Abstract
Objective
While higher lead surgeon volume has been associated with lower mortality following open abdominal aortic aneurysm (AAA) repair, little is known about the impact of using an attending surgeon as assistant surgeon. The aim of this study was to determine if the presence of an assistant surgeon, particularly a high-volume assistant, mitigates the relationship between lead surgeon volume and outcomes.
Methods
We evaluated all Medicare beneficiaries who underwent intact, open AAA repair between 2003-2008 and constructed nested regression models to evaluate the relationship between surgeon and assistant volume and perioperative mortality, adjusting for comorbid conditions and hospital volume.
Results
We studied 28,590 repairs, of which 19,284 (67.5%) were performed by a single surgeon and 9,306 (32.5%) included an assistant surgeon. Of cases with an assistant, 12.3% included a high-volume assistant surgeon. Lower volume surgeons more frequently used an assistant (Lead Surgeon Q1 Volume: 40%, Q2: 36%, Q3: 34%, Q4: 29%, Q5: 27%, P < .01). In cases with no assistant, adjusted perioperative mortality varied monotonically with surgeon volume (Q1: 4.7%, Q2: 4.4%, Q3: 4.1%, Q4: 3.3%, Q5: 3.2%). However, the use of a high- or a low-volume assistant surgeon, compared to no attending surgeon as assistant, was not associated with lower perioperative mortality in any lead surgeon volume quintile, even among those operations performed by the lowest volume lead surgeons.
Conclusions
Employing an assistant surgeon does not improve outcomes amongst any quintile of volume of the lead surgeon. As surgeons perform fewer open AAA repairs in the modern era, these data imply that even the help of a high-volume assistant surgeon may not mitigate the detrimental effect of a lower-volume surgeon.
Introduction
Several national and international studies have identified lower mortality following open repair of intact abdominal aortic aneurysms (AAA) by high-volume compared to low-volume surgeons.1-7 This volume-outcome relationship plays a particularly important role in the modern endovascular era, in which open repairs comprise < 20% of intact, infrarenal AAA repairs.8, 9 As surgeons expand endovascular techniques into increasingly complex aortic aneurysms, their experience with open AAA repair (OAR) will continue to decline. Dua et al. predicted that < 3,000 OARs will be performed in the United States in the year 2020, with the average vascular surgery fellowship trainee completing only 1-2 open repairs throughout their training.9
As their open surgical experience declines, young surgeons may be ill-prepared to perform open AAA repair. In an effort to compensate for their own lack of experience, many surgeons may enlist the aid of an assistant surgeon during their high-risk cases. However, it is unclear whether this strategy improves mortality outcomes for lower-volume lead surgeons. Therefore, we sought to evaluate the association between use of an assistant surgeon and outcomes following open AAA repair, and to determine if assistant surgeon volume is associated with perioperative mortality, particularly in the case of low-volume lead surgeons.
Methods
Study Population
We used comprehensive data from the Medicare program to identify all cases of open AAA repair in patients 67 years and older that occurred between 2003 and 2008. As in our prior studies of Medicare beneficiaries,10, 11 we identified patients with a discharge diagnosis by International Classification of Diseases, 9th version, clinical modifications (ICD-9-CM) of AAA without rupture (441.4) who also had a procedure code for open surgical repair, including 38.44 (resection of abdominal aorta with replacement) and 39.25 (aorto-iliac-femoral bypass). We excluded all those with diagnosis codes for aneurysm rupture (441.3), thoracic aneurysm (441.1, 441.2), thoracoabdominal aortic aneurysm (441.6, 441.7), or aortic dissection (441.0), or those with procedure codes for repair of the thoracic (38.35, 38.45, 39.73) or visceral aorta (38.46, 39.24, 39.26). Only those beneficiaries with both Part A (hospital insurance and inpatient care) and Part B (medical insurance, including doctors’ services, preventative care, laboratory and radiographic tests) coverage were included. While we only included intact AAA repairs for our outcomes analysis, we accounted for all open repairs of both intact and ruptured abdominal and thoracoabdominal aneurysms for the purpose of our volume counts.
Lead Surgeon, Assistant Surgeon, and Hospital Volume
We limited our analysis to cases with no more than two surgeons (<1% had more). We identified the performing physician using the unique physician identification number or national provider identification number listed on each patient’s Medicare claim. The Healthcare Common Procedure Coding System (HCPCS) modifier code 80 or 82, which indicate full assistance by a board-eligible surgeon to the primary surgeon, distinguished assistant surgeon from lead surgeon. Surgeon and hospital volume were assessed over the two-year period preceding each operation. We obtained hospital volume from the provider of service codes contained in the Medicare administrative database.
We divided lead surgeon volume into quintiles using cut points that most closely separated the patients into groups of equal size. The quintiles were set using average two-year volume across all the years, which would allow for a shift in volume over time as less open repairs were performed. Frequency of lead (A) and assistant surgeons (B) for each two-year volume are demonstrated in Figure 1. For certain analyses, we defined a high-volume lead surgeon as one in quintile 5 (≥ 13 cases/year), and a low-volume lead surgeon as anyone with < 13 cases/year (Table I). We stratified assistant volume into high-volume (≥ 13 cases/year) versus low-volume (< 13 cases/year) to correspond with the thresholds used for lead surgeon volume.
Figure 1.

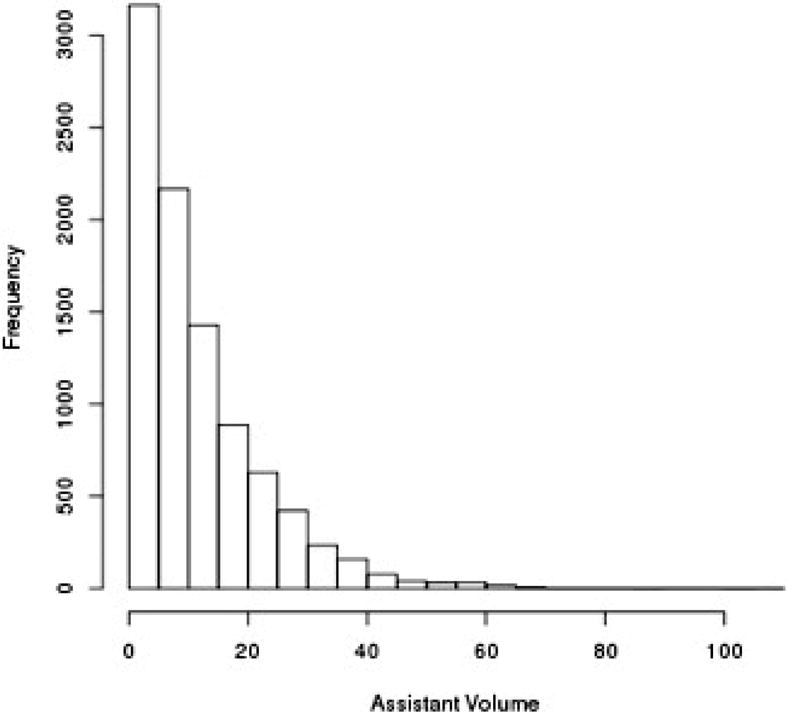
Number of lead surgeons (A) and assistant surgeons (B) with each 2-year volume count.
Table I.
Lead and assistant surgeon two-year volume thresholds, with high-volume for a lead surgeon defined as quintile 5 only. The same threshold was used for high-volume assistant (≥ 25 cases/2-years), with 1,140 assistants (12.3%) being high-volume.
| Quintile | Lead Surgeon | # in Category | Assistant Surgeon | # in Category |
|---|---|---|---|---|
| Q1 | 1-5 | 5,107 | 1-3 | 2,026 |
| Q2 | 6-10 | 6,683 | 4-6 | 1,619 |
| Q3 | 11-15 | 5,422 | 7-11 | 2,023 |
| Q4 | 16-24 | 5,896 | 12-19 | 1,831 |
| Q5 | ≥ 25 | 5,482 | ≥ 20 | 1,807 |
Outcomes Assessment
Our primary outcome was perioperative mortality, defined as death within the index hospitalization, including contiguous transfers to other acute care facilities, or within thirty days of the date the procedure was performed. We assessed mortality using the Medicare Beneficiary Summary File.
Statistical Analysis
We examined surgeon and assistant surgeon volumes for open AAA repair over the years 2003-2008 and then compared the admission characteristics of those patients who had an assistant surgeon to those without an assistant surgeon using the chi-square test for categorical variables and the Student T-test for continuous variables. We then constructed nested multivariable models adjusting for hospital, lead surgeon, and assistant surgeon volume. These models included baseline beneficiary demographic and clinical characteristics obtained from claims during the one-year period prior to but not including the index admission. We measured clinical comorbidities using a version of the Elixhauser algorithm that was adapted to also include diagnoses that occurred only in the outpatient setting.12, 13 We calculated adjusted perioperative mortality rates for each combination of lead and assistant surgeon volume, and performed a type III sum of squares test to evaluate the statistical significance of the interaction-effect between surgeon and assistant surgeon volume. Finally, within each quadrant of hospital volume (high vs. low) and lead surgeon volume (high vs. low), we calculated the independent effect of a low-volume and high-volume assistant surgeon on mortality, as compared to those cases with no assistant surgeon. We completed all statistical analyses using SAS (version 9.4). The Internal Review Board of Harvard Medical School approved this study and waived consent due to the retrospective nature of the study.
Results
We identified a total of 28,590 patients who underwent elective, intact open AAA repair between 2003-2008, of which 9,306 (32.5%) involved an assistant surgeon. With each higher quintile of lead surgeon volume, assistant surgeon use decreased (Q1: 40%, Q2: 36%, Q3: 34%, Q4: 29%, Q5: 27%, P < .01). The baseline characteristics of patients undergoing open AAA repair with and without an assistant surgeon appear in Table II. There were no differences in baseline demographics and comorbidities, including age, sex, chronic obstructive pulmonary disease, and renal failure. The yearly volume of open AAA repairs declined monotonically from 8,445 in 2001 to 2,651 in 2008, corresponding to a 70% reduction (Table III). Despite this decline in overall volume, the proportion of cases that involved an assistant surgeon decreased each year as well, from 38.3% of cases in 2001 to 27.1% of cases in 2008 (P < .001).
Table II.
Baseline characteristics of patients undergoing cases with or without an assistant surgeon.
| With Assistant Surgeon | No Assistant Surgeon | P-Value of Difference | |
|---|---|---|---|
| N = 9,306 (33%) | N = 19,284 (67%) | ||
| Age | .23 | ||
| 67-69 | 1,435 (15.4%) | 3,170 (16.4%) | |
| 70-74 | 2,940 (31.6%) | 6,094 (31.6%) | |
| 75-79 | 2,900 (31.2%) | 5,879 (30.5%) | |
| 80-84 | 1,589 (17.1%) | 3,218 (16.7%) | |
| 85+ | 442 (4.7%) | 923 (4.8%) | |
| Male Sex | 6,831 (73.4%) | 14,009 (72.6%) | .18 |
| Cerebrovascular Disease | 1,332 (14.3%) | 2,760 (14.3%) | 1.0 |
| MI, past 6 months | 125 (1.3%) | 306 (1.6%) | .11 |
| Valve Disease | 770 (8.3%) | 1,565 (8.1%) | .65 |
| Congestive Heart Failure | 948 (10.2%) | 2,023 (10.5%) | .43 |
| Hypertension | 6,039 (64.9%) | 12,471 (64.7%) | .71 |
| Peripheral Vascular | 1,924 (20.7%) | 4,071 (21.1%) | .40 |
| Disease | |||
| COPD | 2,546 (27.4%) | 5,435 (28.2%) | .15 |
| Renal Failure | 548 (5.9%) | 1,149 (6.0%) | .82 |
| ESRD | 26 (0.3%) | 70 (0.4%) | .25 |
| Diabetes | 1,464 (15.7%) | 2,992 (15.5%) | .64 |
Table III.
The number of AAA cases overall has dropped dramatically, as well as the proportion of cases using an assistant surgeon (P < .001 for the trend).
| Year | With Assistant Surgeon | No Assistant Surgeon |
|---|---|---|
| N (%) | N (%) | |
| 2001 | 3,235 (38.3%) | 5,210 (61.7%) |
| 2002 | 2,698 (37.1%) | 4,568 (62.9%) |
| 2003 | 2,385 (35.7%) | 4,293 (64.3%) |
| 2004 | 2,072 (33.1%) | 4,194 (66.9%) |
| 2005 | 1,690 (31.7%) | 3,637 (68.3%) |
| 2006 | 1,400 (32.7%) | 2,881 (67.3%) |
| 2007 | 1,041 (30.7%) | 2,346 (69.3%) |
| 2008 | 718 (27.1%) | 1,933 (72.9%) |
Adjusted perioperative mortality rates with each combination of lead surgeon volume quintile and assistant surgeon volume (categorized as none vs. low-volume vs. high volume) are shown in Table IV. Of cases with an assistant surgeon, 1,140 (12.3%) had a high-volume assistant. Among cases with no assistant surgeon, perioperative mortality varied inversely with higher surgeon volume quintiles (Q1: 4.7%, Q2: 4.4%, Q3: 4.1%, Q4: 3.3%, Q5: 3.2%; P < .001). However, within each quintile of lead surgeon volume, there was no difference in mortality for cases with either a low-volume or high-volume assistant surgeon (P = .74). The odds ratios of mortality with an assistant versus without an assistant in each quadrant of high- versus low-volume hospital and lead surgeon are displayed in Figure 2.
Table IV.
Adjusted 30-day postoperative mortality by lead surgeon volume and assistant surgeon use and volume (P = .74).
| Lead Surgeon Volume | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| No Assistant Surgeon | 4.7% | 4.4% | 4.1% | 3.3% | 3.2% |
| Low-Volume Assistant Surgeon | 5.2% | 4.6% | 3.6% | 4.2% | 3.0% |
| High-Volume Assistant Surgeon | 7.3% | 2.4% | 7.2% | 4.4% | 2.9% |
Figure 2.
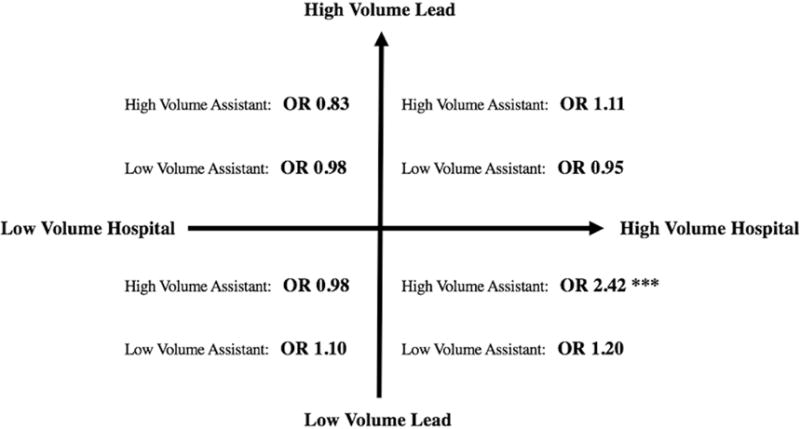
With low-volume lead surgeons in high-volume hospitals, cases with a high-volume assistant have a > 2-fold higher mortality (P < .01). However, among each other quadrant of high-vs. low-volume hospital and lead surgeon, there is no effect from the addition of either a high-volume or a low-volume assistant surgeon, compared to no assistant surgeon (all P > .05).
Among low-volume lead surgeons in high-volume hospitals, cases with a high-volume assistant have a > 2-fold higher mortality (OR 2.42, P < .01). However, in each other quadrant of high- vs. low-volume hospital and lead surgeon, there is no effect from the addition of either a high-volume or a low-volume assistant surgeon, compared to no assistant surgeon (all P > .05). Within the subset of lowest-volume (Q1) lead surgeons, adding a high- or a low-volume assistant surgeon did not affect outcomes (High: OR 1.56, P = .42; Low: OR 1.12, P = .33), although high hospital volume was associated with lower mortality (OR 0.53, P = .046).
Discussion
We used comprehensive data from nearly 30,000 open AAA repairs to evaluate the relationship between assistant surgeon use and volume and outcomes following intact open AAA repair. In all quintiles of lead surgeon volume, the use of an assistant surgeon failed to improve mortality, even if that assistant surgeon was a higher-volume assistant surgeon. Although high lead surgeon volume and high hospital volume were associated with lower perioperative mortality, the use of an assistant surgeon, or a high-volume assistant surgeon, was not. However, we are unable to account for operative complexity, and the mortality in those cases with an assistant may have been even higher had an assistant not been used. In fact, the use of an assistant in the case of a low-volume lead surgeon at a high-volume hospital is associated with a greater than 2-fold higher perioperative mortality, which is most likely a marker of case complexity. These data should not, therefore, discourage the use of an assistant surgeon, although a low-volume surgeon with an assistant still underperforms relative to a high-volume surgeon.
These data imply important, but unfortunate, consequences in the modern endovascular era. We and others consistently demonstrate that patients experience worse outcomes following open AAA repair with low volume surgeons.1, 3, 5-7, 14-19 Indeed, the lowest-volume surgeons (those performing one or fewer cases per year), have incrementally worse outcomes than even other low-volume surgeons.20 The impact of surgeon volume on outcomes occurs above and beyond the effect of hospital volume, and is particular salient when the primary surgeon is low volume.7 These data led many to advocate centralizing open AAA repair to only high-volume providers.21-25 However, as endograft repair of AAA increases and the national rates of open AAA repair dramatically declines, most surgeons are becoming lower volume.26, 27 Vascular surgery trainees will be particularly affected, with recent estimates suggesting that vascular surgery fellowship trainees will complete only 1-2 open AAA repairs, and integrated vascular surgery residents only 2-3 cases throughout training.9 Our data imply that these case numbers result in unacceptably poor outcomes in an already-trained surgeon with potentially many prior years of experience, let alone trainees.28
We hypothesized that the use of an assistant surgeon, particularly an experienced, high-volume assistant surgeon, could mitigate some of the disparity in outcomes seen in the lowest volume surgeons. Unfortunately, our data suggest that this may not be the case. While we cannot predict why an assistant surgeon is used, we suspect that in many cases, assistant surgeons participate in cases thought to be particularly difficult operations, or are called in when the lead surgeon runs into difficulty. Patients with and without an assistant surgeon did not differ in terms of comorbidities; however, we are unable to account for anatomic differences and case complexity. The fact that mortality is higher in cases with a high-volume assistant compared to without, when the lead surgeon is low-volume and the hospital is high-volume, suggests that such a mechanism may be operating, at least in some cases. We suspect that lead surgeon volume primarily dictates operative outcomes, and the use of an assistant may help to lower mortality in particularly complicated cases from where it may have been without an assistant. However, we are unable to confirm this given the limitations of the Medicare data.
In 1992, Archie published a series of 179 consecutive open AAA repairs performed by him with either a board-certified surgeon, or with an experienced surgical nurse.29 Notably, the choice of assistant surgeon in his series depended solely on availability, and not on case complexity. He found no difference in any perioperative outcomes between types of assistant. However, this was a small series from a single surgeon, and, to our knowledge, no other studies evaluated the use of an assistant surgeon in major vascular surgery procedures, and none evaluated volume-outcomes relationships by the assistant surgeon. We believe this is an important area of future study, with implications for surgical credentialing and staffing, because increasingly fewer surgeons will be able to achieve adequate volume and therefore competence to perform open aortic aneurysm repair.
We also understand that surgeon volume alone does not account for all outcomes disparities. We have previously identified hospital and center volume as predictive of outcomes following open infrarenal AAA repair.7 Higher-volume centers likely are better equipped for the preoperative optimization and postoperative care necessary for these medically complex patients undergoing high risk operation, including well-trained anesthesia and intensive care unit providers. Because of this, we accounted for hospital volume in all our analyses, and did again identify an inverse correlation between hospital volume and postoperative mortality.
This study should be interpreted in the context of the study design. Administrative data sources, such as Medicare, have the potential for coding errors, missing data, and data variability, including, perhaps, errors in the coding of surgeon and assistant surgeon. Additionally, this database lacks anatomic detail, including aneurysm diameter and extent, data regarding prior abdominal interventions or incisional approach, and operative time, as well as other procedure-specific variables such as blood loss and blood transfusion, all of which may be related to case complexity, and which may also contribute to the use of an assistant surgeon. We are unable to determine the reason for the use of an assistant surgeon, whether for training purposes in the case of a senior surgeon helping a junior surgeon, or because an assistant was called in due to the complexity of the case. Also, the use of vascular surgery trainees, including fellows and residents, are not accounted for in this analysis. Furthermore, we cannot account for cumulative surgeon volume over their entire career, which is likely to play in an integral role in the relationship between surgeon volume and outcomes.
Conclusions
While lead surgeon volume is inversely proportional to perioperative mortality following intact, open AAA repair, the use of an assistant surgeon, even a high-volume assistant surgeon, is not associated with improved outcomes. These data enhance other studies of volume-outcomes relationships in vascular surgery by demonstrating that even assistance from another surgeon may not fully compensate for a low volume primary surgeon. Further study of the role of an assistant surgeon on outcomes will be important in the endovascular era, as the number of open AAA repairs performed continues to decline.
What This Paper Adds to the Current Literature.
This is the first large scale study to evaluate the role of an assistant surgeon in the volume-outcomes relationship following open repair of abdominal aortic aneurysms (AAA). While we have confirmed prior studies showing lower perioperative mortality following AAA repair among high-volume surgeons, we now also show that the addition of an attending surgeon as assistant, even amongst low-volume lead surgeons, does not improve outcomes, which adds to the existing volume-outcomes literature for aortic disease.
Acknowledgments
Funding
SD, TO, SZ, and KS are supported by the Harvard-Longwood Research Training in Vascular Surgery NIH T32 Grant 5T32HL007734-22. BL and MS are supported by the National Heart, Lung, and Blood Institute of the National Institutes for Health Grant 5R01HL105453-03.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
MLS is a consultant for Abbott, Medtronic, and Endologix.
References
- 1.Dimick JB, Cowan JA, Jr, Stanley JC, Henke PK, Pronovost PJ, Upchurch GR., Jr Surgeon specialty and provider volumes are related to outcome of intact abdominal aortic aneurysm repair in the United States. J Vasc Surg. 2003;38:739–44. doi: 10.1016/s0741-5214(03)00470-1. [DOI] [PubMed] [Google Scholar]
- 2.McPhee JT, Robinson WP, 3rd, Eslami MH, Arous EJ, Messina LM, Schanzer A. Surgeon case volume, not institution case volume, is the primary determinant of in-hospital mortality after elective open abdominal aortic aneurysm repair. J Vasc Surg. 2011;53:591–9.e2. doi: 10.1016/j.jvs.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 3.Holt PJ, Poloniecki JD, Gerrard D, Loftus IM, Thompson MM. Meta-analysis and systematic review of the relationship between volume and outcome in abdominal aortic aneurysm surgery. Br J Surg. 2007;94:395–403. doi: 10.1002/bjs.5710. [DOI] [PubMed] [Google Scholar]
- 4.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 5.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–37. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landon BE, O’Malley AJ, Giles K, Cotterill P, Schermerhorn ML. Volume-outcome relationships and abdominal aortic aneurysm repair. Circulation. 2010;122:1290–7. doi: 10.1161/CIRCULATIONAHA.110.949172. [DOI] [PubMed] [Google Scholar]
- 7.Zettervall SL, Schermerhorn ML, Soden PA, McCallum JC, Shean KE, Deery SE, et al. The effect of surgeon and hospital volume on mortality after open and endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2017;65:626–34. doi: 10.1016/j.jvs.2016.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deery SE, Soden PA, Zettervall SL, Shean KE, Bodewes TC, Pothof AB, et al. Sex differences in mortality and morbidity following repair of intact abdominal aortic aneurysms. J Vasc Surg. 2017;65:1006–13. doi: 10.1016/j.jvs.2016.08.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dua A, Koprowski S, Upchurch G, Lee CJ, Desai SS. Progressive shortfall in open aneurysm experience for vascular surgery trainees with the impact of fenestrated and branched endovascular technology. J Vasc Surg. 2017;65:257–61. doi: 10.1016/j.jvs.2016.08.075. [DOI] [PubMed] [Google Scholar]
- 10.Schermerhorn ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J, et al. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N Engl J Med. 2015;373:328–38. doi: 10.1056/NEJMoa1405778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358:464–74. doi: 10.1056/NEJMoa0707348. [DOI] [PubMed] [Google Scholar]
- 12.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin LM, Klabunde CN, Green P, Barlow W, Wright G. In search of the perfect comorbidity measure for use with administrative claims data: does it exist? Med Care. 2006;44:745–53. doi: 10.1097/01.mlr.0000223475.70440.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimick JB, Upchurch GR., Jr Endovascular technology, hospital volume, and mortality with abdominal aortic aneurysm surgery. J Vasc Surg. 2008;47:1150–4. doi: 10.1016/j.jvs.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 15.Karthikesalingam A, Hinchliffe RJ, Loftus IM, Thompson MM, Holt PJ. Volume-outcome relationships in vascular surgery: the current status. J Endovasc Ther. 2010;17:356–65. doi: 10.1583/10-3035.1. [DOI] [PubMed] [Google Scholar]
- 16.Marlow NE, Barraclough B, Collier NA, Dickinson IC, Fawcett J, Graham JC, et al. Effect of hospital and surgeon volume on patient outcomes following treatment of abdominal aortic aneurysms: a systematic review. Eur J Vasc Endovasc Surg. 2010;40:572–9. doi: 10.1016/j.ejvs.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 17.McPhee JT, Robinson WP, 3rd, Eslami MH, Arous EJ, Messina LM, Schanzer A. Surgeon case volume, not institution case volume, is the primary determinant of in-hospital mortality after elective open abdominal aortic aneurysm repair. J Vasc Surg. 2011;53:591–9.e2. doi: 10.1016/j.jvs.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 18.Meltzer AJ, Connolly PH, Schneider DB, Sedrakyan A. Impact of surgeon and hospital experience on outcomes of abdominal aortic aneurysm repair in New York State. J Vasc Surg. 2017;66:728–34.e2. doi: 10.1016/j.jvs.2016.12.115. [DOI] [PubMed] [Google Scholar]
- 19.Reames BN, Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and operative mortality in the modern era. Ann Surg. 2014;260:244–51. doi: 10.1097/SLA.0000000000000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao J, Goodney P, Cronenwett J, Sedrakyan A. Association of Very Low-Volume Practice With Vascular Surgery Outcomes in New York. JAMA Surg. 2017;152:759–66. doi: 10.1001/jamasurg.2017.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deery SE, Schermerhorn ML. Optimal vs Feasible Volume Thresholds in Vascular Surgery. JAMA Surg. 2017;152:766–7. doi: 10.1001/jamasurg.2017.1081. [DOI] [PubMed] [Google Scholar]
- 22.Karthikesalingam A, Hinchliffe RJ, Poloniecki JD, Loftus IM, Thompson MM, Holt PJ. Centralization harnessing volume-outcome relationships in vascular surgery and aortic aneurysm care should not focus solely on threshold operative caseload. Vasc Endovascular Surg. 2010;44:556–9. doi: 10.1177/1538574410375130. [DOI] [PubMed] [Google Scholar]
- 23.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364–9. doi: 10.1056/NEJM197912203012503. [DOI] [PubMed] [Google Scholar]
- 24.Thompson M, Holt P, Loftus I, Forbes TL. Debate: whether abdominal aortic aneurysm surgery should be centralized at higher-volume centers. J Vasc Surg. 2011;54:1208–14. doi: 10.1016/j.jvs.2011.07.064. [DOI] [PubMed] [Google Scholar]
- 25.Urbach DR. Pledging to Eliminate Low-Volume Surgery. N Engl J Med. 2015;373:1388–90. doi: 10.1056/NEJMp1508472. [DOI] [PubMed] [Google Scholar]
- 26.Dua A, Kuy S, Lee CJ, Upchurch GR, Jr, Desai SS. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. J Vasc Surg. 2014;59:1512–7. doi: 10.1016/j.jvs.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Schermerhorn ML, Bensley RP, Giles KA, Hurks R, O’Malley AJ, Cotterill P, et al. Changes in abdominal aortic aneurysm rupture and short-term mortality, 1995-2008: a retrospective observational study. Ann Surg. 2012;256:651–8. doi: 10.1097/SLA.0b013e31826b4f91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao J, Goodney P, Cronenwett J, Sedrakyan A. Association of Very Low-Volume Practice With Vascular Surgery Outcomes in New York. JAMA Surg. 2017;152:759–66. doi: 10.1001/jamasurg.2017.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Archie JP., Jr Influence of the first assistant on abdominal aortic aneurysm surgery. Tex Heart Inst J. 1992;19:4–8. [PMC free article] [PubMed] [Google Scholar]


