Figure 1.
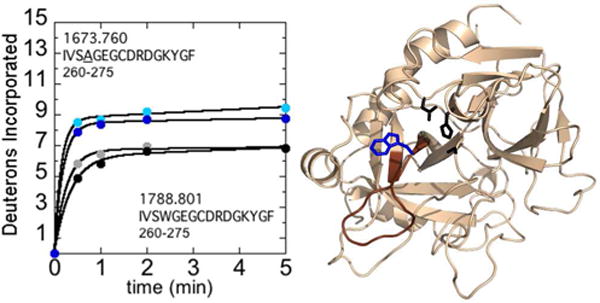
A) Deuterium incorporation into residues 212-227CT (residues 260-275; MH+ 1673.760 and 1788.801 for W215A and WT respectively) over 5 min is shown for WT thrombin at 100 mM NaCl (grey) and 300 mM NaCl (black), and for the W215A mutant at 100 mM NaCl (cyan) and 300 mM NaCl (blue). The mutant residue, is underlined in the peptide sequence shown. B) Structure of WT thrombin (PDB 1PPB) highlighting residues 212-227CT (residues 260-275; brown). The sidechains of Trp215CT (blue) and the catalytic triad (black) are shown as sticks.
