (VgM + UtE⇨vaginal differentiation), and uterine mesenchyme induces vaginal epithelium to undergo uterine epithelial differentiation (UtM + VgE⇨uterine differentiation). These remarkable mesenchyme-induced transformations in epithelial differentiation involve morphological as well as molecular changes in epithelial differentiation (Table 1). For example, induction of vaginal epithelial differentiation in VgM + UtE tissue recombinants involves epithelial induction of ΔNp63 and KRT14 in uterine epithelium (Kurita et al., 2001, 2004).
Fig. 1.
Schematic of tissue recombinants between epithelium and mesenchyme of the neonatal mouse uterus and vagina. UtM = uterine mesenchyme, VgM = vaginal mesenchyme, UtE = uterine epithelium. VgE = vaginal epithelium.
Table 1.
Epithelial differentiation markers in vagina and uterus and in heterotypic vaginal/uterine tissue recombinants prepared with mouse tissues.
| Vagina | VgM + UtE recombinants | Uterus | UtM + VgE recombinants | Reference |
|---|---|---|---|---|
| Stratified squamous | Stratified squamous | Simple columnar | Simple columnar | (Cunha, 1976) |
| Stratified mucifieda | Stratified mucifieda | N/A | N/A | (Cunha, 1976) |
| Trp63(+) | Trp63(+) | Trp63(−) | Trp63(−) | (Kurita and Cunha, 2001; Kurita et al., 2004) |
| Keratin 14(+) | Keratin 14(+) | Keratin 8/18 | Keratin 8/18 | (Kurita and Cunha, 2001) |
| Keratin 10(+)b | Keratin 10(+)b | Keratin 10(−) | Keratin 10(−) | (Buchanan et al., 1998) |
| Progesterone receptor negative following ovex. | Progesterone receptor negative following ovex. | Progesterone receptor positive following ovex. | Progesterone receptor positive following ovex. | (Kurita et al., 2001) |
| Progesterone receptor positive following ovex + E2 | Progesterone receptor positive following ovex + E2 | Progesterone receptor negative following ovex + E2 | Progesterone receptor negative following ovex + E2 | (Kurita et al., 2001) |
| Low molecular weight syndecan | Low molecular weight syndecan | High molecular weight syndecan | High molecular weight syndecan | (Boutin et al., 1991) |
Abbreviations: E2 = estradiol, ovex. = ovariectomy.
following a combination of estradiol plus progesterone.
following short-term estrogen treatment.
Studies from Kurita’s lab have documented the molecular mechanisms underlying mesenchymal induction of vaginal epithelial differentiation. Mouse genetic studies established that ΔNp63 is the determining transcription factor of cervicovaginal epithelial cell fate. Thus, in Trp63 null mice, the epithelium of the Müllerian vagina remains simple columnar epithelium and expresses uterine instead of cervicovaginal markers (Kurita et al., 2004; Laronda et al., 2013). During normal development vaginal mesenchyme induces ΔNp63 expression in MDE and subsequent vaginal epithelial differentiation by activating 3 independent and essential signaling pathways present in MDE: (a) BMP4-SMAD, (b) activin A-RUNX1 (runt-related transcription factor 1) and (c) FGF7/10-MAPK pathways (Kurita et al., 2004; Laronda et al., 2013; Terakawa et al., 2016). When BMP4-SMAD, activin A, RUNX1 or FGF7/10-MAPK pathways were disrupted in Müllerian epithelium by conditional deletion of Smad4, Runx1, or Fgfr2, epithelial cells of the cervix and vagina failed to express ΔNp63, remained simple columnar and expressed uterine markers.
In adulthood, 17β-estradiol and progesterone elicit transient effects in organs of the female reproductive tract (vagina, cervix, uterus, and uterine tube) contingent upon the continued presence of the stimulatory hormone(s). While the responsiveness of female reproductive organs to ovarian steroids is fundamental to female reproductive function, organogenesis of female reproductive tracts occurs independent of estrogen and progesterone receptors based upon examination of mutant mice null for estrogen receptor alpha (ESR1), estrogen receptor beta (ESR2) or progesterone receptor (PGR) (Lubahn et al., 1993; Lydon et al., 1995; Krege et al., 1998). In both human and mouse female reproductive organs, estrogen receptor α/ESR1 is the dominant receptor for estrogen (Matsuzaki et al., 1999; Dupont et al., 2000). The ontogeny of ESR1 has been described in considerable detail in laboratory animals in both the developing uterus and vagina (Greco et al., 1991; Nielsen et al., 2000; Lemmen et al., 1999; Kurita et al., 2001; Korach et al., 1988; Yamashita et al., 1990; Bigsby et al., 1990). ESR1 is initially expressed in mesenchyme of the embryonic Müllerian duct in mice followed postnatally by its expression in the epithelium. While a comprehensive ontogeny of ESR1 has not been described for the human fetal human female reproductive tract, estrogen receptors were first described in steroid autoradiographic studies within vaginal mesenchymal cells (Taguchi et al., 1986). Epithelial estrogen receptors were observed only in specimens whose vaginal development and differentiation was advanced. Thus, mesenchyme appears to be the initial estrogen target tissue within the developing human vagina and may play a fundamental role in estrogen-induced teratogenesis of the human genital tract. These findings have been confirmed recently in a report of ESR1 immunostaining in mesenchyme (but not the epithelium) of the human fetal uterus (Glatstein and Yeh, 1995). In the neonatal mouse uterus, the progesterone receptor (PGR) is initially expressed in the epithelium followed subsequently in uterine mesenchyme (Kurita et al., 2000b; Kurita and Nakamura, 2008). The ontogeny of PGR has not been described in the developing human female reproductive tract. Permanent deleterious effects of exogenous estrogen and progestin on the developing female mouse reproductive tract are mediated via ESR1 and presumably PGR (Bern and Talamantes, 1981; Cooke et al., 2012, 2013; Filant et al., 2012; Couse et al., 2001; Couse and Korach, 2004). Given the reproductive tract malformations elicited by in utero exposure to diethylstilbestrol (DES) in human females (Jefferies et al., 1984; Titus-Ernstoff et al., 2010; Kaufman et al., 1980; Kaufman and Adam, 2002; Robboy et al., 1984), the ontogeny of steroid receptors (especially estrogen receptors) in the human fetal female reproductive tract is of paramount importance.
While a great deal is known about the morphogenesis, differentiation and the molecular biology of the developing female mouse reproductive tract, human female reproductive tract development is poorly understood and is based primarily upon classical morphological studies (Koff, 1933; Bloomfield, 1927; Bulmer, 1959a, b; Müller, 1830; Mijsberg, 1924) with little known about human molecular pathways and whether similar molecular pathways are present during mouse versus human female reproductive tract development. Using immunohistochemistry, we report in this paper a remarkable similarity in the molecular pathways during development of the female reproductive tracts of humans and mice as well as some notable differences.
2. Materials and methods
Forty-eight first and second trimester human fetal specimens were collected devoid of patient identifiers after elective termination of pregnancy approved by the Committee on Human Research at UCSF, IRB# 12–08813. Gestational age could only be estimated using heel-toe length (Drey et al., 2005). Gender was determined in most specimens by virtue of Wolffian and Müllerian duct morphology, but for the youngest specimens sex was determined by PCR of X and Y-chromosomal sequences as previously described (Li et al., 2014). Internal genitalia were identified using a dissecting microscope. Human female reproductive tract specimens were serially sectioned, and every 20th section was stained with hematoxylin and eosin (H& E). Paraffin sections were immunostained with antibodies to a variety of proteins as described previously (Rodriguez et al., 2012) (Table 2). Immunostaining was detected using horseradish peroxidase based Vectastain kits (Vector Laboratories, Burlingame, CA). Negative controls were based upon deletion of the primary antibody. This study is based upon analysis of 48 human fetal specimens 8–21 weeks of gestation.
Table 2.
Antibodies used in this study.
| Antibody | Source | Catalogue # | Concentration | Reacts with: |
|---|---|---|---|---|
| α-actin | Sigma | A2547 | 1/2000 | Smooth muscle |
| Estrogen receptor alpha (ESR1) | Abcam | Ab16660 | 1/100 | Estrogen receptor |
| HOXA11 | Sigma | HPA035623 | 1/400 | Uterine mesenchyme |
| ISL1 | Abcam | Ab20670 | 1/200 | Vaginal mesenchyme |
| Keratin 6 | Acris Antibodies | AM21068PU-S | 1/200 | Basal cells of stratified epithelia |
| Keratin 7 | E.B. Lanea | LP1K | 1/10 | Simple columnar (glandular) epithelia |
| Keratin 8 | E.B. Lanea | LE41 | 1/10 | Simple columnar (glandular) epithelia |
| Keratin 10 | Dako | M7002 | 1/50 | Terminally differentiated squamous cells |
| Keratin 14 | BioGenex | LL002 | 1/100 | Basal cells of stratified epithelia |
| Keratin 19 | E.B. Lanea | LP2K | 1/10 | Simple columnar (glandular) epithelia |
| TP63 | Santa Cruz Biotechnology | sc-8343 | 1/100 | Transcription factor |
| Progesterone receptor | Abcam | Ab16661 | 1/100 | Progesterone receptor |
| Androgen receptor | Genetex | GTX62599 | 1/100 | Androgen receptor |
| Runx1-3 | Abcam | Ab92336 | 1/100 | Transcription factor |
| Uroplakin1 | T. T. Sunb | 1/100 | Urothelium |
Institute of Medical Biology, Singapore.
New York University, New York.
Given the disrupted state of abortus specimens, gestational age can only be estimated by heel-toe length. Since foot size is variable from individual to individual, estimates of gestational age assessed by heeltoe length will necessarily be subject to some variability. In the final analysis, the basis of embryonic and fetal age determination is patient interview, which is of questionable value particularly for fetal specimens. As stated in the accompanying paper (Robboy et al., 2017), ages of embryonic and fetal human specimens should not be taken literally as they are estimates of variable accuracy. This caveat should be kept in mind when reading the results. Another caveat regarding the analysis of fetal specimens is the ambiguity of boundaries between the vagina, uterine cervix and uterine corpus, which cannot be discerned anatomically in most specimens between 8 to 16 weeks of gestation, as reported previously (Fritsch et al., 2012; Fritsch et al., 2013; Robboy et al., 2017). The boundary between the cervix and vagina can only be discerned with certainty when the vaginal fornices become evident at ~18 weeks. For these reasons, the expression patterns of proteins are sometimes described by their relative position within the female reproductive tract rather than by the organ.
3. Results
3.1. Immunohistochemistry of cytokeratins (Table 3)
Table 3.
Differentiation markers in the human female fetal reproductive tract at 14–21 weeks of gestation.
| Uterine tube | Uterine corpus | Uterine cervix | Vagina | Bladder | Urethra | Introitus | |
|---|---|---|---|---|---|---|---|
| Epithelium | |||||||
| ESR1 | + + | −b | − | + | − | − | − |
| PR | − | −c | − | −, +g | − | − | − |
| AR | + | − | − | − | − | + | ND |
| KRT6 | − | − | − & +i | + + | + + | + + | + + |
| KRT7 | + + | + + | + + | − | + | + | − |
| KRT8 | + + | + + | + + | + a | + + | + + | − |
| KRT14 | − | − | −, −/+e | + + | + + | + + | + + |
| KRT10 | − | − | − | +h | − | − | + + |
| KRT19 | + | + | + + | + + & − | + + | + + | + + |
| TP63 | − | −d | + + | + + | + + | + + | + + |
| RUNX1 | − | + + | + + | + | + | + | − |
| Uroplakin | − | − | − | − | + | + | + |
| Mesenchyme | |||||||
| ESR1 | + | + | + | + | − | − | − |
| PR | − | − | − | + & − | − | − | − |
| AR | − | − | − | + | − | + | ND |
| α-actin | + | + + | + + | + | + + | + + | − |
| ISL1 | − | − | + | + + | − | − | ND |
| HOXA11 | − | + + | +/−f | − | − | − | − |
| Runx1 | − | − | −/+ | + | − | − | − |
apical epithelial cells only;
rare ESR1 patches in older specimens;
rare PGR patches in older specimens;
uterine fundus = negative;
exocervix = (−/+), endocervix = (−);
upper cervix, (positive), lower cervix (negative);
PR present at 18–21 weeks;
KRT10 only at 21 weeks;
KRT6 negative versus positive dependent on region; ND = not done.
3.1.1. General
Most of the female reproductive tract is derived from the simple columnar epithelium of the Müllerian ducts. This simple columnar phenotype is maintained into adulthood in the uterine tube, uterine corpus and endocervix, while stratified squamous differentiation occurs in the exocervix and vagina. Simple columnar epithelia are known to express certain cytokeratins (for example, KRT7, KRT8, KRT18, KRT19), while stratified epithelia are known to express a different set of cytokeratins (for example, KRT6, KRT14, KRT10) (Moll et al., 1982, 2008). Given the expected shift from the simple columnar to stratified phenotype in the developing exocervix and vagina, we have examined a spectrum of cytokeratins to assess changes in these proteins that are integral to epithelial differentiation, namely KRT7, KRT8, and KRT19 as representative of simple columnar epithelia, and KRT6, KRT14 and KRT10 as representative of stratified epithelia.
3.1.2. Cytokeratins of simple columnar epithelia
The female reproductive tract derives in large part from the simple columnar epithelium of the fused Müllerian ducts (uterovaginal canal) that give rise to the uterine tubes, uterine corpus and endocervix (all retaining simple columnar epithelia into adulthood). The simple columnar epithelium of the caudal aspect of the uterovaginal canal “becomes converted into a ribbon-like solid plate by stratification and fusion of the original cells lining the lumen” (Koff, 1933). According to Koff (1933) UGE cells “fuse insensibly to form the primitive vaginal plate” (Koff, 1933; Robboy et al., 2017; Bulmer, 1959a). The relative contribution of MDE versus UGE to the solid vaginal plate changes dramatically with development (Koff, 1933; Robboy et al., 2017; Bulmer, 1957). At 12 weeks most of the vaginal plate is composed of PAX2-reactive MDE, but by 21 weeks the epithelium of the entire vagina and exocervix expresses FOXA1, a marker of endodermal UGE (Robboy et al., 2017). Thus, MDE in the cranial segments of the female reproductive tract (uterine tube, uterine corpus and endocervix) retains its original simple columnar phenotype, while caudal segments of the female reproductive tract (exocervix and vagina) undergo stratified squamous differentiation. To generate a baseline of epithelial differentiation in the developing human female reproductive tract, we explored the ontogenic changes in cytokeratin expression.
3.1.2.1. Keratins 7 and 8
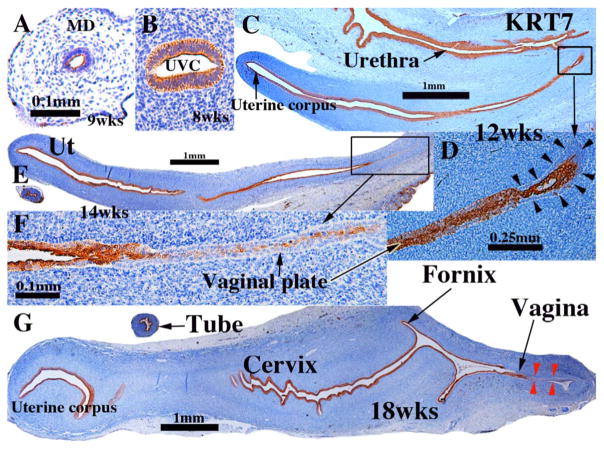
Keratin 7 (KRT7) was detected from 8 and 12 weeks of gestation in simple columnar epithelia of the undifferentiated Müllerian and Wolffian ducts (not illustrated) and uterovaginal canal (Fig. 2A & B). In stages in which a solid vaginal plate was present, KRT7 was expressed in centrally situated epithelial cells (but not in basal cells) in the cranial portion of the solid vaginal plate (Fig. 2C–F), and less so or not at all in the caudal portion of the solid vaginal plate (Fig. 2C–F) (Table 3). From 12–21 weeks KRT7 was expressed in simple columnar epithelia of the developing uterine tube (Fig. 2E & G), uterine corpus (Fig. 2C, E & G) and endocervix (Fig. 2G). KRT 7 was also expressed in apical epithelial cell layers of certain stratified epithelia, namely the bladder (not illustrated), urethra (Fig. 2C), and vagina (Fig. 2G). At 18 weeks of gestation KRT7 was expressed only in epithelium in the caudal aspect of the vagina (Fig. 2G, red arrowheads). At 21 weeks when the vaginal epithelium is many layers thick and stratified squamous, KRT7 was not expressed in vaginal epithelium (not illustrated). Expression of KRT8 was virtually identical to that of KRT7 (not illustrated). KRT18, which normally pairs with KRT8, was not examined.
Fig. 2.
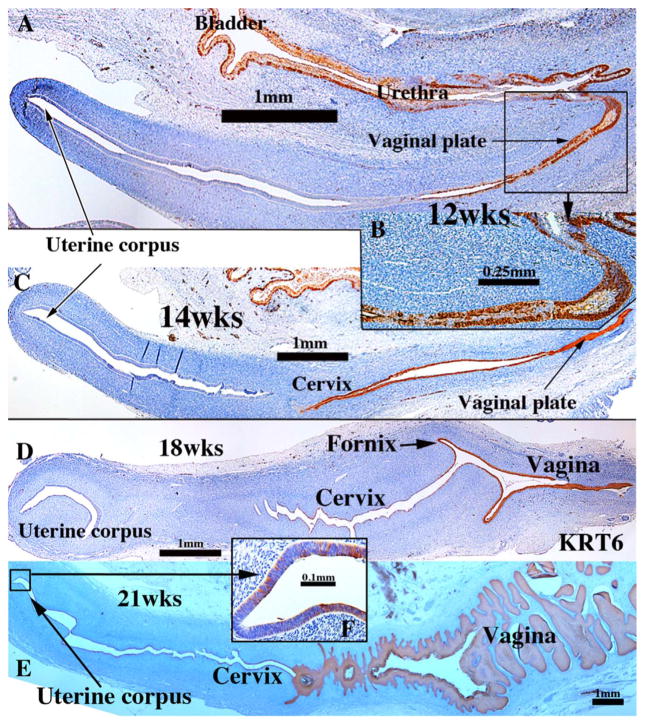
Keratin 7 immunohistochemistry of human fetal female reproductive tract development. (A & B) Müllerian duct and uterovaginal canal in 8 to 9-week human fetuses as indicated. (C & D) Sagittal sections of the human fetal female reproductive tract at 12 weeks of gestation. Note absence of staining of basal epithelial cells (arrowheads in D) at the caudal end of the vaginal plate. (E & F) Sagittal sections of the human fetal female reproductive tract at 14 weeks of gestation. Note continuous KRT7 staining from the uterine corpus to the solid vaginal plate, which shows reduced staining (F). (G) Sagittal section of the human fetal female reproductive tract at 18 weeks of gestation. KRT7 is expressed strongly in epithelium throughout the female reproductive tract except for the caudal portion of the vagina (red arrowheads).
3.1.2.2. Keratin 19
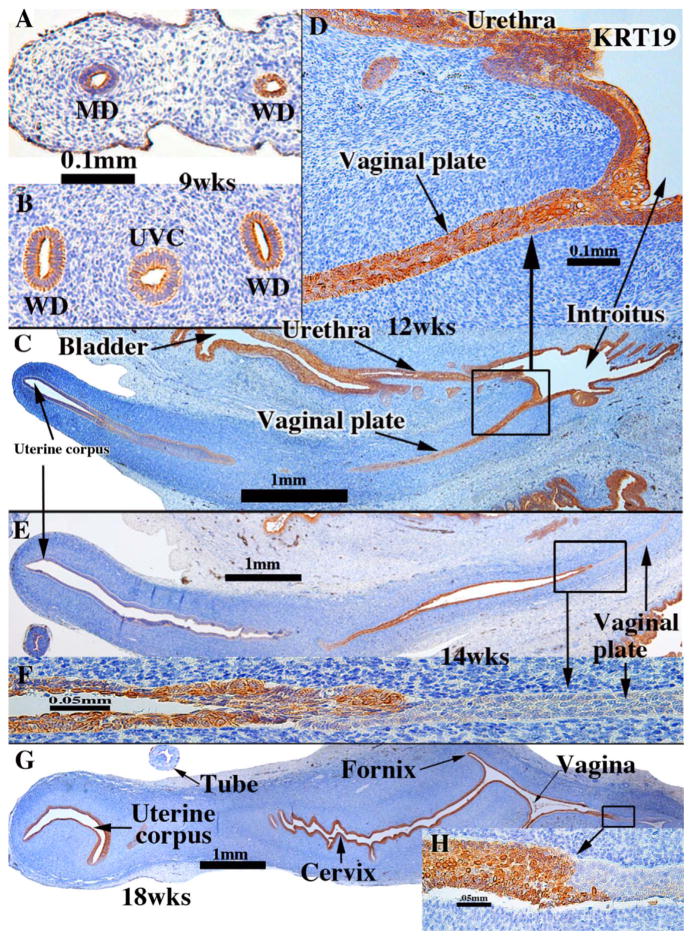
KRT19 was detected at 9 weeks of gestation in simple columnar epithelia of the undifferentiated Müllerian and Wolffian ducts and uterovaginal canal (Fig. 3A & B) (Table 3). At 12–14 weeks KRT19 was weakly expressed (if at all) in epithelium of the uterine corpus (Fig. 3C, E). At 18 weeks (Fig. 3G) KRT19 was prominently expressed from the uterine corpus to the vagina (Fig. 3G) with the exception of the caudal vaginal epithelium (Fig. 3G–H).
Fig. 3.
Keratin 19 immunohistochemistry of human fetal female reproductive tract development. (A & B) Müllerian duct (A) and uterovaginal canal (UVC) in a 9-week human fetus. (C & D) Sagittal Section of the human fetal female reproductive tract at 12 weeks of gestation. KRT19 is expressed in epithelia of the bladder, urethra and most of the female reproductive tract, including the junction of the vaginal plate and the urethra (D). (E & F) Sagittal sections of the human fetal female reproductive tract at 14 weeks of gestation. Note absence of KRT19 staining in the vaginal plate (F). (G & H) Sagittal sections of the human fetal female reproductive tract at 18 weeks of gestation. Strong KRT19 staining is observed in the cervical region, and now the vaginal plate exhibits weak to moderate KRT19 staining. (H) KRT19 staining is seen in the vagina, even though the most caudal vaginal epithelium is KRT19-negative.
The ontogenic profile of keratin expression in the developing vaginal plate appears to be quite dynamic from 12 to 21 weeks and differs amongst KRT7, KRT8 and KRT19. At 12 weeks of gestation KRT19 expression in the vaginal plate merged uninterrupted with KRT19 expression in epithelium of the urethra and introitus (Fig. 3C& D), which differs from the pattern of KRT7 and KRT8. At this stage the caudal most portion of the vaginal plate was KRT7- and KRT8-negative (Fig. 2C & D). Referring to Fig. 3E & F (14 week specimen), note that epithelia of the uterine corpus and vaginal plate are negative for KRT19, while the intervening segment is strongly KRT19-positive.
At 18 weeks, prior to proliferation and differentiation of a “mature” stratified squamous vaginal epithelium (see Fig. 13 for an example of squamous differentiation of vaginal epithelium), KRT19 was detected in the cervical, fornical and most of the cranial vaginal epithelia, but was absent from caudal vaginal epithelium (Fig. 3G & H). Epithelium of the uterine corpus was strongly KRT19-positive at 18 weeks (Fig. 3G) (Table 3). At 21 weeks, when endogenous estrogens had elicited formation of a thick mature vaginal epithelium (see figure 20 in Robboy et al., 2017 and Fig. 13, current paper), KRT19 was expressed in epithelia of the entire female reproductive tract (uterine corpus to vagina) (not illustrated).
Fig. 13.
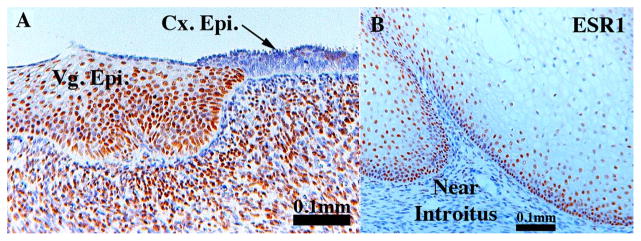
Sections of vaginal epithelium immunostained for ESR1. Section (A) is at the junction of vaginal/cervical border. Note ESR1 expression throughout the vaginal epithelium and the high percentage of ESR1-positive mesenchymal cells. Section (B) is vaginal epithelium near the introitus stained for ESR1. Note the paucity of ESR1-positive mesenchymal cells. Vg. Epi. = vaginal epithelium, Cx. Epi. = cervical epithelium.
3.2. Cytokeratins of stratified epithelia
3.2.1. Keratin 6
Keratin 6 (KRT6) is typically expressed in basal cells of stratified epithelia. Accordingly, KRT6 was detected in stratified epithelium of the UGS, but not in the simple columnar epithelium of the Müllerian and Wolffian ducts and uterovaginal canal in 8 to 9 week specimens (not illustrated). When the solid vaginal plate formed in 11 to 12 week specimens, KRT6 was detected in basal and suprabasal epithelial cells of the solid vaginal plate (Fig. 4A & B). Additionally, KRT6 was expressed in basal cells of urethral epithelium and in a subset of basal cells of the urinary bladder (Fig. 4A), but not in simple columnar epithelia of the uterine tube (not illustrated), uterine corpus and the presumed endocervix (Fig. 4A, C, D). At 14 weeks KRT6 was expressed in epithelium of the solid vaginal plate and in epithelium undergoing stratification in the “cervico-vaginal” region (Fig. 4C), and at 18 weeks KRT6 was detected in epithelium of the vagina, the fornices and the cervical canal (Fig. 4D), but not in the uterine corpus (Fig. 4D) or uterine tube (not illustrated) (Table 3). Finally, at 21 weeks KRT6 was prominently expressed in the thick mature vaginal epithelium (Fig. 4E) and in patchy fashion in the endocervix and uterine corpus (Fig. 4F).
Fig. 4.
Keratin 6 immunohistochemistry of human fetal female reproductive tract development. (A & B) Sagittal sections of the human fetal female reproductive tract at 12 weeks of gestation. Note KRT6 expression in epithelia of the bladder, urethra and basal cells of the vaginal plate. (C) Sagittal section of the human fetal female reproductive tract at 14 weeks of gestation. KRT6 is expressed in stratified epithelia of the cervix, vagina and vaginal plate. (D) Sagittal section of the human fetal female reproductive tract at 18 weeks of gestation. KRT6 is seen in epithelia of the cervical canal, fornix and vagina, but not in the uterine corpus. (E) Sagittal section of the human fetal female reproductive tract at 21 weeks of gestation. (G) Note prominent KRT6 expression in vaginal epithelium and patchy KRT6 expression in the uterine corpus (F).
3.2.2. Keratin 14
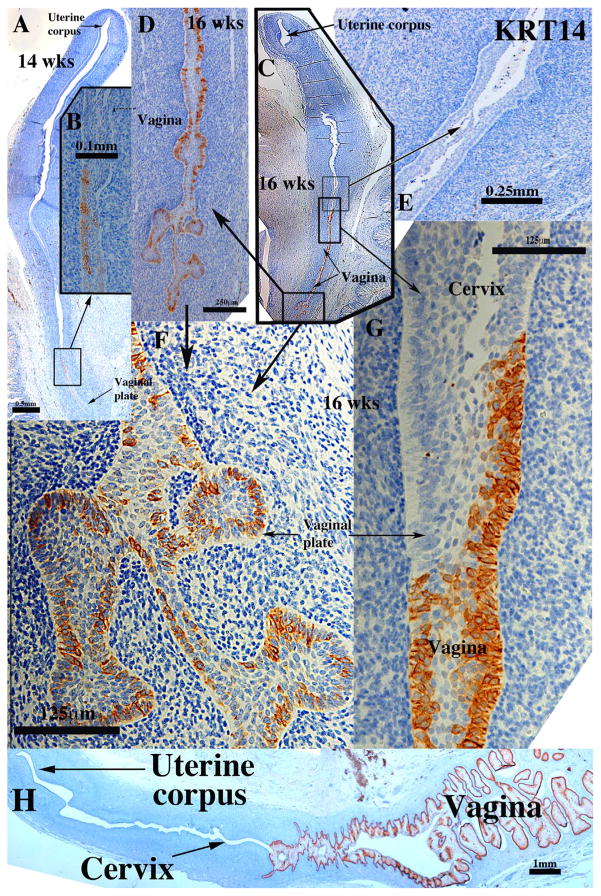
Keratin 14 (KRT14), a marker of basal cells of stratified epithelia, had an ontogenic expression similar to that of KRT6 but the timing and location of KRT14 differed substantially from that of KRT6. As expected KRT14 was not detected in the simple columnar epithelia of the uterine corpus, endocervix and uterine tube at all stages examined (Fig. 5A, C & E). KRT14 was first detected in patches of basal epithelial cells within the vaginal plate at 14 weeks (Fig. 5A–B) becoming more prominent at 16 weeks of gestation (Fig. 5C–G). At the presumed vaginal-exocervical junction KRT14 was detected in vaginal epithelium, but not in the stratified epithelium of the presumed exocervix (Fig. 5C, E & G) (Table 3). At 18 weeks, prior to proliferation and differentiation of a “mature” stratified squamous vaginal epithelium, KRT14 was only detected in basal epithelial cells of the vagina and exocervix (not illustrated). At 21 weeks, when endogenous estrogens had elicited formation of a thick mature vaginal epithelium, KRT14 was prominently expressed in basal cells and adjacent suprabasal cells of the multilayered vaginal epithelium (Fig. 5H). Epithelial cells of the endocervix and uterine corpus were generally devoid of KRT14 staining with the exception of extremely rare KRT14-positive epithelial cells (not illustrated).
Fig. 5.
Keratin 14 immunohistochemistry of human fetal female reproductive tracts in sagittal section. (A & B) Sagittal sections of the human fetal female reproductive tract at 14 weeks of gestation. KRT14 is only seen in patches within the vaginal plate (B). (C–G) Sagittal sections of the human fetal female reproductive tract at 16 weeks of gestation. KRT14 is more strongly expressed in the vaginal plate (C, D, F & G), but not in the presumed cervical epithelium (C, E, G). (H) Sagittal Sections of the human fetal female reproductive tract at 21 weeks of gestation. KRT14 is expressed in basal and suprabasal cells of the vagina.
3.2.3. Keratin 10
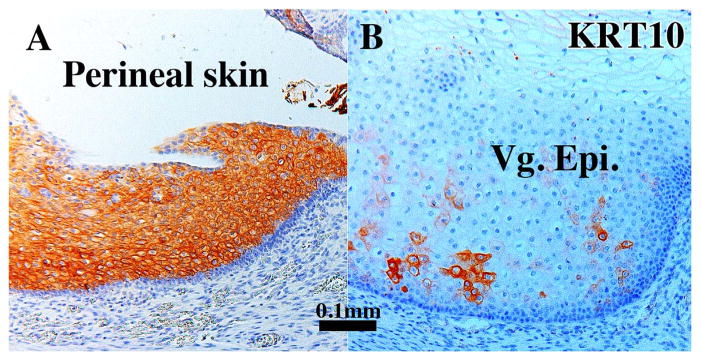
Keratin 10 (KRT10), a terminal differentiation marker of epidermis, was strongly expressed in suprabasal cells of perineal epidermis (Fig. 6A), but not in internal organs of the female reproductive tract with the exception of mature vaginal epithelium. At 21 weeks when the vaginal epithelium had matured into a many-layered stratified squamous epithelium, KRT10 was expressed in patchy fashion within the stratified epithelium (Fig. 6B) (Table 3).
Fig. 6.
Sections immunostained for KRT10 Perineal skin (A) and vaginal epithelium of a 21-week human fetal female reproductive tract (B). In Perineal skin near the introitus KRT10 is expressed in suprabasal epithelial cells (A). Within the vagina (B), whose epithelium is many cell layers thick, KRT10 is expressed in patches.
In summary, the 3 keratins associated with stratified epithelia each had a unique pattern and ontogeny of expression. KRT6 was broadly expressed in the full thickness of epithelia of the exocervix, vagina, and solid vaginal plate over a broad developmental period. KRT10 was expressed in suprabasal cells of perineal epidermis and in a patchy fashion in “mature” vaginal epithelium at 21 weeks, but not in other female reproductive organs. KRT14 was expressed in basal epithelial cells of the vagina and vaginal plate, and in basal cells of “mature” vaginal epithelium at 21 weeks.
3.3. Immunohistochemistry of uroplakin
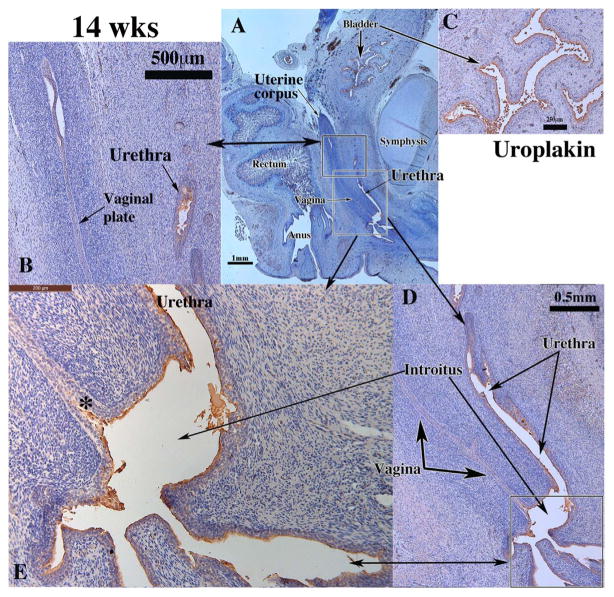
As the female reproductive tract is related anatomically with the urethra, with both the vagina and urethra opening into the vaginal introitus, we explored the expression of uroplakin in this region with special emphasis on the vaginal introitus, a region believed to be of UGS origin (Bulmer, 1957; Koff, 1933). As expected, uroplakin, a marker unique to urothelium, was detected in the epithelium of the human fetal bladder and urethra and throughout the epithelium of the vaginal introitus (Fig. 7D–E) (Table 3) (Figs. 7A, C–E). Uroplakin was absent throughout the developing human fetal female reproductive tract with the exception the vaginal plate at its junction with the introitus (Fig. 7E, asterisk).
Fig. 7.
Uroplakin immunohistochemistry of a 14-week human fetal female reproductive tract in sagittal sections. Uroplakin was not detected in epithelium of the uterine tube (not illustrated), uterine corpus, uterine cervix, vagina and vaginal plate (A, B, D), but was expressed in epithelium of the bladder (A & C), urethra (B, D, E), in the vaginal introitus (D–E) and in vaginal plate at its junction with the urethra (E, asterisk).
3.4. Immunohistochemistry of mesenchymal differentiation markers
The expression of α-actin, a marker of smooth muscle cells, is described in the accompanied paper and is particularly prominent in the developing myometrium (Robboy et al., 2017). An early event in uterovaginal development is specification of organ or regional identity within the mesenchymal wall, which is believed to be determined by region-specific expression of homeobox genes. Accordingly, we assessed the expression patterns of two homeobox gene products, ISL1 (ISL LIM homeobox 1) and HOXA11 in the human fetal female reproductive tract.
3.4.1. ISL1
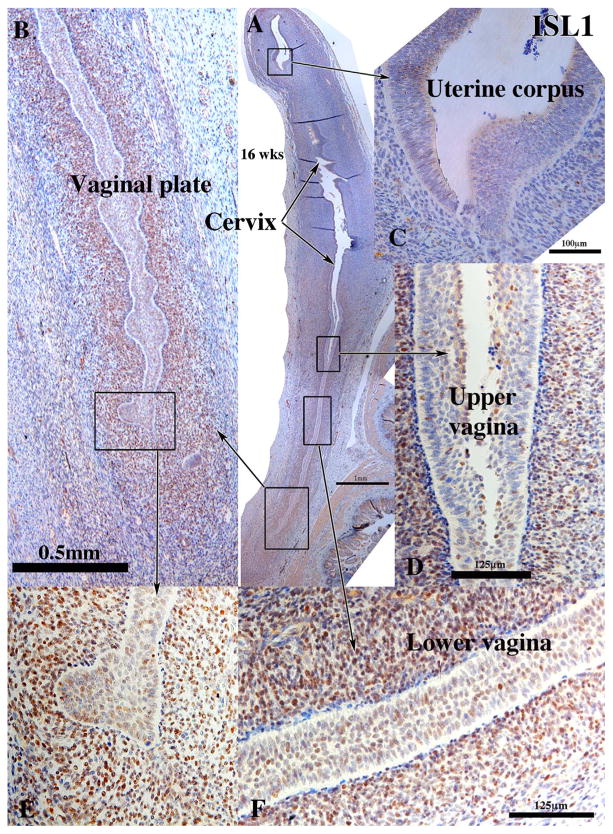
ISL1 is an essential transcription factor for heart development (Cai et al., 2003). Although its role in female reproductive tract is unknown, in mice ISL1 is enriched in vaginal, but not uterine mesenchyme (Gene Expression Omnibus (GEO) Series GSE44697) (Laronda et al., 2013) (Kurita unpublished). Similar to mice, ISL1 was detected in the nuclei of mesenchymal cells in the vagina and cervix from 11 weeks and thereafter (Fig. 8), but rarely if at all in the uterine tube (not illustrated) or uterine corpus (Fig. 8) (See also Fig. 14 in Robboy et al., 2017) (Table 3). At 21 weeks ISL1 continued to be expressed in vaginal mesenchyme (not illustrated).
Fig. 8.
ISL1 immunohistochemistry of a 16-week human fetal female reproductive tract in sagittal sections. ISL1 is expressed in mesenchymal cells associated with the vaginal plate (A, B, E), the vagina (D & F), but not in the mesenchymal cells associated with the cervix (A), uterine corpus (A & C) and the uterine tube (not illustrated).
3.4.2. HOXA11
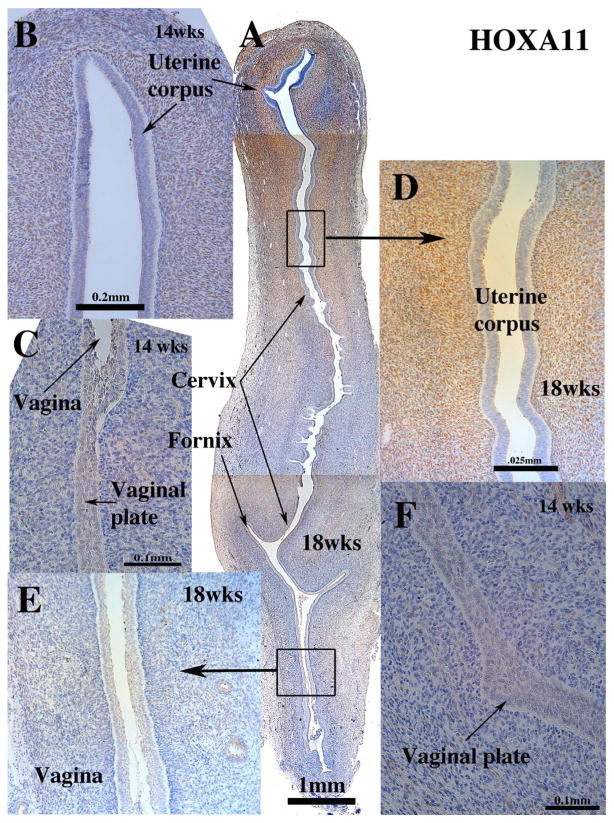
In mice, homeobox genes are expressed in a cranial-caudal gradient throughout the developing female reproductive tract (Dolle et al., 1991). Hoxa10 and Hoxa11 are expressed in uterine mesenchyme during murine development, and in adult mice Hoxa11 is observed in uterine epithelium and mesenchyme (Ma et al., 1998; Hsieh-Li et al., 1995; Wong et al., 2004). The expression pattern of HOXA11 protein in the developing human reproductive tract has not been previously reported. As in mice, HOXA11 was detected in mesenchymal cells of the cranial (but not the caudal) aspect of the uterovaginal canal at 9 weeks and in mesenchyme of the developing human uterine corpus after 11 weeks, but not in mesenchyme of the uterine tube, vagina and cervix (Fig. 9) (Table 3). Examination of adjacent sections stained for HOXA11 and ISL1 demonstrate little overlap in HOXA11 and ISL1 expression (Compare Figs. 8 and 9). At 21 weeks HOXA11 continued to be expressed in uterine mesenchyme (not illustrated).
Fig. 9.
HOXA11 immunohistochemistry of 14- and 18-week human fetal female reproductive tracts in sagittal section. (Sagittal sections of the human fetal female reproductive tract at 14 weeks of gestation B, C & F). Sagittal sections of the human fetal female reproductive tract at 18 weeks of gestation (A, D & E). Note nuclear staining of uterine mesenchymal cells, but not vaginal mesenchymal cells.
3.5. Immunocytochemistry of epithelial and mesenchymal transcription factors
3.5.1. RUNX1
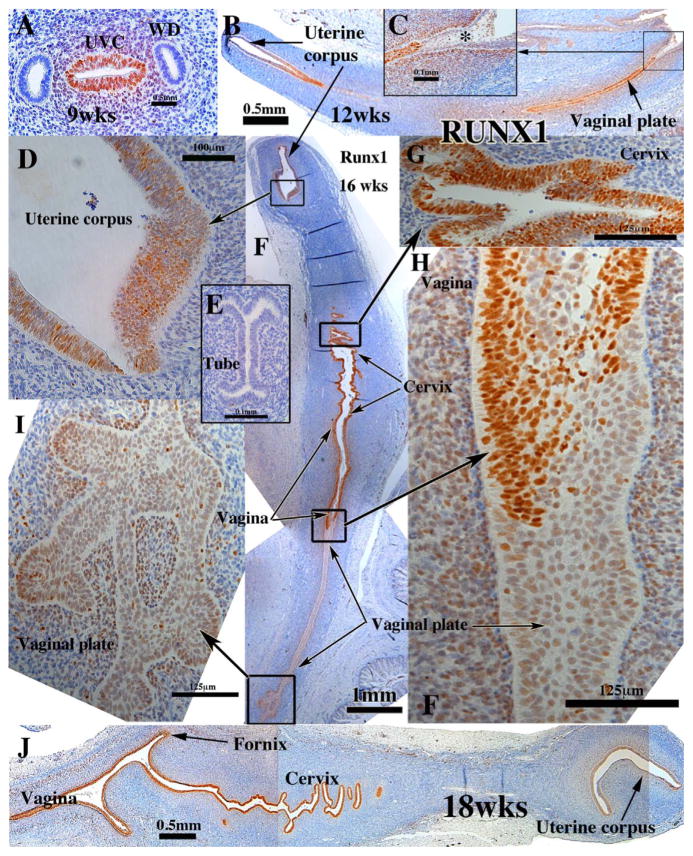
In mice, RUNX1 is precedent to and essential for the expression of ΔNp63 in Müllerian duct epithelium (Laronda et al., 2013). Thus, in the mouse RUNX1 is expressed in epithelium of the vagina and cervix and not in uterine epithelium (Laronda et al., 2013). In the developing human female reproductive tract RUNX1 had a wider distribution than that of the mouse. RUNX1 was detected in the simple columnar epithelium of the uterovaginal canal as early as 9 weeks of gestation (Fig. 10A), but not in that portion of the Müllerian duct destined to become the uterine tube (Fig. 9E) or in the Wolffian duct (Fig. 9A). RUNX1 was also expressed in the mesenchyme associated with the uterovaginal canal in 9 to 11 week specimens (Fig. 10A). At 12 weeks RUNX1 was expressed weakly in epithelium of the uterine corpus, and more strongly in caudal segments including the solid vaginal plate (Fig. 9B), but not in the final caudal segment of the vaginal plate near its junction with the urethral epithelium (Fig. 10B–C, asterisk). This pattern was also seen in 14-week specimens (not illustrated). At 16 weeks the pattern of RUNX1 exhibited heterogeneity of staining intensity with the most intense staining of the epithelium in the middle 2/4ths of the female reproductive tract (cervix) and reduced staining intensity in the upper uterine region and lower vaginal regions (Fig. 10F–I). At 18 weeks uniform strong epithelial staining for RUNX1 was seen in epithelium of the uterine corpus caudally to the vagina (Fig. 10J). Uterine tube epithelium was consistently negative for RUNX1 (Fig. 10E). At 21 weeks when the vaginal epithelium is many layers thick and stratified squamous, RUNX1 is expressed prominently in basal and suprabasal cell of the thick stratified vaginal epithelium (not illustrated). Epithelium of the cervix and uterine corpus remained RUNX1-positive at 21 weeks (not illustrated) (Table 3).
Fig. 10.
RUNX1 immunohistochemistry of human fetal female reproductive tracts in sagittal section. (A) Transverse section of the human fetal female reproductive tract at 9 weeks of gestation showing RUNX1 staining of the uterovaginal canal (UVC) and surrounding mesenchyme and absence of RUNX1 staining in the Wolffian ducts (WD). Sagittal sections of the human fetal female reproductive tract at 12 weeks of gestation (B & C). RUNX1 is expressed in epithelia throughout the female reproductive tract with the exception of weak staining of uterine epithelium (B) and an absence of staining in the vaginal plate at its junction with the urethra (C, asterisk). Sections of the human fetal female reproductive tract at 16 weeks of gestation (D, F–I). RUNX1 is expressed in epithelia throughout the female reproductive tract but at vastly different levels. (J) Sagittal sections of the human fetal female reproductive tract at 18 weeks of gestation. RUNX1 is expressed in epithelia throughout the female reproductive tract and highest in vaginal and cervical epithelia.
3.5.2. TP63/TRP63
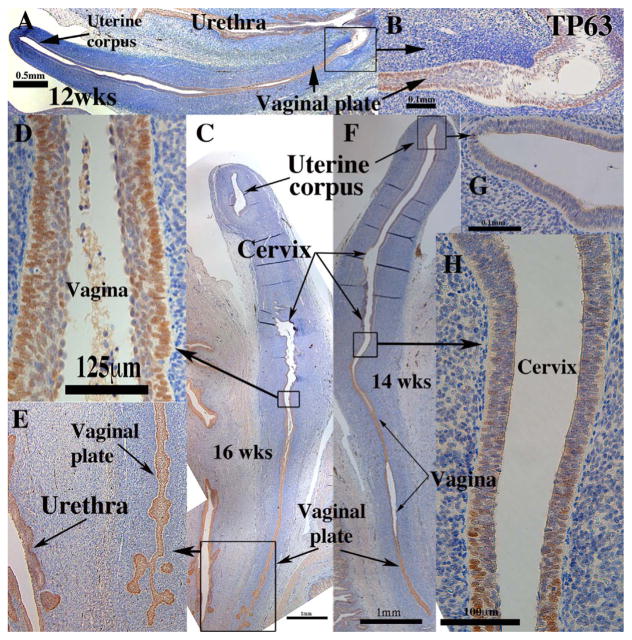
In mice TRP63 expression specifies cervicovaginal epithelial cell fate in the Müllerian vagina. Expression of KRT14 depends on the prior expression of TRP63 (Kurita and Cunha, 2001). TP63 immunostaining was consistently expressed strongly in all stratified (but not simple columnar) epithelia of the human female reproductive tract. Thus, at 8–12 weeks the simple columnar epithelia of the Müllerian duct and uterovaginal canal were negative for TP63 (not illustrated), while the stratified epithelia of the urethra, UGS and vaginal plate were TP63-positive (Fig. 11A&B). From 12–21 weeks all stratified epithelia of the vaginal plate, cervix and vagina were strongly TP63-positive, while the simple columnar epithelia of the uterine fundus (Fig. 11C, F & G) and tube (not illustrated) were TP63 negative. Note that at 16 weeks and earlier the boundaries between uterine corpus, endocervix, exocervix and vagina are difficult/impossible to discern. However, strong nuclear TP63 staining in the vaginal region (Fig. 11C–F) diminished to patchy single cell expression within the pseudostratified columnar epithelium at the presumed transition between the endocervix and lower uterine corpus (Fig. 11H) (Table 3). At 21 weeks TP63 was expressed in basal and suprabasal vaginal epithelial cells as well as in basal cells of the exocervix; rare TP63-positive epithelial cells were seen in the lower uterine corpus (not illustrated). These scattered TP63-positive cells in the presumed endocervix and lower uterine corpus may be due to diffuse mesenchymal signals at the uterine corpus/cervical boundary. For example, patchy expression of TRP63 in columnar epithelium of postnatal day 1 mouse Müllerian vagina disappeared when the epithelium was combined with uterine mesenchyme (Kurita et al., 2004). Estrogen-induced squamous metaplasia of endometrium may be caused by retention of these TRP63/TP63-positive epithelial cells in the uterine corpus (Fritsch et al., 2013; Kurita, 2011). Clearly, the expression of TP63 during the human female reproductive development differs from that seen in mice.
Fig. 11.
TP63 immunohistochemistry of human fetal female reproductive tracts in sagittal sections. (A & B) Sagittal sections of the human fetal female reproductive tract at 12 weeks of gestation. TP63 is expressed in stratified epithelia of the vagina and vaginal plate to its junction with the urethra (B). (C–E) Sagittal sections of the human fetal female reproductive tract at 16 weeks of gestation. TP63 is expressed only in stratified epithelia of the vagina (D) and vaginal plate (E). (F–H) Sagittal sections of the human fetal female reproductive tract at 14 weeks of gestation. TP63 is expressed in stratified epithelia of the vaginal plate (E), vagina (F) and in single cell patches in the cervix (H).
3.6. Immunocytochemistry of steroid receptors
3.6.1. Estrogen receptor alpha (ESR1)
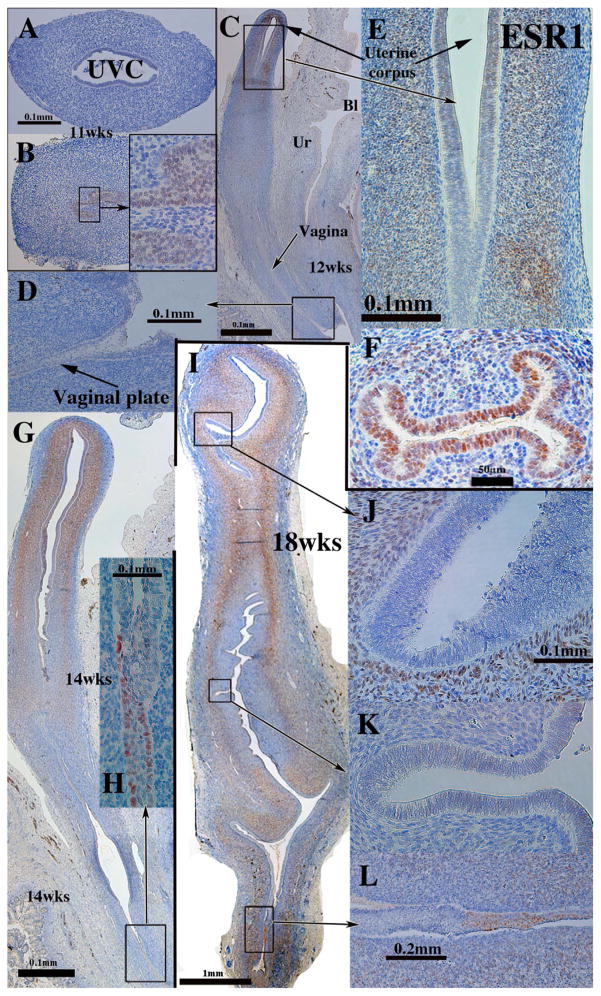
ESR1 has a unique ontogenic profile that varied on an organ-by-organ basis. At 8–11 weeks of gestation ESR1 was not detected in either the mesenchyme or epithelium of the Müllerian ducts and the uterovaginal canal, but was expressed in UGE (Fig. 12A–B). ESR1 became prominent in the mesenchyme of the uterine corpus at 12 weeks of gestation (Fig. 12C & E) (Table 4), but was undetectable in epithelium of the vagina plate and its associated mesenchyme (Fig. 12C–D). At 14 and 16 weeks ESR1 was strongly expressed in epithelium of the uterine tube and maintained thereafter (Fig. 12F). At 14–18 weeks the epithelium of the uterine fundus was for the most part ESR1-negative with the exception of weak staining in rare epithelial cells (Fig. 12G). ESR1 was strongly expressed in mesenchymal cells of the developing endometrial stroma and myometrium from 12 to 21 weeks (Fig. 12C, E, G & I). At 18–21 weeks ESR1 expression in the simple columnar epithelium of the endocervix and uterine corpus varied from large patches to other areas where single ESR1-reactive epithelial cells were interspersed amongst ESR1-negative epithelial cells. Tubal epithelium remained ESR1-positive at 21 weeks (not illustrated). As seen in younger specimens, mesenchymal cells associated with the uterine corpus and cervix remained ESR1-positive at 21 weeks.
Fig. 12.
ESR1 immunohistochemistry of human fetal female reproductive tracts. (A & B). Sections of the human fetal female reproductive tract at 11 weeks of gestation. (A) the uterovaginal canal (UVC) and its mesenchyme are ESR1-negative, but urogenital sinus epithelium is ESR1-positive (B and inset). (C–E) Sagittal sections of the human fetal female reproductive tract at 12 weeks of gestation. (C) Uterine mesenchyme is the only ESR1-positive tissue. (F) Uterine tube at 16 weeks of gestation, whose epithelium and mesenchyme are ESR1-positive. (G–H) Sagittal sections of the human fetal female reproductive tract at 14 weeks of gestation. (G) ESR1 is seen in uterine and cervical mesenchyme and in scattered epithelial cells in the vaginal plate (H). (I–L) Sagittal sections of the human fetal female reproductive tract at 18 weeks of gestation. (I–K) ESR1 is observed in mesenchyme throughout the female reproductive tract, but not in epithelium. Patches of ESR1-positive cells were seen in the caudal portion of the vagina (I & L).
Table 4.
Ontogeny of ESR1 in the human female fetal reproductive tract.
| Uterovaginal canal | Uterine tube | Ut-tube junct. | Uterine corpus | Uterine cervix | Vagina (with lumen) | |
|---|---|---|---|---|---|---|
| Epithelium | ||||||
| 8wks | − | − | NA | NA | NA | NA |
| 9wks | − | − | NA | NA | NA | NA |
| 11wks | − | − | NA | NA | NA | NA |
| 12wks | − | − | ND | − | − | NA |
| 13wks | − | − | − | − | − | NA |
| 14wks | NA | + | +/− | −/+ a | −/+ a | − |
| 16wks | NA | + | + | − | − | − |
| 18wks | NA | + | +/− | +/− | +/− | |
| 21wks | NA | ND | ND | + | −/+c | + |
| Mesenchymeb | ||||||
| 8wks | − | − | NA | NA | NA | NA |
| 9wks | − | − | NA | NA | NA | NA |
| 11wks | − | − | ND | NA | NA | NA |
| 12wks | + | ND | + | + | + | NA |
| 13wks | + | ND | ND | + | + | NA |
| 14wks | NA | + | ND | + | −/+ | NA |
| 16wks | NA | − | −/+ | + | + | −/+ |
| 18wks | NA | − | ND | + | −/+ | − |
| 21wks | NA | ND | ND | + | + | + |
rare ESR1-positivie cells.
= mesenchymal cells associated with epithelial structures as indicated.
Patchy expression; NA = Not applicable, ND = not done, Ut-tube junct. = uterotubal junction, wks = weeks.
ESR1 expression in vaginal development deserves special attention. At 14 weeks ESR1 was expressed in small patches of vaginal plate epithelium (Fig. 12G–H) and at 18 weeks larger coherent patches of ESR1 were observed in developing vaginal epithelium (Fig. 12I & L). At 14–18 weeks of gestation ESR1 was not detected in vaginal mesenchymal cells (Fig. 12G, H & L). At 21 weeks, when the developing female reproductive tract is stimulated with endogenous estrogen and the vaginal epithelium is many layers thick and stratified, ESR1 is expressed in basal and suprabasal cells of the thick stratified vaginal epithelium (Fig. 13). Near the vaginal introitus mesenchymal cells associated with the hyperplastic vaginal epithelium were mostly ESR1-negative with rare ESR1-positive mesenchymal cells present (Fig. 13B), while near the vaginal/cervical boundary a large percentage of the mesenchyme cells were ESR1-positive (Fig. 13A).
3.6.2. Progesterone receptor (PGR)
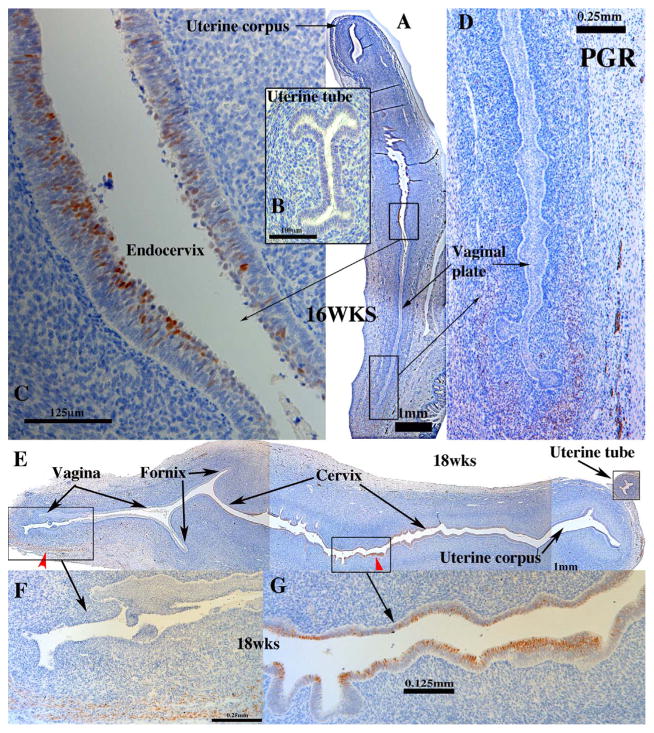
Before 14 weeks PGR was undetectable throughout the entire developing human female reproductive tract. At 16 weeks of gestation PGR was first detected in small patches of epithelial cells in the region of the lower uterine corpus/uterine cervix (Fig. 14A & C) and in a subset of mesenchymal cells near the lower vaginal plate (Fig. 14A& D). At 18 weeks of gestation PGR was prominently expressed in cervical epithelium (Fig. 14E & G), but not in epithelium of the vagina, uterine corpus or uterine tube (Fig. 14E–G). Patches of PR-positive vaginal stromal cells appeared at 16 and 18 weeks of gestation (Fig. 14D & F). At 21 weeks PGR was expressed uniformally in basal and suprabasal epithelial cells of the vagina. At this stage mesenchymal expression was noted in some areas in close association with vaginal epithelium, while in other areas of vagina mesenchymal cells were completely devoid of PGR immunostaining (not illustrated). At 21 weeks epithelium of the uterine corpus and cervix were generally PGR-negative with the exception of small pockets of PGR-positive epithelial cells (not illustrated). Mesenchymal cells associated with the uterine corpus and cervix were PGR-negative (Table 3).
Fig. 14.
Progesterone receptor (PGR) immunohistochemistry of fetal female reproductive tract. (A–D) PGR immunostaining was initially seen at 16 weeks as patches of PGR-positive epithelial cells of the endocervix (A & C). (E–G) At 18 weeks of gestation PGR was detected in epithelium of the cervix (E and G) and in scattered mesenchymal cells associated with the lower vagina (F).
3.6.3. Androgen receptor (AR)
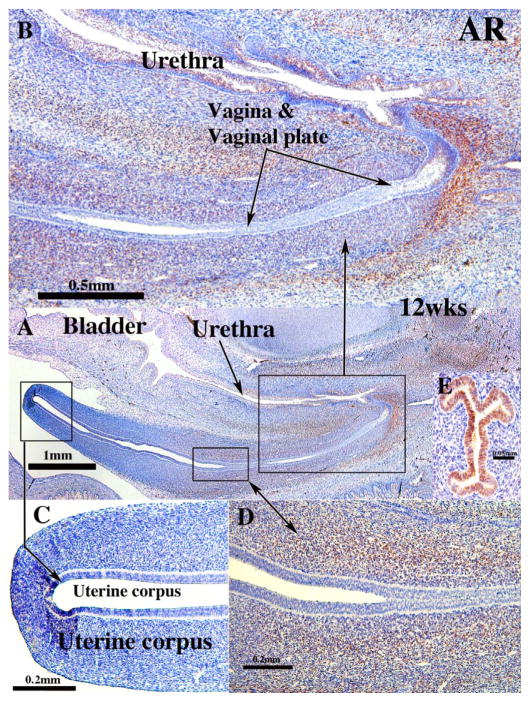
At 8 and 9 weeks AR was not detected in the uterovaginal canal. At 9 weeks AR was expressed in the mesenchyme associated with the UGS (not illustrated), becoming more extensive in mesenchyme associated with the UGS, the solid vaginal plate, vagina and the urethra as indicated in the 12-week specimen and thereafter (Fig. 15). The pattern of AR expression in the mesenchyme was similar to that of ISL1 (compare with Fig. 8). Mesenchyme at the junction of the solid vaginal plate and the urethra exhibited particularly strong AR staining (Fig. 15A–B). Epithelium of the vagina, vaginal plate, uterine corpus and endocervix were AR-negative (Fig. 15A & C), while epithelium of the uterine tube was AR-positive (Fig. 15E). Urethral epithelium had AR-negative basal cells and AR-positive apical cells (Fig. 15B). At 21 weeks AR was detected in mesenchyme associated with the AR-negative vaginal epithelium, while epithelium of the uterine corpus and cervix exhibited small patches of AR positive epithelial cells, and the mesenchyme of these organs contained rare AR-positive cells (not illustrated).
Fig. 15.
Androgen receptor immunohistochemistry of human fetal female reproductive tracts. (A) low power overview sagittal section. Note AR staining in mesenchyme associated with the vaginal plate and vagina. (B) Higher magnification view of vaginal region showing mesenchymal AR staining. (C) Absence of AR staining in uterine corpus A & C). Intense mesenchymal AR staining associated with the junction of the cervix and vagina (D). (E) Uterine tube of a 16-week fetus showing strong AR immuno-reactivity.
4. Discussion
Forty years ago, Cunha demonstrated for the first time that epithelial morphology unique to the uterus and vagina is induced by organ-specific mesenchyme (Cunha, 1976), a finding verified in a series of studies from the same group (Cooke et al., 1987; Boutin et al., 1991; Kurita et al., 2001). Mesenchymal cells associated with MDE establish their organ-specific identity according to their cranial-caudal position, and homeobox genes are candidate factors that provide positional information along the cranial-caudal axis of the developing female reproductive tract (Dolle et al., 1991). In mice abdominal B Hox genes are expressed in a spatially coordinated pattern along the cranial-caudal axis of the developing Müllerian duct and its derivatives (Kobayashi and Behringer, 2003). For example, in the mouse Hoxa9 to Hoxa13 genes are expressed in the following pattern: Hoxa9 from the isthmus of the oviduct to the utero-cervical junction; Hoxa10 from the utero-tubal junction to the cervix; Hoxa11 in the uterus and cervix; and Hoxa13 in the cervix and vagina. The significance of this particular pattern of expression of Hox genes is demonstrated in the phenotype of Hoxa10−/− mice, in which the cranial end of the uterus transforms into an oviduct-like structure (Benson et al., 1996). Similarly, humans with HOXA13 mutations have malformations of Müllerian duct derivatives (de Santa Barbara and Roberts, 2002; Mortlock and Innis, 1997). Our study confirms a similar expression pattern of HOXA11 in developing female reproductive tracts of human and mouse. In both cases, HOXA11 is expressed in mesenchyme of uterine corpus, to a lesser extent in the endocervix and not at all in the vagina. Conversely, ISL1, another homeobox transcription factor, is expressed in more caudal regions in mesenchymal cells of the human vagina and cervix and not in the uterine corpus. Comparison of adjacent sections stained for HOXA1 and ISL1 demonstrate very little overlap in the expression patterns of these two transcription factors. These observations suggest that the expression of homeobox genes defines the segment-specific identity of mesenchymal cells in both human and mouse, which in turn specifies the differentiation of associated epithelium in developing female reproductive organs.
The ontogeny of ESR1 protein in the developing human fetal reproductive tract is remarkably similar to that reported for mice and rats in so far as the initial ESR1 expression is confined to the mesenchyme of the fetal Müllerian duct (Greco et al., 1991; Nielsen et al., 2000; Lemmen et al., 1999) followed subsequently by its expression in uterine epithelium (Jefferson et al., 2000; Korach et al., 1988; Yamashita et al., 1990; Bigsby et al., 1990). Taguchi et al were the first report of estrogen receptors in the developing human female reproductive tract using steroid autoradiography (Taguchi et al., 1986). To our knowledge there are no reports of an absence of ESR1 immunostaining in Müllerian duct mesenchyme of embryonic mice, perhaps because early stages have not yet been examined. However, at 8–11 weeks of gestation we noted a complete absence of mesenchymal (and epithelial) ESR1 expression in the human fetal female reproductive tract. ESR1 was initially detected in mesenchyme of the uterovaginal canal at 12 weeks of gestation (when the Müllerian epithelium remained ESR1-negative). Mesenchymal ESR1 expression was maintained thereafter in a substantial subset of mesenchymal cells throughout the upper female reproductive tract (uterine corpus and uterine cervix) in all specimens examined (12–21 weeks of gestation). Thus, mesenchymal ESR1 was expressed prior to epithelial ESR1 and maintained thereafter in mice, rats and humans.
The ontogeny of epithelial expression of ESR1 was also similar in mice and humans. With few exceptions ESR1 was undetectable in epithelium of the embryonic female mouse Müllerian duct. Only one study reports ESR1 in epithelium of the fetal mouse Müllerian duct (Greco et al., 1991), but this finding was not confirmed by others (Nielsen et al., 2000). In rats the expression of ESR1 in epithelium of the fetal Müllerian duct was observed, but only in cranial segments of the Müllerian duct and not in middle and caudal segments (Okada et al., 2002). Thus, the cranial-caudal position of observations may be a factor in detecting ESR1 in epithelium of the fetal Müllerian duct. Significantly, the expression of ESR1 in epithelium of the cranial segment of the rat Müllerian duct, the oviductal precursor, correlates with the consistently strong ESR1 expression in epithelium of the human fetal uterine tube. At birth ESR1 is initially undetectable in mouse uterine epithelium but appears at 3–6 days postnatal depending on the mouse strain (Jefferson et al., 2000; Korach et al., 1988; Yamashita et al., 1990; Bigsby et al., 1990). A similar pattern of ESR1 expression was observed in the epithelium of developing human female reproductive tract: Epithelial ESR1 expression was completely negative or single cell patchy in epithelium of the uterine corpus, cervix and upper vagina throughout most stages. However, strong epithelial ESR1 expression was observed in the uterine tube and the solid vaginal plate from 14 weeks of gestation and thereafter. By 21 weeks of gestation ESR1 was detected in epithelium of the vagina, uterine corpus and uterine cervix, even though in the latter two organs epithelial expression of ESR1 was patchy (Table 4). While epithelium of the human uterine corpus, cervix and upper vagina express ESR1 in adulthood, the ontogeny of ESR1 expression in epithelium of these organs requires further study.
The presence of ESR1 in mesenchyme coupled with the absence of ESR1 in epithelium of organs of the early human female reproductive tracts support a concept established by our tissue recombination studies. Namely, that mesenchymal cells first establish their organ identity and molecular characteristics, and subsequently instruct associated epithelial cells to express organ specific phenotypes. It is possible that the appearance of epithelial ESR1 in the developing human female reproductive tract depends upon paracrine signals from mesenchyme, as mesenchymal induction/regulation has been reported for epithelial AR, ESR1 and PGR (Cunha et al., 1980; Kurita et al., 1998, 2000a).
PRG was first detected at 16 weeks of gestation in small epithelial patches, and at 21 weeks was likewise detected in patches in epithelium and mesenchyme in the uterine corpus and uterine cervix. In contrast, in the vagina PGR was expressed uniformly in basal and suprabasal cells at 21 weeks. PGR is known to be induced by estrogen signaling via ESR1. The minimal expression of PGR seen at 16 and 18 weeks of gestation may be related to the gradual ontogeny of uterine/cervical epithelial ESR1 expression and/or inadequate endogenous estrogen levels. Administration of a maximally stimulatory dose of diethylstilbestrol (DES) to xenografts of human fetal reproductive tracts (see Cunha et al., 2017, companion paper in virtual special issue) strongly induces PGR globally within epithelial and mesenchymal cells throughout the human fetal reproductive tract. This observation confirms estrogenic induction of PGR in the developing human female reproductive tract. While the level of endogenous estrogen at 21 weeks is sufficient to induce prominent PRG in vaginal epithelium, it is not sufficient to induce strong uniform PGR expression in uterine and cervical epithelia, suggesting a significant difference in estrogenic sensitivity of vaginal versus uterine/cervical epithelial cells and their associated mesenchymal cells.
The expression of epithelial and mesenchymal ESR1 correlates with the congenital malformations elicited by DES in women whose mothers were administered this potent estrogen during pregnancy (Herbst et al., 1975; Herbst and Bern, 1981). In women exposed in utero to DES, a range of epithelial and stromal abnormalities have been described in the uterine tube, uterotubal junction, uterine corpus, cervix and vagina (Titus-Ernstoff et al., 2010; Robboy et al., 1977, 1984; Hoover et al., 2011). Similar malformations of the female reproductive tract have been reported in mice treated perinatally with exogenous estrogens including DES (Bern et al., 1976, 1984; McLachlan et al., 1980; Newbold et al., 1983; Newbold and MaLachlan, 1985; Newbold, 2004; Forsberg, 1969; Forsberg and Kalland, 1981). Female reproductive tract malformations (including adenosis) in mice elicited by exogenous estrogens are mediated via ESR1 (Couse et al., 2001; Couse and Korach, 2004). One of the lesions elicited by DES in the developing vagina of humans and mice is adenosis, aberrant simple columnar glands in the vagina (and cervix) (Emens, 1984; Forsberg, 1976; Herbst et al., 1975; Iguchi et al., 1986; Newbold and McLachlan, 1982; Plapinger and Bern, 1979; Robboy et al., 1982; Taguchi et al., 1983; Robboy, 1983). Studies in mice demonstrated that perinatal DES exposure elicits differentiation of simple columnar Müllerian epithelium (adenosis) in the vagina (expressing uterine markers) by repressing ΔNp63 through epithelial ESR1 (Kurita et al., 2004; Laronda et al., 2012, 2013). In mice, RUNX1 is essential for expression of ΔNp63 in cervicovaginal epithelial cells. Exposure to DES at the time of the epithelial cell fate decision in Müllerian vaginal epithelium represses expression of RUNX1, suggesting that RUNX1 is the target of DES in the pathogenesis of cervicovaginal adenosis (Kurita et al., 2004; Laronda et al., 2012, 2013). The current study is the first to demonstrate expression of RUNX1 in epithelium of the human fetal cervix and vagina and thus implies a role for RUNX1 in normal and abnormal development of the human female reproductive tract including the pathogenesis of vaginal adenosis in humans. In this regard, epithelial RUNX1 is expressed globally in the developing female reproductive tract beginning with the uterovaginal canal at 8–9 weeks of gestation, as well as in all epithelial derivatives of the uterovaginal canal up to and including developing organs at 21 weeks of gestation. The one exception to this finding is uterine tube epithelium, in which RUNX1 was never detected at any stage. A novel finding in our study is the expression of RUNX1 in mesenchymal cells of developing human female reproductive tract organs. The significance of this finding remains to be determined. However, what is of interest is that 8–9 weeks of gestation is the period when DES administration initiates its deleterious disruptive teratogenic effects in humans (Robboy et al., 1977; Herbst et al., 1974).
The strong androgen receptor immunostaining at the junction of the vaginal plate and the endoderm-derived urethra deserves special comment. The position of the junction of the Müllerian ducts with the endodermal UGS differs radically in males versus females. In males the fused Müllerian ducts (prostatic utricle) join the UGS in a cranial position immediately below the bladder neck at the summit of the verumontanum (Clemente, 1985). In contrast, in females the Müllerian epithelium of the uterovaginal canal contacts the endodermal epithelium of the urethra in a caudal position near the vaginal introitus. In mice the cranial positioning of the Müllerian duct-UGS junction in males is due to signaling through androgen receptors in the mesenchyme (Larkins et al., 2016). Patients with congenital adrenal hyperplasia (and thus elevated androgen levels) are masculinized to variable degrees (Speiser et al., 2010), and in many cases the vagina does not terminate caudally in the vaginal vestibule, but instead is attached to the urethra in a more cranial position (Larkins et al., 2016). The localization of AR observed in mesenchyme associated with the human vaginal plate/urethral junction is in the appropriate position to alter the Müllerian duct-UGS junction should androgen levels be elevated. AR was also noted in the epithelium (but not the mesenchyme) of the uterine tube. Based upon a PUBMED search, there are no reports of AR in the mammalian oviduct/uterine tube.
Uroplakins are membrane proteins unique to urothelium of the ureter, bladder and urethra (Yu et al., 1990; Riedel et al., 2005). Urothelium lining the ureter is derived from the mesoderm germ layer (Saxen, 1987), while the urothelium lining the bladder and urethra are derived from the endodermal UGS (Moore and Persaud, 2003). Accordingly, uroplakins are not expected to be expressed in the female reproductive tract, and this prediction was confirmed in this study.
The vaginal introitus is a space into which the vagina and the urethra open in the perineum (Clemente, 1985). While there are no previous reports of uroplakin expression in the vaginal introitus, immunostaining demonstrates uroplakin expression extending from the urethral epithelium into the vaginal introitus, which also expresses FOXA1, an endodermal marker (Robboy et al., 2017). This suggests that epithelia of both the urethra and vaginal introitus are derived from the endodermal UGS. This finding has implications regarding the development of the hymen. “The hymen is a thin fold of mucous membrane situated at the orifice of the vagina” (Clemente, 1985), and accordingly represents the most caudal portion of the vagina as it opens into the vestibule. Koff proposes that the hymen is derived from the UGS portion of the vagina (Koff, 1933). The expression of uroplakin and FOXA1 in the vaginal vestibule is consistent with this interpretation.
KRT8 and KRT18 are normally expressed in simple epithelia, and have been reported previously in the developing Müllerian duct epithelium (Fritsch et al., 2013; Magro and Grasso, 1995; Viebahn et al., 1987) as well as in adult human tubal, uterine and cervical epithelia (Moll et al., 1982, 2008). KRT 7 and KRT19 are also frequently expressed in simple epithelia or in the apical layers of stratified urothelium and vaginal epithelium (Moll et al., 2008). KRT 7 and KRT19 have been reported previously in the embryonic Müllerian duct of golden hamster (Viebahn et al., 1987). In the present study KRT8 and KRT7 were detected as expected in the simple columnar epithelium of the uterine tube and the upper portion of the uterovaginal canal (uterine corpus). However, at 12–14 weeks the junction of the lower 1/3 and upper 2/3 s of the uterovaginal canal, the epithelium transitions from a simple columnar (KRT7-positive) to a stratified (KRT7-negative) epithelium. This “stratified/simple columnar junction” likely represents the boundary between the stratified epithelium of the exocervix and the simple columnar epithelium of the endocervix (Clemente, 1985; Hendrickson and Kempson, 1992).
KRT19 is also frequently expressed in simple epithelium and in apical layers of certain stratified epithelia (bladder and vagina) (Moll et al., 1982, 2008). As expected, KRT19 was detected in epithelium of the developing human urinary bladder and urethra. At 8–9 weeks of gestation KRT19 was expressed in epithelia of the Müllerian ducts and uterovaginal canal and subsequently in the solid vaginal plate. In the course of development KRT19 expression diminished in the uterine tube and uterine corpus, but was strongly expressed in stratified squamous epithelium lining the vagina and exocervix. At 14 weeks the cervico-vaginal region consists of two different morphologies. Caudally is the solid vaginal plate, which merges cranially with a stratified epithelium lining a lumen. The solid vaginal plate at 14 and 16 weeks of gestation is FOXA1-positive and KRT19-negative (and thus is derived from UGE), while the stratified epithelium lining the lumen is PAX2-positive and KRT19-positive (and thus is derived from Müllerian epithelium) (Robboy et al., 2017). At 18 weeks of gestation, before vaginal epithelium undergoes estrogen-induced maturation, immunostaining demonstrated KRT19 in the cranial (but not the caudal) aspect of the vagina. The abrupt change in KRT19 immunostaining occurs precisely at the point within vaginal epithelium of this age in which there is an abrupt change from PAX2-positive epithelial cells cranially to FOXA1-positive epithelial cells caudally (see figure 19 in Robboy et al., 2017). Thus, the pattern of KRT19 expression appears to track with the PAX2/HOXA11 boundary.
For virtually all vertebrates the Müllerian duct is the progenitor of female reproductive tract organs. Accordingly, animal models such as mouse and rat have been used to explore the molecular mechanisms of female reproductive tract development with the tacit, but unproven assumption, that concepts derived from animal models are relevant to human development. This idea is supported by classic histological studies and by a small number of human molecular and immunohistochemical studies mentioned above. Expanding and extending previous reports we have examined a wide spectrum of differentiation and molecular markers that demonstrate a remarkable similarity in the morphogenetic and molecular pathways common to mouse and human female reproductive tract development and have highlighted some of the notable exceptions in mouse versus human female reproductive tract development.
Acknowledgments
This work was supported by NIH grant RO1 DK0581050 to L. Baskin and NIH grant RO1 CA154358 to T. Kurita. The authors thank Drs. EB Lane and T. T. Sun for providing antibodies.
Abbreviations
- MD
Müllerian duct
- WD
Wolffian duct
- UGS
urogenital sinus
- UGE
urogenital sinus epithelium
- MDE
Müllerian epithelium
- ESR1
estrogen receptor alpha
- PGR
progesterone receptor
- AR
androgen receptor
- KRT
keratin
- H& E
hematoxylin and eosin
References
- Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- Bern HA, Jones LA, Mills KT. Use of the neonatal mouse in studying long-term effects of early exposure to hormones and other agents. J Toxicol Environ Health Suppl. 1976;1:103–116. [PubMed] [Google Scholar]
- Bern HA, Mills KT, Ostrander PL, Schoenrock B, Graveline B, Plapinger L. Cervicovaginal abnormalities in BALB/c mice treated neonatally with sex hormones. Teratology. 1984;30:267–274. doi: 10.1002/tera.1420300214. [DOI] [PubMed] [Google Scholar]
- Bern HA, Talamantes FJ. Neonatal mouse models and their relation to disease in the humal female. In: Herbst A, Bern HA, editors. Developmental Effects of Diethylstilbestrol (DES) in Pregnancy. Thieme Stratton Inc; New York: 1981. pp. 129–147. [Google Scholar]
- Bigsby RM, Li AX, Luo K, Cunha GR. Strain differences in the ontogeny of estrogen receptors in murine uterine epithelium. Endocrinology. 1990;126:2592–2596. doi: 10.1210/endo-126-5-2592. [DOI] [PubMed] [Google Scholar]
- Bloomfield A. The Development of the Lower End of the Vagina. J Anat. 1927;62:9–32. [PMC free article] [PubMed] [Google Scholar]
- Boutin EL, Sanderson RD, Bernfield M, Cunha GR. Epithelial-mesenchymal interactions in uterus and vagina alter the expression of the cell surface proteoglycan, syndecan. Dev Biol. 1991;148:63–74. doi: 10.1016/0012-1606(91)90317-v. [DOI] [PubMed] [Google Scholar]
- Buchanan DL, Kurita T, Taylor JA, Lubahn DL, Cunha GR, Cooke PS. Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification and cornification. Endocrinology. 1998;139:4345–4352. doi: 10.1210/endo.139.10.6241. [DOI] [PubMed] [Google Scholar]
- Bulmer D. The development of the human vagina. J Anat. 1957;91:490–509. [PMC free article] [PubMed] [Google Scholar]
- Bulmer D. The epithelium of the urogenital sinus in female human foetuses. J Anat. 1959a;93:491–498. [PMC free article] [PubMed] [Google Scholar]
- Bulmer D. Histochemical observations on the foetal vaginal epithelium. J Anat. 1959b;93:36–42. [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente CD. Gray’s Anatomy. Lea and Febiger; Philadelphia: 1985. [Google Scholar]
- Cooke PS, Ekman GC, Kaur J, Davila J, Bagchi IC, Clark SG, Dziuk PJ, Hayashi K, Bartol FF. Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice. Biol Reprod. 2012;86:63. doi: 10.1095/biolreprod.111.097188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Fujii DK, Cunha GR. Vaginal and uterine stroma maintain their inductive properties following primary culture. Vitr Cell Dev Biol. 1987;23:159–166. doi: 10.1007/BF02623575. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Spencer TE, Bartol FF, Hayashi K. Uterine glands: development, function and experimental model systems. Mol Hum Reprod. 2013;19:547–558. doi: 10.1093/molehr/gat031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Dixon D, Yates M, Moore AB, Ma L, Maas R, Korach KS. Estrogen receptor-alpha knockout mice exhibit resistance to the developmental effects of neonatal diethylstilbestrol exposure on the female reproductive tract. Dev Biol. 2001;238:224–238. doi: 10.1006/dbio.2001.0413. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor-alpha mediates the detrimental effects of neonatal diethylstilbestrol (DES) exposure in the murine reproductive tract. Toxicology. 2004;205:55–63. doi: 10.1016/j.tox.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J Exp Zool. 1976;196:361–370. doi: 10.1002/jez.1401960310. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Reese BA, Sekkingstad M. Induction of nuclear androgen-binding sites in epithelium of the embryonic urinary bladder by mesenchyme of the urogenital sinus of embryonic mice. Endocrinology. 1980;107:1767–1770. doi: 10.1210/endo-107-6-1767. [DOI] [PubMed] [Google Scholar]
- de Santa Barbara P, Roberts DJ. Tail gut endoderm and gut/genitourinary/tail development: a new tissue-specific role for Hoxa13. Development. 2002;129:551–561. doi: 10.1242/dev.129.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle P, Izpisua-Belmonte JC, Brown JM, Tickle C, Duboule D. HOX-4 genes and the morphogenesis of mammalian genitalia. Genes Dev. 1991;5:1767–1777. doi: 10.1101/gad.5.10.1767. [DOI] [PubMed] [Google Scholar]
- Drey EA, Kang MS, McFarland W, Darney PD. Improving the accuracy of fetal foot length to confirm gestational duration. Obstet Gynecol. 2005;105:773–778. doi: 10.1097/01.AOG.0000154159.75022.11. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Emens M. Vaginal adenosis and diethylstilboestrol. Br J Hosp Med. 1984;31:42–48. [PubMed] [Google Scholar]
- Filant J, Zhou H, Spencer TE. Progesterone inhibits uterine gland development in the neonatal mouse uterus. Biol Reprod. 2012;86(146):141–149. doi: 10.1095/biolreprod.111.097089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg JG. The development of atypical epithelium in the mouse uterine cervix and vaginal fornix after neonatal oestradiol treatment. Br J Exp Pathol. 1969;50:187–195. [PMC free article] [PubMed] [Google Scholar]
- Forsberg JG. Animal model of human disease: adenosis and clear-cell carcinomas of vagina and cervix. Am J Pathol. 1976;84:669–672. [PMC free article] [PubMed] [Google Scholar]
- Forsberg JG, Kalland T. Neonatal estrogen treatment and epithelial abnormalities in the cervicovaginal epithelium of adult mice. Cancer Res. 1981;41:721–734. [PubMed] [Google Scholar]
- Fritsch H, Hoermann R, Bitsche M, Pechriggl E, Reich O. Development of epithelial and mesenchymal regionalization of the human fetal utero-vaginal anlagen. J Anat. 2013;222:462–472. doi: 10.1111/joa.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch H, Richter E, Adam N. Molecular characteristics and alterations during early development of the human vagina. J Anat. 2012;220:363–371. doi: 10.1111/j.1469-7580.2011.01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatstein IZ, Yeh J. Ontogeny of the estrogen receptor in the human fetal uterus. J Clin Endocrinol Metab. 1995;80:958–964. doi: 10.1210/jcem.80.3.7883857. [DOI] [PubMed] [Google Scholar]
- Greco TL, Furlow JD, Duello TM, Gorski J. Immunodetection of estrogen receptors in fetal and neonatal female mouse reproductive tracts. Endocrinology. 1991;129:1326–1332. doi: 10.1210/endo-129-3-1326. [DOI] [PubMed] [Google Scholar]
- Guioli S, Sekido R, Lovell-Badge R. The origin of the Mullerian duct in chick and mouse. Dev Biol. 2007;302:389–398. doi: 10.1016/j.ydbio.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Hendrickson MR, Kempson RL. Uterus and fallopian tubes. In: Sternberg SS, editor. Histology for Pathologists. Raven Press; New York: 1992. pp. 797–834. [Google Scholar]
- Herbst A, Bern H. Developmental effects of DES in Pregnancy. Thieme Stratton; New York: 1981. [Google Scholar]
- Herbst AL, Robboy SJ, Scully RE, Poskanzer DC. Clear-cell adenocarcinoma of the vagina and cervix in girls: analysis of 170 registry cases. Am J Obstet Gynecol. 1974;119:713–724. [PubMed] [Google Scholar]
- Herbst AL, Scully RE, Robboy SJ. Vaginal adenosis and other diethylstilbestrol-related abnormalities. Clin Obstet Gynecol. 1975;18:185–194. doi: 10.1097/00003081-197509000-00021. [DOI] [PubMed] [Google Scholar]
- Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, Colton T, Hartge P, Hatch EE, Herbst AL, Karlan BY, Kaufman R, Noller KL, Palmer JR, Robboy SJ, Saal RC, Strohsnitter W, Titus-Ernstoff L, Troisi R. Adverse health outcomes in women exposed in utero to diethylstilbestrol. New Engl J Med. 2011;365:1304–1314. doi: 10.1056/NEJMoa1013961. [DOI] [PubMed] [Google Scholar]
- Hsieh-Li HM, Witte DP, Weinstein M, Branford W, Li H, Small K, Potter SS. Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development. 1995;121:1373–1385. doi: 10.1242/dev.121.5.1373. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Takase M, Takasugi N. Development of vaginal adenosis-like lesions and uterine epithelial stratification in mice exposed perinatally to diethylstilbestrol. Proc Soc Exp Biol Med. 1986;181:59–65. doi: 10.3181/00379727-181-42224. [DOI] [PubMed] [Google Scholar]
- Jaubert A, Robboy SJ, Mutter GL, MF . Embryology. In: Mutter G, Robboy SJ, Mutte G, Prat J, Bentley P, Russell P, Anderson M, editors. Robboy’s Pathology of the Female Reproductive Tract. Churchill Livingstone; London: 2009. pp. 1–22. [Google Scholar]
- Jefferies JA, Robboy SJ, O’Brien PC, Bergstralh EJ, Labarthe DR, Barnes AB, Noller KL, Hatab PA, Kaufman RH, Townsend DE. Structural anomalies of the cervix and vagina in women enrolled in the Diethylstilbestrol Adenosis (DESAD) Project. Am J Obstet Gynecol. 1984;148:59–66. doi: 10.1016/s0002-9378(84)80033-2. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Couse JF, Banks EP, Korach KS, Newbold RR. Expression of estrogen receptor beta is developmentally regulated in reproductive tissues of male and female mice. Biol Reprod. 2000;62:310–317. doi: 10.1095/biolreprod62.2.310. [DOI] [PubMed] [Google Scholar]
- Kaufman RH, Adam E. Findings in female offspring of women exposed in utero to diethylstilbestrol. Obstet Gynecol. 2002;99:197–200. doi: 10.1016/s0029-7844(01)01682-9. [DOI] [PubMed] [Google Scholar]
- Kaufman RH, Adam E, Binder GL, Gerthoffer E. Upper genital tract changes and pregnancy outcome in offspring exposed in utero to diethylstilbestrol. Am J Obstet Gynecol. 1980;137:299–308. doi: 10.1016/0002-9378(80)90913-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet. 2003;4:969–980. doi: 10.1038/nrg1225. [DOI] [PubMed] [Google Scholar]
- Koff AK. Development of the vagina in the human fetus. Contrib Embryol Carne Inst Wash. 1933;24:59–90. [PubMed] [Google Scholar]
- Korach KS, Horigome T, Tomooka Y, Yamashita S, Newbold RR, McLachlan JA. Immunodetection of estrogen receptor in epithelial and stromal tissues of neonatal mouse uterus. Proc Natl Acad Sci USA. 1988;85:3334–3337. doi: 10.1073/pnas.85.10.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T. Developmental origin of vaginal epithelium. Differ; Res Biol Divers. 2010;80:99–105. doi: 10.1016/j.diff.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T. Normal and abnormal epithelial differentiation in the female reproductive tract. Differ; Res Biol Divers. 2011;82:117–126. doi: 10.1016/j.diff.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Mullerian) epithelial differentiation. Dev Biol. 2001;240:194–211. doi: 10.1006/dbio.2001.0458. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cunha GR. Roles of p63 in differentiation of Mullerian duct epithelial cells. Ann N Y Acad Sci. 2001;948:9–12. doi: 10.1111/j.1749-6632.2001.tb03982.x. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cunha GR, Robboy SJ, Mills AA, Medina RT. Differential expression of p63 isoforms in female reproductive organs. Mech Dev. 2005;122:1043–1055. doi: 10.1016/j.mod.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee K-J, Cooke PS, Lydon JP, Cunha GR. Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biol Reprod. 2000a;62:831–838. doi: 10.1095/biolreprod62.4.831. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, Cunha GR. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000b;62:821–830. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- Kurita T, Mills AA, Cunha GR. Roles of p63 in the diethylstilbestrol-induced cervicovaginal adenosis. Development. 2004;131:1639–1649. doi: 10.1242/dev.01038. [DOI] [PubMed] [Google Scholar]
- Kurita T, Nakamura H. Embryology of the uterus. In: Aplin JD, Fazleabas AT, Glasser SR, Giudice LC, editors. Endometrium. Informa UK Ltd; London: 2008. pp. 1–18. [Google Scholar]
- Kurita T, Young P, Brody J, Lydon JP, O’Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell (UtE) proliferation. Endocrinology. 1998;139:4708–4713. doi: 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- Larkins CE, Enriquez AB, Cohn MJ. Spatiotemporal dynamics of androgen signaling underlie sexual differentiation and congenital malformations of the urethra and vagina. Proc Natl Acad Sci USA. 2016;113:E7510–E7517. doi: 10.1073/pnas.1610471113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laronda MM, Unno K, Butler LM, Kurita T. The development of cervical and vaginal adenosis as a result of diethylstilbestrol exposure in utero. Differ; Res Biol Divers. 2012;84:252–260. doi: 10.1016/j.diff.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laronda MM, Unno K, Ishi K, Serna VA, Butler LM, Mills AA, Orvis GD, Behringer RR, Deng C, Sinha S, Kurita T. Diethylstilbestrol induces vaginal adenosis by disrupting SMAD/RUNX1-mediated cell fate decision in the Mullerian duct epithelium. Dev Biol. 2013;381:5–16. doi: 10.1016/j.ydbio.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmen JG, Broekhof JL, Kuiper GG, Gustafsson JA, van der Saag PT, van der Burg B. Expression of estrogen receptor alpha and beta during mouse embryogenesis. Mech Dev. 1999;81:163–167. doi: 10.1016/s0925-4773(98)00223-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Sinclair A, Cao M, Shen J, Choudhry S, Botta S, Cunha G, Baskin L. Canalization of the urethral plate precedes fusion of the urethral folds during male penile urethral development: the Double Zipper Hypothesis. J Urol. 2014 doi: 10.1016/j.juro.2014.09.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Ma L, Benson GV, Lim H, Dey SK, Maas RL. Abdominal B (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in mullerian duct by the synthetic estrogen diethylstilbestrol (DES) Dev Biol. 1998;197:141–154. doi: 10.1006/dbio.1998.8907. [DOI] [PubMed] [Google Scholar]
- Magro G, Grasso S. Expression of cytokeratins, vimentin and basement membrane components in human fetal male mullerian duct and perimullerian mesenchyme. Acta Histochem. 1995;97:13–18. doi: 10.1016/S0065-1281(11)80202-3. [DOI] [PubMed] [Google Scholar]
- Matsuzaki S, Fukaya T, Suzuki T, Murakami T, Sasano H, Yajima A. Oestrogen receptor alpha and beta mRNA expression in human endometrium throughout the menstrual cycle. Mol Hum Reprod. 1999;5:559–564. doi: 10.1093/molehr/5.6.559. [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Bullock B. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980;40:3988–3999. [PubMed] [Google Scholar]
- Mijsberg W. Uber die Entwicklung der vagina, des Hymen und des Sinus urogenitalis beim Menchen. Z Anat Entw-Gesch. 1924;74:684–760. [Google Scholar]
- Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol. 2008;129:705–733. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratin polypeptides: patterns of expression of specific cytokeratins in normal epithelia, tumors, and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moore KL, Persaud TVN. The Developing Human. Saunders; Philadelphia: 2003. [Google Scholar]
- Mortlock DP, Innis JW. Mutation of HOXA13 in hand-foot-genital syndrome. Nat Genet. 1997;15:179–180. doi: 10.1038/ng0297-179. [DOI] [PubMed] [Google Scholar]
- Müller J. Bildungsgeschichte der Genitalien. Arnz; Dusseldorf: 1830. [Google Scholar]
- Mutter GL, Robboy SJ. Embryology. In: Mutter GS, Prat J, editors. Pathology of the Female Reproductive Tract. Churchill Livingston; London: 2014. pp. 1–17. [Google Scholar]
- Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199:142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Newbold RR, MaLachlan JA. Diethylstilbestrol-associated defects in murine genital tract development. In: McLachlan JA, editor. Estrogens in the Environment II. Elsevier; New York: 1985. pp. 288–318. [Google Scholar]
- Newbold RR, McLachlan JA. Vaginal adenosis and adenocarcinoma in mice exposed prenatally or neonatally to diethylstilbestrol. Cancer Res. 1982;42:2003–2011. [PubMed] [Google Scholar]
- Newbold RR, Tyrey S, Haney AF, McLachlan JA. Developmentally arrested oviduct: a structural and functional defect in mice following prenatal exposure to diethystilbestrol. Teratology. 1983;27:417–426. doi: 10.1002/tera.1420270316. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Bjornsdottir S, Hoyer PE, Byskov AG. Ontogeny of oestrogen receptor alpha in gonads and sex ducts of fetal and newborn mice. J Reprod Fertil. 2000;118:195–204. doi: 10.1530/jrf.0.1180195. [DOI] [PubMed] [Google Scholar]
- Okada A, Ohta Y, Buchanan DL, Sato T, Inoue S, Hiroi H, Muramatsu M, Iguchi T. Changes in ontogenetic expression of estrogen receptor alpha and not of estrogen receptor beta in the female rat reproductive tract. J Mol Endocrinol. 2002;28:87–97. doi: 10.1677/jme.0.0280087. [DOI] [PubMed] [Google Scholar]
- Orvis GD, Behringer RR. Cellular mechanisms of Mullerian duct formation in the mouse. Dev Biol. 2007;306:493–504. doi: 10.1016/j.ydbio.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plapinger L, Bern HA. Adenosis-like lesions and other cervicovaginal abnormalities in mice treated perinatally with estrogen. J Natl Cancer Inst. 1979;63:507–518. [PubMed] [Google Scholar]
- Riedel I, Liang FX, Deng FM, Tu L, Kreibich G, Wu XR, Sun TT, Hergt M, Moll R. Urothelial umbrella cells of human ureter are heterogeneous with respect to their uroplakin composition: different degrees of urothelial maturity in ureter and bladder? Eur. J Cell Biol. 2005;84:393–405. doi: 10.1016/j.ejcb.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Robboy SJ. A hypothetic mechanism of diethylstilbestrol(DES)-induced anomalies in exposed progeny. Hum Pathol. 1983;14:831–833. doi: 10.1016/s0046-8177(83)80157-9. [DOI] [PubMed] [Google Scholar]
- Robboy SJ, Kurita T, Baskin L, Cunha GR. New insights insights into human female reproductive tract development. Differ; Res Biol Divers. 2017 doi: 10.1016/j.diff.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robboy SJ, Mutter GL. Disorders of sexual development. In: Mutter GS, Prat J, editors. Pathology of the Female Reproductive Tract. Churchill Livingston; London: 2014. pp. 18–47. [Google Scholar]
- Robboy SJ, Noller KL, RHK, Barnes AB, Townsend D, Gunderson JH, Kurland L, Nash S. An atlas of findings in the human female after intrauterine exposure to diethylstilbestrol. DHEW publication. 1984:84–2344. [Google Scholar]
- Robboy SJ, Scully RE, Welch WR, Herbst AL. Intrauterine diethylstilbestrol exposure and its consequences: pathologic characteristics of vaginal adenosis, clear cell adenocarcinoma, and related lesions. Arch Pathol Lab Med. 1977;101:1–5. [PubMed] [Google Scholar]
- Robboy SJ, Welch WR, Young RH, Truslow GY, Herbst AL, Scully RE. Topographic relation of cervical ectropion and vaginal adenosis to clear cell adenocarcinoma. Obstet Gynecol. 1982;60:546–551. [PubMed] [Google Scholar]
- Rodriguez E, Jr, Weiss DA, Ferretti M, Wang H, Menshenia J, Risbridger G, Handelsman D, Cunha G, Baskin L. Specific morphogenetic events in mouse external genitalia sex differentiation are responsive/dependent upon androgens and/or estrogens. Differ; Res Biol Divers. 2012;84:269–279. doi: 10.1016/j.diff.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxen L. Organogenesis of the Kidney. Cambridge University Press; New York: 1987. [Google Scholar]
- Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC, Endocrine S. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi O, Cunha GR, Robboy SJ. Experimental study of the effect of diethylstilbestrol on the development of the human female reproductive tract. Int J Biol Res Pregnancy. 1983;4:56–70. [PubMed] [Google Scholar]
- Taguchi O, Cunha GR, Robboy SJ. Expression of nuclear estrogen-binding sites within developing human fetal vagina and urogenital sinus. Am J Anat. 1986;177:473–480. doi: 10.1002/aja.1001770405. [DOI] [PubMed] [Google Scholar]
- Terakawa J, Rocchi A, Serna VA, Bottinger EP, Graff JM, Kurita T. FGFR2IIIb-MAPK Activity Is Required for Epithelial Cell Fate Decision in the Lower Mullerian Duct. Mol Endocrinol. 2016;30:783–795. doi: 10.1210/me.2016-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus-Ernstoff L, Troisi R, Hatch EE, Palmer JR, Hyer M, Kaufman R, Adam E, Noller K, Hoover RN. Birth defects in the sons and daughters of women who were exposed in utero to diethylstilbestrol (DES) Int J Androl. 2010;33:377–384. doi: 10.1111/j.1365-2605.2009.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viebahn C, Lane EB, Ramaekers FC. The mesonephric (wolffian) and paramesonephric (mullerian) ducts of golden hamsters express different intermediate-filament proteins during development. Differ; Res Biol Divers. 1987;34:175–188. doi: 10.1111/j.1432-0436.1987.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Wong KH, Wintch HD, Capecchi MR. Hoxa11 regulates stromal cell death and proliferation during neonatal uterine development. Mol Endocrinol. 2004;18:184–193. doi: 10.1210/me.2003-0222. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Newbold RR, McLachlan JA, Korach KS. The role of the estrogen receptor in uterine epithelial proliferation and cytodifferentiation in neonatal mice. Endocrinology. 1990;127:2456–2463. doi: 10.1210/endo-127-5-2456. [DOI] [PubMed] [Google Scholar]
- Yu J, Manabe M, Wu XR, Xu C, Surya B, Sun TT. Uroplakin I: a 27-kD protein associated with the asymmetric unit membrane of mammalian urothelium. J Cell Biol. 1990;111:1207–1216. doi: 10.1083/jcb.111.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]