Abstract
Background
Previous studies have demonstrated that earlier epinephrine administration is associated with improved survival from out-of-hospital cardiac arrest (OHCA) with shockable initial rhythms. However, the effect of epinephrine timing on patients with non-shockable initial rhythms is unclear. The objective of this study was to measure the association between time to epinephrine administration and survival in adults and children with EMS-treated OHCA with non-shockable initial rhythms.
Methods
We performed a secondary analysis of OHCAs prospectively identified by the Resuscitation Outcomes Consortium (ROC) network from June 4, 2011 to June 30, 2015. We included patients of all ages with an EMS-treated OHCA and an initial non-shockable rhythm. We excluded those with return of spontaneous circulation in < 10 minutes. We conducted a subgroup analysis involving patients < 18 years. The primary exposure was time (minutes) from arrival of the first EMS agency to the first dose of epinephrine. Secondary exposure was time to epinephrine dichotomized as “early” (<10 minutes) or “late” (≥10 minutes). The primary outcome was survival to hospital discharge. We adjusted for Utstein covariates and ROC study site.
Results
From 55,568 EMS-treated OHCAs, 32,101 patients with initial non-shockable rhythms were included. There were 12,238 in the “early” group, 14,517 in the “late” group, and 5346 not treated with epinephrine. After adjusting for potential confounders, each minute from EMS arrival to epinephrine administration was associated with a 4% decrease in odds of survival for adults, OR = 0.96 (95% CI 0.95, 0.98). A subgroup analysis (n=13,290) examining neurological outcomes showed a similar association (adjusted OR 0.94 per minute; 95%CI 0.89–0.98). When epinephrine was given late compared to early, odds of survival were 18% lower (OR 0.82; 95% CI 0.68–0.98). In a pediatric analysis (n=595), odds of survival were 9% lower (OR 0.91; 95%CI 0.81–1.01) for each minute delay in epinephrine.
Conclusions
Among OHCA’s with non-shockable initial rhythms, the majority of patients were administered epinephrine > 10 minutes after EMS arrival. Each minute delay in epinephrine administration was associated with decreased survival and unfavorable neurological outcomes. EMS agencies should consider strategies to reduce epinephrine administration times in patients with initial non-shockable rhythms.
Keywords: Resuscitation, Cardiopulmonary Resuscitation, Cardiac Arrest, Epinephrine
Introduction
Out-of-hospital cardiac arrest (OHCA) is an important public health problem affecting at least 390,000 people per year in North America alone.1 Professional organizations including the International Liaison Committee on Resuscitation (ILCOR) as well as the American Heart Association (AHA) recommend epinephrine as the first-line drug treatment for OHCA.2 Epinephrine improved the rate of return of spontaneous circulation (ROSC) in two small randomized trials including patients with both shockable and non-shockable rhythms.3,4 Several observational studies have demonstrated that the effects of epinephrine may be time-dependent, with earlier epinephrine associated with improved outcomes in OHCA with shockable initial cardiac rhythms.5–7 However, the majority of OHCA patients present with non-shockable initial cardiac rhythms where epinephrine and advanced airway management are the primary advanced life support (ALS) interventions available to EMS. However, the ideal timing of epinephrine administration is unclear. One observational study in Japan reported that among witnessed arrests with pulseless electrical activity (PEA), epinephrine given before 20 minutes was associated with improved survival, however the optimal time for delivery was not explored and these findings may also not be generalizable to other EMS systems.8
Epinephrine is thought to benefit patients in cardiac arrest by increasing coronary perfusion pressure potentially enhancing cardiac function, but may also reduce cerebral flow and can cause increased myocardial oxygen demand.9,10 The timing of drug delivery in relationship to the onset of the arrest may affect the balance of the potential benefits or harms of epinephrine.
The objective of this study is to evaluate the association between timing of the first dose of epinephrine and survival to hospital discharge among OHCA patients with initial non-shockable rhythms. Our primary hypothesis was that delays in epinephrine administration would be associated with decreased odds of survival.
METHODS
The data from this study will be made available publicly through the NHLBI Biologic Specimen and Data Repository Information Coordinating Center, with anticipated release in 2018. IRB/ethics approval was obtained from all study sites and waivers of consent were granted for study subjects.
Study Setting
The Resuscitation Outcomes Consortium (ROC) research network includes 10 sites in North America (7 in the United States and 3 in Canada), 260 EMS agencies, and 287 participating hospitals, serving a combined population of approximately 24 million people.11 The network was created to study the treatment and outcomes of patients with cardiac arrest and severe traumatic injuries focusing on interventions that take place in the out-of-hospital setting. The ROC Epistry is a prospective epidemiologic registry of consecutive OHCA patients in the network for whom there is an organized EMS response. To be enrolled in the Epistry, patients must receive chest compressions by EMS personnel or defibrillation by EMS or bystanders, including use of an automated external defibrillator (AED). Data collection for the ROC Epistry was approved by the Institutional Review Board or Research Ethics Boards at each participating site.
Study Population
In this analysis we included EMS-treated patients of all ages from June 4, 2011 to June 30, 2015 with OHCA presenting with initial non-shockable rhythms defined as PEA or asystole. We excluded patients who had epinephrine administered but were missing the timing of administration, those who had received epinephrine before an EMS-witnessed arrest, and those who received epinephrine after a re-arrest following initial ROSC without epinephrine. We also excluded patients who achieved early ROSC, defined as within 10 minutes of arrival of the first EMS agency specifically to mitigate bias caused by including patients resuscitated successfully before epinephrine could feasibly be delivered. Patients with initial non-shockable rhythms who did not receive any epinephrine were not included in the primary sample, but were included in a sensitivity analysis. We included patients in the primary analysis regardless of whether or not complete CPR process files were available, since CPR process measurement varies by monitor manufacturer. CPR process files contain data collected by the electronic patient monitor in conjunction with an attached CPR accelerometer and allow measurement of chest compression rate, depth, and fraction (percent of resuscitation in which CPR was being performed).
Data Quality
The 10 sites used several strategies to identify the cardiac arrest cases including electronic queries of EMS records, manual sorting of paper EMS charts, and telephone notification of each treated case by EMS personnel.12 After incident cases were identified, trained research assistants at each site abstracted data into a standardized electronic database managed by the network. Each site developed specific data quality assurance processes, including training of the EMS providers in data collection, variable definitions and review of randomly selected records for data quality both by the site and by the network clinical coordinating center. Multiple logic checks on the data were also included in the electronic data entry and transmission processes. The data coordinating center conducted annual audits of random charts from each site including EMS and hospital data both remotely and in-person, allowing sites to ensure high data quality and make improvements to data abstraction as needed. The ROC Epistry registry was used to collect EMS and outcomes data for all ROC clinical trials.13–15
Main Exposures and Outcomes
The primary exposure was time in minutes from the first EMS agency arrival on scene to the first administration of epinephrine. The ROC data abstraction form records timing and dose of each medication. In addition to the minute by minute classification, we dichotomized the epinephrine administration based on who received initial epinephrine “early” (defined as less than 10 minutes) versus “late” (defined as greater than or equal to 10 minutes), consistent with time-group classification used in the published studies on shockable rhythms.6
Outcome
The primary outcome for this study was survival to hospital discharge. Although neurological status at discharge was not recorded for all cases, a subset of patients in the registry was included in a randomized trial of CPR in OHCA and neurologic outcomes were ascertained in this group.15 We performed a subgroup analysis of patients from this trial defining a favorable neurological outcome as Modified Rankin Scale (MRS) <3 (slight disability or better). (MRS range: 0–6; 0 = no symptoms, 6 = death)16
Covariates
We categorized the presenting rhythm as ventricular fibrillation/ventricular tachycardia, PEA, asystole, not shockable without available rhythm strip (AED only), and cannot determine. We grouped together PEA, asystole, and non-shockable rhythms for this analysis. Other covariates in logistic regression analysis are detailed below.
Statistical Analysis
We used descriptive statistics to characterize groups by timing of epinephrine administration. Survival to hospital discharge proportions were examined at one minute intervals.
To examine the relationship between survival and epinephrine timing, we evaluated epinephrine timing as a continuous variable in multivariate logistic regression models adjusted for age (years); sex; witness status (EMS/bystander/none); bystander CPR (yes/no); initial rhythm (PEA/Asystole); time from dispatch to first EMS arrival (minutes); vascular access success (Intravenous [IV]/Intraosseous [IO]/both/neither); successfully placed advanced airway (Endotracheal intubation [ETI[/Laryngeal tube [LT]/both/none); arrest location (public/private); etiology of arrest (cardiac/non-cardiac); and ROC site. Successful advanced airway was defined as an airway for which placement was confirmed by EMS per local protocols. We could not account for airway device attempts, removal, or abandonment the device while in the field since these were not required data points. We planned a similar analysis for the pediatric patients (<18 years) using the same exposure, outcomes, and covariates.
We conducted a series of sensitivity analyses to evaluate the robustness of the relationship between epinephrine timing and survival. For early vs. late epinephrine, we conducted a sensitivity analysis which included patients not administered epinephrine as part of the “late” administration group. For continuous time to epinephrine, we conducted additional sensitivity analyses using the time from arrival of the first ALS-capable agency on scene (Sensitivity A), and time from EMS dispatch to epinephrine (Sensitivity B) as the exposures. In a third sensitivity analysis (Sensitivity C), we eliminated the exclusion criterion of ROSC in < 10 minutes and included all patients who had received epinephrine. Additionally, we performed an analysis in the cohort of patients who had CPR process measures available including chest compression fraction (percentage of time compressions were being performed during resuscitation), average chest compression depth, chest compression rate (100–120, <100 or >120 per minute) (Sensitivity D). We performed several subgroup analyses by including interaction terms in the full models for early vs. late epinephrine. We assessed interactions for early vs. late epinephrine with chest compression fraction (<0.8 vs. >/= 0.8), rate (100–120 vs <100 or >120), depth (37–51 mm vs <31 or >51 mm), PEA vs asystole initial rhythms, and witnessed vs unwitnessed arrests. Arrests with missing or unknown covariate data were excluded from all analyses. For the pediatric subgroups we assessed age by subcategories of 0–1 year, 1–11 years, and 11–18 years. We conducted an additional analysis that included those with ROSC in <10 minutes. Previous data has demonstrated differences in epinephrine administration between and within ROC EMS agencies. In addition, to account for potential differences between EMS agencies, rather than study site which encompassed multiple agencies, we conducted a separate analysis using Generalized Estimating Equations to control for clustering at the EMS agency.
We analyzed separate models using a categorical term for epinephrine timing, splines for epinephrine timing and an indicator that epinephrine was administered prior to/after successful airway application. Finally, we plotted adjusted survival probabilities for epinephrine times between 0 and 20 minutes. Using the fitted model, we set covariate values to represent the average patient in the sample and then systematically varied epinephrine timing. We conducted all analyses using S-Plus version 6.2.1 (TIBCO Software Inc. Palo Alto, California, USA), and Stata version 11 (StataCorp, College Station, Texas, USA).
RESULTS
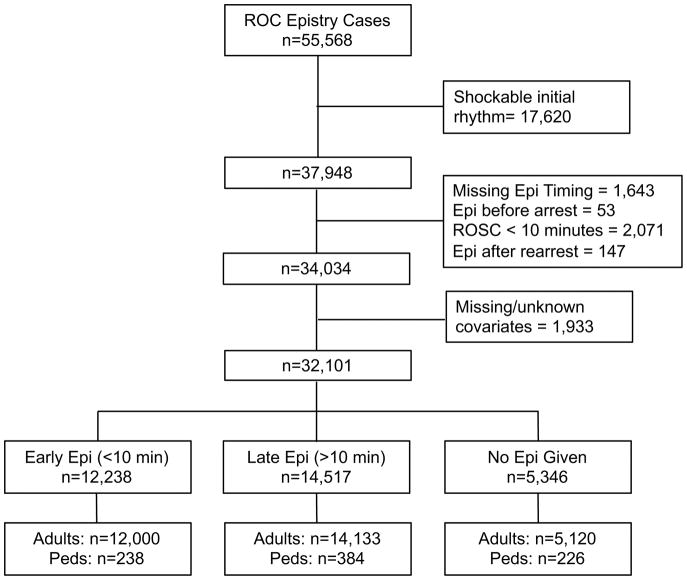
From 55,568 EMS-treated cardiac arrests, we excluded 17,620 with shockable rhythms. We then excluded 2,271 due to population definitions noted above and 1,643 due to missing epinephrine timing data (Figure 1). After applying all exclusions the sample of patients who received epinephrine by EMS consisted of 26,755 patients. Using the 10-minute cutoff for time from first EMS arrival to epinephrine administration, the final population included 12,238 in the “early” group and 14,417 in the “late” Group. Patient characteristics are shown in Table 1. The early and late epinephrine groups were similar though EMS witnessed arrests were twice as likely to receive epinephrine early. Among patients receiving epinephrine, the majority received epinephrine >10 minutes from EMS arrival (54.2%).
Figure 1.
Study inclusion and exclusion
Table 1.
Patient Characteristics
| Epi Given | Early Epi (<10min) | Late Epi (≥10min) | No Epi Given | |
|---|---|---|---|---|
| n | 26755 | 12238 | 14517 | 5346 |
|
| ||||
| Age | ||||
| Median (IQR) | 67.0 (54.0, 80.0) | 67.0 (54.0, 80.0) | 67.0 (54.0, 80.0) | 73.0 (57.0, 84.0) |
| 0–1 years, n (%) | 294 (1.1%) | 104 (0.8%) | 204 (1.4%) | 147 (2.7%) |
| 1–11 years, n (%) | 179 (0.7%) | 80 (0.7%) | 103 (0.7%) | 61 (1.1%) |
| 11–18 years, n (%) | 149 (0.6%) | 60 (0.5%) | 91 (0.6%) | 18 (0.3%) |
| 18–40 years, n (%) | 2237 (8.4%) | 1016 (8.3%) | 1225 (8.4%) | 345 (6.5%) |
| 40–60 years, n (%) | 6415 (24.0%) | 2997 (24.4%) | 3432 (23.6%) | 965 (18.0%) |
| ≥60 years, n (%) | 17481 (65.3%) | 8007 (65.3%) | 9509 (65.3%) | 3810 (71.3%) |
|
| ||||
| Sex | ||||
| Male, n (%) | 16187 (60.5%) | 7705 (63.0%) | 8482 (58.4%) | 2942 (55.0%) |
|
| ||||
| Initial rhythm | ||||
| PEA, n (%) | 8307 (31.0%) | 3870 (31.6%) | 4437 (30.6%) | 1444 (27.0%) |
| Asystole, n (%) | 18448 (69.0%) | 8368 (68.4%) | 10080 (69.4%) | 3902 (73.0%) |
|
| ||||
| Witness Status | ||||
| EMS, n (%) | 2776 (10.4%) | 1807 (14.8%) | 969 (6.7%) | 656 (12.3%) |
| Bystander, n (%) | 8212 (30.7%) | 3433 (28.1%) | 4779 (32.9%) | 1270 (23.7%) |
| None, n (%) | 15767 (58.9%) | 6998 (57.2%) | 8769 (60.4%) | 3420 (64.0%) |
|
| ||||
| Bystander CPR, n(%) | 11242 (42.0%) | 5335 (43.6%) | 5907 (40.7%) | 1875 (35.1%) |
|
| ||||
| Public location, n(%) | 2368 (8.9%) | 1101 (9.0%) | 1267 (8.7%) | 316 (5.9%) |
|
| ||||
| Obvious non-cardiac etiology, n(%) | 2128 (8.0%) | 933 (7.6%) | 1195 (8.2%) | 612 (11.4%) |
|
| ||||
| Site | ||||
| A, n (%) | 3148 (11.8%) | 1531 (12.5%) | 1617 (11.1%) | 890 (16.6%) |
| B, n (%) | 5174 (19.3%) | 2254 (18.4%) | 2920 (20.1%) | 391 (7.3%) |
| C, n (%) | 2626 (9.8%) | 1228 (10.0%) | 1398 (9.6%) | 548 (10.2%) |
| D, n (%) | 555 (2.1%) | 245 (2.0%) | 310 (2.1%) | 166 (3.1%) |
| E, n (%) | 2816 (10.5%) | 889 (7.3%) | 1927 (13.3%) | 688 (12.9%) |
| F, n (%) | 1962 (7.3%) | 1138 (9.3%) | 824 (5.7%) | 118 (2.2%) |
| G, n (%) | 1930 (7.2%) | 1363 (11.1%) | 567 (3.9%) | 151 (2.8%) |
| H, n (%) | 6639 (24.8%) | 2287 (18.7%) | 4352 (30.0%) | 2079 (38.9%) |
| I, n (%) | 1157 (4.3%) | 815 (6.7%) | 342 (2.4%) | 250 (4.7%) |
| J, n (%) | 748 (2.8%) | 488 (4.0%) | 260 (1.8%) | 65 (1.2%) |
Unadjusted Outcomes
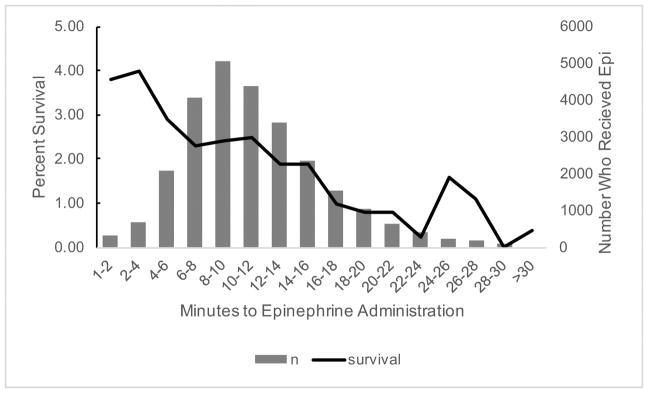
Overall, unadjusted survival to discharge was 2.6% in the “early” group and 1.7% in the “late” group. For patients with initial PEA, unadjusted survival was 4.9% in the “early” group and 4.8% in the “late” group. For patients with initial asystole, unadjusted survival was 1.5% in the “early” group and 1.0% in the “late” group. In Figure 2, we display the unadjusted survival to hospital discharge using 2 minute intervals of time from EMS arrival to epinephrine administration. The highest survival was noted when epinephrine was given before 4 minutes, which occurred in 7% of patients.
Figure 2.
Unadjusted survival and number of patients that received epinephrine by 2-minute intervals
Adjusted outcomes
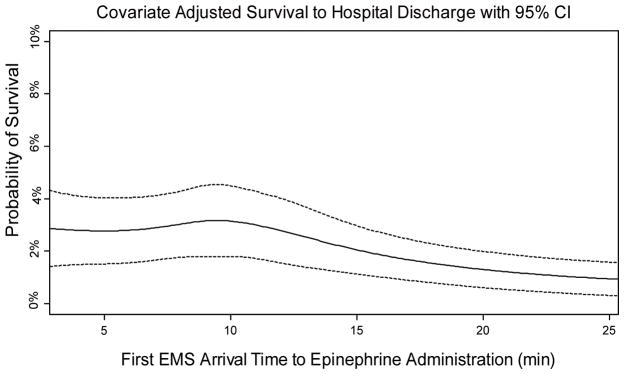
After adjusting for potential confounders, each additional minute of time from EMS arrival to epinephrine administration was associated with 4% decrease in the odds of survival to hospital discharge (OR 0.96, 95% CI 0.95–0.98) (Table 2). The results of four sensitivity analyses are also displayed in Table 2 and demonstrate similar results. When epinephrine was given late vs. early, the odds of survival decreased 18% (OR 0.82, 95% CI 0.68–0.98) and this result was stable when those where were not given epinephrine were included in the late group. In Figure 3, we plot the covariate adjusted expected probability of survival to discharge with varying epinephrine administration times and demonstrate the highest survival was when epinephrine is given within 10 minutes of EMS arrival. In the analysis that included patients who received epinephrine and had ROSC in <10 minutes, the results favored early epinephrine with larger effect sizes than those in the primary analysis (continuous analysis OR 0.93, 95% CI 0.91–0.95; categorical anlysis OR 0.61 95% CI 0.52–0.71). In the Generalized Estimating Equations analysis in which we controlled for clustering by each EMS agency, rather than study site, we found similar results in direction and significance to the primary continuous and early vs. late analyses (continuous analysis OR 0.95, 95% CI 0.93–0.97; categorical analysis OR 0.67, 95% CI 0.57–0.79).
Table 2.
Primary and sensitivity multivariable analyses
| Primary: 1st EMS arrival to epi | Sensitivity A: 1st ALS arrival to epi | Sensitivity B: EMS dispatch to epi | Sensitivity D: Includes ROSC <10 minutes* | Sensitivity D: Includes CPR process* | |
|---|---|---|---|---|---|
| n | 26,133 | 26,089 | 26,133 | 26990 | 22,869 |
|
| |||||
| Odds of survival for 1 minute delay in epi OR(95%CI) | 0.96 (0.95–0.98) | 0.97 (0.95–0.99) | 0.96(0.95–0.98) | 0.95(0.94–0.97) | 0.97 (0.94–0.98) |
These models include adults only
Figure 3.
This analysis was conducted using average values for all covariates based on adjusted analyses and was fit using spline regression. (NOTE TO EDITORS: this sentence is the legend at the bottom of the figure, the title is included in this figure)
We found a significant interaction between early vs late epinephrine and arrest witness status (p=0.02). In an exploratory subgroup analysis of witnessed vs. unwitnessed arrests, late epinephrine was significantly associated with lower odds of survival compared to early epinephrine in unwitnessed arrests (OR 0.63, 95% CI 0.48 – 0.83), but there was no association in the witnessed arrest group (OR 0.93, 95% CI 0.75 – 1.14). Similarly, we found a significant interaction between early vs. late epinephrine and initial rhythm. In an exploratory subgroup analysis of PEA vs. asystole, late epinephrine was significantly associated with lower odds of survival in asystole compared to early epinephrine (OR 0.62, 95% CI 0.47 – 0.81), but there was no association in the PEA group (OR 0.96, 95% CI 0.77 – 1.19). The CPR process variable interaction terms were not statistically significant.
Epinephrine was administered via the IV route 70% of the time in adults while given via the IO route 71% of the time in children. After adjustment for other covariates, in both the primary adult and pediatric models, the route of epinephrine administration was not significantly associated with survival.
Neurologic Outcomes
In the subgroup analysis of 13,290 patients from the randomized trial requiring neurologic outcome determination, odd of survival with MRS <3 were reduced 6% (OR 0.94, 95% CI 0.89–0.98) for each minute delay in epinephrine administration.
Pediatric analysis
In the 595 pediatric patients, odds of survival were decreased 9% (OR 0.91, 95% CI 0.81–1.01) for each minute delay in epinephrine administration. Odds of survival were decreased 57% (OR 0.43, 95% CI 0.16–1.14) when epinephrine was given late compared to early. Results were similar when children who were not given epinephrine were included in the late group.
DISCUSSION
In this North American observational study of 26,755 adults and children with OHCA and initial non-shockable rhythms, each minute of delay to epinephrine delivery was associated with reduced survival to hospital discharge after controlling for multiple known confounders. In a subgroup analysis, we also found each minute delay in epinephrine was also associated with worsened neurologic outcome. Overall, our results suggest that earlier administration of epinephrine may improve the probability of neurologically-intact survival in patients with non-shockable initial rhythms. The association between time to epinephrine and survival was stable across several sensitivity analyses. The association between timing of epinephrine administration and survival was modified by witness status and initial rhythm. Results were similar for pediatric patients, though the smaller sample size may have contributed to lack of statistical significance. Together, these results suggest that in non-shockable rhythms, it may be important to focus on delivering epinephrine earlier during the course of resuscitation.
The results of this study are similar to those of Ewy et al. who assessed the effect of time from dispatch to epinephrine administration on 3469 patients in Arizona with witnessed arrests, 42% of whom had an initial shockable rhythm.7 The odds ratio for survival to hospital discharge for each minute delay in epinephrine administration was 0.95, though this was only significant in patients with initial shockable rhythms.7 Recent studies of in-hospital cardiac arrests, two in adults and another in pediatric patients, found that shorter times to first epinephrine were also associated with improved survival.17–19 Our study adds to the scientific body supporting early epinephrine administration by demonstrating the importance of early epinephrine in non-shockable initial OHCA rhythms, including asystolic and unwitnessed arrests, in a large and geographically diverse patient cohort.
Previous studies have consistently found that epinephrine improves the rate of ROSC during cardiac arrest.3–5,20,21 The primary physiologic mechanism for improving ROSC is thought to be through the alpha effect of epinephrine improving aortic diastolic pressure which leads to an increase in blood flow to the left ventricle mediated by an increase in coronary perfusion pressure.22–25 However, vital organs such as the brain and heart become more ischemic with longer CPR times, and the beneficial effects of epinephrine on left ventricular blood flow may be countered by a reduction in cerebral blood flow, worsened brain ischemia, reduced microcirculatory flow, and increased myocardial oxygen demand.9,10,26
We found that more than half of the patients in this study received epinephrine more than 10 minutes from first EMS arrival, although there was significant variability across the sites. Our findings suggest that a focus on earlier epinephrine administration in patients with non-shockable initial cardiac rhythms is feasible since some sites have successfully accomplished this. Furthermore, earlier epinephrine administration may also improve neurologic outcomes in these patients. Though overall survival from OHCA with non-shockable initial rhythms is poor, these rhythms are initially present in the majority of OHCA patients, so small increases in survival rates could have a significant public health impact.
We also found that in adults, epinephrine was given via the IV route 70% of the time, while given via the IO route in pediatric patients 71% of the time. There was no association between route of administration of epinephrine and survival. Using a powered device, providers are able to achieve IO access with more than 95% success in less than 30 seconds, after undergoing minimal training.27 Currently, vascular access procedures and IV medications are generally ALS procedures which may be a limiting factor in time to epinephrine delivery in certain EMS systems. Incorporating IO vascular access and epinephrine administration into BLS training could improve epinephrine delivery times in some EMS systems. However, it is unknown in human cardiac arrest if the pharmacokinetics of epinephrine are the same when using the IV and or various IO routes (e.g. proximal tibia vs. proximal humerus), and this should be a consideration for future studies.28
Limitations
This was a secondary analysis of a cardiac arrest registry, and thus we are only able to comment on association rather than causation. There is the potential for residual confounding, as previous research has shown that traditional Utstein variables do not completely explain the variability in outcomes after out-of-hospital cardiac arrest.29 Earlier epinephrine administration may also be a marker for higher quality ALS care overall. Controlling for study site in the primary models, and for agency in the GEE analysis in an effort to reduce confounding due to differences in EMS care and patient populations, did not change our findings. Though reassuring, these analyses may not fully account for ALS care variability within individual EMS agencies.30 Though the ROC sites are geographically diverse, they represent mature, high-functioning EMS systems that may not be completely generalizable, especially to EMS systems outside North America. We were also unable to assess the specific aspects of post-resuscitation hospital care (e.g., targeted temperature management and early coronary angiography) that can impact patient outcomes, though these factors seem less likely to be associated with time to epinephrine administration and are most notable among patients with an initial rhythm of ventricular fibrillation/tachycardia.
CONCLUSIONS
We found an association between each minute delay in epinephrine administration and decreased survival to hospital discharge as well as survival with favorable neurological outcomes in adult and pediatric patients with non-shockable OHCA. EMS agencies should consider prioritizing early administration of epinephrine among patients presenting with PEA or asystole in their training and protocols.
Clinical Perspective.
What is new?
This study finds that in both adults and children with out-of-hospital cardiac arrest from a non-shockable initial rhythm, faster time from arrival of Emergency Medical Services (EMS) providers to the first dose of epinephrine is associated with improved survival.
Each minute delay was associated with worsened odds of survival to discharge and neurologically intact survival in continuous analysis of time to epinephrine delivery.
In a categorical analysis, epinephrine delivery in less than 10 minutes was also associated with significantly improved odds of survival.
What are the clinical implications?
Currently, the majority of initial doses of epinephrine are delivered more than 10 minutes from EMS arrival for patients with out-of-hospital cardiac arrest with initial non-shockable rhythms.
However, initial epinephrine dosing in less than 10 minutes is associated with significantly improved survival.
Providers should consider ways to deliver epinephrine as early as feasible during resuscitation for out-of-hospital cardiac arrest with initial non-shockable rhythms.
Acknowledgments
Funding Sources
The Resuscitation Outcomes Consortium is supported by a series of cooperative agreements to nine regional clinical centers and one Data Coordinating Center (5U01 HL077863 – University of Washington Data Coordinating Center, HL077866 – Medical College of Wisconsin, HL077867 – University of Washington, HL077871 – University of Pittsburgh, HL077872 – St. Michael’s Hospital, HL077873 – Oregon Health and Science University, HL077881 – University of Alabama at Birmingham, HL077885 – Ottawa Hospital Research Institute, HL077887 – University of Texas SW Medical Center/Dallas, HL077908 – University of California San Diego) from the National Heart, Lung and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, U.S. Army Medical Research & Material Command, The Canadian Institutes of Health Research (CIHR) – Institute of Circulatory and Respiratory Health, Defense Research and Development Canada and the Heart, Stroke Foundation of Canada and the American Heart Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
Footnotes
Conflict of Interest Disclosures:
None
References
- 1.Cardiac Arrest Statistics [Internet] [cited 2017 Aug 7];Available from: http://cpr.heart.org/AHAECC/CPRAndECC/General/UCM_477263_Cardiac-Arrest-Statistics.jsp.
- 2.Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, Neumar RW, O’Neil BJ, Paxton JH, Silvers SM, White RD, Yannopoulos D, Donnino MW. Part 7: Adult Advanced Cardiovascular Life Support. Circulation. 2015;132:S444–S464. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 3.Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA. 2009;302:2222–2229. doi: 10.1001/jama.2009.1729. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs IG, Finn JC, Jelinek GA, Oxer HF, Thompson PL. Effect of adrenaline on survival in out-of-hospital cardiac arrest: A randomised double-blind placebo-controlled trial. Resuscitation. 2011;82:1138–1143. doi: 10.1016/j.resuscitation.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 5.Hagihara A, Hasegawa M, Abe T, Nagata T, Wakata Y, Miyazaki S. Prehospital Epinephrine Use and Survival Among Patients With Out-of-Hospital Cardiac Arrest. JAMA. 2012;307:1161–1168. doi: 10.1001/jama.2012.294. [DOI] [PubMed] [Google Scholar]
- 6.Nakahara S, Tomio J, Takahashi H, Ichikawa M, Nishida M, Morimura N, Sakamoto T. Evaluation of pre-hospital administration of adrenaline (epinephrine) by emergency medical services for patients with out of hospital cardiac arrest in Japan: controlled propensity matched retrospective cohort study. BMJ. 2013;347:f6829. doi: 10.1136/bmj.f6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewy GA, Bobrow BJ, Chikani V, Sanders AB, Otto CW, Spaite DW, Kern KB. The time dependent association of adrenaline administration and survival from out-of-hospital cardiac arrest. Resuscitation. 2015;96:180–185. doi: 10.1016/j.resuscitation.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Goto Y, Maeda T, Goto Y. Impact of dispatcher-assisted bystander cardiopulmonary resuscitation on neurological outcomes in children with out-of-hospital cardiac arrests: a prospective, nationwide, population-based cohort study. J Am Heart Assoc. 2014;3:e000499. doi: 10.1161/JAHA.113.000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ristagno G, Tang W, Huang L, Fymat A, Chang Y-T, Sun S, Castillo C, Weil MH. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit Care Med. 2009;37:1408–1415. doi: 10.1097/CCM.0b013e31819cedc9. [DOI] [PubMed] [Google Scholar]
- 10.Ditchey RV, Lindenfeld J. Failure of epinephrine to improve the balance between myocardial oxygen supply and demand during closed-chest resuscitation in dogs. Circulation. 1988;78:382–389. doi: 10.1161/01.cir.78.2.382. [DOI] [PubMed] [Google Scholar]
- 11.Davis DP, Garberson LA, Andrusiek DL, Hostler D, Daya M, Pirrallo R, Craig A, Stephens S, Larsen J, Drum AF, Fowler R. A Descriptive Analysis of Emergency Medical Service Systems Participating in the Resuscitation Outcomes Consortium (ROC) Network. Prehosp Emerg Care. 2007;11:369–382. doi: 10.1080/10903120701537147. [DOI] [PubMed] [Google Scholar]
- 12.Morrison LJ, Nichol G, Rea TD, Christenson J, Callaway CW, Stephens S, Pirrallo RG, Atkins DL, Davis DP, Idris AH, Newgard C ROC Investigators. Rationale, development and implementation of the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Resuscitation. 2008;78:161–169. doi: 10.1016/j.resuscitation.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aufderheide TP, Nichol G, Rea TD, Brown SP, Leroux BG, Pepe PE, Kudenchuk PJ, Christenson J, Daya MR, Dorian P, Callaway CW, Idris AH, Andrusiek D, Stephens SW, Hostler D, Davis DP, Dunford JV, Pirrallo RG, Stiell IG, Clement CM, Craig A, Van Ottingham L, Schmidt TA, Wang HE, Weisfeldt ML, Ornato JP, Sopko G. A Trial of an Impedance Threshold Device in Out-of-Hospital Cardiac Arrest. N Engl J Med. 2011;365:798–806. doi: 10.1056/NEJMoa1010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudenchuk PJ, Brown SP, Daya M, Rea T, Nichol G, Morrison LJ, Leroux B, Vaillancourt C, Wittwer L, Callaway CW, Christenson J, Egan D, Ornato JP, Weisfeldt ML, Stiell IG, Idris AH, Aufderheide TP, Dunford JV, Colella MR, Vilke GM, Brienza AM, Desvigne-Nickens P, Gray PC, Gray R, Seals N, Straight R, Dorian P. Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest. N Engl J Med. 2016;374:1711–1722. doi: 10.1056/NEJMoa1514204. [DOI] [PubMed] [Google Scholar]
- 15.Nichol G, Leroux B, Wang H, Callaway CW, Sopko G, Weisfeldt M, Stiell I, Morrison LJ, Aufderheide TP, Cheskes S, Christenson J, Kudenchuk P, Vaillancourt C, Rea TD, Idris AH, Colella R, Isaacs M, Straight R, Stephens S, Richardson J, Condle J, Schmicker RH, Egan D, May S, Ornato JP. Trial of Continuous or Interrupted Chest Compressions during CPR. N Engl J Med. 2015;373:2203–2214. doi: 10.1056/NEJMoa1509139. [DOI] [PubMed] [Google Scholar]
- 16.Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D, Bossaert LL, Brett SJ, Chamberlain D, de Caen AR, Deakin CD, Finn JC, Gräsner J-T, Hazinski MF, Iwami T, Koster RW, Lim SH, Huei-Ming Ma M, McNally BF, Morley PT, Morrison LJ, Monsieurs KG, Montgomery W, Nichol G, Okada K, Eng Hock Ong M, Travers AH, Nolan JP Utstein Collaborators. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation. 2015;132:1286–1300. doi: 10.1161/CIR.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 17.Andersen LW, Berg KM, Saindon BZ, Massaro JM, Raymond TT, Berg RA, Nadkarni VM, Donnino MW American Heart Association Get With the Guidelines–Resuscitation Investigators. Time to Epinephrine and Survival After Pediatric In-Hospital Cardiac Arrest. JAMA. 2015;314:802–810. doi: 10.1001/jama.2015.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnino MW, Salciccioli JD, Howell MD, Cocchi MN, Giberson B, Berg K, Gautam S, Callaway C American Heart Association’s Get With The Guidelines-Resuscitation Investigators. Time to administration of epinephrine and outcome after in-hospital cardiac arrest with non-shockable rhythms: retrospective analysis of large in-hospital data registry. BMJ. 2014;348:g3028. doi: 10.1136/bmj.g3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khera R, Chan PS, Donnino M, Girotra S American Heart Association’s Get With The Guidelines-Resuscitation Investigators. Hospital Variation in Time to Epinephrine for Nonshockable In-Hospital Cardiac Arrest. Circulation. 2016;134:2105–2114. doi: 10.1161/CIRCULATIONAHA.116.025459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ono Y, Hayakawa M, Wada T, Sawamura A, Gando S. Effects of prehospital epinephrine administration on neurological outcomes in patients with out-of-hospital cardiac arrest. J Intensive Care [Internet] 2015:3. doi: 10.1186/s40560-015-0094-3. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4478688/ [DOI] [PMC free article] [PubMed]
- 21.Goto Y, Maeda T, Goto Y. Effects of prehospital epinephrine during out-of-hospital cardiac arrest with initial non-shockable rhythm: an observational cohort study. Crit Care Lond Engl. 2013;17:R188. doi: 10.1186/cc12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindner KH, Strohmenger HU, Prengel AW, Ensinger H, Goertz A, Weichel T. Hemodynamic and metabolic effects of epinephrine during cardiopulmonary resuscitation in a pig model. Crit Care Med. 1992;20:1020–1026. doi: 10.1097/00003246-199207000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Paradis NA, Martin GB, Rivers EP, Goetting MG, Appleton TJ, Feingold M, Nowak RM. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–1113. [PubMed] [Google Scholar]
- 24.Callaway CW. Epinephrine for cardiac arrest. Curr Opin Cardiol. 2013;28:36–42. doi: 10.1097/HCO.0b013e32835b0979. [DOI] [PubMed] [Google Scholar]
- 25.Otto CW. Cardiovascular pharmacology. II: The use of catecholamines, pressor agents, digitalis, and corticosteroids in CPR and emergency cardiac care. Circulation. 1986;74:IV80–85. [PubMed] [Google Scholar]
- 26.Microcirculation during cardiac arrest and resuscitation: Critical Care Medicine [Internet] LWW; [cited 2017 Aug 7];Available from: http://journals.lww.com/ccmjournal/Fulltext/2006/12001/Microcirculation_during_cardiac_arrest_and.8.aspx. [Google Scholar]
- 27.Levitan RM, Bortle CD, Snyder TA, Nitsch DA, Pisaturo JT, Butler KH. Use of a battery-operated needle driver for intraosseous access by novice users: skill acquisition with cadavers. Ann Emerg Med. 2009;54:692–694. doi: 10.1016/j.annemergmed.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Feinstein BA, Stubbs BA, Rea T, Kudenchuk PJ. Intraosseous compared to intravenous drug resuscitation in out-of-hospital cardiac arrest. Resuscitation. 2017;117:91–96. doi: 10.1016/j.resuscitation.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I Resuscitation Outcomes Consortium Investigators. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rea TD, Cook AJ, Stiell IG, Powell J, Bigham B, Callaway CW, Chugh S, Aufderheide TP, Morrison L, Terndrup TE, Beaudoin T, Wittwer L, Davis D, Idris A, Nichol G Resuscitation Outcomes Consortium Investigators. Predicting survival after out-of-hospital cardiac arrest: role of the Utstein data elements. Ann Emerg Med. 2010;55:249–257. doi: 10.1016/j.annemergmed.2009.09.018. [DOI] [PubMed] [Google Scholar]