Abstract
Contrary to the apoptosis-necrosis binary view of cell death, recent experimental evidence demonstrates that several forms of necrosis, represented by necroptosis, are regulated or programmed in nature. Multiple death stimuli known to be associated with cardiovascular disease are capable of causing either apoptosis or necroptosis. Whether a cell dies from apoptosis or necroptosis has distinct consequences on inflammation. It is known that apoptosis, a non-lytic form of death mediated by the caspase family of proteases, does not generally evoke an immune response. Necroptosis, on the other hand, is a lytic form of cell death. Due to the rapid loss of plasma membrane integrity, cells dying from necroptosis release proinflammatory intracellular contents and subsequently cause inflammation. Our review delineates various genetic and biochemical evidence that demonstrates a compelling role of necroptosis in the pathogenesis and/or progression of cardiovascular disease including myocardial infarction, atherosclerosis, and aortic aneurysm. Through recent studies of necroptosis in cardiovascular diseases, we attempt to discuss the role of necroptosis in vascular inflammation as well as the potential of necroptosis inhibitors in future clinical management of cardiovascular events. Inhibiting necroptosis in the vasculature has an overall protective role and necroptosis may represent a new therapeutic target to prevent the development and progression of cardiovascular diseases.
Keywords: Apoptosis, atherosclerosis, aneurysm, cardiovascular, inhibitors, necroptosis, RIP3, RIP1
Introduction
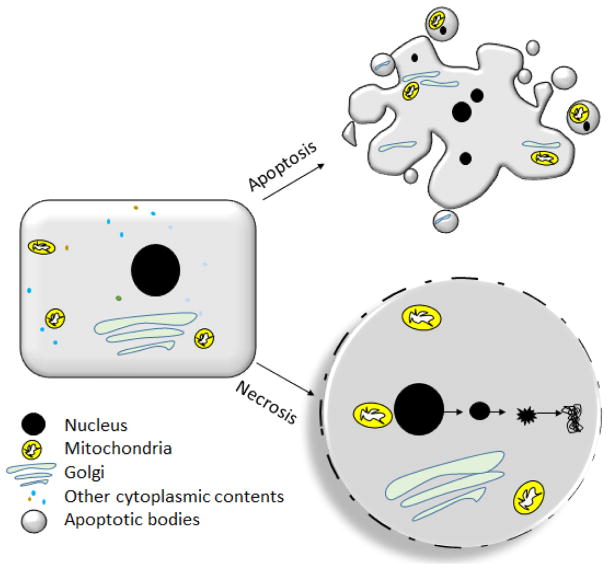
A fine balance between cell proliferation and cell death is essential for healthy physiological processes. Disruption to this life and death balance is frequently observed in pathological events including major cardiovascular diseases (CVDs). For decades, apoptosis was depicted as the only mode of programmed cell death whereas necrosis was viewed as incidental. While sequential activation of caspases is the molecular signature of apoptosis, non-lytic nature is the morphological characteristic of apoptosis. Based on studies in various animal models, the importance of apoptosis in health and disease is well recognized [1–3]. Contrasting to apoptosis, necrosis involves rapid loss of plasma membrane-integrity and is frequently associated with extreme environmental stress (Figure 1). Major morphological differences between apoptotic and necroptotic cells are also found in intracellular organelles like mitochondria, Golgi and the nucleus (Figure 1) [4]. In addition to the morphological differences, apoptosis and necrosis are regulated through distinct signaling mechanisms and have drastically different impacts on inflammation [5], as discussed in detail in the review.
Figure 1.
Schematic diagram depicting the key morphological differences between apoptosis and necrosis. Apoptotic cells have shrunken nucleus, un-ruptured plasma membrane, shrunken mitochondria and golgi. Necrotic cells have ruptured plasma membrane, enlarged organelles and an overall increase in cell-volume.
The concept of necrosis being passive and unregulated was first challenged about fifteen years ago [6]. Several seminal works reported forms of cell death with morphological features of necrosis and yet regulated by well-orchestrated signaling networks that are distinct from the caspase cascade [7] [8] [6] [9] [10] (Table 1). Ever since, studies of programmed necrosis have led to description of several mechanisms of regulated necrosis including necroptosis, cyclophilin D-mediated mitochondrial permeability transition [11] [12], glutamate-induced oxytosis [13], parthanatos [14], ferroptosis [15], NETosis [16], pyronecrosis and pyroptosis [17]. Among them, necroptosis, which is defined as necrosis mediated by receptor interacting protein kinase-3 (RIP3), has been most extensively studied [18–20]. Results generated from animal models suggest that inhibiting necroptosis may serve as a novel strategy for the treatment of many pathological processes such as ischemia-reperfusion injury [21], neurodegenerative diseases [22] and cardiovascular diseases [23–26].
Table 1.
Comparison of the biochemical, morphological and immunological features accompanying apoptosis and necroptosis.
| Apoptosis | Necroptosis | |
|---|---|---|
| Biochemical Features | Caspase activation; intrinsic or extrinsic pathways | Activation of RIP1/RIP3/MLKL |
| Exposure of PS to the cell-exterior | ROS production | |
| Loss of mitochondrial membrane potential | Cathepsin activation | |
| Cytochrome c release | Ceramide and Sphingosine overproduction | |
| ROS production and formation of DNA/Protein adducts | RIP3 dependent ROS production | |
| Activation of DNases | Unknown | |
| ATP consumption decreases; production unchanged | ATP production decreases; consumption unchanged | |
| Cell-Morphology | Plasma membrane blebbing | Rupture of plasma membrane |
| Overall shrinkage in cell-volume; increased opacity | Overall swelling; increased transparency | |
| Condensation and fragmentation of chromatin | Overall no known change in chromatin condensation or nuclear shrinkage | |
| Formation of apoptotic bodies and shedding of intracellular organelles | ||
| Mitochondria usually normal | Swelling of Mitochondria occurs | |
| Immunogenecity | Negligible release of DAMPs like ATP, HMGB1, ROS or dsDNA | Considered highly immunogenic due to the release of DAMPs |
| Efficient clearance by phagocytosis | Poor clearance | |
| Extracellular PS exposure facilitates clearance | Clearance mechanisms include macropinocytosis | |
| Non-amplificatory in nature | Amplificatory in nature |
PS, phosphatidylserine; ROS, reactive oxygen species; DAMPs, damage associated molecular patterns; HMGB1, High mobility group box 1 protein; dsDNA, double stranded DNA
1. Molecular and Cellular Basics of Necroptosis
Triggers of Necroptosis
The precise stimuli of necroptosis across various human disease conditions remain obscure, although several chemical “triggers” of necroptosis have been suggested (Figure 2). Necroptosis triggers include but are not limited to ligands to death receptors (such as TNFα, FAS ligand) [27] [28] [8], genotoxic stress [29], pathogen-derived double strand DNAs/RNAs [30] and interferons [31] (Figure 2). Necroptosis can also be triggered by damage associated molecular patterns (DAMPs), nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RGRs), ripoptosome and protein kinase R (PKR) complexes as reviewed in detail by Berghe et al. [32]. Those triggers are thought to individually or jointly induce necroptosis in complicated disease conditions. Regardless of the upstream triggers, RIP3 plays a decisive role in necroptosis and its expression is directly linked to the susceptibility of cells to undergo necroptosis. The expression of RIP3 occurs basally in many tissue types as reported by databases like ENCODE, the human protein atlas and also in primary literature. RIP3 expression becomes noticeably elevated during infection, tissue injury or disease [33] [34]. Structurally, RIP3 is a RHIM domain containing protein. Unlike other members of the RIP kinase family which includes RIP1, RIP3 lacks a death-domain [35] [36]. The death domain is required for interacting with the death-domain containing adaptors like FADD and TRADD. Many pro-necroptosis triggers are also capable of inducing apoptosis. How does a cell decide whether to die through apoptosis or necroptosis? In addition, why do we need multiple cell death mechanisms? Thus far, these fundamental questions are best addressed in the context of host defense. The importance of necroptosis in host defense was first demonstrated by Cho and colleagues who described severely impaired virus-induced tissue necrosis, inflammation, and control of viral replication in Rip3 gene deficient mice [27]. The current view is that necroptosis functions as an alternative mechanism to eliminate infected cells when the default apoptotic mechanism is compromised [37]. It remains unclear how the necroptosis cell fate is decided in non-sterile conditions. Experimentally, one can “reveal” or “unleash” necroptosis activities in cells or tissues by deleting caspase 8, which normally suppresses necroptosis by cleaving RIP3 [38]or by using caspase inhibitors such as Z-Val-Ala-Asp-fluoromethylketone/carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone (zVAD-fmk) [39].
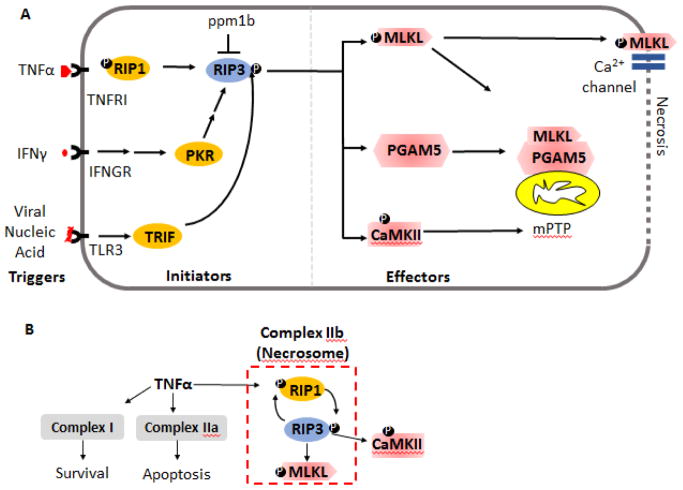
Figure 2.
Signal-transduction events in necroptosis. A) The necroptosis pathway may be categorized into the triggers, initiators and effectors of necroptosis. The initiator molecules involve cytokines like TNFα, IFNγ or viral nucleic acids which via their cognate receptors activate the initiator molecules. These initiators converge on activating RIP3, for instance via phosphorylation. Activated RIP3 is currently known to function via at least three downstream effectors-- MLKL PGAM5 and CaMKII, which are the effector molecules leading to necrosis. Protein phosphatase ppm1b is found to de-phosphorylate RIP3 and prevent necroptosis. Ⓟ denotes phosphorylation. B) The complexes involved in cell-death are depicted. Depending on the cell-type and cellular-context, TNFα may promote formation of a pro-survival complex (complex I), a pro-apoptotic complex (complex IIa) or depending on the inhibition of caspase 8 and presence of RIP3, a pro-necroptotic complex (complex IIb). The components of the pro-necroptosis complex II are depicted.
PKR: Protein Kinase R; TRIF: TIR-domain-containing adapter-inducing interferon-β, MLKL: Mixed Lineage Kinase domain Like pseudokinase; CaMKII: Ca2+/calmodulin-dependent protein kinase II; ppm1b: Protein Phosphatase, Mg2+/Mn2+ Dependent 1B; mPTP: mitochondrial Permeability Transition Pore complex; PGAM5: Phosphoglycerate Mutase Family Member 5
Morphological Features of Necroptotic Cells
In textbooks, the term “necrosis” frequently refers to cells that fail to maintain membrane integrity. Histologically, necrotic cells appear swollen and show increased eosinophila in hematoxylin and eosin (H&E) stains [40]. Electron microscopy reveals many ultrastructure changes of necrosis, exemplified by disrupted plasma membrane and fragmentation or swelling of organelles [4]. The disrupted plasma membrane allows large molecules such as Evan’s blue or propidium iodide (PI) to enter the intracellular compartment. Excluded from healthy cells and apoptotic cells, these dyes are commonly used to identify cells dying from necrosis [41].
Because cells dying from necroptosis display typical necrotic features, they are currently indistinguishable under standard histological inspections from cells that die truly incidentally. Apoptotic cells, on the other hand, are recognized by their shrinkage, membrane blebs, and chromatin condensations (Figure 1 and Table 1). There are also many reliable biochemical approaches to detect apoptosis in vitro and in vivo such as terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), which identifies apoptotic cells by detecting DNA fragmentation. Biochemical markers for necroptosis are under development. Currently, there are no commonly used definite biomarkers for necroptosis. Our lab uses the proximal ligation assay to detect the formation of pronecroptosis complexes also called necrosome as a method of identification of necroptosis in cells and tissues in which protein-protein interaction between RIP3 and RIP1 is required for the onset of necroptosis (unpublished work). Phosphorylation of RIP3 substrate is also used to measure necroptosis by multiple investigative groups [42] [43] [44].
Intracellular Signaling during Necroptosis
Parallel to the caspase activation cascade, activation of RIP3 is the molecular signature of necroptosis. However, comparing to the extensive information in regards to caspase signaling and regulation, our knowledge of RIP3 activation, its interacting partners, and downstream substrates is primitive. Since the necroptosis initiation mechanism has been discussed in details by multiple review articles including the recent ones by Liu [45] and Grootjans [46], we will be brief in this regard. Essentially, the molecular mechanisms underlying necroptosis initiation (by initiator proteins (Figure 2A) is largely derived from studies of tumor necrosis factor α (TNFα)-mediated necroptosis. TNFα is a potent inducer of cell death but may also promote survival under certain conditions. Additionally, it has a major role during inflammation in infection or tissue injury. The divergent functions of TNFα are accomplished through distinct intracellular signaling pathways triggered by binding of TNFα to TNF receptor 1 (TNFR1), illustrated as three signaling complexes (Figure 2B) that may lead cell survival, apoptosis or necroptosis [19, 20]. Complex I is pro-survival and includes TNF-R1, TNFR-associated death domain (TRADD), cellular inhibitors of apoptosis, and RIP1 among others. Complex IIa is pro-apoptotic and results from the de-ubiquitination of RIP1. Complex IIa also contains caspase-8 that activates downstream caspases but also suppresses the initiation of necrotic events by cleaving RIP1 and RIP3. Under pathological or experimental conditions in which either RIP3 expression is upregulated or caspase-8 is inhibited, Complex IIb also called necrosome is formed (Figure 2B) [47]. Whether TNFα produces pro-survival, pro-apoptotic or pro-necroptotic protein complexes is influenced by cell type, cellular contents and regulatory mechanisms such as ubiquitination and phosphorylation. Phosphorylation of RIP1 and RIP3 is crucial to the assembly and activity of the necrosome. Mass spectroscopy data suggest RIP1 may be autophosphorylated upon necrosis initiation [48] [49]. Inhibiting the kinase activity of RIP1 can successfully block necrosome assembly [27, 50]. In vitro, RIP3 can directly phosphorylate RIP1, and Rip3 gene deficiency abolishes necrosis-associated RIP1 phosphorylation [27]. The phosphatase Ppm1b interacts with phosphorylated RIP3 and dephosphorylates it, preventing necroptosis in this capacity [51].
Mouse knock-out models have contributed substantially towards our understanding of the necroptosis pathway and the interplay between RIP kinases and caspases. Mice deficient in the Rip3 gene are viable and fertile [52]. However, under pathological conditions such as mouse models of atherosclerosis [23] and photoreceptor degeneration [53] [54], RIP3 deficiency prevents necrosis but not apoptosis. Moreover, cells harvested from Rip3−/− mice display similar sensitivity to a variety of apoptotic stimuli as the wild-type cells [55], suggesting that apoptosis and necroptosis are somewhat modular in their signaling. Contrary to this notion, the kinase dead mutant D161N mice (Ripk3D161N/D161N) die at the embryonic state due to the spontaneous induction of Caspase 8-dependent apoptosis [52]. Apoptosis can also be triggered when RIP3 kinase activity is perturbed by high concentrations of certain inhibitors [19]. Mandal et al. postulated that RIP3 exists in two conformations: a kinase-dependent form leading to necroptosis and a kinase-independent form that recruits RIP1, FADD, and Caspase 8 which subsequently activates apoptosis [19]. However, not all RIP3 kinase dead mutants cause apoptosis. Unlike the pro-apoptotic activity of RIP3D161N, RIP3 kinase dead mutants K51A, D143N and D161G did not trigger apoptosis in vitro [19]. Furthermore, Rip3K51A/K51A kinase-mutant knock-in mice are viable and fertile.
Unlike Rip3 knock-out mice, mice lacking the Rip1 gene die shortly after birth [56] [57]. However, this perinatal lethality is independent of RIP1 kinase activity. Several groups have generated RIP1 kinase-dead knock-in mice Ripk1K45A (which bear a point mutation in exon 3 corresponding to the catalytic lysine of the Ripk1 gene) [56] and Ripk1D138N/D138N (where conserved aspartate is mutated to asparagine at position 138) [57]. These RIP1 kinase dead mutant mice are viable and healthy, indicating that RIP1 kinase activity is dispensable for development. However, both strains of RIP1 kinase dead mutants show abrogated pro-necroptotic activities. A growing body of evidence also suggests that the kinase activity of RIP1 underlies some pathological events independent of RIP3, such as IL-1α production in hematopoietic cells [50]. Therefore, RIP1 kinase dead mouse line is an important tool to dissect such functions of RIP1 kinase in disease models.
RIP3 may regulate certain physiological and pathological events independent of RIP1. Although RIP1 and RIP3 often act in synergy to promote cell death, RIP3-dependent necroptosis can occur in the absence of RIP1 under certain conditions such as DAI-induced necroptosis triggered by mouse cytomegalovirus infection [58] and Toll like receptor 3 (TLR3)-induced necroptosis triggered by poly (I:C) in the presence of pan-caspase inhibitor [59]. The pseudokinase mixed lineage kinase domain-like (MLKL) was identified by Wang’s group in 2012 as a substrate of RIP3 in necroptosis pathway [9] (Figure 2A). Phosphorylation of MLKL by RIP3 is essential for necroptosis execution with different executioner mechanisms of MLKL proposed. Vandenabeele’s group and Wang’s group independently report that phosphorylated MLKL forms an oligomer that binds to negatively charged phosphatidylinositol phosphates and acts as a pore-forming complex, directly causing necrotic membrane disruption [60, 61]. Other studies suggest that following its phosphorylation, MLKL translocates to the plasma membrane causing an influx calcium or sodium which leads to necrotic membrane rupture due to the increase in osmotic pressure [55, 62]. Consistent with the role of MLKL in the execution of necroptosis, MLKL knock-out mice display resistance to necroptosis in vitro and in-vivo using a pancreatitis model, without affecting apoptosis.
Mitochondria together with ROS is known to be associated with cell death [63] and several mechanisms implicate these in necroptosis. Zhang [64] reported a lack of phosphorylation of MLKL during myocardial necroptosis in response to ischemia and oxidative stress. The authors demonstrated Ca2+-calmodulin-dependent protein kinase (CaMKII) being an alternative effector. RIP3 activates CaMKII by phosphorylation and oxidation triggering the opening of the mitochondrial permeability transition pore (mPTP) and myocardial necroptosis [64] (Figure 2A). Whether and how MLKL and CaMKII may functionally interact with one another remains unclear. ROS was also found to be produced in RIP3 expressing 3T3 cells but not RIP3 lacking cells and was required for the induction of necrosis; in this report, it was demonstrated that the decision to execute necroptosis over apoptosis was closely tied with mitochondrial ROS production [65]. The mitochondrial phosphatase PGAM5 is also involved in the execution of necroptosis and is found to be associated with RIP1/RIP3 complex (Figure 2A) [66]. It functions downstream of the necrosome as well as intrinsic triggers like ROS. PGAM5 serves to recruit the mitochondrial fission protein Drp1, which is required to fragment the mitochondria. This appears to be a pre-requisite for necroptosis, as attenuating PGAM5 inhibits necroptosis [66]. Others have reported a dispensable role of Drp1 in a RIP3 dimerization-dependent model for necroptosis, which may be owing to the difference in the trigger for necroptosis in the latter system (i.e. independent of TNFα) [67].
2. Pharmacological targeting of necroptosis
With the discovery of a unique set of proteins that constitute the necroptosis pathway, great efforts have been devoted towards developing small-molecule inhibitors that target these proteins. The loss-of-function phenotypes in mice as discussed above have provided a proof of concept that inhibiting the necroptotic machinery can have across the board benefits for many diseases, including CVDs. In the following section, we discuss the development of various inhibitors targeting necroptosis in general while the application in CVDs is discussed later in a disease-specific manner.
RIP1 inhibitors
In an effort to define the non-apoptotic pathway triggered by activation of death receptors, Yuan and her group screened a chemical library of 15,000 compounds for chemical inhibitors of the necrotic death of human monocytic U937 cells induced by TNFα and zVAD.fmk. The effort led to the discovery of Necrostatin 1s (Nec-1s) [6]. Since this hallmark discovery in 2005, Nec-1 has been applied widely to necroptosis in various cell types and disease process. Later, the same group identified RIP1 as the primary intracellular target of Nec-1 [68]. Using a comprehensive loss and gain of function mutant study and computational modelling of RIP1, Degterev et al established Nec-1’s mode of action as an activation loop based inhibition [68]. The crystal structure of Nec-1 bound to the RIP1 kinase domain also suggests that Nec-1 interacts with conserved amino acids in the activation loop thus stabilizes RIP1 in an inactive conformation [35]. The potential use of Nec-1 to treat human disease has been investigated in mouse models of neuronal loss, photoreceptor loss, ischemic brain injury, myocardial infarction, and most recently atherosclerosis [6, 18, 23, 33] [69]. Necrostatin-1s (Nec-1s) is a modified version of Nec-1 with significant improvement in target specificity and stability [70]. Interestingly, low doses of Nec-1 sensitizes mice to lethality during TNF-induced systemic inflammatory response syndrome (SIRS) [71] [72]. This toxicity has not been observed with Nec-1s, suggesting Nec-1 may sensitize systemic inflammation through targets other than RIP1.
In addition to Nec-1 and other necrostatins, several other RIP1 inhibitors have been identified in chemical screens. Among the RIP1 inhibitors, GSK2982772 is in phase 2a clinical trial for psoriasis, rheumatoid arthritis, and ulcerative colitis [73]. GSK2982772 shows excellent selectivity for RIP1 [73] and it blocks inflammation induced by TNFα through blocking cytokine production [73]. The potential application of GSK2982772 has not been tested in CVDs, although in principle it should be beneficial since inflammation is a large component of CVDs. As with Nec-1, excellent structure activity relationship (SAR) data for GSK2982772 may lead to the development of analogs for additional pre-clinical and clinical applications [73].
RIP3 inhibitors
Conceptually, RIP3 is a better target than RIP1 because Rip3 gene knockout mice are viable and displayed disease protection even with deletion of one gene copy at least in some pathological models [23, 41]. In addition, RIP3 has been shown to regulate inflammatory gene-expression [74] [41] [75]. Through chemical library screening with human recombinant RIP3 kinase domain, Mocarski’s group identified several compounds including GSK’840, GSK’843 and GSK’872 that bind and inhibit RIP3 at sub-nM concentrations [76, 77]. While effective in suppressing necroptosis, these inhibitors alone induce apoptosis in a concentration dependent manner. The cytotoxicity of GSK’840 and other related inhibitors led to the appreciation of RIP3 kinase-independent function in apoptosis [76]. Despite the known cytotoxicity, GSK’872 was applied to a mouse cerebral ischemic-reperfusion model for 72 hours and found to produce a ~30% reduction in infarct volume and reduced phosphorylation of MLKL [78]. Recently, a class of inhibitors for B-Raf (V600E), traditionally used in treating metastatic melanoma, has demonstrated promising results for RIP3 inhibition where several in-class compound (represented by dabrafenib) show RIP3 binding [79]. Dabrafenib potently inhibited RIP3 activity in-vitro and in-vivo in a B-Raf independent manner by competing with ATP binding. Dabrafenib also showed activity in protecting against necroptosis in ex-vivo and in-vivo models, emerging as a leading inhibitor for necroptosis although its effect on CVDs remains untested.
Hybrid inhibitors
FDA-approved anti-cancer drugs ponatinib and pazopanib are recently found to inhibit necroptosis in vitro [80]. Ponatinib inhibits both RIPK1 and RIPK3, while pazopanib preferentially inhibits RIP1. However, tyrosine kinase inhibitors ponatinib and pazopanib are also associated with cardiovascular toxicities, which precludes in vivo studies [81].
Najjar et. al. from the Degeterev group reported a “hybrid” inhibitor that combined features of Nec-1 and ponatinib [82]. The investigators asserted that the “hybrid” inhibitors target RIP1 with a high potency and inhibited TNF-induced injury in-vivo, which was caused via tail vein injection of 5 ug/mouse with TNFα [82].
MLKL inhibitors
Relative to RIP1 and RIP3, few inhibitors have been reported for MLKL despite of the fact that MLKL is essential for the execution of necrosis and therefore may serve as an excellent candidate target for preventing necroptosis and the subsequent non-sterile inflammation. To this, necrosulphonamide (NSA) is an inhibitor of human MLKL that was found to bind to MLKL in an affinity probe and blocked necroptosis [9]. NSA may be a promising drug candidate since it has found to prevent necroptosis in a variety of disease, although mostly using ex-vivo/cell-culture based systems. Its potential application in pre-clinical animal models is prevented by its specificity for human MLKL.
3. Necroptosis and inflammation
Necroptosis and inflammation are intimately associated. Inflamed tissues often contain necrotic cells. Many pro-inflammatory cytokines such as TNFα are also capable of triggering necroptosis. The disruption of cellular membranes during necroptosis leads to massive release of intracellular contents. Some of these cellular components are danger-associated molecular patterns (DAMPs) which are pro-inflammatory when present outside the cells. By contrast, the preserved plasma membrane and orderly disassembly of intracellular contents prevents or limits apoptotic cells from releasing DAMPs [83, 84]. The chromatin associated protein high-mobility group box 1 (HMGB1) is a well-established DAMP that acts on several immune cells to trigger inflammatory responses. Known receptors for HMGB1 include toll like receptor 2 (TLR2), TLR4 and receptor for advanced glycation end products RAGE [85]. HMGB1 can induce dendritic cell maturation [86], induce production of other pro-inflammatory cytokines (IL-1, TNFα, IL-6, IL-8) [87] as well as upregulate expression of cell adhesion molecules (ICAM-1, VCAM-1) on endothelial cells [88]. Other DAMP molecules include DNA and RNA outside of nuclei or mitochondria, S100 proteins and purine metabolites [89].
The intracellular mediators of necroptosis such as RIP1 and RIP3 can promote pathological inflammatory processes independent of their functions in cell death. Since Wallach and colleagues provided an extensive overview of this topic in their recent review [90], we will discuss only a few developments. For example, inflammasome activation and IL-1 maturation in LPS-primed macrophages requires RIP3 [20]. While caspase 8 deficiency in dendritic cells depends on the function of RIP3, they also require RIP1, MLKL and PGAM5 [91]. Lawlor et al. confirm the role of RIP3 in inflammasome and IL-1 maturation in vivo using in a rheumatoid arthritis mouse model [92]. Interestingly, the authors found that RIP3 promotes the inflammatory responses without needing MLKL [92], further underscoring the presence of additional RIP3 substrates or effectors. In our own study in vascular smooth muscle cells (VSMCs), we demonstrated that RIP3 regulates the expression of pro-inflammatory cytokines and adhesion molecules through the NF-κB pathway [41]. We also noted that inhibition of RIP1 with Nec-1s attenuated macrophage migration toward MCP1 however failed to influence cytokine expression [93], indicating diverse functions of RIP1 and RIP3 in regulation of inflammatory response,
4. Targeting necroptosis in Cardiovascular Diseases
Accumulating animal work proves the involvement of necroptosis in pathogenesis and progression of cardiovascular diseases including atherosclerosis, myocardial infarction, restenosis and abdominal aortic aneurysms. In the following paragraphs, we attempt to offer a brief summary of findings regarding necroptosis in several major cardiovascular events.
Myocardial infarction
Acute myocardial infarction, commonly referred as heart attack, is a leading cause of death worldwide. Myocardial infarction occurs when blood flow to a section of heart suddenly becomes blocked. If the blocked coronary circulation is not restored on a timely fashion, cardiomyocytes in the affected area die and result in irreversible cell loss [94]. Timely administration of thrombolytic medicines or/and percutaneous coronary interventions is effective to minimize heart muscle damage. However, the process of restoring blood flow to ischemic myocardial tissue can also induce cell injury and death, a process that is termed as ischemic reperfusion (I/R) injury. Therefore, prevention of ischemic reperfusion injury is a pressing concern for modern cardiology.
The involvement of necroptosis in myocardial infarction was first suggested by Luedde et al, who discovered up-regulated levels of RIP3 protein in ischemic mouse hearts. The same authors reported that mice deficient for RIP3 recovered from an experimental infarction resulted from permanent left anterior descending coronary artery (LAD) ligation with a significantly higher ejection fraction and less hypertrophy compared to their wildtype counterparts. The functional improvements were accompanied by diminished infiltrating T cells in the Rip3−/− hearts [18]. A more recent study by Zhang et al. using an acute I/R injury model (30 min ischemia/4 h reperfusion) affirms that RIP3 deficiency protects mouse hearts from IR-induced necrosis and significantly reduces infarct size [64]. The benefit of Rip3 knockout extended to long term cardiac remodeling reflected by improvements in fibrosis, hypertrophy, ejection fraction, and mortality measured 8 weeks after a temporary LAD ligation. Mechanistically, Zhang and colleagues asserted that (1) elevated RIP3 is responsible for myocardial necroptosis, as well as apoptosis and inflammation and (2) RIP3 signals through CaMKII instead of MLKL in cardiomyocytes (Figure 3B) [64].
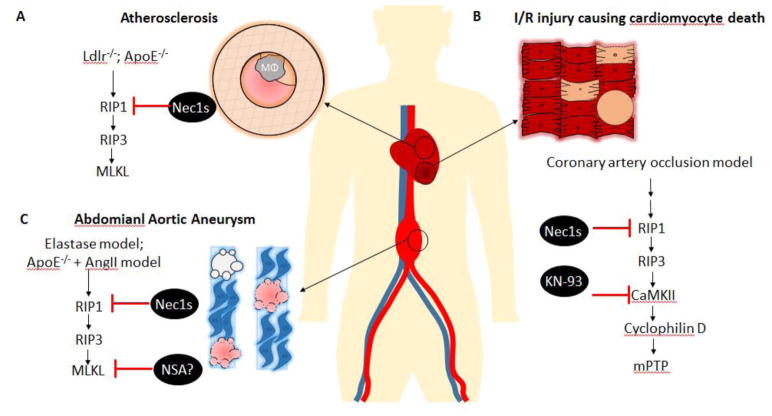
Figure 3.
Schematic outline of the involvement of necroptosis in elimination of cells of the cardiovascular system and reported inhibition strategies based on necroptosis pathway. A) Role of Nec1s in treatment of atherosclerosis in response to inflammation and necroptotic death of cells including macrophages and VSMCs. B) Ischemia-reperfusion related injury has been found to eliminate cardiomyocytes, notably via necroptotic machinery somewhat different from the conventional signaling pathway. C) Treatment of abdominal aortic aneurysms by Nec1s and potentially NSA by prevention of VSMC-necroptosis as a therapeutic strategy. ApoE−/−, apolipoprotein E knockout; Ldlr−/−, low-density lipoprotein receptor knockout; Mφ, macrophage; I/R, ischemia reperfusion; mPTP, mitochondrial permeability transition pore
The concept that pharmacological targeting of the necroptosis pathway is cardio-protective against reperfusion injury was first established by Smith et al. who demonstrated that the RIP1 inhibitor Nec-1 inhibits myocardial cell death and reduces infarct size in mice [95], thus establishing pharmacological inhibition of the necroptosis pathway as a potential cardio-protective therapy against I/R induced cardiac injury. It is worthwhile to note while Nec-1 at low concentration (30 μM) significantly reduced infarct size, administration of high concentration of Nec-1(100 μM) increased infarct size, potentially through off target effects [95]. A study by Dmitriev et al. also revealed that intraperitoneal injections of Nec-1 or Nec-5 prior to reperfusion of isolated heart from rats also reduced the infarction zone caused by 30 min global ischemia and 120-min reperfusion [96]. Importantly, Nec-1 prevents both short and long-term effects of myocardial ischemia, including diminished necrotic cell death and reduced myocardial infarct size, as well as preservation of long-term cardiac function and preventing adverse cardiac remodeling [97]. Mechanistically, Oerlemans showed an increased RIP1/3 phosphorylation after myocardial ischemia and reperfusion [97]. Administration of Nec-1 significantly decreased RIP1 phosphorylation, RIP1-RIP3 binding, and MLKL phosphorylation, the necessary signaling steps leading to necrotic cell death. Results from Oerlemans’ study in regards to MLKL appears to be inconsistent from what reported by Zhang et al. who showed that siRNA-mediated knockdown of MLKL in cardiac myocytes produced little effect on necroptosis [64]. The precise role of MLKL remains to be further tested.
The aforementioned studies do not exclude the critical role of apoptosis. The apoptotic pathway has long been established as an underlying mechanism responsible for the irreversible loss of cardiomyocytes due to infarction [98]. The notion that both caspase and RIP pathways contribute to cellular damages caused by I/R injury was supported by finding that combined administration of a pan caspase inhibitor zVAD-fmk and Nec-1 leads to an even further reduction in infarct size, compared to Nec-1 alone [99]. Since RIP1 participates in the signal-cascade leading to apoptosis [59] as well as necroptosis, Nec-1-mediated RIP1 inhibition is found to reduce both necroptosis and apoptosis of cardiac myocytes [99] [97]. Of note, cardiac apoptosis, resulting from either ischemic injury or I/R injury, involves both intrinsic (mediated by caspase 9) and extrinsic (mediated by caspase 8) pathways [99, 100]. The precise role of RIP1 in cardiac apoptosis remains elusive.
Atherosclerosis
The formation of atherosclerotic plaques in arteries reduces blood flow to major organs and contributes to ischemia of major organs as well as limbs. Rupture of atherosclerotic plaque leads to the formation of an occluding thrombus on the surface of the plaque which is the most common cause of myocardial infarction and stroke [101]. Various lipid-lowering drugs represented by statins have been used successfully in reducing the major cardiovascular events [102, 103]. However, detection of unstable plaques and preventing them from ruptures continues to be a challenge in the management of millions of atherosclerotic patients.
Histologically, atherosclerotic plaques that are prone to rupture are characterized by thinning of the fibrotic cap coupled with expanded necrotic core. VSMC apoptosis as well as secondary necrosis resulted from untimely clearance of apoptotic cells has been actively studied in the context of plaque rupture [101, 104]. In comparison, investigations of necroptosis in atherosclerosis are emerging only recently. Using murine models of atherosclerosis, Lin et al. demonstrated the presence of necroptotic cells and elevated RIP3 in the necrotic core of advanced lesions[23]. Meng and colleagues subsequently showed phosphorylated RIP3 (serine 232) in atherosclerotic lesions of ApoE knockout mice fed with high fat diet [105]. More recently, Karunakaran et al. reported enhanced RIP3 and MLKL as well as levels of phosphorylated MLKL (used as an indication of activation of RIP3) in human carotid atheroma [33].
To more directly test the role of RIP3 thus necroptosis in atherosclerosis, Lin and colleagues bred Rip3−/− mice into atherosclerosis-prone Ldlr−/− and Apoe−/− backgrounds. The resultant mice were fed with a Western-type diet for either 8 or 16 weeks. The lack of RIP3 was found to dose-dependently reduce the lesion areas and diminish the features of plaque vulnerability including the necrotic core size, disruption of collagen, thinning of the fibrous cap, and elastin depletion in mice that was on the Western diet for 16 weeks [23]. A similar athero-protective effect of Rip3 gene deficiency was also reported by Meng et al. Using the bone marrow transplantation approach, Lin and co-authors showed that loss of RIP3 expression from bone-marrow-derived cells is responsible for the reduced plaque maturation. Interestingly, Rip3 gene deficiency produced little effect on lesion sizes at the 8-week time point [23], suggesting that necroptosis is dispensable during the formation atherosclerotic plaques. This notion that necroptosis may drive plaque instability is further highlighted by Karunakaran who reversed plaque maturation of established atherosclerosis in Apoe−/− mice with the RIP1 inhibition Nec-1 (Figure 3A) [33].
A major functional distinction of necroptosis from apoptosis is its pro-inflammatory property. Indeed, mouse bone marrow derived macrophages responded to necroptosis inducing-conditions such as TNFα combined with Smac mimetic and the pan-caspase inhibitor z-VAD-fmk (TSZ) with profound release of IL-1α and Il-1β in a RIP3-dependent manner [105]. This RIP3-sensitive production of cytokines is thought to be secondary of necroptosis because Rip3 knockout has little effects on cytokine release triggered by stimuli that do not cause necroptosis [105]. Along the same line of argument, the diminished vascular inflammation detected in the lesion prone areas of fat-fed Rip3 KO mice in Ldlr−/− or Apoe−/− backgrounds is most likely resulted from inhibition of necroptosis. However, lineage tracing studies demonstrate that as high as 40% of foam cells in the atherosclerotic plaques originated from VSMCs [106]. Whether RIP3 has a direct role in regulation of inflammatory response of VSMC-derived macrophage like cells remains to be tested. Pertinently, our group showed that RIP3 may regulate cytokine expression in VSMCs through a cell death-independent mechanism [41].
Contrasting to the cardiomyocyte study which suggests that RIP3 contributes to both necroptosis and apoptosis [64], Lin’s work indicates the role of RIP3 in macrophages is primarily necrosis. The authors believe that the high necrotic activity in advanced atherosclerotic lesions is most likely a consequence of elevated RIP3 expression lesion macrophages because RIP3 overexpression in cultured macrophages skewed the effect of oxidized LDL from apoptosis to necroptosis [23]. We believe whether high RIP3 drives necroptosis or both necroptosis and apoptosis depends on the nature and context of the cell. In support of this notion, adenovirus-mediated overexpression of RIP3 triggered VSMC apoptosis (and suppressed VSMC proliferation) in balloon injured rat carotid arteries [107]. The same investigative group also demonstrated RIP3 gene silencing in cultured VSMCs potentiated mitogen-induced Akt activation and cell proliferation [107].
Abdominal aortic aneurysm
Abdominal aortic aneurysm (AAA) is defined clinically as a full-thickness dilatation of the vessel wall of the abdominal aorta exceeding the normal vessel diameter by 50% [108]. Ruptured AAAs frequently lead to lethality [109, 110]. Although small aneurysms do occasionally rupture, the risk of rupture is found to be associated with larger aneurysm size [111]. Currently, surgical interventions, including open surgical and endovascular aneurysm repairs, are the only effective treatments to prevent rupture in patients with AAAs larger than 5.5cm (male) or 5.0cm (female) [111]. However, the majority (90%) of AAA cases identified by diagnostic screening is small, asymptomatic aneurysm (<3.5cm in diameter) [112] and is left untreated due to the low benefit-to-risk ratio of surgical interventions in this patient population [113, 114]. The clinical need of pharmacological treatments for small AAAs has motivated active investigations which have identified several key pathophysiological processes, including chronic vascular inflammation, excessive local production of matrix-degrading proteases, progressive destruction of structural matrix proteins, particularly elastin and collagen, as well as depletion of medial VSMCs [108, 115]. VSMC apoptosis has been noted in human aneurysmal tissue for a long time [116–120]. Several anti-inflammatory strategies, such as removing mast cells[120], deletion of TNFα [121], or blocking angiotensin II (Ang II) [122], were found to prevent VSMC loss, suggesting that inflammation is an important cause of VSMC death at least in experimental models of aortic aneurysm. To determine whether cell apoptosis is a contributing, pathological event in aortic aneurysm, our group treated Apoe−/− mice with a pan caspase inhibitor (z-QVD-fmk) in an Angiontensin II-induced aortic aneurysm model. While the administration of caspase inhibitor at the onset of Ang II infusion successfully prevented aneurysm formation, administration of the inhibitor one week after Ang II pump installation failed to stop aneurysm growth [93]. We postulated that the contribution of apoptosis is somewhat limited to the early phase of aneurysm formation in aneurysm models. Indeed, time course studies indicate that TUNEL positive cells (a marker of apoptosis) as well as caspase 3 activation peaked before aortic dilation reaches aneurysmal threshold in multiple models including Ang II/Apoe−/− model, CaCl2 and elastase models [41, 93, 100, 123]. In human, early stages of aneurysm tissues are not accessible. Although apoptosis markers were detected in advanced human aneurysmal tissues, it is difficult to appreciate the prevalence of apoptosis in the absence of tissue specimens from various disease stages of AAAs.
Since inhibiting apoptosis is not sufficient to reduce aneurysm growth, we turned to necroptosis. We found protein levels of necroptosis mediators RIP1 and RIP3 were elevated in human AAA tissues compared to normal aortae [41]. The upregulation of RIP3 as well as complex formation between RIP1 and RIP3 in VSMCs was also detected in mouse aneurysm models. The direct experimental evidence supporting the role of necroptosis in this preclinical model was provided by Rip3 gene deficient mice. Deletion of a single or both copies of Rip3 genes protected mice from aneurysm formation [41]. Two weeks after the elastase-perfusion, the aortic walls of Rip3−/− mice appeared normal, populated by contractile protein-expressing VSMCs and free of infiltrated inflammatory cells. The protection appears to be intrinsic to the vessel wall because a Rip3−/− aorta retains its aneurysm resistant property even when transplanted to the abdominal aortic circulation of a wild type mouse [41], which suggests that the lack of RIP3-mediated necroptosis and inflammatory response of vascular cells most likely VSMCs is a major mechanism underlying aneurysm protection provided by Rip3 gene deficiency.
More recently, we used the optimized RIP1 inhibitor necrostatin-1s (Nec-1s) to test the concept that pharmacologically targeting necroptosis may serve as a new and effective way for treating small aneurysm progression [93]. We started treatment with RIP1 inhibitors 7 days after elastase perfusion, a time point at which the mean aortic dilation is slightly below the aneurysm threshold (defined in this model as 100% over the diameter prior elastase perfusion). Mice were randomized to three groups to receive daily IP injections of either DMSO (post-elastase+DMSO), Nec-1 (3.2 mg/kg/day, post-elastase+Nec-1) or Nec-1s (1.6mg/kg/day, post-elastase+Nec-1s), respectively. Upon sacrifice 14 days after elastase perfusion, mice in the post-elastase + DMSO group exhibited a mean aortic expansion of 172.80±13.68% (1.408±0.068mm) as compared to the day 7 measurement of 100.70±4.37%, indicating progressive aneurysm growth. In contrast, mice in the post-elastase+Nec-1s group had a mean aortic expansion of 64.12±4.80% (0.864±0.032 mm), indicating no aneurysm growth.
However, mice in the post-elastase + Nec-1 group displayed the mean aortic expansion of 121.60±10.40% (1.133±0.052mm), which was smaller than mice treated with DMSO, though this difference was not statistically significant. While it remains to be determined whether Nec-1 failed to block aneurysm growth in our study due to insufficient dosing, results produced by Nec-1s are encouraging (Figure 3C) [93].
Histologically, Nec-1s treated aortic wall showed resolved inflammation and improved tissue integrity [93], which is consistent with what was observed in RIP3 knockout mice. In cultured VSMCs, inhibition of RIP1 with Nec-1 or Nec-1s or siRNA attenuate necroptosis triggered by TNFα in the presence of zVAD, indicating the essential role of RIP1 in regulation of necroptosis of VSMCs. However, RIP3 appears to regulate the cytokine expression by VSMCs independent of RIP1 because inhibition of RIP1 does not affect TNFα-induced cytokine expression. Intriguingly, Nec-1 prevents macrophage from migrating toward MCP-1, suggesting a potential role of RIP1 in regulation of inflammatory infiltration, an area warrants further investigations.
Summary and future perspective
Primary necrotic cell death has long been observed in human pathological conditions, however, its relevance and importance in human diseases has only recently been highlighted. In contrast to the conventional thought that primary necrosis is passive and unregulated, recent discoveries revealed different types of regulated necrosis including necroptosis. The discovery and use of Nec-1 in small animal studies prove the concept that intervening necroptosis is efficacious in treating cardiovascular disease at least in mice. However, similar studies need to be repeated particularly with inhibitors against RIP3.
Although the experimental data are strong in establishing the pathological involvements of RIP1, RIP3 and MLKL in cardiovascular disease, the majority of these studies were performed using a “preventive” approach, i.e. inhibitors were administered or genes were deleted prior to disease induction, conditions that are far away from clinical situation. Even in the few “treatment” studies such as those performed by Karunakaran and by our own group young and healthy mice were used, which is unlike the typical cardiovascular patients. Therefore, future studies are warranted not only in the areas of discovering new components in the necroptosis signaling but also in the areas of translational studies that focuses on development of new inhibitors with high specificity/low toxicity as well as new animal models that are better replicates of clinical realities.
Highlights.
RIP3-mediated necrosis (necroptosis) is implicated in cardiovascular diseases
Necroptosis eliminates essential tissue and causes non-sterile inflammation
VSMC, cardiomyocytes and macrophages are major targets of necroptosis in CVD
Many proteins in the necroptosis pathway can be targeted by inhibitors
Targeting necroptosis is beneficial in CVD; clinical efficacy of inhibitors unknown
Acknowledgments
Funding
This study was supported by the National Institute of Health R01HL088447 (BL) and R01HL122562 (BL), and American Heart Association 17PRE33670082 (KG) and14PRE18560035 (QW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosures
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graham APFW, Brickell P, Lumsden A. The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature. 1994:372. doi: 10.1038/372684a0. [DOI] [PubMed] [Google Scholar]
- 2.Horvitz HHE, Sternberg P. Programmed cell death in nematode development. Neurosci. 1982:55–6. [Google Scholar]
- 3.Kuida K, TSZ, Na S, Kuan C-Y, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–72. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 4.Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14:727–36. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- 5.Chan FK, Luz NF, Moriwaki K. Programmed necrosis in the cross talk of cell death and inflammation. Annu Rev Immunol. 2015;33:79–106. doi: 10.1146/annurev-immunol-032414-112248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 7.Stanger BZ, Leder P, Lee TH, Kim E, Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81:513–23. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 8.He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–11. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Kung G, Konstantinidis K, Kitsis RN. Programmed necrosis, not apoptosis, in the heart. Circ Res. 2011;108:1017–36. doi: 10.1161/CIRCRESAHA.110.225730. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa TSS, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 12.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–62. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 13.Tan SSD, Maher P. Oxytosis: A novel form of programmed cell death. Curr Top Med Chem. 2001;1:497–506. doi: 10.2174/1568026013394741. [DOI] [PubMed] [Google Scholar]
- 14.David KKAS, Dawson TM, Dawson VL. Parthanatos, a messenger of death. Front Biosci. 2009;14:28. doi: 10.2741/3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon SL, KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, Stockwell BR., 3rd Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinkmann VRU, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil Extracellular Traps Kill Bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 17.Bergsbaken T, Cookson BT. Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3:e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luedde MLM, Carter N, Sosna J, Jacoby C, Vucur M, Gautheron J, Roderburg C, Borg N, Reisinger F, Hippe HJ, Linkermann A, Wolf MJ, Rose-John S, Lüllmann-Rauch R, Adam D2, Flögel U, Heikenwalder M, Luedde T, Frey N. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovascular research. 2014;103:206–16. doi: 10.1093/cvr/cvu146. [DOI] [PubMed] [Google Scholar]
- 19.Mandal PBS, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger JKV, Votta B, Ouellette M, King BW, Wisnoski D, Lakdawala AS, DeMartino MP, Casillas LN, Haile PA, Sehon CA, Marquis RW, Upton J, Daley-Bauer LP, Roback L, Ramia N, Dovey CM, Carette JE, Chan FK, Bertin J, Gough PJ, Mocarski ES, Kaiser WJ. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–95. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriwaki K, Chan FK. RIP3: a molecular switch for necrosis and inflammation. Genes Dev. 2013;27:1640–9. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linkermann A, Hackl MJ, Kunzendorf U, Walczak H, Krautwald S, Jevnikar AM. Necroptosis in immunity and ischemia-reperfusion injury. Am J Transplant. 2013;13:2797–804. doi: 10.1111/ajt.12448. [DOI] [PubMed] [Google Scholar]
- 22.Ofengeim D, Ito Y, Najafov A, Zhang Y, Shan B, DeWitt JP, et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 2015;10:1836–49. doi: 10.1016/j.celrep.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J, Li H, Yang M, Ren J, Huang Z, Han F, Huang J, Ma J, Zhang D, Zhang Z, Wu J, et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013;3:200–10. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol. 2014;35:14–23. doi: 10.1016/j.semcdb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Linkermann A, Hackl MJ, Kunzendorf U, Walczak H, Krautwald S, Jevnikar AM. Necroptosis in immunity and ischemia-reperfusion injury. Am J Transplant. 2013;13:2797–804. doi: 10.1111/ajt.12448. [DOI] [PubMed] [Google Scholar]
- 26.Zhao H, Jaffer T, Eguchi S, Wang Z, Linkermann A, Ma D. Role of necroptosis in the pathogenesis of solid organ injury. Cell Death Dis. 2015;6:e1975. doi: 10.1038/cddis.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho YSCS, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–23. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van Herreweghe F, et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19:2003–14. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–48. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser WJSH, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–79. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A. 2013;110:E3109–18. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nature reviews. 2014;15:135–47. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 33.Karunakaran DGM, Wei L, Gan W, Richards L, Shangari P, DeKemp EM, Beanlands RA, Perisic L, Maegdefessel L, Hedin U, Sad S, Guo L, Kolodgie FD, Virmani R, Ruddy T, Rayner KJ. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci Adv. 2016:2. doi: 10.1126/sciadv.1600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koo GB, Morgan MJ, Lee DG, Kim WJ, Yoon JH, Koo JS, et al. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res. 2015;25:707–25. doi: 10.1038/cr.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie T, Peng W, Yan C, Wu J, Gong X, Shi Y. Structural insights into RIP3-mediated necroptotic signaling. Cell Rep. 2013;5:70–8. doi: 10.1016/j.celrep.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 36.Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem. 2002;277:9505–11. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 37.Mocarski ES, Guo H, Kaiser WJ. Necroptosis: The Trojan horse in cell autonomous antiviral host defense. Virology. 2015;479–480:160–6. doi: 10.1016/j.virol.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–67. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Wang CYMM, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–3. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 40.Elmore SA, Dixon D, Hailey JR, Harada T, Herbert RA, Maronpot RR, et al. Recommendations from the INHAND Apoptosis/Necrosis Working Group. Toxicol Pathol. 2016;44:173–88. doi: 10.1177/0192623315625859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Q, Liu Z, Ren J, Morgan S, Assa C, Liu B. Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation. Circ Res. 2015;116:600–11. doi: 10.1161/CIRCRESAHA.116.304899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFalpha-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS One. 2013;8:e76841. doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang SH, Lee DK, Shin J, Lee S, Baek S, Kim J, et al. Nec-1 alleviates cognitive impairment with reduction of Abeta and tau abnormalities in APP/PS1 mice. EMBO Mol Med. 2017;9:61–77. doi: 10.15252/emmm.201606566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szobi A, Goncalvesova E, Varga ZV, Leszek P, Kusmierczyk M, Hulman M, et al. Analysis of necroptotic proteins in failing human hearts. J Transl Med. 2017;15:86. doi: 10.1186/s12967-017-1189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Shi F, Li Y, Yu X, Peng S, Li W, et al. Post-translational modifications as key regulators of TNF-induced necroptosis. Cell Death Dis. 2016;7:e2293. doi: 10.1038/cddis.2016.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grootjans S, Vanden Berghe T, Vandenabeele P. Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ. 2017;24:1184–95. doi: 10.1038/cdd.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22:263–8. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Almagro MC, Goncharov T, Izrael-Tomasevic A, Duttler S, Kist M, Varfolomeev E, et al. Coordinated ubiquitination and phosphorylation of RIP1 regulates necroptotic cell death. Cell Death Differ. 2017;24:26–37. doi: 10.1038/cdd.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Su SS, Zhao S, Yang Z, Zhong CQ, Chen X, et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat Commun. 2017;8:14329. doi: 10.1038/ncomms14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lukens JR, Vogel P, Johnson GR, Kelliher MA, Iwakura Y, Lamkanfi M, Kanneganti TD. RIP1-driven autoinflammation targets IL-1alpha independently of inflammasomes and RIP3. Nature. 2013;498:224–7. doi: 10.1038/nature12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen W, Wu J, Li L, Zhang Z, Ren J, Liang Y, et al. Ppm1b negatively regulates necroptosis through dephosphorylating Rip3. Nat Cell Biol. 2015;17:434–44. doi: 10.1038/ncb3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newton KDD, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, Roose-Girma M, Warming S, Dixit VM. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–60. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 53.Viringipurampeer IA, Shan X, Gregory-Evans K, Zhang JP, Mohammadi Z, Gregory-Evans CY. Rip3 knockdown rescues photoreceptor cell death in blind pde6c zebrafish. Cell Death Differ. 2014;21:665–75. doi: 10.1038/cdd.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kataoka K, Matsumoto H, Kaneko H, Notomi S, Takeuchi K, Sweigard JH, et al. Macrophage- and RIP3-dependent inflammasome activation exacerbates retinal detachment-induced photoreceptor cell death. Cell Death Dis. 2015;6:e1731. doi: 10.1038/cddis.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P, Han J. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105–21. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berger SB, Kasparcova V, Hoffman S, Swift B, Dare L, Schaeffer M, et al. Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol. 2014;192:5476–80. doi: 10.4049/jimmunol.1400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polykratis A, Hermance N, Zelic M, Roderick J, Kim C, Van TM, et al. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J Immunol. 2014;193:1539–43. doi: 10.4049/jimmunol.1400590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–7. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han J, Zhong CQ, Zhang DW. Programmed necrosis: backup to and competitor with apoptosis in the immune system. Nat Immunol. 2011;12:1143–9. doi: 10.1038/ni.2159. [DOI] [PubMed] [Google Scholar]
- 60.Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, Hulpiau P, Weber K, Sehon CA, Marquis RW, Bertin J, Gough PJ, Savvides S, Martinou JC, Bertrand MJ, Vandenabeele P. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–81. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 61.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–46. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gupta K, Madan E, Sayyid M, Arias-Pulido H, Moreno E, Kuppusamy P, et al. Oxygen regulates molecular mechanisms of cancer progression and metastasis. Cancer Metastasis Rev. 2014;33:183–215. doi: 10.1007/s10555-013-9464-2. [DOI] [PubMed] [Google Scholar]
- 64.Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, Liu Y, Zheng W, Shang H, Zhang J, Zhang M, et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22:175–82. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- 65.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–6. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–43. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 67.Moujalled DM, Cook WD, Murphy JM, Vaux DL. Necroptosis induced by RIPK3 requires MLKL but not Drp1. Cell Death Dis. 2014;5:e1086. doi: 10.1038/cddis.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Degterev AHJ, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–21. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dong K, Zhu H, Song Z, Gong Y, Wang F, Wang W, et al. Necrostatin-1 protects photoreceptors from cell death and improves functional outcome after experimental retinal detachment. Am J Pathol. 2012;181:1634–41. doi: 10.1016/j.ajpath.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 70.Degterev A, Maki JL, Yuan J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 2013;20:366. doi: 10.1038/cdd.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vandenabeele P, Grootjans S, Callewaert N, Takahashi N. Necrostatin-1 blocks both RIPK1 and IDO: consequences for the study of cell death in experimental disease models. Cell Death Differ. 2013;20:185–7. doi: 10.1038/cdd.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takahashi N, Duprez L, Grootjans S, Cauwels A, Nerinckx W, DuHadaway JB, et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3:e437. doi: 10.1038/cddis.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harris PA, Berger SB, Jeong JU, Nagilla R, Bandyopadhyay D, Campobasso N, et al. Discovery of a First-in-Class Receptor Interacting Protein 1 (RIP1) Kinase Specific Clinical Candidate (GSK2982772) for the Treatment of Inflammatory Diseases. J Med Chem. 2017;60:1247–61. doi: 10.1021/acs.jmedchem.6b01751. [DOI] [PubMed] [Google Scholar]
- 74.Moriwaki K, Balaji S, Bertin J, Gough PJ, Chan FK. Distinct Kinase-Independent Role of RIPK3 in CD11c(+) Mononuclear Phagocytes in Cytokine-Induced Tissue Repair. Cell Rep. 2017;18:2441–51. doi: 10.1016/j.celrep.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moriwaki K, Balaji S, McQuade T, Malhotra N, Kang J, Chan FK. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity. 2014;41:567–78. doi: 10.1016/j.immuni.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–95. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–79. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang XS, Yi TL, Zhang S, Xu ZW, Yu ZQ, Sun HT, et al. Hypoxia-inducible factor-1 alpha is involved in RIP-induced necroptosis caused by in vitro and in vivo ischemic brain injury. Sci Rep. 2017;7:5818. doi: 10.1038/s41598-017-06088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li JX, Feng JM, Wang Y, Li XH, Chen XX, Su Y, et al. The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell Death Dis. 2014;5:e1278. doi: 10.1038/cddis.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fauster A, Rebsamen M, Huber KV, Bigenzahn JW, Stukalov A, Lardeau CH, Scorzoni S, Bruckner M, Gridling M, Parapatics K, Colinge J, Bennett KL, et al. A cellular screen identifies ponatinib and pazopanib as inhibitors of necroptosis. Cell Death Dis. 2015;6:e1767. doi: 10.1038/cddis.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, Nicolini FE, Apperley JF, Khoury HJ, Talpaz M, DiPersio J, DeAngelo DJ, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–96. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Najjar M, Suebsuwong C, Ray SS, Thapa RJ, Maki JL, Nogusa S, et al. Structure guided design of potent and selective ponatinib-based hybrid inhibitors for RIPK1. Cell Rep. 2015;10:1850–60. doi: 10.1016/j.celrep.2015.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–20. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 84.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–23. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 85.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–24. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 86.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173:307–13. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 87.Wahamaa H, Schierbeck H, Hreggvidsdottir HS, Palmblad K, Aveberger AC, Andersson U, et al. High mobility group box protein 1 in complex with lipopolysaccharide or IL-1 promotes an increased inflammatory phenotype in synovial fibroblasts. Arthritis Res Ther. 2011;13:R136. doi: 10.1186/ar3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–60. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 89.Srikrishna G, Freeze HH. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11:615–28. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wallach D, Kang TB, Dillon CP, Green DR. Programmed necrosis in inflammation: Toward identification of the effector molecules. Science. 2016;352:aaf2154. doi: 10.1126/science.aaf2154. [DOI] [PubMed] [Google Scholar]
- 91.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 92.Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D’Cruz AA, Hall C, Kaur Spall S, Anderton H, Masters SL, Rashidi M, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Q, Zhou T, Liu Z, Ren J, Phan N, Gupta K, Stewart DM, Morgan S, Assa C, Kent KC, Liu B. Inhibition of Receptor-Interacting Protein Kinase 1 with Necrostatin-1s ameliorates disease progression in elastase-induced mouse abdominal aortic aneurysm model. Sci Rep. 2017;7:42159. doi: 10.1038/srep42159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mozaffarian D, Benjamin, Emelia J, Go, Alan S, Arnett Donna K, Blaha Michael J, Cushman Mary, de Ferranti Sarah, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, et al. Executive Summary: Heart Disease and Stroke Statistics—2015 Update. Circulation. 2015;131:434–41. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 95.Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther. 2007;21:227–33. doi: 10.1007/s10557-007-6035-1. [DOI] [PubMed] [Google Scholar]
- 96.Dmitriev YV, Minasian SM, Demchenko EA, Galagudza MM. Study of cardioprotective effects of necroptosis inhibitors on isolated rat heart subjected to global ischemia-reperfusion. Bulletin of experimental biology and medicine. 2013;155:245–8. doi: 10.1007/s10517-013-2124-2. [DOI] [PubMed] [Google Scholar]
- 97.Oerlemans MI, Liu J, Arslan F, den Ouden K, van Middelaar BJ, Doevendans PA, Sluijter JP. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol. 2012;107:270. doi: 10.1007/s00395-012-0270-8. [DOI] [PubMed] [Google Scholar]
- 98.Oerlemans MI, Koudstaal S, Chamuleau SA, de Kleijn DP, Doevendans PA, Sluijter JP. Targeting cell death in the reperfused heart: pharmacological approaches for cardioprotection. International journal of cardiology. 2013;165:410–22. doi: 10.1016/j.ijcard.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 99.Koshinuma S, Miyamae M, Kaneda K, Kotani J, Figueredo VM. Combination of necroptosis and apoptosis inhibition enhances cardioprotection against myocardial ischemia-reperfusion injury. Journal of anesthesia. 2014;28:235–41. doi: 10.1007/s00540-013-1716-3. [DOI] [PubMed] [Google Scholar]
- 100.Yamanouchi D, Morgan S, Kato K, Lengfeld J, Zhang F, Liu B. Effects of caspase inhibitor on angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30:702–7. doi: 10.1161/ATVBAHA.109.200527. [DOI] [PubMed] [Google Scholar]
- 101.Wang D, Wang Z, Zhang L, Wang Y. Roles of Cells from the Arterial Vessel Wall in Atherosclerosis. Mediators Inflamm. 2017;2017:8135934. doi: 10.1155/2017/8135934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013:CD004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lonn EM, Bosch J, Lopez-Jaramillo P, Zhu J, Liu L, Pais P, Diaz R, Xavier D, Sliwa K, Dans A, Avezum A, et al. Blood-Pressure Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med. 2016;374:2009–20. doi: 10.1056/NEJMoa1600175. [DOI] [PubMed] [Google Scholar]
- 104.Zheng Y, Gardner SE, Clarke MC. Cell death, damage-associated molecular patterns, and sterile inflammation in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2011;31:2781–6. doi: 10.1161/ATVBAHA.111.224907. [DOI] [PubMed] [Google Scholar]
- 105.Meng L, Jin W, Wang X. RIP3-mediated necrotic cell death accelerates systematic inflammation and mortality. Proc Natl Acad Sci U S A. 2015;112:11007–12. doi: 10.1073/pnas.1514730112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Q, Li G, Lan X, Zheng M, Chen KH, Cao CM, Xiao RP. Receptor interacting protein 3 suppresses vascular smooth muscle cell growth by inhibition of the phosphoinositide 3-kinase-Akt axis. J Biol Chem. 2010;285:9535–44. doi: 10.1074/jbc.M109.071332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Golledge J, Muller J, Daugherty A, Norman Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26:2605–13. doi: 10.1161/01.ATV.0000245819.32762.cb. [DOI] [PubMed] [Google Scholar]
- 109.Baxter BT, Terrin MC, Dalman RL. Medical management of small abdominal aortic aneurysms. Circulation. 2008;117:1883–9. doi: 10.1161/CIRCULATIONAHA.107.735274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weintraub NL. Understanding abdominal aortic aneurysm. N Engl J Med. 2009;361:1114–6. doi: 10.1056/NEJMcibr0905244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101–8. doi: 10.1056/NEJMcp1401430. [DOI] [PubMed] [Google Scholar]
- 112.Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Annals of internal medicine. 1997;126:441–9. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- 113.Baxter BT, Terrin MC, Dalman RL. Medical Management of Small Abdominal Aortic Aneurysms. Circulation. 2008;117:1883–9. doi: 10.1161/CIRCULATIONAHA.107.735274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brewster DC, Cronenwett JL, Hallett JW, Jr, Johnston KW, Krupski WC, Matsumura JS. Guidelines for the treatment of abdominal aortic aneurysms: Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. Journal of Vascular Surgery. 2003;37:1106–17. doi: 10.1067/mva.2003.363. [DOI] [PubMed] [Google Scholar]
- 115.Daugherty AC, LA Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004:24. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 116.Sinha I, Sinha-Hikim AP, Hannawa KK, Henke PK, Eagleton MJ, Stanley JC, Upchurch GR., Jr Mitochondrial-dependent apoptosis in experimental rodent abdominal aortic aneurysms. Surgery. 2005;138:806–11. doi: 10.1016/j.surg.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 117.Henderson ELGY, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of Smooth Muscle Cells and Expression of Mediators of Apoptosis by T Lymphocytes in Human Abdominal Aortic Aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- 118.López-Candales AHD, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997:150. [PMC free article] [PubMed] [Google Scholar]
- 119.Walton LJFI, Bayston T, Brown LC, Greenhalgh RM, Taylor GW, Powell JT. Inhibition of Prostaglandin E2 Synthesis in Abdominal Aortic Aneurysms: Implications for Smooth Muscle Cell Viability, Inflammatory Processes, and the Expansion of Abdominal Aortic Aneurysms. Circulation. 1999;100:48–54. doi: 10.1161/01.cir.100.1.48. [DOI] [PubMed] [Google Scholar]
- 120.Sun JSG, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, Shi GP. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. The Journal of Clinical Investigation. 2007;117:3359–68. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xiong WMJ, Knispel R, Worth J, Persidsky Y, Baxter BT. Blocking TNF-alpha attenuates aneurysm formation in a murine model. J Immunol. 2009;183:2741–6. doi: 10.4049/jimmunol.0803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaschina ESF, Sommerfeld M, Kemnitz UR, Grzesiak A, Krikov M, Unger T. Telmisartan prevents aneurysm progression in the rat by inhibiting proteolysis, apoptosis and inflammation. Journal of hypertension. 2008;26:2361–73. doi: 10.1097/HJH.0b013e328313e547. [DOI] [PubMed] [Google Scholar]
- 123.Yamanouchi D, Morgan S, Stair C, Seedial S, Lengfeld J, Kent KC, Liu B. Accelerated aneurysmal dilation associated with apoptosis and inflammation in a newly developed calcium phosphate rodent abdominal aortic aneurysm model. J Vasc Surg. 2012;56:455–61. doi: 10.1016/j.jvs.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]