Abstract
Humanized mice, i.e. animals engrafted with human tissues and/or expressing human genes, have been instrumental in improving our understanding of the pathogenesis and immunological processes that define some of the most challenging human-tropic viruses. In particular, mice engrafted with components of a human immune system (HIS) offer unprecedented opportunities for mechanistic studies of human immune responses to infection. Here, we provide a brief overview of the current panel of HIS mouse models available and cite recent examples of how such humanized animals have been used to study immune responses and pathogenesis elicited by human-tropic viruses. Finally, we will outline some of the challenges that lay ahead and strategies to improve and refine humanized mice with the goal of more accurately recapitulating human immune responses to viral infection.
Keywords: Humanized mice, virus infection, immunity, pathogenesis, host tropism
Graphical Abstract
Introduction
Human-tropic viruses, such as human immunodeficiency virus (HIV), hepatitis B virus (HBV) and Dengue virus (DENV), are causes of major health and economic concerns worldwide [1]. However, the restricted human tropism of these viruses, along with the scarcity of permissive animal models, has significantly hampered our ability to study the life cycles of these viruses and, ultimately, to develop anti-viral therapies and vaccines [2]. Characterizing the pathogenesis of these viruses and the immune response they invoke in human patients has also proven challenging. With the exception of the peripheral blood, human tissues are not readily accessible, and comprehensive data analysis is complicated by a high number of variables, including, but not limited to, intra-human genetic variation, age, gender, co-morbidities, nutritional and microbiome status. Over the past three decades, immunodeficient mice engrafted with components of a human immune system (HIS) have been an effective tool for studying the life cycle of a broad range of human-tropic pathogens in controlled experimental settings. Among these pathogens are HIV [3], human cytomegalovirus [4], Epstein Barr virus (EBV) [5,6], DENV [7–12], human T-cell leukemia virus (HTLV) [13], Karposi sarcoma-associated herpes virus (KSHV) [14], yellow fever virus vaccine strain 17D (YFV-17D) [15], Salmonella typhi [16], Borrelia hermsii [17] and Mycobacterium tuberculosis [18,19]. By modeling critical aspects of pathogenesis or immunological features observed in infected human patients, humanized mice have allowed major breakthroughs in our understanding of the interactions between viruses of strong clinical relevance and their human host.
Human-hemato lymphoid system mice models for studying viral pathogenesis and immunity
The accurate modeling of virus-human interactions in humanized mice is heavily dependent on the generation of mouse models able to support human hematopoietic stem cell (HSC) engraftment, recapitulate the complexity of human hematopoiesis and mount human-like immune responses, both in terms of function and spatial organization. To meet these requirements, suitable xenorecipient strains need to have 1) limited human graft rejection and 2) a favorable environment for human hematopoiesis.
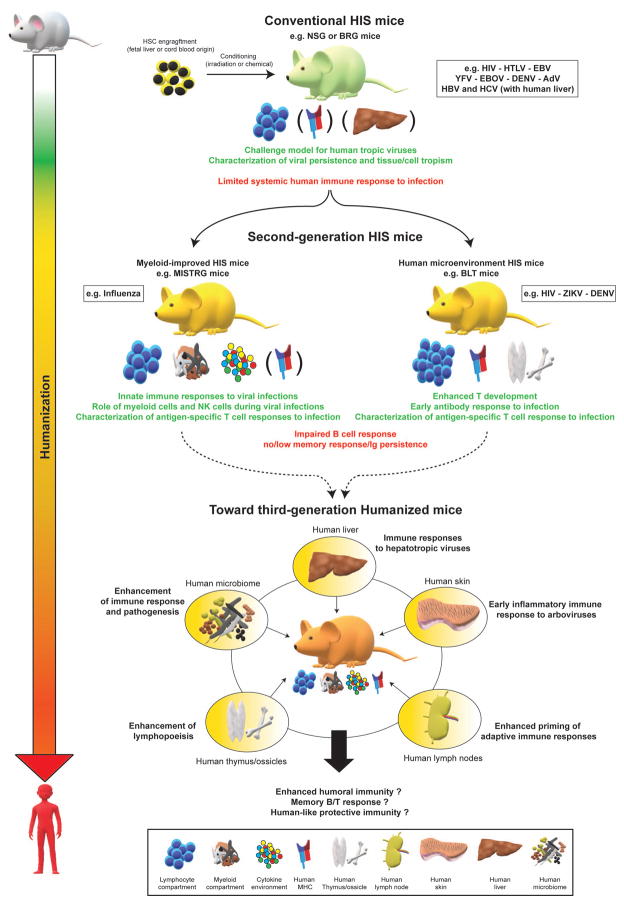
Key mutations or genetic deficiencies that strongly attenuate rejection of transplanted human HSCs by the murine immune system have been identified, such as Scid or Rag1/Rag2 mutations that prevent mouse B/T cell maturation [20–28] and an IL-2 receptor γ mutation that disrupts IL-15 mediated signaling and natural killer (NK) cell development [27,29–31]. A polymorphism discovered in the signal regulatory protein α (SIRPα) in the non-obese diabetic (NOD) genetic background can promote mouse macrophage tolerance to human cells upon HSC engraftment of HIS mice [23,24,32,33]. Up till now, the most commonly used xenorecipient strains for generating HIS mice utilized different combinations of these three mutations, such as NOD-Scid [23,24,34], NOD-Scid IL2rγnull (NSG or NOG) [29–31], BALB-c-Rag2−/−IL2rγnull (BRG) [27] or NOD- Rag2−/−IL2rγnull (NRG) [35,36]. These conventional HIS mice (Figure 1) all support efficient human hematolymphoid engraftment, including a broad variety of lymphoid cells and some myeloid cells.
Figure 1. Current and future humanized mouse models for the study of viral infection and immunity.
Advantages (green text) and limitations (red text) of conventional HIS mice and second-generation HIS mice (myeloid-improved HIS mice and human-microenvironment HIS mice) are shown. Across the entire figure, humanization levels are symbolized by a color gradient from white (unaltered/wildtype mice) to red (humans). Examples of viral pathogens are listed in a box beside the respective model in which they have been studied. Pictograms representing the specific characteristics and/or improvements of each category are shown (legend at bottom of figure). Characteristics/improvements not ubiquitously present across all HIS mice models of a given category (conventional or second-generation) are indicated in parentheses. Putative improvements of second-generation HIS mice toward the generation of third-generation HIS mice are depicted in yellow circles. HSC, human hematopoietic cells.
However, although there is evidence that the engrafted HIS becomes activated upon microbial challenge in such strains, the immune response is generally weak [5,19,37–39]. This limited immune functionality is due to numerous factors, including aberrant organization of secondary lymphoid structures, the lack of human MHC molecules on non-hematopoietic tissues, and the underrepresentation of critical human immune cell lineages, which are key in activating the adaptive immune response.
In particular, the scarcity of human dendritic cells (DCs) – a central cell type bridging innate and adaptive immune responses [40] – and other myeloid lineages and NK cells decreases the functionality of the engrafted HIS. The low frequency of these cell populations can be explained by the limited biological cross-reactivity of non-redundant cytokines that promote lineage differentiation [41,42].
To address such caveats, there have been continuous efforts to further refine xenorecipient strains and humanization protocols, hence leading to improved, second-generation HIS mouse models (Figure 1). Recent work has particularly focused on combining an immunodeficient genetic background with additional mutations to promote a more favorable environment for human hematopoiesis and immune response. Mutations in the mouse gene encoding c-kit improved HIS reconstitution in non-irradiated BRG or NSG mice [43,44]. Additionally, the transgenic or knock-in expression of one or several human cytokines (such as IL-3, TPO, GM-CSF or Flt3L) [45–53] enhanced engraftment and differentiation of multiple myeloid subsets and NK cells, which have been poorly represented in conventional humanized NSG and BRG mice (Figure 1). HIS mice expressing human MHC molecules have also been used to facilitate tracking of human antigen-specific T cells [5,6,38,54].
Additionally, the reconstitution of non-immune human microenvironments in HIS mice can also promote more physiological hematopoiesis (Figure 1). For instance, the generation of a bone marrow microenvironment by co-engraftment of human HSCs with ossicles created ex vivo from bone marrow-derived mesenchymal stromal cells (MSCs) enhanced human-like hematopoiesis [55–59]. Furthermore, HIS mice engrafted with small fragments of fetal liver and thymus, namely bone marrow-liver-thymus (BLT) mice [10,12,60–62], or with embryonic stem cell-derived thymic epithelial progenitors [63], can stimulate human lymphopoiesis and enhance T cell education.
Recent findings in viral pathogenesis and immune responses to infection using humanized mice
Some of the most challenging human viruses display a very narrow tropism restricted to humans and a few primate species [2]. Hence, HIS mice often represent the only and most appropriate alternative to characterize the life cycle of these viruses in vivo. Here, we present recent selected findings that highlight the utility of HIS mice for uncovering virus-host interactions that regulate viral pathogenesis and immune responses in humans. Specifically, we will discuss three distinct groups of human-tropic viruses that are of great significance to human health and for which in vivo investigation has been considerably challenging: lymphotropic viruses, flaviviruses and hepatotropic viruses.
Lymphotropic viruses
Many lymphotropic viruses, such as HIV, HTLV and EBV, cause significant mortality and morbidity worldwide [64–66]. HIS mice models have been instrumental in unveiling molecular interactions between these viruses and their target cells in vivo. In the case of HIV, humanized mice have a long-standing track record as the only small animal model for testing preclinically the efficacy of anti-retroviral therapies and vaccination approaches [67,68]. While HIV infection can now be controlled in patients through administration of highly active anti-retroviral therapy (HAART) [69], a cure for HIV and a prophylactic vaccine protecting against HIV remain elusive. To develop more effective, possibly curative, therapies, a better understanding is needed of the dynamic interactions between HIV and the human immune system in vivo. Towards this goal, HIS mice have provided mechanistic insights into HIV latency and anti-viral immunity following viral reactivation. Work with humanized NOD/SCID mice demonstrated that tissue macrophages constitute a latent HIV reservoir in vivo, highlighting that cell types besides CD4+ T cells allow HIV persistence after long-term suppressive antiretroviral therapy [70]. Reactivated latent viruses have also been found that harbor mutations which promote escape of the CD8+ T cell response. In HIS mice, stimulation of specific CD8+ T cell clonal populations able to recognize latent HIV viruses induced the elimination of the reactivated viruses [71].
The role of type I interferons (IFNs) in HIV replication and pathogenesis has also been explored in HIS mice. Although HIV does not induce type I IFN production in infected CD4+ T cells, a recent report showed that TREX1 (TRanscription-Export complex 1) downregulation in CD4+ T cells of the genital mucosa increases type I IFN in vivo and inhibits early HIV replication in BLT-NSG mice [72]. Importantly, intravaginal injection of recombinant type I IFN had similar effects, but systemic injection of recombinant type I IFN enhanced viral replication and dissemination. However, this is still a matter of debate. Other groups have reported that type I IFN signaling and type I IFN produced by plasmacytoid DCs inhibit viral replication but promote HIV-induced cell death in HIS mice [73,74], suggesting a differential effect of type I IFNs depending on the concentration and/or spatio-temporal dynamics of induction.
Furthermore, the anti-HIV activity of specific host proteins has also been investigated in HIS mice. Two host factors, APOBEC3 and BST2, strongly inhibit HIV replication, evolution and dissemination [75,76], thereby highlighting the importance of HIV’s ability to antagonize these restriction factors in vivo for the establishment of chronic infection and pathogenesis. Additionally, IL-21-mediated upregulation of micro-RNA129 in HIS mice inhibited early HIV infection [77].
Another lymphotropic virus is EBV, a γ-herpesvirus widely prevalent in the human population that replicates in B cells and can cause B cell lymphomas [78]. The scarcity of animal models for EBV has not only impeded the development of treatments but has also hampered studies of EBV-associated disease [79]. HIS mice have emerged as a versatile experimental platform for not only analyzing immune responses and EBV-associated pathogenesis but also for defining the role of specific EBV viral proteins in vivo. For example, it was recently shown that the EBV large tegument protein BPLF1 is an important promoter of B-cell transformation and subsequent tumor formation in HIS mice [80]. Humanized mice used to model a clinically relevant co-infection of EBV and KSHV demonstrated that persistent KSHV infection can enhance EBV-associated tumor formation in vivo [81], highlighting the synergy between the pathogenesis processes of these two tumor viruses. Additionally, with their utility for modeling EBV-associated tumorigenesis, HIS mice also represent a pre-clinical platform for therapeutic strategies against EBV. Recently, a group demonstrated that T-cell responses against EBV can be enhanced via a PD-1/CTLA-4 pathway blockade, leading to a reduction of EBV-infected cells and better control of EBV-induced lymphoma in HIS-NSG mice [82].
Flaviviruses
Arthropod-borne flaviviruses, such as DENV, Zika virus (ZIKV) and YFV, pose significant clinical health concerns [83–87]. Although flaviviruses causing disease in humans usually display a broad cellular tropism and can replicate in a variety of non-human primate tissues [88–90], the pathogenic processes associated with these viruses mostly result from specific interactions with human specific-components [88,91–93].
With no treatment available, DENV is responsible for 50 to 100 million infections with clinical manifestations every year [83]. Dengue fever, caused by DENV infection, is usually non-lethal, but re-infection with heterologous DENV serotypes can lead to severe hemorrhagic disease [94] as a result of antibody-dependent enhancement (ADE). Inbred immunocompetent mice are not permissive to DENV infection, presumably due to the inability of DENV to evade murine antiviral responses [95]. When specific murine innate immune signaling pathways are blunted, infection with mouse-adapted DENV strains can be established [89]. However, the utility of such immunocompromised mouse models is limited by the partial functionality of the immune system and having to study replication of a human virus in the murine cellular environment [89]. Consequently, HIS mice have proven useful for exploring the in vivo replication of not only lab-adapted but also patient-derived viruses and the intricate role of the immune response in controlling or promoting DENV-pathogenesis [12,89].
In humanized NSG mice, human NK cells were shown to be critical for controlling DENV infection in vivo, limiting viral replication, thrombocytopenia and liver damage [96]. Additionally, contacts between NK cells and DENV-infected monocyte-derived DCs were crucial for NK cell activation and control of infection. As DENV is a mosquito-borne virus, early skin-specific inflammatory events at the site of infection are particularly important for the DENV life cycle and development of immunopathogenesis. Although DENV infections in advanced HIS mouse models dually engrafted with allogenic skin explants have yet to be performed, previous studies have reported that DENV disease and replication are more severe in conventionally humanized NSG mice after mosquito-mediated infection than after intra-dermal injection [97]. HIS mice have also been used to test the efficacy of several anti-viral strategies, such as antibody-based therapies [98].
Beyond conventional humanized mice, BLT-NSG mice have also been a valuable platform for studying DENV infection, mounting a more extensive adaptive immune response to infection. BLT-NSG mice can support sustained DENV replication, mount an HLA-A2-restricted human T-cell response and produce DENV-neutralizing IgM antibodies upon infection [10,12,99]. Nevertheless, with limited evidence for class-switching and affinity maturation, the quality of the humoral response and, ultimately, the neutralizing capacity of virus-specific antibodies in BLT mice is still not vastly different from those in conventional HIS mouse models. These aspects limit proper characterization of e.g. DENV-induced ADE in vivo and highlight the need for further immunological improvements in HIS mice to better understand key flavivirus-mediated immunopathogenesis processes. More recently, humanized BLT mice have also been employed as a challenge model for ZIKV infection and were shown to support long-term replication of the virus [100].
With a significantly higher mortality rate than DENV, YFV is responsible for around 200,000 new infections and 30,000 deaths each year [86]. Although YFV was one of the major infectious diseases in the 18th and 19th centuries, the generation of a potent, live-attenuated vaccine in the 1930s [101], YFV-17D, significantly constrained the spread of the virus, which is endemic in only South America and Africa. However, the host mechanisms governing YFV pathogenesis and the potent immunogenicity of YFV-17D remain poorly understood. Additionally, recent vaccine shortages and low vaccination coverage in at-risk areas create ideal conditions for YFV re-emergence and outbreaks of significant health concern [102–104]. Altogether, this situation highlights the urgent need for improving our understanding of the pathogenesis and immunological processes that govern YFV infection in vivo.
Similar to DENV, YFV replication is inhibited by the murine innate immune response in immunocompetent mouse models [36,105], and in vivo infection is restricted to a few primate species. Recently, our group provided proof-of-concept that HIS mice are permissive to YFV-17D infection [36]. Effective viral replication in the blood and in lymphoid tissues was dependent on the presence of a HIS, and we identified a set of human-specific, spatio-temporal interactions between particular immune cell subsets and YFV-17D. Hence, HIS mice represent ideal platforms for understanding how virulent YFV strains and YFV-17D differentially interact with the HIS, which could ultimately open important avenues for the design of novel vaccine or immunotherapy strategies against flavivirus infections.
Hepatotropic viruses
An estimated 330–350 million people are persistently infected with HBV, hepatitis delta (HDV) and/or hepatitis C virus (HCV) [106,107]. Chronic carriers are at risk of developing severe liver disease including fibrosis, cirrhosis and hepatocellular carcinoma. Despite significant advances in prevention and treatment, hepatitis viruses remain a medical problem. There is no vaccine protecting against HCV, but direct-acting antivirals can now cure chronic hepatitis C in a majority of patients. Conversely, an effective prophylactic vaccine for HBV and HDV does exist, but there are no approved treatments for treating chronic hepatitis delta and current HBV therapies that can suppress viremia are rarely curative. The unique human-tropism and liver-specific replication cycle of these viruses [108,109] have posed challenges for creating small animal models suitable for studying immune responses and pathogenesis of these viruses and ultimately develop drug and vaccine candidates. Mice engrafted with human hepatocytes, so-called human liver chimeric mice, remain the gold standard and have proven suitable model for chronic HBV, HCV, HDV and, most recently, hepatitis E virus infection [110–119]. However, the highly immunocompromised status of these engrafted mice precludes the study of immune-mediated pathogenesis by human hepatitis viruses.
Ongoing efforts are thus aimed at engrafting suitable xenorecipients with both human hepatocytes and components of a HIS (HIS-huHEP mice) [120–122]. Such dually humanized mice initially gained traction for studying immune responses and liver disease progression in the context of HCV infection [123]. More recently, HIS-huHEP mice were shown to support persistent HBV infection over several months [124–126]. Liver inflammation and fibrosis were observed in infected HIS-huHEP mice, and increased M2 macrophage infiltration of the liver potentially promoted liver disease [124]. Unlike immunodeficient human liver only-engrafted mice, HIS-huHEP mice exhibit partial immune control of HBV infection with detectable antigen-specific IgGs and liver-infiltrating Kupffer cells, NK cells and CD4+ T cells [126]. Nevertheless, the immune response to HBV remains low in HIS-huHEP mice [124,126] as most of the mouse strains employed thus far to generate HIS-huHEP mice are conventional strains with low myeloid and NK cell engraftment. In addition to HSC transfer, a recent modified humanization protocol enhanced donor-matched fetal hepatoblast engraftment by human oncostatin-M administration [125]. Interestingly, the more robust hepatic reconstitution also led to higher frequencies of human monocytes and NK cells model in comparison to conventional HIS mice, underscoring the important crosstalk between the liver and immune system even in steady state. Upon HBV infection, NK cell frequencies increased significantly, and NK cells acquired an activated phenotype.
HIS mice models for studying virus infection: Promises and upcoming challenges
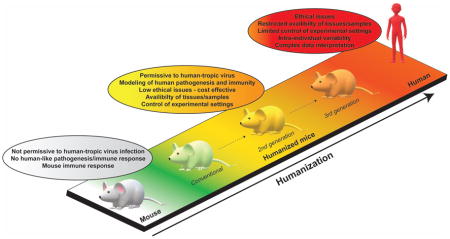
Understanding the pathogenesis and immune responses elicited by human-tropic pathogens presents considerable challenges. Over the past decades, humanized mice have proven susceptible to a large number of human-tropic pathogens and have thus emerged as valuable platforms to model human-specific infectious processes in vivo. However, despite the ability of HIS mice to support the complete replication cycle of multiple human-tropic viruses [127], recapitulating the full panel of interactions between these viruses and the HIS as well as the complex immunological mechanisms that result from them remains challenging. Such limitations hamper our understanding of the molecular mechanisms governing the frontier between virus-induced protective immunity and viral pathogenicity/chronicity.
Protocols have been developed that can yield humanized mice with high human hematopoietic chimerism. However, in conventional models, important human subsets, in particular those of the erythro-myeloid lineage and NK cells, are not generated at physiological levels. This can be attributed to the absence or limited biological cross-reactivity of cytokines crucial for the development of these subsets. Consequently, immune functionality is impaired, and there is limited evidence that even the most advanced HIS mice models can mount a potent human-like immune response. Despite recent accomplishments in promoting superior engraftment of key myeloid/NK cell subsets and/or superior hematopoiesis [45–53,128], more refined HIS mice models displaying robust memory B- and T-cell responses, human-like neutralizing titers and long-term protective immunity remain a major challenge.
In the future, overcoming these caveats would open unique opportunities for i.) a better understanding of how the immune response is evaded and exhausted during chronic and latent viral infection; ii.) exploring human-specific immunopathogenesis processes (such as DENV-induced ADE) during hemorrhagic fever virus infection; iii.) establishing correlates of protection by in-depth study of effective human vaccines, such as the YFV vaccine, and test novel vaccine strategies; and iv.) understanding the impact of pre-existing heterologous immunity on human immune function to other human pathogens.
For the generation of third-generation HIS mice, one promising approach could co-engraft key human tissues that would work in synergy to enhance human hematopoiesis and immune response into a mouse genetic background modeling a favorable human cytokine environment by expression of one or more human cytokines (Figure 1). For instance, the co-engraftment of human HSCs along with human lymph nodes in a mouse genetic background promoting human myeloid and NK cell subset expansion likely represents a valuable strategy to enhance B-cell effector and memory response. Additionally, the co-engraftment of HSCs and human hepatocytes in a similar such genetic background would open novel avenues for better understanding the immune response to human hepatotropic viruses in vivo (Figure 1). Humanized mice have been continually refined over the last three decades and have matured from simple challenge models for human-tropic viruses to experimental platforms for studying aspects of human immunity in vivo. The future refinements of these models undoubtedly hold great promise for uncovering novel molecular mechanisms regulating viral pathogenicity and immunity.
Highlights.
Studying human-tropic virus has been challenging due to their often-narrow host range.
HIS mice have been instrumental for characterizing human-tropic virus infection in vivo.
Key virus-induced pathogenesis and immunological processes have been uncovered.
However, HIS mice still exhibit only a partial human immune response.
Future refinements of HIS mice models will be critical for further discoveries.
Acknowledgments
The authors thank Jenna Gaska for edits and critical discussion of the manuscript. We apologize to all colleagues whose work could not be cited due to space constraints.
Funding sources
Work in the laboratory is supported in part by grants from the National Institutes of Health (R01 AI079031, R01 AI107301), a Research Scholar Grant from the American Cancer Society, the Grand Challenge Program of Princeton University and an Investigator in Pathogenesis Award by the Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* of interest, ** of special interest
- 1.Morens DM, Fauci AS. Emerging infectious diseases: threats to human health and global stability. PLoS Pathog. 2013;9:e1003467. doi: 10.1371/journal.ppat.1003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douam F, Gaska JM, Winer BY, Ding Q, von Schaewen M, Ploss A. Genetic Dissection of the Host Tropism of Human-Tropic Pathogens. Annu Rev Genet. 2015;49:21–45. doi: 10.1146/annurev-genet-112414-054823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berges BK, Wheat WH, Palmer BE, Connick E, Akkina R. HIV-1 infection and CD4 T cell depletion in the humanized Rag2−/−gamma c−/− (RAG-hu) mouse model. Retrovirology. 2006;3:76. doi: 10.1186/1742-4690-3-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith MS, Goldman DC, Bailey AS, Pfaffle DL, Kreklywich CN, Spencer DB, Othieno FA, Streblow DN, Garcia JV, Fleming WH, et al. Granulocyte-colony stimulating factor reactivates human cytomegalovirus in a latently infected humanized mouse model. Cell Host Microbe. 2010;8:284–291. doi: 10.1016/j.chom.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5**.Strowig T, Gurer C, Ploss A, Liu YF, Arrey F, Sashihara J, Koo G, Rice CM, Young JW, Chadburn A, et al. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med. 2009;206:1423–1434. doi: 10.1084/jem.20081720. First evidence priming of virus-specific, HLA-restricted CD8+ T cells in humanized mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, Doi T, Sone A, Suzuki N, Fujiwara H, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A. 2010;107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bente DA, Melkus MW, Garcia JV, Rico-Hesse R. Dengue fever in humanized NOD/SCID mice. J Virol. 2005;79:13797–13799. doi: 10.1128/JVI.79.21.13797-13799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaumier CM, Jaiswal S, West KY, Friberg H, Mathew A, Rothman AL. Differential in vivo clearance and response to secondary heterologous infections by H2(b)-restricted dengue virus-specific CD8+ T cells. Viral Immunol. 2010;23:477–485. doi: 10.1089/vim.2010.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanya S, Kim SS, Abraham S, Yao J, Kumar M, Kumar P, Haridas V, Lee SK, Shultz LD, Greiner D, et al. Targeted delivery of small interfering RNA to human dendritic cells to suppress dengue virus infection and associated proinflammatory cytokine production. J Virol. 2010;84:2490–2501. doi: 10.1128/JVI.02105-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaiswal S, Pazoles P, Woda M, Shultz LD, Greiner DL, Brehm MA, Mathew A. Enhanced humoral and HLA-A2-restricted dengue virus-specific T-cell responses in humanized BLT NSG mice. Immunology. 2012;136:334–343. doi: 10.1111/j.1365-2567.2012.03585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sridharan A, Chen Q, Tang KF, Ooi EE, Hibberd ML, Chen J. Inhibition of megakaryocyte development in the bone marrow underlies dengue virus-induced thrombocytopenia in humanized mice. J Virol. 2013;87:11648–11658. doi: 10.1128/JVI.01156-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frias-Staheli N, Dorner M, Marukian S, Billerbeck E, Labitt RN, Rice CM, Ploss A. Utility of humanized BLT mice for analysis of dengue virus infection and antiviral drug testing. J Virol. 2014;88:2205–2218. doi: 10.1128/JVI.03085-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villaudy J, Wencker M, Gadot N, Gillet NA, Scoazec JY, Gazzolo L, Manz MG, Bangham CR, Dodon MD. HTLV-1 propels thymic human T cell development in “human immune system” Rag2(−)/(−) gamma c(−)/(−) mice. PLoS Pathog. 2011;7:e1002231. doi: 10.1371/journal.ppat.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dittmer D, Stoddart C, Renne R, Linquist-Stepps V, Moreno ME, Bare C, McCune JM, Ganem D. Experimental transmission of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) to SCID-hu Thy/Liv mice. J Exp Med. 1999;190:1857–1868. doi: 10.1084/jem.190.12.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Douam F, Hrebikova G, Soto Albrecht YE, Sellau J, Sharon Y, Ding Q, Ploss A. Single-cell tracking of flavivirus RNA uncovers species-specific interactions with the immune system dictating disease outcome. Nat Communications. 2017 doi: 10.1038/ncomms14781. In Press. Dissection of host and tissue tropism of the yellow fever virus 17D vaccine strain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song J, Willinger T, Rongvaux A, Eynon EE, Stevens S, Manz MG, Flavell RA, Galan JE. A mouse model for the human pathogen Salmonella typhi. Cell Host Microbe. 2010;8:369–376. doi: 10.1016/j.chom.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vuyyuru R, Liu H, Manser T, Alugupalli KR. Characteristics of Borrelia hermsii infection in human hematopoietic stem cell-engrafted mice mirror those of human relapsing fever. Proc Natl Acad Sci U S A. 2011;108:20707–20712. doi: 10.1073/pnas.1108776109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calderon VE, Valbuena G, Goez Y, Judy BM, Huante MB, Sutjita P, Johnston RK, Estes DM, Hunter RL, Actor JK, et al. A humanized mouse model of tuberculosis. PLoS One. 2013;8:e63331. doi: 10.1371/journal.pone.0063331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Brehm MA, Greiner D, Shultz LD, Kornfeld H. Engrafted human cells generate adaptive immune responses to Mycobacterium bovis BCG infection in humanized mice. BMC Immunol. 2013;14:53. doi: 10.1186/1471-2172-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 21.Lapidot T, Pflumio F, Doedens M, Murdoch B, Williams DE, Dick JE. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992;255:1137–1141. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- 22.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 23.Lowry PA, Shultz LD, Greiner DL, Hesselton RM, Kittler EL, Tiarks CY, Rao SS, Reilly J, Leif JH, Ramshaw H, et al. Improved engraftment of human cord blood stem cells in NOD/LtSz-scid/scid mice after irradiation or multiple-day injections into unirradiated recipients. Biol Blood Marrow Transplant. 1996;2:15–23. [PubMed] [Google Scholar]
- 24.Pflumio F, Izac B, Katz A, Shultz LD, Vainchenker W, Coulombel L. Phenotype and function of human hematopoietic cells engrafting immune-deficient CB17-severe combined immunodeficiency mice and nonobese diabetic-severe combined immunodeficiency mice after transplantation of human cord blood mononuclear cells. Blood. 1996;88:3731–3740. [PubMed] [Google Scholar]
- 25.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 26.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 27**.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. Study demonstrates superior human hematolymphoid engraftment in Rag2−/− IL2RgNULL doubly deficient mice. [DOI] [PubMed] [Google Scholar]
- 28.Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 29.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 30*.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. Reference30 & 31 demonstrate efficient human hematolymphoid engrafted in irradiation-conditioned NOD SCID IL2Rgamma NULL mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. Reference30 & 31 demonstrate efficient human hematolymphoid engrafted in irradiation-conditioned NOD SCID IL2Rgamma NULL mice. [DOI] [PubMed] [Google Scholar]
- 32*.Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. Study provides a mechanistic explanation why immunodeficient mice on the NOD background are particularly good recipients for human hematolymphoid cells. [DOI] [PubMed] [Google Scholar]
- 33.Strowig T, Rongvaux A, Rathinam C, Takizawa H, Borsotti C, Philbrick W, Eynon EE, Manz MG, Flavell RA. Transgenic expression of human signal regulatory protein alpha in Rag2−/−gamma(c)−/− mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci U S A. 2011;108:13218–13223. doi: 10.1073/pnas.1109769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hesselton RM, Greiner DL, Mordes JP, Rajan TV, Sullivan JL, Shultz LD. High levels of human peripheral blood mononuclear cell engraftment and enhanced susceptibility to human immunodeficiency virus type 1 infection in NOD/LtSz-scid/scid mice. J Infect Dis. 1995;172:974–982. doi: 10.1093/infdis/172.4.974. [DOI] [PubMed] [Google Scholar]
- 35.Harris DT, Badowski M, Balamurugan A, Yang OO. Long-term human immune system reconstitution in non-obese diabetic (NOD)-Rag (−)-gamma chain (−) (NRG) mice is similar but not identical to the original stem cell donor. Clin Exp Immunol. 2013;174:402–413. doi: 10.1111/cei.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douam F, Hrebikova G, Albrecht YE, Sellau J, Sharon Y, Ding Q, Ploss A. Single-cell tracking of flavivirus RNA uncovers species-specific interactions with the immune system dictating disease outcome. Nat Commun. 2017;8:14781. doi: 10.1038/ncomms14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Dudek TE, No DC, Seung E, Vrbanac VD, Fadda L, Bhoumik P, Boutwell CL, Power KA, Gladden AD, Battis L, et al. Rapid evolution of HIV-1 to functional CD8(+) T cell responses in humanized BLT mice. Sci Transl Med. 2012;4:143ra198. doi: 10.1126/scitranslmed.3003984. Evidence of selection pressure on HIV by the engrafted human immune system in humanized mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Billerbeck E, Horwitz JA, Labitt RN, Donovan BM, Vega K, Budell WC, Koo GC, Rice CM, Ploss A. Characterization of human antiviral adaptive immune responses during hepatotropic virus infection in HLA-transgenic human immune system mice. J Immunol. 2013;191:1753–1764. doi: 10.4049/jimmunol.1201518. Characterization of adenovirus-specific immune responses in MHC class I & II double transgenic humanized mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baenziger S, Tussiwand R, Schlaepfer E, Mazzucchelli L, Heikenwalder M, Kurrer MO, Behnke S, Frey J, Oxenius A, Joller H, et al. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−gamma c−/− mice. Proc Natl Acad Sci U S A. 2006;103:15951–15956. doi: 10.1073/pnas.0604493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinman RM. Decisions About Dendritic Cells: Past, Present, and Future. Annual Review of Immunology. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 41.Theocharides AP, Rongvaux A, Fritsch K, Flavell RA, Manz MG. Humanized hemato-lymphoid system mice. Haematologica. 2016;101:5–19. doi: 10.3324/haematol.2014.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rongvaux A, Takizawa H, Strowig T, Willinger T, Eynon EE, Flavell RA, Manz MG. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol. 2013;31:635–674. doi: 10.1146/annurev-immunol-032712-095921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Cosgun KN, Rahmig S, Mende N, Reinke S, Hauber I, Schafer C, Petzold A, Weisbach H, Heidkamp G, Purbojo A, et al. Kit regulates HSC engraftment across the human-mouse species barrier. Cell Stem Cell. 2014;15:227–238. doi: 10.1016/j.stem.2014.06.001. Study demonstrates that mutation of the Kit receptor enables robust, uniform, sustained, and serially transplantable engraftment of human HSCs in adult mice without a requirement for irradiation conditioning. [DOI] [PubMed] [Google Scholar]
- 44.McIntosh BE, Brown ME, Duffin BM, Maufort JP, Vereide DT, Slukvin II, Thomson JA. Nonirradiated NOD,B6. SCID Il2rgamma−/− Kit(W41/W41) (NBSGW) mice support multilineage engraftment of human hematopoietic cells. Stem Cell Reports. 2015;4:171–180. doi: 10.1016/j.stemcr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rongvaux A, Willinger T, Takizawa H, Rathinam C, Auerbach W, Murphy AJ, Valenzuela DM, Yancopoulos GD, Eynon EE, Stevens S, et al. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc Natl Acad Sci U S A. 2011;108:2378–2383. doi: 10.1073/pnas.1019524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014;32:364–372. doi: 10.1038/nbt.2858. Study provides careful characterization of human hematopoiesis in xenorecipients harboring multiple human cytokine knock-ins (“MISTRG” mice) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willinger T, Rongvaux A, Takizawa H, Yancopoulos GD, Valenzuela DM, Murphy AJ, Auerbach W, Eynon EE, Stevens S, Manz MG, et al. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci U S A. 2011;108:2390–2395. doi: 10.1073/pnas.1019682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brehm MA, Racki WJ, Leif J, Burzenski L, Hosur V, Wetmore A, Gott B, Herlihy M, Ignotz R, Dunn R, et al. Engraftment of human HSCs in nonirradiated newborn NOD-scid IL2rgamma null mice is enhanced by transgenic expression of membrane-bound human SCF. Blood. 2012;119:2778–2788. doi: 10.1182/blood-2011-05-353243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takagi S, Saito Y, Hijikata A, Tanaka S, Watanabe T, Hasegawa T, Mochizuki S, Kunisawa J, Kiyono H, Koseki H, et al. Membrane-bound human SCF/KL promotes in vivo human hematopoietic engraftment and myeloid differentiation. Blood. 2012;119:2768–2777. doi: 10.1182/blood-2011-05-353201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ito R, Takahashi T, Katano I, Kawai K, Kamisako T, Ogura T, Ida-Tanaka M, Suemizu H, Nunomura S, Ra C, et al. Establishment of a human allergy model using human IL-3/GM-CSF-transgenic NOG mice. J Immunol. 2013;191:2890–2899. doi: 10.4049/jimmunol.1203543. [DOI] [PubMed] [Google Scholar]
- 51.Nicolini FE, Cashman JD, Hogge DE, Humphries RK, Eaves CJ. NOD/SCID mice engineered to express human IL-3, GM-CSF and Steel factor constitutively mobilize engrafted human progenitors and compromise human stem cell regeneration. Leukemia. 2004;18:341–347. doi: 10.1038/sj.leu.2403222. [DOI] [PubMed] [Google Scholar]
- 52.Rathinam C, Poueymirou WT, Rojas J, Murphy AJ, Valenzuela DM, Yancopoulos GD, Rongvaux A, Eynon EE, Manz MG, Flavell RA. Efficient differentiation and function of human macrophages in humanized CSF-1 mice. Blood. 2011;118:3119–3128. doi: 10.1182/blood-2010-12-326926. [DOI] [PubMed] [Google Scholar]
- 53.Billerbeck E, Barry WT, Mu K, Dorner M, Rice CM, Ploss A. Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rgamma(null) humanized mice. Blood. 2011;117:3076–3086. doi: 10.1182/blood-2010-08-301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaiswal S, Pearson T, Friberg H, Shultz LD, Greiner DL, Rothman AL, Mathew A. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rgammanull mice. PLoS One. 2009;4:e7251. doi: 10.1371/journal.pone.0007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reinisch A, Etchart N, Thomas D, Hofmann NA, Fruehwirth M, Sinha S, Chan CK, Senarath-Yapa K, Seo EY, Wearda T, et al. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood. 2015;125:249–260. doi: 10.1182/blood-2014-04-572255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holzapfel BM, Hutmacher DW, Nowlan B, Barbier V, Thibaudeau L, Theodoropoulos C, Hooper JD, Loessner D, Clements JA, Russell PJ, et al. Tissue engineered humanized bone supports human hematopoiesis in vivo. Biomaterials. 2015;61:103–114. doi: 10.1016/j.biomaterials.2015.04.057. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y, Jacamo R, Shi YX, Wang RY, Battula VL, Konoplev S, Strunk D, Hofmann NA, Reinisch A, Konopleva M, et al. Human extramedullary bone marrow in mice: a novel in vivo model of genetically controlled hematopoietic microenvironment. Blood. 2012;119:4971–4980. doi: 10.1182/blood-2011-11-389957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groen RW, Noort WA, Raymakers RA, Prins HJ, Aalders L, Hofhuis FM, Moerer P, van Velzen JF, Bloem AC, van Kessel B, et al. Reconstructing the human hematopoietic niche in immunodeficient mice: opportunities for studying primary multiple myeloma. Blood. 2012;120:e9–e16. doi: 10.1182/blood-2012-03-414920. [DOI] [PubMed] [Google Scholar]
- 59.Reinisch A, Hernandez DC, Schallmoser K, Majeti R. Generation and use of a humanized bone-marrow-ossicle niche for hematopoietic xenotransplantation into mice. Nat Protoc. 2017;12:2169–2188. doi: 10.1038/nprot.2017.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 61**.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–1322. doi: 10.1038/nm1431. Evidence that humanized BLT-mice mount more robust human immune responses to viral pathogens and superantigens. [DOI] [PubMed] [Google Scholar]
- 62.Covassin L, Jangalwe S, Jouvet N, Laning J, Burzenski L, Shultz LD, Brehm MA. Human immune system development and survival of non-obese diabetic (NOD)-scid IL2rgamma(null) (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clin Exp Immunol. 2013;174:372–388. doi: 10.1111/cei.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parent AV, Russ HA, Khan IS, LaFlam TN, Metzger TC, Anderson MS, Hebrok M. Generation of functional thymic epithelium from human embryonic stem cells that supports host T cell development. Cell Stem Cell. 2013;13:219–229. doi: 10.1016/j.stem.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384:258–271. doi: 10.1016/S0140-6736(14)60164-1. [DOI] [PubMed] [Google Scholar]
- 65.Goncalves DU, Proietti FA, Ribas JG, Araujo MG, Pinheiro SR, Guedes AC, Carneiro-Proietti AB. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev. 2010;23:577–589. doi: 10.1128/CMR.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young LS, Yap LF, Murray PG. Epstein-Barr virus: more than 50 years old and still providing surprises. Nat Rev Cancer. 2016;16:789–802. doi: 10.1038/nrc.2016.92. [DOI] [PubMed] [Google Scholar]
- 67.Marsden MD, Zack JA. Humanized Mouse Models for Human Immunodeficiency Virus Infection. Annu Rev Virol. 2017;4:393–412. doi: 10.1146/annurev-virology-101416-041703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Victor Garcia J. Humanized mice for HIV and AIDS research. Curr Opin Virol. 2016;19:56–64. doi: 10.1016/j.coviro.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cihlar T, Fordyce M. Current status and prospects of HIV treatment. Curr Opin Virol. 2016;18:50–56. doi: 10.1016/j.coviro.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Honeycutt JB, Thayer WO, Baker CE, Ribeiro RM, Lada SM, Cao Y, Cleary RA, Hudgens MG, Richman DD, Garcia JV. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat Med. 2017;23:638–643. doi: 10.1038/nm.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, Lai J, McHugh HL, Hao H, Zhang H, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517:381–385. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wheeler LA, Trifonova RT, Vrbanac V, Barteneva NS, Liu X, Bollman B, Onofrey L, Mulik S, Ranjbar S, Luster AD, et al. TREX1 Knockdown Induces an Interferon Response to HIV that Delays Viral Infection in Humanized Mice. Cell Rep. 2016;15:1715–1727. doi: 10.1016/j.celrep.2016.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng L, Yu H, Li G, Li F, Ma J, Li J, Chi L, Zhang L, Su L. Type I interferons suppress viral replication but contribute to T cell depletion and dysfunction during chronic HIV-1 infection. JCI Insight. 2017:2. doi: 10.1172/jci.insight.94366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li G, Cheng M, Nunoya J, Cheng L, Guo H, Yu H, Liu YJ, Su L, Zhang L. Plasmacytoid dendritic cells suppress HIV-1 replication but contribute to HIV-1 induced immunopathogenesis in humanized mice. PLoS Pathog. 2014;10:e1004291. doi: 10.1371/journal.ppat.1004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dave VP, Hajjar F, Dieng MM, Haddad E, Cohen EA. Efficient BST2 antagonism by Vpu is critical for early HIV-1 dissemination in humanized mice. Retrovirology. 2013;10:128. doi: 10.1186/1742-4690-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sato K, Takeuchi JS, Misawa N, Izumi T, Kobayashi T, Kimura Y, Iwami S, Takaori-Kondo A, Hu WS, Aihara K, et al. APOBEC3D and APOBEC3F potently promote HIV-1 diversification and evolution in humanized mouse model. PLoS Pathog. 2014;10:e1004453. doi: 10.1371/journal.ppat.1004453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adoro S, Cubillos-Ruiz JR, Chen X, Deruaz M, Vrbanac VD, Song M, Park S, Murooka TT, Dudek TE, Luster AD, et al. IL-21 induces antiviral microRNA-29 in CD4 T cells to limit HIV-1 infection. Nat Commun. 2015;6:7562. doi: 10.1038/ncomms8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen MR. Epstein-barr virus, the immune system, and associated diseases. Front Microbiol. 2011;2:5. doi: 10.3389/fmicb.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Munz C. Humanized mouse models for Epstein Barr virus infection. Curr Opin Virol. 2017;25:113–118. doi: 10.1016/j.coviro.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 80.Whitehurst CB, Li G, Montgomery SA, Montgomery ND, Su L, Pagano JS. Knockout of Epstein-Barr virus BPLF1 retards B-cell transformation and lymphoma formation in humanized mice. MBio. 2015;6:e01574–01515. doi: 10.1128/mBio.01574-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McHugh D, Caduff N, Barros MHM, Ramer PC, Raykova A, Murer A, Landtwing V, Quast I, Styles CT, Spohn M, et al. Persistent KSHV Infection Increases EBV-Associated Tumor Formation In Vivo via Enhanced EBV Lytic Gene Expression. Cell Host Microbe. 2017;22:61–73. e67. doi: 10.1016/j.chom.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 82.Ma SD, Xu X, Jones R, Delecluse HJ, Zumwalde NA, Sharma A, Gumperz JE, Kenney SC. PD-1/CTLA-4 Blockade Inhibits Epstein-Barr Virus-Induced Lymphoma Growth in a Cord Blood Humanized-Mouse Model. PLoS Pathog. 2016;12:e1005642. doi: 10.1371/journal.ppat.1005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cucunawangsih, Lugito NPH. Trends of Dengue Disease Epidemiology. Virology (Auckl) 2017;8:1178122–X17695836. doi: 10.1177/1178122X17695836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zika virus infection. global update on epidemiology and potentially associated clinical manifestations. Wkly Epidemiol Rec. 2016;91:73–81. [PubMed] [Google Scholar]
- 85.Yun SI, Lee YM. Zika virus: An emerging flavivirus. J Microbiol. 2017;55:204–219. doi: 10.1007/s12275-017-7063-6. [DOI] [PubMed] [Google Scholar]
- 86.Monath TP, Vasconcelos PF. Yellow fever. J Clin Virol. 2015;64:160–173. doi: 10.1016/j.jcv.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 87.Vasconcelos PF, Monath TP. Yellow Fever Remains a Potential Threat to Public Health. Vector Borne Zoonotic Dis. 2016;16:566–567. doi: 10.1089/vbz.2016.2031. [DOI] [PubMed] [Google Scholar]
- 88.Miner JJ, Diamond MS. Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe. 2017;21:134–142. doi: 10.1016/j.chom.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chan KW, Watanabe S, Kavishna R, Alonso S, Vasudevan SG. Animal models for studying dengue pathogenesis and therapy. Antiviral Res. 2015;123:5–14. doi: 10.1016/j.antiviral.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 90.Julander JG. Animal models of yellow fever and their application in clinical research. Curr Opin Virol. 2016;18:64–69. doi: 10.1016/j.coviro.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 91.Diamond MS, Pierson TC. Molecular Insight into Dengue Virus Pathogenesis and Its Implications for Disease Control. Cell. 2015;162:488–492. doi: 10.1016/j.cell.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fernandez-Garcia MD, Mazzon M, Jacobs M, Amara A. Pathogenesis of flavivirus infections: using and abusing the host cell. Cell Host Microbe. 2009;5:318–328. doi: 10.1016/j.chom.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 93.Quaresma JA, Pagliari C, Medeiros DB, Duarte MI, Vasconcelos PF. Immunity and immune response, pathology and pathologic changes: progress and challenges in the immunopathology of yellow fever. Rev Med Virol. 2013;23:305–318. doi: 10.1002/rmv.1752. [DOI] [PubMed] [Google Scholar]
- 94.Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22:564–581. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aguirre S, Maestre AM, Pagni S, Patel JR, Savage T, Gutman D, Maringer K, Bernal-Rubio D, Shabman RS, Simon V, et al. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog. 2012;8:e1002934. doi: 10.1371/journal.ppat.1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Costa VV, Ye W, Chen Q, Teixeira MM, Preiser P, Ooi EE, Chen J. Dengue Virus-Infected Dendritic Cells, but Not Monocytes, Activate Natural Killer Cells through a Contact-Dependent Mechanism Involving Adhesion Molecules. MBio. 2017:8. doi: 10.1128/mBio.00741-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cox J, Mota J, Sukupolvi-Petty S, Diamond MS, Rico-Hesse R. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J Virol. 2012;86:7637–7649. doi: 10.1128/JVI.00534-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Robinson LN, Tharakaraman K, Rowley KJ, Costa VV, Chan KR, Wong YH, Ong LC, Tan HC, Koch T, Cain D, et al. Structure-Guided Design of an Anti-dengue Antibody Directed to a Non-immunodominant Epitope. Cell. 2015;162:493–504. doi: 10.1016/j.cell.2015.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jaiswal S, Smith K, Ramirez A, Woda M, Pazoles P, Shultz LD, Greiner DL, Brehm MA, Mathew A. Dengue virus infection induces broadly cross-reactive human IgM antibodies that recognize intact virions in humanized BLT-NSG mice. Exp Biol Med (Maywood) 2015;240:67–78. doi: 10.1177/1535370214546273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schmitt K, Charlins P, Veselinovic M, Kinner-Bibeau L, Hu S, Curlin J, Remling-Mulder L, Olson KE, Aboellail T, Akkina R. Zika viral infection and neutralizing human antibody response in a BLT humanized mouse model. Virology. 2018;515:235–242. doi: 10.1016/j.virol.2017.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Theiler M, Smith HH. The Use of Yellow Fever Virus Modified by in Vitro Cultivation for Human Immunization. J Exp Med. 1937;65:787–800. doi: 10.1084/jem.65.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shearer FM, Moyes CL, Pigott DM, Brady OJ, Marinho F, Deshpande A, Longbottom J, Browne AJ, Kraemer MUG, O’Reilly KM, et al. Global yellow fever vaccination coverage from 1970 to 2016: an adjusted retrospective analysis. Lancet Infect Dis. 2017 doi: 10.1016/S1473-3099(17)30419-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kupferschmidt K. INFECTIOUS DISEASES. Yellow fever outbreak triggers vaccine alarm. Science. 2016;352:128–129. doi: 10.1126/science.352.6282.128. [DOI] [PubMed] [Google Scholar]
- 104.Paules CI, Fauci AS. Yellow Fever - Once Again on the Radar Screen in the Americas. N Engl J Med. 2017;376:1397–1399. doi: 10.1056/NEJMp1702172. [DOI] [PubMed] [Google Scholar]
- 105.Douam F, Soto Albrecht YE, Hrebikova G, Sadimin E, Davidson C, Kotenko SV, Ploss A. Type III Interferon-Mediated Signaling Is Critical for Controlling Live Attenuated Yellow Fever Virus Infection In Vivo. MBio. 2017:8. doi: 10.1128/mBio.00819-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 107.MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med. 2015;5:a021410. doi: 10.1101/cshperspect.a021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Watashi K, Wakita T. Hepatitis B Virus and Hepatitis D Virus Entry, Species Specificity, and Tissue Tropism. Cold Spring Harb Perspect Med. 2015;5:a021378. doi: 10.1101/cshperspect.a021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ding Q, von Schaewen M, Ploss A. The impact of hepatitis C virus entry on viral tropism. Cell Host Microbe. 2014;16:562–568. doi: 10.1016/j.chom.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110*.Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JR, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. First pioneering study demonstrating the utility of human liver chimeric mice for study hepatotropic pathogens, here specifically hepatitis C virus. [DOI] [PubMed] [Google Scholar]
- 111.Bissig KD, Wieland SF, Tran P, Isogawa M, Le TT, Chisari FV, Verma IM. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest. 2010;120:924–930. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lutgehetmann M, Mancke LV, Volz T, Helbig M, Allweiss L, Bornscheuer T, Pollok JM, Lohse AW, Petersen J, Urban S, et al. Humanized chimeric uPA mouse model for the study of hepatitis B and D virus interactions and preclinical drug evaluation. Hepatology. 2012;55:685–694. doi: 10.1002/hep.24758. [DOI] [PubMed] [Google Scholar]
- 113.de Jong YP, Dorner M, Mommersteeg MC, Xiao JW, Balazs AB, Robbins JB, Winer BY, Gerges S, Vega K, Labitt RN, et al. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci Transl Med. 2014;6:254ra129. doi: 10.1126/scitranslmed.3009512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Winer BY, Huang T, Low BE, Avery C, Pais MA, Hrebikova G, Siu E, Chiriboga L, Wiles MV, Ploss A. Recapitulation of treatment response patterns in a novel humanized mouse model for chronic hepatitis B virus infection. Virology. 2016;502:63–72. doi: 10.1016/j.virol.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carpentier A, Tesfaye A, Chu V, Nimgaonkar I, Zhang F, Lee SB, Thorgeirsson SS, Feinstone SM, Liang TJ. Engrafted human stem cell-derived hepatocytes establish an infectious HCV murine model. J Clin Invest. 2014;124:4953–4964. doi: 10.1172/JCI75456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Allweiss L, Gass S, Giersch K, Groth A, Kah J, Volz T, Rapp G, Schobel A, Lohse AW, Polywka S, et al. Human liver chimeric mice as a new model of chronic hepatitis E virus infection and preclinical drug evaluation. J Hepatol. 2016;64:1033–1040. doi: 10.1016/j.jhep.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 117.Sayed IM, Foquet L, Verhoye L, Abravanel F, Farhoudi A, Leroux-Roels G, Izopet J, Meuleman P. Transmission of hepatitis E virus infection to human-liver chimeric FRG mice using patient plasma. Antiviral Res. 2017;141:150–154. doi: 10.1016/j.antiviral.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 118.Sayed IM, Verhoye L, Cocquerel L, Abravanel F, Foquet L, Montpellier C, Debing Y, Farhoudi A, Wychowski C, Dubuisson J, et al. Study of hepatitis E virus infection of genotype 1 and 3 in mice with humanised liver. Gut. 2017;66:920–929. doi: 10.1136/gutjnl-2015-311109. [DOI] [PubMed] [Google Scholar]
- 119.van de Garde MD, Pas SD, van der Net G, de Man RA, Osterhaus AD, Haagmans BL, Boonstra A, Vanwolleghem T. Hepatitis E Virus (HEV) Genotype 3 Infection of Human Liver Chimeric Mice as a Model for Chronic HEV Infection. J Virol. 2016;90:4394–4401. doi: 10.1128/JVI.00114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilson EM, Bial J, Tarlow B, Bial G, Jensen B, Greiner DL, Brehm MA, Grompe M. Extensive double humanization of both liver and hematopoiesis in FRGN mice. Stem Cell Res. 2014;13:404–412. doi: 10.1016/j.scr.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Strick-Marchand H, Dusseaux M, Darche S, Huntington ND, Legrand N, Masse-Ranson G, Corcuff E, Ahodantin J, Weijer K, Spits H, et al. A novel mouse model for stable engraftment of a human immune system and human hepatocytes. PLoS One. 2015;10:e0119820. doi: 10.1371/journal.pone.0119820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gutti TL, Knibbe JS, Makarov E, Zhang J, Yannam GR, Gorantla S, Sun Y, Mercer DF, Suemizu H, Wisecarver JL, et al. Human hepatocytes and hematolymphoid dual reconstitution in treosulfan-conditioned uPA-NOG mice. Am J Pathol. 2014;184:101–109. doi: 10.1016/j.ajpath.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123*.Washburn ML, Bility MT, Zhang L, Kovalev GI, Buntzman A, Frelinger JA, Barry W, Ploss A, Rice CM, Su L. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 2011;140:1334–1344. doi: 10.1053/j.gastro.2011.01.001. First study providing evidence for immune-mediated liver disease in dually humanized mice induced by a chronic hepatitis viruses infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124*.Bility MT, Cheng L, Zhang Z, Luan Y, Li F, Chi L, Zhang L, Tu Z, Gao Y, Fu Y, et al. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014;10:e1004032. doi: 10.1371/journal.ppat.1004032. Evidence of HBV-associated immunopathogenesis in dually engrafted humanized mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Billerbeck E, Mommersteeg MC, Shlomai A, Xiao JW, Andrus L, Bhatta A, Vercauteren K, Michailidis E, Dorner M, Krishnan A, et al. Humanized mice efficiently engrafted with fetal hepatoblasts and syngeneic immune cells develop human monocytes and NK cells. J Hepatol. 2016;65:334–343. doi: 10.1016/j.jhep.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dusseaux M, Masse-Ranson G, Darche S, Ahodantin J, Li Y, Fiquet O, Beaumont E, Moreau P, Riviere L, Neuveut C, et al. Viral Load Affects the Immune Response to HBV in Mice With Humanized Immune System and Liver. Gastroenterology. 2017;153:1647–1661. e1649. doi: 10.1053/j.gastro.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gaska JM, Ploss A. Study of viral pathogenesis in humanized mice. Curr Opin Virol. 2015;11:14–20. doi: 10.1016/j.coviro.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li Y, Mention JJ, Court N, Masse-Ranson G, Toubert A, Spits H, Legrand N, Corcuff E, Strick-Marchand H, Di Santo JP. A novel Flt3-deficient HIS mouse model with selective enhancement of human DC development. Eur J Immunol. 2016;46:1291–1299. doi: 10.1002/eji.201546132. [DOI] [PubMed] [Google Scholar]