Introduction
Dizziness and imbalance are the most common symptoms of vestibular dysfunction, which is estimated to occur in approximately 11% of US adults in a given year1. People with vestibular deficits have an increased risk of falling due to postural instability, especially in scenarios involving low-lighting or compliant surfaces1–3. Herdman et al. demonstrated increased incidence of falls in people with unilateral vestibular hypofunction (UVH) (41%) compared with community-dwelling older adults (25%)3. Studies have also shown postural control abnormalities in people with UVH, especially when vision and/or somatosensory inputs were degraded during quiet stance4–6. People with vestibular dysfunction are typically prescribed vestibular rehabilitation therapy7 and many opt to use canes or other mobility aids during activities of daily living to obtain cues about their bodies’ vertical orientation8. Given that people with vestibular dysfunction do not necessarily require physical support from mobility aids, researchers have developed and assessed wearable sensory augmentation and substitution technologies9–13 over the past 20 years as an alterative option for decreasing fall risk and improving the quality of life2.
Sensory augmentation and sensory substitution devices use one or more alternative functional sensory modality(ies) to supplement or replace, respectively, one or more non-functional sensory modality(ies)14. Sensory augmentation/substitution devices typically comprise one or more sensors (e.g., inertial measurement unit, force transducer) to measure body kinematic or kinetic information, a processor, and a feedback display10. Several different types of feedback displays, including vibrotactile9,10, auditory12,15, electrotactile11, and multimodal13 have been used to provide people with UVH with cues about verticality or sway information during quiet and perturbed stance conditions10,13. Vibrotactile feedback (VTF) is one such balance aid, which uses vibration to provide the information about body orientation to replace impaired vestibular function10. Vibrotactle feedback (VTF) has been found to significantly decrease RMS sway for people with UVH or bilateral vestibular deficits during tandem stance or a multi-directional surface perturbation scenario10,16–18. VTF also improves balance control during certain sensory integration conditions (i.e., sway-referenced surface or/and sway-referenced visual surround) in people with vestibular disorders19,20. The use of VTF may provide one solution to decrease fall risk in people with UVH21.
In real-life settings, people usually perform balance tasks and cognitive tasks concurrently, such as talking or texting while maintaining balance. These additional activities have a measurable influence on balance control22–24. Dual-task paradigms have been introduced to test the allocation of attention between balance tasks and cognitive tasks25. Redfern et al. indicated that people with compensated vestibular disorders require more attention for balance compared to healthy controls, as demonstrated by increased auditory choice reaction times, despite balance performance that was no different than controls26. Redfern et al. also suggested that the increase in attentional demand was due to the increased attentional requirements in the sensory selection process. The same results also were found in people with uncompensated vestibular disorders27.
While vibrotactile feedback provides additional sensory information for postural orientation10, the ability to use VTF may be considered in and of itself as a secondary cognitive task, especially when the person is learning how to use the VTF. Lin et al. have shown that using VTF increases reaction times in both younger and older adults which suggests that using VTF requires additional attentional resources28. People with vestibular disorders may be able to successfully use VTF during a single balance task under fully controlled laboratory settings. However, using VTF under dual-task conditions may be problematic in people with vestibular disorders since the attentional demand is high during postural control.26 Moreover, people with vestibular disorders showed postural control deficits during multi-sensory integration balance conditions.29 It is unknown how people with vestibular disorders respond to the competition between postural tasks and cognitive tasks, and also utilize the external sensory feedback, such as VTF. Prior to potential use of sensory augmentation technologies as real-time balance aids, it is important to understand the attentional demand associated with using a sensory augmentation device and people’s ability to use the device under attentionally demanding conditions. In order to assess the attentional demands of VTF, we used a choice reaction time task to examine the interaction between a secondary cognitive task and use of VTF in people with uncompensated unilateral vestibular hypofunction (UVH). We hypothesized that people with UVH would have increased postural sway and slower reaction times while using VTF during dual-task conditions compared with age-matched controls.
Methods
Subjects
Nine people with unilateral vestibular hypofunction (UVH) who were seeking medical care from either a neurologist or physical therapist specializing in the management of individuals with vestibular disorders and nine aged-matched controls enrolled in this study. The dizziness and imbalance symptoms of individuals with UVH varied in acuity and intensity, but all resulted in need for medical consultation at a tertiary care clinic. Table 1 summarizes the demographics and clinical presentation of the study participants. Exclusion criteria consisted of a diagnosis of a neurologic or orthopedic disorder, known pregnancy, binocular visual acuity with corrective lenses worse than 20/40, or impaired sensation with the Semmes-Weinstein monofilament test (0.07g)30. Additional exclusion criteria for the control group were scores less than 19 on the Dynamic Gait Index31, scores less than 22 on the Functional Gait Assessment32, or abnormal age-corrected audiometric function. All participants were consented prior to the initiation of the study. The Institutional Review Board at the University of Pittsburgh approved the protocol.
Table 1.
The study participants’ characteristics, vestibular function tests and performance on clinical tests of balance.
| Group | ID | Age | Gender | Lesion Side | Caloric test | RT | Duration (wks) | Dizziness Rating* | Gait Speed | DGI | FGA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | F | L | + | + | 1 | 6 | 0.91 | 19 | 19 | |
| 2 | 29 | F | R | + | 7 | 3 | 0.97 | 21 | 21 | ||
| 3 | 56 | F | R | + | + | 28 | 2 | 1.07 | 20 | 21 | |
| 4 | 55 | F | L | + | 265 | 1 | 0.86 | 18 | 16 | ||
| UVH | 5 | 59 | F | L | + | 282 | 0 | 1.04 | 21 | 22 | |
| 6 | 64 | M | L | + | + | 5 | 3 | 0.84 | 12 | 12 | |
| 7 | 59 | M | L | + | + | 4 | 2 | 1.47 | 21 | 24 | |
| 8 | 52 | F | L | + | 260 | 1 | 1.23 | 21 | 24 | ||
| 9 | 60 | F | L | + | 7 | 1 | 1.12 | 22 | 27 | ||
|
| |||||||||||
| Mean | 55 ± 11 | 1.07 ± 0.21 | 19 ± 3 | 20 ± 5 | |||||||
|
| |||||||||||
| 1 | 65 | F | 1.03 | 23 | 28 | ||||||
| 2 | 29 | F | 0.98 | 22 | 27 | ||||||
| 3 | 55 | F | 1.38 | 23 | 28 | ||||||
| 4 | 55 | M | 1.31 | 22 | 27 | ||||||
| Controls | 5 | 58 | F | 1.41 | 24 | 28 | |||||
| 6 | 64 | M | 1.19 | 24 | 28 | ||||||
| 7 | 58 | M | 1.18 | 23 | 26 | ||||||
| 8 | 50 | F | 1.21 | 24 | 29 | ||||||
| 9 | 59 | F | 1.15 | 22 | 27 | ||||||
|
| |||||||||||
| Mean | 55 ± 10 | 1.20 ± 0.14 | 23 ± 1 | 28± 1 | |||||||
|
| |||||||||||
| p value | 0.67a | 0.60b | 0.16a | <0.01a | <0.01a | ||||||
Mann-Whitney test;
Chi-square test
UVH: Unilateral vestibular hypofunction; RT: Rotational chair (+: positive results in RT); DGI: Dynamic Gait Index; FGA: Functional Gait Assessment
Caloric test: (+) > 25% weakness on lesion side compared with sound side
Dizziness rating: 0 to 10 scale, 10 is the maximum intensity of dizziness; during the first experimental visit
Instrumentation
A customized VTF system28 and a computerized dynamic posturography platform (Smart EquiTest™; Neurocom, Inc) were used in this study. The customized VTF system included a waist belt, an IMU (Xsens Technologies B.V., Enschede, Netherlands), eight vibrating tactors (C-2; Engineering Acoustics, Casselberry, FL, USA), and a laptop computer. The two rows by four columns tactor array was set to align with the midline front, midline back, right and left sides of the body. The IMU which was attached to the belt at the level of the fourth lumbar vertebra was used to detect the subject’s trunk angular position and angular velocity in the anteroposterior (AP) and mediolateral (ML) directions. Vibrotactile feedback at a frequency of 250 Hz was provided when the proportional-plus-derivative feedback control signal was equal to the trunk angular position value (degrees) plus 0.5 (seconds) times the trunk angular velocity (degrees/second).16,33 The threshold for the lower-row of tactors was set to 1.5° for the anterior direction and 0.5° for the posterior directions, right, and left directions. The threshold for the upper-row of tactors was set to 3° for the anterior direction and 1.5° for the posterior directions, right, and left directions. One tactor was activated at a time which was regulated by “the nearest neighbor” principle.16 The subjects were instructed to null the vibration by moving in the opposite direction of the vibrotactile cue.
A secondary auditory choice reaction time (CRT) task was delivered by a customized program (Labview, National Instruments). Two different tones (560 Hz and 980 Hz at 80 dB for 250 ms) were randomly transmitted through a set of earphones (E·A·RTONE®) every 2 to 6 seconds during a 2 minute period. The participants were asked to press a microswitch button in the dominant hand for the higher pitch tone and a microswitch button in the non-dominant hand for the lower pitch tone. Twenty-five to twenty-nine auditory stimuli were presented in each trial. The onset of the switch activation relative to the stimulus (i.e. reaction time) was recorded using a computer hardware timing mechanism with a temporal resolution of 1 ms.
Experimental procedure
Each participant completed one screening and training visit and two experimental visits. A physical therapist evaluated the participant’s gait speed, Dynamic Gait Index (DGI)31 and Functional Gait Assessment (FGA)32 during the screening and training visit. After the balance evaluation, the participant was briefly trained to perform the CRT tasks, use the VTF in four different sensory integration conditions, and perform the CRT tasks while using the VTF. The four sensory integration conditions included standing on a fixed platform with eyes open in the light (Fixed/EO), standing on a fixed platform with eyes open in the dark (Fixed/EOD), standing on a sway-referenced platform with eyes open in the light (SR/EO) and standing on a sway-referenced platform with eyes open in the dark (SR/EOD). The participants also practiced one additional trial of standing on a SR platform with EO while performing the CRT tasks. The participants wore darkened goggles during the EOD conditions. Each training condition lasted for 120 seconds. During the two experimental visits, a short training period was held before the experimental test, which repeated the VTF training conditions, but with a shorter duration to avoid fatigue. A total of sixteen two-minute trials were performed, including all combinations of VTF on/off, CRT task on/off, and sensory conditions (Fixed/EO, Fixed/EOD, SR/EO and SR/EOD). The participants performed the experimental conditions in random order during the experimental visits.
Outcome measures
The center of pressure (COP) was recorded in the anterior-posterior (AP) and medial-lateral (ML) directions for all 16 trials. However, only the AP direction of COP data was used because sway-referencing only occurred in the AP direction because of the design of the Smart Equi-Test. The root-mean square (RMS) of COP was calculated via a customized Matlab (MathWorks, Natick, MA) program. The median reaction times (RTs) were calculated from each of the eight trials which included the CRT task. Prior to extracting the median reaction time in each CRT condition, the first RT response was excluded due to the increase in latency on the first response28, as were RTs less than 100 ms and RTs greater than 1000 ms.
Statistical Analysis
All data were examined for normality via the Shapiro-Wilk test. The Mann-Whitney test was used to examine group differences in age, gait speed, DGI and FGA. A chi-squared test was used to assess group differences in gender. A repeated measures analysis of variance (ANOVA) was conducted to examine the effect of Group, Condition, VTF, and CRT on RMS COP during all conditions. However, two out of nine people with UVH were not able to perform the balance task during SR/EOD condition so only three levels of sensory integration conditions (Fixed/EO, Fixed/EOD and SR/EO) were included in our model. Moreover, the statistical analysis only included the data from experimental visit 1 due to three subjects dropping out of the study after the first experimental visit. The postural sway data (RMS COP) was logarithmically transformed to meet the normality assumption of a repeated measures ANOVA. A Bonferroni correction was applied if post-hoc analysis was needed for the Condition variable. The significance level of α = 0.017 was set for post-hoc analysis using Bonferroni correction. In addition, because of the small sample size, we also performed an analysis of the Group effect using the Mann-Whitney test. Similarly, we investigated the effect of Group, Condition and VTF on reaction times. Using the repeated measures ANOVA, we also examined two- and three-way interactions between Group and the other independent variables. All statistical analyses were performed using IBM® SPSS® Statistics, Release Version 19 (IBM, Armonk, NY). A significance level of α = 0.05 was used for between group and within-subject variables.
Results
The sample consisted of seven females and two males in the UVH group and six females and three males in the age-matched control group. There was no statistically significant difference between the two groups in age (p =0.67) or gender (p = 0.60). Significant differences between UVH and controls were found in DGI (p < 0.01) and FGA (p < 0.01) scores (Table 1). Our study participants in the UVH group were uncompensated, as 8 out of 9 reported dizziness, and all participants’ demonstrated impairments during the DGI and FGA tests.
Postural Sway
Exemplar COP raw data from an individual with UVH is presented in Figure 1, where the increase in COP across the three conditions (i. e. columns) is evident. The repeated measures ANOVA showed that there was no significant difference between people with UVH and age-matched controls on RMS COP (p = 0.40, effect size = 0.05) across all conditions (Table 2). These results were confirmed using a non-parametric Mann-Whitney test.
Figure 1.
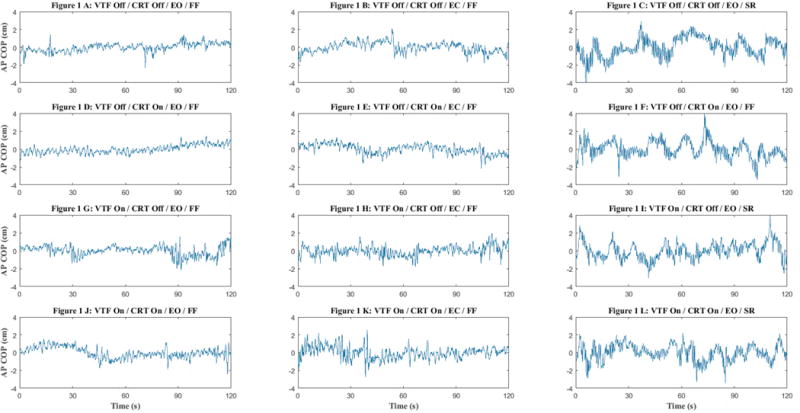
A-L The sample raw data of center of pressure (COP) in different conditions in an individual with unilateral vestibular hypofunction.
• VTF: vibrotactile feedback; CRT: choice reaction time task; EO: eyes open; EC: eyes closed; FF: Fixed forceplate; SR: sway-referenced foreceplate
Table 2.
Repeated measures ANOVA results testing the effects of Group, Condition, Vibrotactile Feedback (VTF), and performance of auditory choice reaction time (CRT) tasks on the root-mean-square of the anterior-posterior center of pressure (RMS COP) for all 16 trials.
| Main Effects | RMS COP (cm) mean ± SD | F and P values | Interaction(s) | F and P values |
|---|---|---|---|---|
| Group | Control: 0.80 ± 0.20 UVH: 0.95 ± 0.36 |
F1, 16 = 0.8, p = 0.40 | VTF*CRT*Group VTF*Condition |
F2, 16 = 5.2, p = 0.04 F2, 32 = 14.1, p < 0.001 |
| Conditiona | Fixed/EO: 0.61 ± 0.23 Fixed/EOD: 0.72 ± 0.28 SR/EO: 1.30 ± 0.50 |
F1.3, 3.4 = 70.2, p < 0.001 | ||
| VTF | Off: 0.86 ± 0.30 On: 0.90 ± 0.33 |
F1, 16 = 2.7, p = 0.12 | ||
| CRT | Off: 0.90 ± 0.36 On: 0.85 ± 0.26 |
F1, 16 = 0.9, p = 0.35 |
Post-hoc test for Condition: all conditions were significantly different from one another, p < 0.01
Fixed: Fixed platform; SR: Sway-referenced platform; EO: Eyes open; EOD: Eyes open in the dark; VTF: Vibrotactile feedback; CRT: auditory choice reaction time; COP: center of pressure; RMS: root-mean square
Condition was the only variable that resulted in significant differences in the RMS COP (p < 0.001) (Table 2). The magnitude of RMS COP increased across the sensory conditions: Fixed/EO, Fixed/EOD to the SR/EO condition. There were no significant differences in RMS COP due to the main effects of VTF or CRT.
A significant interaction between VTF, CRT, and Group was found for RMS COP (p = 0.04). The UVH group had a significant reduction in RMS COP when performing the CRT task without VTF, but there was virtually no difference in RMS COP due to the CRT when VTF was being used. Age-matched controls did not demonstrate differences in RMS COP due to the CRT in either of the VTF conditions. (Figure 2)
Figure 2.
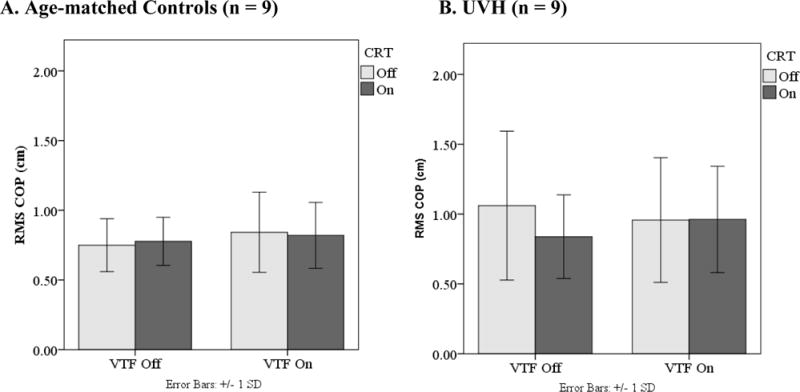
Interaction between VTF*CRT*Group on the root-mean-square of the anterior-posterior center of pressure (RMS COP). Individuals with unilateral vestibular hypofunction (UVH) showed increased RMS COP in the condition without vibrotactile feedback and not performing the CRT task while the control group showed no change in RMS in the same condition.
• COP: center of pressure; RMS: root-mean-square; VTF: Vibrotactile feedback; CRT: Choice reaction time task
• Error bars: +/− 1SD
Reaction Time
Reaction time was used to examine the attention demands of the postural task. No RT responses were less than 100 ms or greater than 1000 ms and therefore all RT responses were used to compute the median RT. A repeated measures ANOVA of the median reaction time (RT) demonstrated significant main effects of VTF (p< 0.001). (Table 3) When VTF was used, the RT increased approximately 44 milliseconds in people with UVH and 21 milliseconds in controls compared with when VTF was not used. No significant interactions between Group and the other variables were found. (Figure 3)
Table 3.
Repeated measures ANOVA results testing the effects of Group, Condition, and Vibrotactile Feedback (VTF) on the median reaction time during performance of the auditory choice reaction time task.
| Main Effects | Reaction Time (ms) mean ± SD |
F and P values | Interaction(s) | F and P values |
|---|---|---|---|---|
| Group | Control: 501 ± 78 UVH: 537 ± 46 |
F1,16 = 1.4, p = 0.26 | None | None |
| Condition | Fixed/EO: 523 ± 59 Fixed/EOD: 527 ± 73 SR/EO: 507 ± 69 |
F2,32 = 2.8, p = 0.08 | ||
| VTF | Off: 483 ± 52 On: 556 ± 80 |
F1,16 = 50.7, p < 0.001 |
Fixed: Fixed platform; SR: Sway-referenced platform; EO: Eyes open; EOD: Eyes open in the dark; VTF: Vibrotactile feedback; CRT: auditory choice reaction time.
Figure 3.
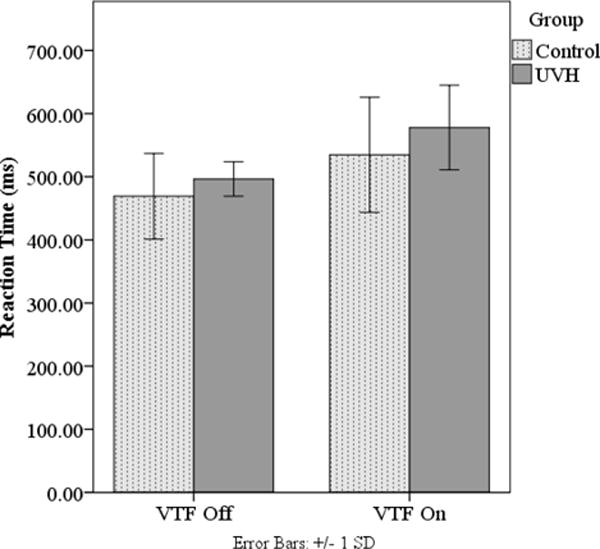
Median reaction times for age-matched controls and individuals with unilateral vestibular hypofunction (UVH) while vibrotactile feedback (VTF) was on and off. Both groups increased reaction times when VTF was on.
• Error bars: +/− 1SD
Discussion
The purpose of this study was to examine if individuals with UVH had different postural performance and reaction times during dual-task conditions when using VTF compared with controls. We did not detect a significant difference between participants with UVH and controls on the magnitude of postural sway while using VTF during the dual-task and different sensory integration conditions, suggesting that individuals with UVH are able to incorporate the external feedback. This finding is consistent with the investigation showing the people with vestibular disorders are able to use external auditory biofeedback to enhance their balance control.12,15 However, using VTF significantly increased choice reaction time in both groups which suggested increase attentional demand for using VTF under dual-task conditions.
The RMS COP during the dual-task conditions were not different between the two groups in our study. The inability to detect a difference may have been a result of the small sample size. However, the results confirmed previous findings of Yardley et al. and Redfern et al.26,27 in individuals with uncompensated and compensated vestibular hypofunction. Redfern et al. found no group differences for postural sway when people with well-compensated vestibular lesions were compared with age-matched controls while they performed different cognitive tasks during balancing.26 Yardley et al. also compared the RMS COP between people with uncompensated vestibular disorders and healthy controls and found no difference for RMS COP on a stable or sway-referenced platform while they performed secondary cognitive tasks27. However, worse equilibrium scores were found in people with vestibular disorders in Yardley et al.’s study while performing secondary cognitive tasks during the Sensory Organization Test27. In contrast to previous studies, where VTF was found to significantly decrease RMS sway for people with UVH10,16–18, we did not find the same effect in this study. However, the previous studies were conducted during single task balance conditions, where subjects were able to focus on the VTF to correct their postural sway, rather than with the current dual-task paradigms where attention was divided.
Comparing the RMS COP data with a previous study28, the RMS COP in the control group was similar to the previous findings. Although the RMS COP in the UVH group was similar with our prior research, the magnitude of the standard deviation was higher compared to Lin et al.’s data. The larger magnitude of the standard deviation indicates that our study group with UVH had more variable performance than controls, perhaps because of the difference in clinical variation of the symptoms among the groups. The RMS COP values in our data was higher than the data presented by Redfern et al.26 during the eyes open and fixed platform condition (0.45 vs 0.67 cm) and the eyes open/sway-referenced condition (1.1 vs 1.41 cm), suggesting that the increase in RMS COP was related to VTF.
Slower RTs of 36 ms were found in individuals with UVH compared to the controls while performing a secondary cognitive task, averaged across all tasks, which may indicate an increased demand on attention in the UVH group while performing the secondary cognitive task. Although there was no statistically significant difference in the current study, the magnitude of difference in RTs was about the same as previous studies that demonstrated a 40 ms difference between well-compensated individuals with UVH and controls26, but smaller than a 75 ms difference between poorly compensated subjects with UVH and controls27. In our study, the people in UVH group were uncompensated subjects. However, when compared to the single test condition, the baseline RT values (no VTF, EO and Fixed platform) were 456 ms in the control group and 495 in UVH group. The difference in RT values were similar to the RT values in the same condition in Redfern et al.’s study26. The RT values with VTF on EO and fixed platform condition were 504 ms in the control group and 574 ms in people with UVH. The difference in RT was 70 ms, which indicated that using VTF increased the demands on attention more in the UVH group than in the control group.
In this study, we also examined how sensory integration affected the dual-task performance while using VTF. Our results illustrated that there was no difference in reaction times during the Fixed/EO, the Fixed/EOD and the SR/EO conditions. The results of Redfern et al. suggested that sway-referenced condition significantly increases reaction times26. Our results showed there was no main effect of Condition on reaction time or any interactions which differs from Lin et al.’s study28, which found that the participants had greatly increased RTs in the SR/EO condition compared with the Fixed/EO condition and SR/EOD compared to other conditions. The SR/EOD condition was not included in the analysis in this study because some participants with UVH could not complete the task. However, our data suggested that the RT increased greatly in SR/EOD condition in those participants who could complete the SR/EOD condition. The lack of a main effect of Condition on reaction time may be also due to the small sample size in our study.
Our study participants may not be able to represent all individuals who have had UVH due to the smaller sample size. Most of our participants with UVH had mild to moderate dizziness symptoms. Four of the subjects had gait speeds below 1.0 m/s. Only one control subject was below 1.0 m/s at 0.98 m/s. In general, the UVH group was on the borderline of fall risks based on their DGI scores. This may affect the generalizability of this study results to people who are higher functioning with UVH. Furthermore, the current study was powered to detect a group difference in sway of 0.15 cm. In order to detect this difference, the study would have required participation of over 33 subjects in each group, which was time- and cost prohibitive.
Conclusion
Differences in RMS COP were not found between individuals with UVH and age-matched controls. The findings suggest that people with UVH were able to use VTF while performing CRT. However, greater attentional resources may be required in individuals with UVH when using vibrotactile feedback, based on the performance in the CRT task.
Acknowledgments
The authors give special thanks to Dr. Beom-Chan Lee for his contributions to the development of the VTF device used in this study. The authors also thank Anita Lieb and Susan Strelinski for their help on this project. We are grateful for the assistance of Cindy Kapelewski, Joe Skledar, and Kareen Congleton in participant recruitment. This material is based upon work supported by the National Science Foundation CAREER program under Grant No. RAPD-0846471 to K.H. Sienko and the National Institute on Deafness and Other Communication Disorders under Grant no. 5R21-DC-012410-02 to K. H. Sienko and S. L. Whitney.
References
- 1.Lin HW, Bhattacharyya N. Impact of dizziness and obesity on the prevalence of falls and fall-related injuries. Laryngoscope. 2014;124(12):2797–2801. doi: 10.1002/lary.24806. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med. 2009;169(10):938–944. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- 3.Herdman SJ, Blatt P, Schubert MC, Tusa RJ. Falls in patients with vestibular deficits. Am J Otol. 2000;21(6):847–851. [PubMed] [Google Scholar]
- 4.Black FO, Shupert CL, Horak FB, Nashner LM. Abnormal postural control associated with peripheral vestibular disorders. Prog Brain Res. 1988;76:263–275. doi: 10.1016/s0079-6123(08)64513-6. [DOI] [PubMed] [Google Scholar]
- 5.Asai M, Watanabe Y, Ohashi N, Mizukoshi K. Evaluation of vestibular function by dynamic posturography and other equilibrium examinations. Acta Otolaryngol Suppl. 1993;504:120–124. doi: 10.3109/00016489309128136. [DOI] [PubMed] [Google Scholar]
- 6.Nashner LM, Black FO, Wall C., Iii Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. J Neurosci. 1982;2(5):536–544. doi: 10.1523/JNEUROSCI.02-05-00536.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horning E, Gorman S. Vestibular rehabilitation decreases fall risk and improves gaze stability for an older individual with unilateral vestibular hypofunction. J Geriatr Phys Ther. 2007;30(3):121–127. doi: 10.1519/00139143-200712000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Sienko KH, Whitney SL, Carender WJ, Wall C. The role of sensory augmentation for people with vestibular deficits: Real-time balance aid and/or rehabilitation device? J Vestib Res. 2017;27(1):63–76. doi: 10.3233/VES-170606. [DOI] [PubMed] [Google Scholar]
- 9.Sienko KH, Vichare VV, Balkwill MD, Wall C., 3rd Assessment of vibrotactile feedback on postural stability during pseudorandom multidirectional platform motion. IEEE Trans Biomed Eng. 2010;57(4):944–952. doi: 10.1109/TBME.2009.2036833. [DOI] [PubMed] [Google Scholar]
- 10.Wall C, 3rd, Weinberg MS, Schmidt PB, Krebs DE. Balance prosthesis based on micromechanical sensors using vibrotactile feedback of tilt. IEEE Trans Biomed Eng. 2001;48(10):1153–1161. doi: 10.1109/10.951518. [DOI] [PubMed] [Google Scholar]
- 11.Danilov Y, Tyler M, Skinner K, Hogle R, Bach-y-Rita P. Efficacy of electrotactile vestibular substitution in patients with peripheral and central vestibular loss. J Vestib Res. 2007;17(2–3):119–130. [PMC free article] [PubMed] [Google Scholar]
- 12.Dozza M, Horak FB, Chiari L. Auditory biofeedback substitutes for loss of sensory information in maintaining stance. Exp Brain Res. 2007;178(1):37–48. doi: 10.1007/s00221-006-0709-y. [DOI] [PubMed] [Google Scholar]
- 13.Allum JH, Carpenter MG, Horslen BC, et al. Improving impaired balance function: real-time versus carry-over effects of prosthetic feedback. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:1314–1318. doi: 10.1109/IEMBS.2011.6090309. [DOI] [PubMed] [Google Scholar]
- 14.Bach-y-Rita P, Collins CC, Saunders FA, White B, Scadden L. Vision substitution by tactile image projection. Nature. 1969;221(5184):963–964. doi: 10.1038/221963a0. [DOI] [PubMed] [Google Scholar]
- 15.Hegeman J, Honegger F, Kupper M, Allum JH. The balance control of bilateral peripheral vestibular loss subjects and its improvement with auditory prosthetic feedback. J Vestib Res. 2005;15(2):109–117. [PubMed] [Google Scholar]
- 16.Sienko KH, Balkwill MD, Oddsson LI, Wall C. Effects of multi-directional vibrotactile feedback on vestibular-deficient postural performance during continuous multi-directional support surface perturbations. J Vestib Res. 2008;18(5–6):273–285. [PubMed] [Google Scholar]
- 17.Sienko KH, Balkwill MD, Wall C., 3rd Biofeedback improves postural control recovery from multi-axis discrete perturbations. J Neuroeng Rehabil. 2012;9:53. doi: 10.1186/1743-0003-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horak FB, Dozza M, Peterka R, Chiari L, Wall C., 3rd Vibrotactile biofeedback improves tandem gait in patients with unilateral vestibular loss. Ann N Y Acad Sci. 2009;1164:279–281. doi: 10.1111/j.1749-6632.2008.03707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wall C, 3rd, Kentala E. Control of sway using vibrotactile feedback of body tilt in patients with moderate and severe postural control deficits. J Vestib Res. 2005;15(5–6):313–325. [PubMed] [Google Scholar]
- 20.Kentala E, Vivas J, Wall C., 3rd Reduction of postural sway by use of a vibrotactile balance prosthesis prototype in subjects with vestibular deficits. Ann Otol Rhinol Laryngol. 2003;112(5):404–409. doi: 10.1177/000348940311200503. [DOI] [PubMed] [Google Scholar]
- 21.Wall C., 3rd Application of vibrotactile feedback of body motion to improve rehabilitation in individuals with imbalance. J Neurol Phys Ther. 2010;34(2):98–104. doi: 10.1097/NPT.0b013e3181dde6f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strubhar AJ, Peterson ML, Aschwege J, Ganske J, Kelley J, Schulte H. The effect of text messaging on reactive balance and the temporal and spatial characteristics of gait. Gait Posture. 2015;42(4):580–583. doi: 10.1016/j.gaitpost.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Schabrun SM, van den Hoorn W, Moorcroft A, Greenland C, Hodges PW. Texting and walking: strategies for postural control and implications for safety. PLoS ONE. 2014;9(1):e84312. doi: 10.1371/journal.pone.0084312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plummer-D’Amato P, Altmann LJ. Relationships between motor function and gait-related dual-task interference after stroke: a pilot study. Gait Posture. 2012;35(1):170–172. doi: 10.1016/j.gaitpost.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Kerr B, Condon SM, McDonald LA. Cognitive spatial processing and the regulation of posture. J Exp Psychol Hum Percept Perform. 1985;11(5):617–622. doi: 10.1037//0096-1523.11.5.617. [DOI] [PubMed] [Google Scholar]
- 26.Redfern MS, Talkowski ME, Jennings JR, Furman JM. Cognitive influences in postural control of patients with unilateral vestibular loss. Gait Posture. 2004;19(2):105–114. doi: 10.1016/S0966-6362(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 27.Yardley L, Gardner M, Bronstein A, Davies R, Buckwell D, Luxon L. Interference between postural control and mental task performance in patients with vestibular disorder and healthy controls. J Neurol Neurosurg Psychiatry. 2001;71(1):48–52. doi: 10.1136/jnnp.71.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin CC, Whitney SL, Loughlin PJ, et al. The effect of age on postural and cognitive task performance while using vibrotactile feedback. J Neurophysiol. 2015;113(7):2127–2136. doi: 10.1152/jn.00083.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shumway-Cook A, Woollacott M. Attentional demands and postural control: the effect of sensory context. J Gerontol A Biol Sci Med Sci. 2000;55(1):M10–16. doi: 10.1093/gerona/55.1.m10. [DOI] [PubMed] [Google Scholar]
- 30.Bell-Krotoski JA, Fess EE, Figarola JH, Hiltz D. Threshold detection and Semmes-Weinstein monofilaments. J Hand Ther. 1995;8(2):155–162. doi: 10.1016/s0894-1130(12)80314-0. [DOI] [PubMed] [Google Scholar]
- 31.Whitney SL, Marchetti GF, Schade A, Wrisley DM. The sensitivity and specificity of the Timed “Up & Go” and the Dynamic Gait Index for self-reported falls in persons with vestibular disorders. J Vestib Res. 2004;14(5):397–409. [PubMed] [Google Scholar]
- 32.Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther. 2004;84(10):906–918. [PubMed] [Google Scholar]
- 33.Goodworth AD, Wall C, 3rd, Peterka RJ. Influence of feedback parameters on performance of a vibrotactile balance prosthesis. IEEE Trans Neural Syst Rehabil Eng. 2009;17(4):397–408. doi: 10.1109/TNSRE.2009.2023309. [DOI] [PMC free article] [PubMed] [Google Scholar]


