Abstract
Neuroticism, a broad personality trait linked to negative emotions, is consistently linked to ill-health when self-report is used to assess health. However, when health risk is assessed with biomarkers, the evidence is inconsistent. Here, we tested the hypothesis that the association between neuroticism and biological health risk is moderated by behavioral adjustment, a propensity to flexibly adjust behaviors to environmental contingencies. Using a U.S.-Japan cross-cultural survey, we found that neuroticism was linked to lower biological health risk for those who are high, but not low, in behavioral adjustment. Importantly, Japanese were higher in behavioral adjustment than European Americans, and as predicted by this cultural difference, neuroticism was linked to lower biological health risk for Japanese, but not for European Americans. Lastly, consistent with prior evidence, neuroticism was associated with worse self-reported health regardless of behavioral adjustment or culture. Discussion focused on the significance of identifying socio-cultural correlates of biological health. (150 words; 150 max)
Keywords: neuroticism, behavioral adjustment, biological health risk, culture
Neuroticism is a broad personality trait that is linked to various negative emotions such as anger, sadness, and disgust (Costa & McCrae, 1987; Goldberg, 1992). It may seem quite intuitive, then, to anticipate that having this trait has maladaptive health effects (Friedman, 2011). In fact, researchers have used self-report measures of health and empirically linked high neuroticism to poor health (T. W. Smith & MacKenzie, 2006; Suls & Bunde, 2005) and low wellbeing (Kessler, Ruscio, Shear, & Wittchen, 2010). Surprisingly, however, recent work has shown that neuroticism is not always linked to increased health risk when health risk is assessed objectively with pro-inflammatory biomarkers such as interleukin-6 (IL-6) and C-reactive protein (CRP). While some studies found positive associations between neuroticism and biological health risk (Armon, Melamed, Shirom, Shapira, & Berliner, 2013; Sutin et al., 2009), the effect appears less systematic and inconsistent across studies (Luchetti, Barkley, Stephan, Terracciano, & Sutin, 2014).
In view of the inconsistency in the findings, we have argued that the association between neuroticism and self-reported health documented in prior work may be due, in no small part, to a valence component that is shared in the two constructs (Kitayama & Park, 2017). That is, since both neuroticism and ill-health are experienced as negative, undesirable, and unpleasant, they are likely to be correlated for this semantic reason. Further, the mixed evidence with objective measures of health may suggest that neuroticism can have positive, as well as negative, effects on biological health depending on certain individual difference variables (Friedman, 2000). Consistent with this, recent evidence suggests that high neuroticism is linked to lower levels of IL-6 for Americans who are high in conscientiousness (Turiano, Mroczek, Moynihan, & Chapman, 2013). Similarly, another recent study conducted in the U.K. showed that higher neuroticism was protective against mortality among people who perceived their health to be poor or fair, but not among those who rated their health as excellent (Gale et al., 2017).
To date, however, other moderators of the link between neuroticism and biological health risk have been left uninvestigated. One main goal of the present work was to address this issue by testing behavioral adjustment as a potentially powerful moderating variable. We anticipated that for those who are both willing and able to flexibly adjust their behaviors to environmental contingencies including potential threats and troubles, neuroticism would be healthy, being inversely associated with biological health risk. Importantly, as we shall see, the mean level of behavioral adjustment varies systematically across cultures. Hence, another main aim of our work was to test a cross-cultural prediction, namely, that the association between neuroticism and lower biological health risk should be more likely in cultures in which the mean level of behavioral adjustment was sufficiently high (vs. low). We also secondarily tested the prediction that because of a substantial semantic overlap between self-reported health measures and neuroticism, the moderation of the link between neuroticism and health by behavioral adjustment or culture is less likely to be observed in subjective measures of health.
Behavioral Adjustment as a Moderator of the Neuroticism-Health Link
Our predictions are based on prior evidence that neuroticism alerts individuals to potential threats in the environment (Derryberry & Reed, 1994; Wilson, MacLeod, Mathews, & Rutherford, 2006). That is, individuals with high (vs. low) neuroticism would be more vigilant of and thus are better able to detect potential dangers and threats in their environment. In response to such signals, individuals are likely to experience different reactions, either challenged or threatened (Mendes, Blascovich, Major, & Seery, 2001; Mendes, Reis, Seery, & Blascovich, 2003), depending on the amount of coping resource they have at their disposal (Lazarus & Folkman, 1984). They may be challenged when they have a sufficient amount of resource to cope with the problem, whereas they may feel threatened when they perceive that the situational demands exceed the amount of their coping resource.
We propose that behavioral adjustment serves as powerful coping resource. Behavioral adjustment refers to each individual’s propensity to adjust one’s behaviors to environmental contingencies. It may therefore be seen as one type of secondary control (a propensity to adjust to the environment). However, secondary control typically refers to cognitive forms of adjustment such as rationalization and reframing (Rothbaum, Weisz, & Snyder, 1982). Behavioral adjustment, in contrast, places a greater emphasis on more active forms of adjustment, involving behavioral changes in accordance with environmental demands (Heckhausen & Schulz 1995).
Since behavioral adjustment enables one to address and preempt potential threats and dangers, it may serve as one potent coping resource that is likely to moderate health effects of immediate threats highlighted by neuroticism. Specifically, people high in behavioral adjustment are both skilled and motivated to address the problems and issues highlighted by high neuroticism, and therefore, will be challenged following the detection of initial threat. The resulting sense of challenge and empowerment would in turn be associated with salubrious health outcomes (Brosschot et al., 1998; Epel et al., 1998). Since the sense of challenge and empowerment occurs only when a threat is readily taken note of and clearly registered, we may expect that for those high in behavioral adjustment, neuroticism should be linked to better biological health. In contrast, people low in behavioral adjustment lack the ability and/or motivation to change their behaviors in accordance with environmental demands. Hence, when alerted to a threat by high neuroticism, they will have no readily available means to cope with the threat and, thus, will feel helpless and threatened. The sense of helplessness or threat, in turn, is likely to result in poor health outcomes (Epel et al., 1998; O’Donovan et al., 2012). Since the sense of helplessness or threat occurs only when a threat is highlighted by neuroticism, we may expect that for those low in behavioral adjustment, neuroticism should be linked to worse biological health.
In short, we predicted a significant Neuroticism × Behavioral adjustment interaction on biological health risk. That is, for individuals who are both willing and able to flexibly adjust their behaviors to environmental contingencies including potential threats and troubles, neuroticism would be salubrious, showing an inverse association with biological health risk, whereas high neuroticism would be linked to increased biological health risk for those who are relatively low in behavioral adjustment.
Culture as a Moderator of the Neuroticism-Health Link
The current analysis on the Neuroticism × Behavioral adjustment interaction on biological health risk offers one important cross-cultural prediction. Previous cross-cultural work suggests that behavioral adjustment is higher among Asians than among European Americans (Markus & Kitayama, 1991). In particular, Asians place a greater value on interdependence of the self with others than European Americans do (Kitayama, Park, Sevincer, Karasawa, & Uskul, 2009). We may thus expect Asians to be more likely to be both skilled and motivated to adjust themselves to social expectations than European Americans. In support of this prediction, Tsai and colleagues (2007) tested adjustment and influence goals between European Americans and Hong Kong Chinese and found that Chinese endorsed adjustment goals more than European Americans. Likewise, Morling and colleagues (2002) asked Japanese and Americans to recall situations involving their adjusting or influencing actions and found that the recalled situations involving influence were more recent than those involving adjustment for Americans, but those involving adjustment were more recent than those involving influence for Japanese, thus indicating that adjustment is more commonplace for Japanese than for Americans. Moreover, a systematic review of prior work shows that Asians are more likely to change behaviors and conform to group expectations than European Americans (Bond & Smith, 1996). Although conformity can occur for a variety of reasons other than adjustment, this cross-cultural evidence is still consistent with the hypothesis that Asians are higher in behavioral adjustment than European Americans.
Remember our prediction is that neuroticism would likely be linked to lower biological health risk among those who are high (vs. low) in behavioral adjustment. Because Japanese are likely to be higher in behavioral adjustment than European Americans, Japanese should show the pattern characteristics of those high in behavioral adjustment. That is, among Japanese, neuroticism should be linked to lower biological health risk, whereas the pattern would be attenuated or even reversed for European Americans who are likely to be low in behavioral adjustment. Thus, we expected that the link between neuroticism and biological health risk would be moderated by culture, resulting in a significant Neuroticism × Culture interaction on biological health risk. Moreover, this interaction effect would be accounted for by the fact that the effect of neuroticism is moderated by behavioral adjustment─i.e., the Neuroticism × Behavioral adjustment interaction.
Present Study
We tested our predictions using large-scale paired surveys conducted in both the United States (Midlife in the US, MIDUS) and Japan (Midlife in Japan, MIDJA). Perceptions of threat and stress are known to increase inflammatory responses, which gradually compromise cardiovascular functioning when sustained over a long period. In combination, inflammation and cardiovascular malfunction are potent predictors of morbidity and mortality (Mozaffarian et al., 2015). We thus targeted, a priori, four biomarkers to define biological health risk based on our prior work (Kitayama et al., 2015), which included two standard biomarkers of inflammation (i.e., IL-6 and CRP) and two biomarkers of cardiovascular malfunction (i.e., systolic blood pressure1 and the ratio of total-to-HDL cholesterol). Our first aim was to test the prediction that neuroticism would be linked to reduced biological health risk for those who are sufficiently high in behavioral adjustment whereas the link would be reversed for those low in behavioral adjustment. Equally important, we tested an additional prediction that Japanese would be higher in behavioral adjustment than European Americans, and as a consequence, the link between neuroticism and reduced biological health risk would be more likely to be observed for Japanese than for European Americans.
In addition to the four biomarkers we chose on an a priori basis, we also tested several self-report measures of health, including self-rated general health, chronic conditions, and functional limitations. As noted earlier, prior work overwhelmingly shows a negative association between neuroticism and subjective appraisals of health. One plausible interpretation for this association is that both neuroticism and ill-health share a common negative valence semantic component (Kitayama & Park, 2017). Given this interpretation, on these measures, neuroticism would be positively associated with ill-health regardless of culture or behavioral adjustment.
Method
Participants
The American participants were a subset from the MIDUS survey. The first wave of this survey was conducted in 1995 based on a national probability sample of English-speaking adults recruited via random digit dialing (MIDUS I; N = 7,108). A subset of the MIDUS I participants completed a follow-up survey in 2004 (MIDUS II; N = 4,963; retention rate = 75%). Biological data were obtained from the MIDUS II participants who participated in an additional overnight biomarker session at one of three General Clinical Research Centers (Madison, WI, Washington, DC, or Los Angeles, CA; N = 1,054; 578 females, Mage = 58.04, SDage = 11.62). After excluding a small subgroup of non-Europeans (32 African Americans, 6 Native Americans, 5 Asian Americans, 1 multi-racial, 30 others, and 4 missing), the final analysis focused on 976 European Americans (531 females, Mage = 58.36, SDage = 11.69). The Japanese data were obtained from a companion survey conducted in Japan in 2008, the MIDJA, based on 1,027 adults randomly selected from the Tokyo metropolitan area. The analysis focused on a subset of the MIDJA participants who travelled to a medical clinic near the University of Tokyo for biomarker data collection (N = 382; 214 females; Mage = 55.47 years, SDage = 14.04). The sample sizes were constrained by the fact that we used the existing datasets. However, given the previous evidence that the same sample sizes were sufficient to demonstrate effects of different socio-cultural variables on biological health markers (Kitayama et al., 2015; Miyamoto et al., 2013), the current sample sizes were deemed sufficient to obtain the predicted effects.
Materials
Biological health risk
Following Kitayama et al. (2015), we assessed both inflammation and cardiovascular malfunction to index biological health risk.
Inflammation
Inflammation was assessed with interleukin-6 (IL-6) and C-reactive protein (CRP). Serum and plasma samples taken during the biomarker session in both countries were frozen and shipped to a single testing laboratory in the U.S. (Biocore Laboratory; University of Wisconsin, Madison, WI). Serum IL-6 levels were determined by high-sensitivity enzyme-linked immunosorbent assay (ELISA; Quantikine, R&D Systems, Minneapolis, MN), with a lower sensitivity of detection at 0.16 pg/mL, while plasma CRP levels were assayed using BNII immunoephelometry (BNII Nephelometer 100 Analyzer; Dade Behring Inc., Deerfield, IL).
Cardiovascular malfunction
Cardiovascular malfunction was assessed with systolic blood pressure (SBP) and the ratio of total-to-HDL cholesterol (Total/HDL cholesterol). Blood pressure was recorded by clinic staff three times in a seated position following a five-minute resting period, with 30 second intervals between assessments. The averaged ratings of the two most similar recordings were used as a single index of SBP. For cholesterol assays, blood samples taken from the biomarker session were frozen and shipped to a separate testing laboratory in each country. The total and HDL cholesterol assays were performed at Meriter Labs (Madison, WI) in the U.S., using a Cobas Integra analyzer (Roche Diagnostics, Indianapolis, IN), and at Showa Medical Science in Japan.
Following prior research (Kitayama et al., 2015; Miyamoto et al., 2013), extreme values on each biomarker were winsorized at ±3 standard deviations from the mean in each culture (IL-6: n = 7, CRP: n = 4, total cholesterol: n = 3, SBP: n = 1) and then log-transformed to reduce skewness. Consistent with the findings that inflammation in the white blood cells is inherently linked to cardiovascular malfunction (Finch, 2010), Kitayama et al. (2015) performed the principal component analysis (PCA) and found that the four biomarkers loaded on a single factor in both cultural groups, accounting for 41.06% and 50.09% of the variance for European Americans and Japanese, respectively. We thus used the factor score obtained from the PCA after collapsing both cultural groups as an index of biological health risk in addition to analyzing each biomarker separately.
Subjective health
We utilized three self-report measures to assess participants’ subjective health, including (a) general health, (b) chronic conditions, and (c) functional limitations. General health was assessed by participants’ ratings about their current health using a 11-point scale (0 = worst, 10 = best). Chronic conditions were assessed based on the number of health problems (maximum of 30; e.g., diabetes, asthma, tuberculosis) participants reported having experienced in the past 12 months. Finally, participants rated (0 = not at all, 10 = a lot) the extent to which their health limits them in doing basic and instrumental activities of daily living (10 items; e.g., bathing or dressing yourself, lifting or carrying groceries). The responses were averaged to create a single index of functional limitations (αs = .94 and .93 for European Americans and Japanese, respectively).
PCA showed that these three measures loaded on a single factor, accounting for 64.29% and 44.40% of the variance for European Americans and Japanese, respectively. We thus used the factor score obtained from the PCA after combining both cultural groups as a composite index of self-reported health in addition to analyzing each measure separately. A higher number on the composite index indicates better subjective health.
Personality traits
Neuroticism was assessed with the items of an abbreviated Big 5 personality trait scale (Rossi, 2001). Participants indicated the extent to which each adjective described them (1 = not at all, 4 = a lot; worrying, nervous, irritable, and calm [reverse-coded]; αs = .75 and .56 for European Americans and Japanese, respectively). The low reliability for Japanese was due to one reverse item (calm), which showed low correlations with the remaining three items. Once this item was excluded, the reliability became reasonable (α = .64). The results did not depend on whether “calm” was included (reported below) or excluded (see Fig. S1).
In addition, we used the same scale and assessed conscientiousness (organized, responsible, hardworking, thorough, and careless [reverse-coded]; αs = .71 and .67 for European Americans and Japanese, respectively) to test the generalizability of the Turiano et al. (2013) finding to Japanese culture (see Exploratory Analysis in the results section).
Behavioral adjustment
Participants completed a 5-item adjustment scale, a newly designed measure of individuals’ preparedness to adjust to certain challenges or difficulties, either social or non-social. They rated the extent to which they agree or disagree with each statement (1 = strongly disagree, 7 = strongly agree; e.g., “When many people have an opinion different from mine, I can adjust mine to theirs”, “Once something has happened, I try to adjust myself to it because it is difficult to change it myself”; αs = .54 and .59 for European Americans and Japanese, respectively). The full-scale items are reported in Appendix.2 The low reliability of this scale presents some concern because it could lower the statistical power of findings involving this scale (thus potentially calling into question the robustness of the findings). We addressed this concern by using structural equation modeling (SEM, see Footnotes 6 and 7).
Control variables
Following prior research (Kitayama et al., 2015; Miyamoto et al., 2013; Turiano et al., 2013), we controlled for potential confounding variables that could influence the relationship between neuroticism and biological health risk, i.e., demographic variables (age, gender, educational attainment), health behaviors (alcohol consumption, smoking), and health status (medication usage, body-mass-index [BMI]).
Demographic variables
Building on prior evidence that the levels of inflammation and cardiovascular risk depend on certain demographic variables such as age, gender, and educational attainment (Coe et al., 2011), we adjusted for these factors in our analysis. Participants’ age at the time of biomarker assessment ranged from 35 to 86 in the U.S. and from 31 to 80 in Japan. For both cultural groups, approximately 55% of the participants were female (54.5% and 56.0% for European Americans and Japanese, respectively). Educational attainment was assessed with a culture-specific scale to reflect cultural differences in the educational system, ranging from 1 (8th grade, junior high school) to 12 (Ph.D., or other professional degree) in the U.S., and from 1 (junior high school graduate) to 8 (graduate school) in Japan. Following previous studies (Curhan et al., 2014; Kitayama et al., 2015; J. Park et al., 2013), these values were rescaled to a 7-point scale for both cultural groups to make the responses comparable across cultures (1 = 8th grade/junior high school, 7 = attended or graduate from graduate school).
Health behaviors
We controlled for two indices of health behaviors (alcohol consumption and smoking status) that are known to be associated with the levels of inflammation and cardiovascular malfunctions (O’Connor et al., 2009; O’Connor & Irwin, 2010). Alcohol consumption was measured as an average number of alcohol drinks participants consumed per week. Extreme values on this variable (n = 26) were winsorized at ±3 standard deviations from the mean within each culture. Smoking status was categorized as never-smoker, former-smoker, and current-smoker and we used two dummy coded variables contrasting either former-smoker or current-smoker with never-smoker as a reference group.
Health status
To adjust for the possible cultural differences in health status, we controlled for two measures of health status linked to biomarkers (medication use and BMI) (see Miyamoto et al., 2013 for a similar approach). Medication use was assessed based on three dummy-coded variables (0 = no, 1 = yes) of the current use of three medications that can influence the level of inflammation and/or cardiovascular risk (antihypertensive, cholesterol-lowering, and steroid medication). BMI was computed from weight and height measurements obtained by clinic staff (kg/m2) and log-transformed to reduce skewness.3,4,5
See Tables 1 and 2 for descriptive statistics and inter-correlations for key variables, respectively.
Table 1.
Descriptive statistics for study variables for European Americans (left) and Japanese (right)
| European Americans
|
Japanese
|
Cultural Differences
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | N | M | SD | N | M | SD | Statistics | P | effect size |
| Demographic variables | |||||||||
| Age | 976 | 58.36 | 11.69 | 382 | 55.47 | 14.04 | F(1, 1356) = 14.98 | < .001 | ηp2= 01 |
| Gender (% female) | 976 | 54.5% | 382 | 56.0% | X2(1) = .25 | .614 | V = .01 | ||
| Educational attainment | 972 | 5.00 | 1.60 | 378 | 4.38 | 1.63 | F(1, 1348) = 41.07 | < .001 | ηp2= 03 |
| Psychological variables | |||||||||
| Neuroticism | 973 | 2.02 | 0.62 | 381 | 2.13 | 0.58 | F(1, 1352) = 9.53 | .002 | ηp2= 01 |
| Conscientiousness | 973 | 3.40 | 0.45 | 381 | 2.65 | 0.55 | F(1, 1352) = 658.52 | < .001 | ηp2= 33 |
| Behavioral adjustment | 975 | 4.11 | 0.83 | 374 | 4.32 | 0.73 | F(1, 1347) = 19.52 | < .001 | ηp2= 01 |
| Health behaviors | |||||||||
| Alcohol consumption | 974 | 3.15 | 5.34 | 379 | 7.24 | 11.75 | F(1, 1351) = 99.26 | < .001 | ηp2= 07 |
| Smoking status (% yes) | 976 | 382 | |||||||
| Never-smoker | 559 | 57.3% | 185 | 48.4% | X2(1) = 8.67 | .003 | V = .08 | ||
| Former-smoker | 318 | 32.6% | 89 | 23.3% | X2(1) = 11.27 | .001 | V = .09 | ||
| Current-smoker | 99 | 10.1% | 82 | 21.5% | X2(1) = 30.47 | < .001 | V = .15 | ||
| Missing | 0 | 0.0% | 26 | 6.8% | X2(1) = 67.63 | < .001 | V = .22 | ||
| Health status | |||||||||
| Medication usage | |||||||||
| Antihypertensive (% yes) | 976 | 35.0% | 374 | 16.0% | X2(1) = 46.67 | < .001 | V = .19 | ||
| Cholesterol (% yes) | 976 | 29.9% | 365 | 9.9% | X2(1) = 57.83 | < .001 | V = .21 | ||
| Steroid (% yes) | 976 | 4.0% | 360 | 0.6% | X2(1) = 10.46 | .001 | V = .09 | ||
| Body-mass-index (BMI) | 975 | 1.46 | 0.08 | 382 | 1.35 | 0.06 | F(1, 1355) = 512.61 | < .001 | ηp2 = .27 |
| Biological health risk | 962 | 0.29 | 0.85 | 382 | −0.78 | 0.96 | F(1, 1342) = 406.68 | < .001 | ηp2 = .23 |
| Interleukin-6 [IL-6] | 968 | 0.32 | 0.32 | 382 | 0.04 | 0.36 | F(1, 1348) = 195.14 | < .001 | ηp2 = .13 |
| C-reactive protein [CRP] | 965 | 0.14 | 0.50 | 382 | −0.45 | 0.42 | F(1, 1345) = 419.48 | < .001 | ηp2 = .24 |
| Systolic blood pressure [SBP] | 975 | 2.11 | 0.06 | 382 | 2.08 | 0.07 | F(1, 1355) = 84.36 | < .001 | ηp2 = .06 |
| Total/HDL cholesterol | 967 | 0.55 | 0.15 | 382 | 0.47 | 0.14 | F(1, 1347) = 64.46 | < .001 | ηp2= 05 |
| Subjective health | 972 | 0.05 | 1.04 | 375 | −0.13 | 0.86 | F(1, 1345) = 8.99 | .003 | ηp2= 01 |
| General health | 975 | 7.61 | 1.43 | 382 | 6.43 | 1.82 | F(1, 1355) = 157.46 | < .001 | ηp2 = .10 |
| Chronic conditions | 976 | 2.28 | 2.33 | 377 | 2.31 | 2.02 | F(1, 1351) = .05 | .822 | ηp2 = .00 |
| Functional limitations | 973 | 1.55 | 0.71 | 380 | 1.33 | 0.64 | F(1, 1351) = 27.10 | < .001 | ηp2 = .02 |
Note. Alcohol consumption, IL-6, CRP, SBP, total cholesterol, and BMI are log-transformed data.
Table 2.
Intercorrelations among key study variables for (A) European Americans, (B) Japanese, and (C) both cultural groups combined
| A. European Americans |
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 1. Neuroticism | – | 0.04 | −0.05 | −0.26*** |
| 2. Behavioral adjustment | – | 0.05 | −0.05 | |
| 3. Biological health risk | – | −0.26*** | ||
| 4. Subjective health | – | |||
|
| ||||
| B. Japanese |
|
|||
| 1 | 2 | 3 | 4 | |
|
| ||||
| 1. Neuroticism | – | 0.01 | −0.17*** | −0.29*** |
| 2. Behavioral adjustment | – | 0.02 | −0.09† | |
| 3. Biological health risk | – | −0.14** | ||
| 4. Subjective health | ||||
|
| ||||
| C. Both cultures combined |
|
|||
| 1 | 2 | 3 | 4 | |
|
| ||||
| 1. Neuroticism | – | 0.04 | −0.12*** | −0.27*** |
| 2. Behavioral adjustment | – | −0.02 | −0.06* | |
| 3. Biological health risk | – | −0.20*** | ||
| 4. Subjective health | – | |||
p ≤ .10,
p ≤ .05,
p ≤ .001.
Results
Analytic Strategies
The first aim of the present work was to test behavioral adjustment as a moderator of the link between neuroticism and biological health risk. We predicted a significant interaction between neuroticism and behavioral adjustment (Analysis 1). The second aim was to test whether the link between neuroticism and biological health risk is moderated by culture. We predicted a significant interaction between neuroticism and culture (Analysis 2). We further tested whether the Neuroticism × Culture interaction would be accounted for by the Neuroticism × Behavioral adjustment interaction.
In each analysis, we tested three different models that varied in the set of control variables that were entered (see Kitayama et al., 2015; Miyamoto et al., 2013; Turiano et al., 2013 for similar approaches). Model 1 was our base model in which we tested our key predictor variables as well as their interaction term (Neuroticism × Behavioral adjustment and Neuroticism × Culture in Analyses 1 and 2, respectively). In Model 2, we entered three demographic variables (age, gender, and educational attainment) as covariates. Model 3 included our measures of health behaviors (alcohol consumption and smoking status) and health status (medication usage and BMI). We first conducted these analyses on the composite index of biological health risk and then performed the same set of analyses on each of the four biomarkers separately.
Next, we examined whether the hypothesized moderating effect of behavioral adjustment or culture is specific to biomarkers or generalizable to subjective health by testing the two key interaction effects on self-reported health (Analysis 3). We tested the composite index of subjective health first and then repeated the same analysis for each measure of self-reported health using the same analytic strategy we used for Analyses 1 and 2.
Finally, we ran an exploratory analysis to test the generalizability of the Turiano et al. (2013) to Japanese culture. Turiano et al. (2013) tested one of the four biomarkers that constituted our measure of biological health risk (IL-6) and found that neuroticism is related to reduced IL-6 for Americans who are high in conscientiousness. We examined whether the same moderation effect is evident among both Americans and Japanese when we tested a composite index of biological health risk.
Analysis 1: Behavioral Adjustment as a Moderator of the Link between Neuroticism and Biological Health Risk
We hypothesized that neuroticism alerts individuals to potential threats. Hence, neuroticism may reduce health risks for individuals who are prepared to make adjustment to the environmental contingencies and thus to preempt the anticipated threats. Moreover, this should be the case across cultures. This hypothesis would receive support if the Neuroticism × Behavioral adjustment interaction were significant. We expected a significant interaction both when the two cultural groups were combined and when each was tested separately.
Combined analysis
We first tested the Neuroticism × Behavioral adjustment interaction with the two cultural groups combined. The pertinent regression coefficients are summarized in Table 3. In support of our prediction, the Neuroticism × Behavioral adjustment interaction proved statistically significant in Model 1, b = −.16, 95% Confidence Interval [CI] [−.28, −.05], t(1275) = −2.78, p = .006. This pattern remained the same in Model 2 in which we controlled for the three demographic variables, b = −.15, 95% CI [−.26, −.04], t(1272) = −2.72, p = .007. Importantly, this interaction remained significant even in Model 3 in which we additionally controlled for health behaviors and health status, b = −.10, 95% CI [−.18, −.02], t(1265) = −2.35, p = .019.6
Table 3.
Regression coefficients in predicting biological health risk as a function of neuroticism and behavioral adjustment
| Biological Health Risk | Model 1
|
Model 2
|
Model 3
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE B | P | B | SE B | P | B | SE B | P | |
| Neuroticism (N) | −0.21 | 0.05 | < 0.001 | −0.07 | 0.05 | 0.120 | −0.03 | 0.04 | 0.332 |
| Behavioral adjustment (BA) | −0.02 | 0.03 | 0.595 | −0.08 | 0.03 | 0.020 | −0.05 | 0.03 | 0.080 |
| N × BA | −0.16 | 0.06 | 0.006 | −0.15 | 0.06 | 0.007 | −0.10 | 0.04 | 0.019 |
| Age | 0.02 | < 0.01 | < 0.001 | 0.02 | < 0.01 | < 0.001 | |||
| Gender | −0.27 | 0.05 | < 0.001 | −0.14 | 0.04 | 0.001 | |||
| Education | −0.03 | 0.02 | 0.048 | −0.02 | 0.01 | 0.069 | |||
| Average alcohol use | −0.01 | < 0.01 | 0.084 | ||||||
| Former smoker | 0.01 | 0.05 | 0.855 | ||||||
| Current smoker | 0.23 | 0.06 | < 0.001 | ||||||
| Medication usage: Antihypertensive | 0.18 | 0.05 | < 0.001 | ||||||
| Medication usage: Cholesterol | −0.16 | 0.05 | 0.002 | ||||||
| Medication usage: Steroid | 0.06 | 0.12 | 0.594 | ||||||
| BMI | 6.59 | 0.24 | < 0.001 | ||||||
|
| |||||||||
| R2 | 0.02 | 0.13 | 0.49 | ||||||
| F for change in R2 | 9.52*** | 52.89*** | 127.84*** | ||||||
Note. N = 1279.
p ≤ .001.
We used Model 3 performed on the combined analysis and plotted biological health risk as a function of neuroticism and behavioral adjustment (see Fig. 1-A). As can be seen, neuroticism was significantly related to lower biological health risk at one standard deviation (SD) above the mean of behavioral adjustment, b = −.11, 95% CI [−.21, −.02], t(1265) = −2.28, p = .023. In contrast, there was a tendency that neuroticism was linked to increased biological health risk at one SD below the mean of behavioral adjustment, although this effect did not reach statistical significance, b = .05, 95% CI [−.05, .14], t(1265) = .99, p = .324.
Figure 1.
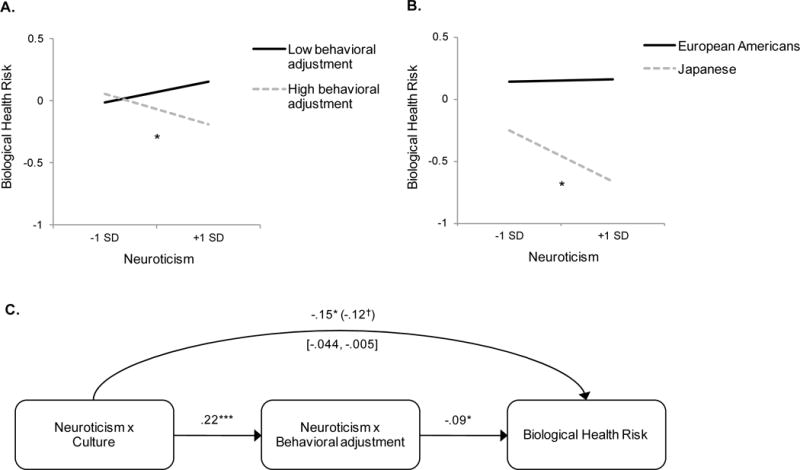
The relationship between neuroticism and biological health risk (defined by inflammation and cardiovascular malfunction). (A) Neuroticism × Behavioral adjustment interaction. (B) Neuroticism × Culture interaction. (C) Mediation analysis demonstrating that the Neuroticism × Culture interaction effect on biological health risk is mediated by the Neuroticism × Behavioral adjustment interaction. This indicates that unlike European Americans, Japanese get health benefits from neuroticism because they are sufficiently high in behavioral adjustment. Demographic variables, health conditions, and health behaviors are controlled. †p ≤ .10, *p ≤ .05, ***p ≤ .001.
Although the effect of neuroticism on biological health risk was statistically negligible at one SD below the mean of behavioral adjustment, our theoretical analysis implies that if we isolate individuals who are even lower in behavioral adjustment, we ought to be able to find a significant association between high neuroticism and higher biological health risk. To test this possibility, we isolated 82 individuals who scored lower than 1.5 SD below the mean of behavioral adjustment. As predicted, in this group, high neuroticism was related to higher biological health risk in Model 3, b = .31, 95% CI [.02, .59], t(67) = 2.13, p = .034.
When each of the four biomarkers was tested individually, the consistent patterns of the interaction effects were found for all outcomes although the effects were somewhat attenuated as may be expected (see Fig. S3). The interaction pattern was always in the same direction and largely significant in Models 1 and 2. Only when health behaviors and health status were controlled (Model 3) did the effect become attenuated for IL-6, SBP, and Total/HDL cholesterol, although it remained in the same direction. The interaction remained significant for CRP (see Table S1-A for regression coefficients of the Neuroticism × Behavioral adjustment interaction effect on four biomarkers separately).
Culture-wise analysis
Next, we tested the Neuroticism × Behavioral adjustment interaction separately for each cultural group. The interaction was significant for European Americans in both Models 1 and 2, b = −.12, 95% CI [−.23, −.01], t(950) = −2.15, p = .032, and b = −.11, 95% CI [−.22, −.00], t(947) = −2.00, p = .046, respectively, although this effect became non-significant when we additionally controlled for health behaviors and health status in Model 3, b = −.07, 95% CI [−.16, .02], t(940) = −1.52, p = .128. For Japanese, the interaction effect was marginal in Model 1, b = −.23, 95% CI [−.48, .03], t(321) = −1.76, p = .079, but was highly significant in both Models 2 and 3, b = −.32, 95% CI [−.53, −.12], t(318) = −3.13, p = .002, and b = −.26, 95% CI [−.44, −.07], t(311) = −2.73, p = .007, respectively. Importantly, the Culture × Neuroticism × Behavioral adjustment 3-way interaction was not significant on biological health risk in all three models, ts < −1.61, ps > .11, suggesting that regardless of cultural groups, those who are adjusting to environmental threats are more likely to enjoy a health benefit of neuroticism.
Analysis 2: Culture as a Moderator of the Link between Neuroticism and Biological Health Risk
Cultural difference in the effect of neuroticism on biological health risk
Next, we tested our cross-cultural prediction. Since behavioral adjustment would be higher for Japanese than for European Americans, neuroticism would more likely be associated with lower biological health risk among Japanese than for European Americans. As predicted, Japanese were higher in behavioral adjustment (M = 4.32, SE = .04, 95% CI [4.24, 4.41]) than European Americans (M = 4.11, SE = .03, 95% CI [4.06, 4.16]), F(1, 1347) = 19.52, p < .001, ηp2 = .01. This cultural difference remained the same even after controlling for all the covariates included in Model 3, F(1, 1282) = 12.85, p < .001, ηp2 = .01.
Subsequently, we tested whether culture would interact with neuroticism to predict biological health risk. We regressed biological health risk on neuroticism, culture, and the interaction between the two. We followed the steps in our Analysis 1 to test three models that varied in the set of controlled variables. As predicted, in all models, the predicted Neuroticism × Culture interaction proved significant (see Table 4). We used Model 3 and plotted biological health risk as a function of neuroticism and culture. As can be seen in Fig. 1-B, neuroticism was significantly related to lower biological health risk among Japanese, b = −.15, 95% CI [−.28, −.02], t(1271) = −2.24, p = .025. This relationship was negligible among European Americans, b = .003, 95% CI [−.07, .08], t(1271) = .09, p = .932.7
Table 4.
Regression coefficients in predicting biological health risk as a function of neuroticism and culture
| Biological Health Risk | Model 1
|
Model 2
|
Model 3
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE B | P | B | SE B | P | B | SE B | P | |
| Neuroticism (N) | −0.07 | 0.05 | 0.106 | 0.01 | 0.04 | 0.841 | 0.00 | 0.04 | 0.932 |
| Culture | −1.08 | 0.06 | <0.001 | −1.06 | 0.05 | <0.001 | −0.53 | 0.05 | <0.001 |
| N × Culture | −0.22 | 0.09 | 0.018 | −0.19 | 0.09 | 0.036 | −0.15 | 0.08 | 0.042 |
| Age | 0.02 | <0.01 | <0.001 | 0.02 | <0.01 | <0.001 | |||
| Gender | −0.31 | 0.05 | <0.001 | −0.17 | 0.04 | <0.001 | |||
| Education | −0.07 | 0.01 | <0.001 | −0.04 | 0.01 | 0.001 | |||
| Average alcohol use | <0.01 | <0.01 | 0.788 | ||||||
| Former smoker | <0.01 | 0.05 | 0.959 | ||||||
| Current smoker | 0.28 | 0.06 | <0.001 | ||||||
| Medication usage: Antihypertensive | 0.16 | 0.05 | 0.001 | ||||||
| Medication usage: Cholesterol | −0.19 | 0.05 | <0.001 | ||||||
| Medication usage: Steroid | 0.01 | 0.11 | 0.904 | ||||||
| BMI | 5.45 | 0.26 | <0.001 | ||||||
|
| |||||||||
| R2 | 0.25 | 0.33 | 0.52 | ||||||
| F for change in R2 | 140.81*** | 55.09*** | 72.68*** | ||||||
Note. N = 1285.
p ≤ .05,
p ≤ .01,
p ≤ .001.
Consistent patterns of the interaction effects were found when each of the four biomarkers was tested individually, although the effects were somewhat attenuated (see Fig. S4). The interaction pattern was always in the same direction and largely significant for the two measures of cardiovascular malfunction. The interaction was weaker for the two indicators of inflammation (see Table S1-B for regression coefficients of the Neuroticism × Culture interaction effect on four biomarkers separately).
Explaining the cultural difference by behavioral adjustment
Combining the two analyses reported so far, we may hypothesize that neuroticism has divergent effects depending on culture (the Neuroticism × Culture interaction) because (i) neuroticism has divergent effects depending on behavioral adjustment (the Neuroticism × Behavioral adjustment interaction) and (ii) culture varies in the level of behavioral adjustment. This conceptual model is illustrated in Fig. 2.
Figure 2.
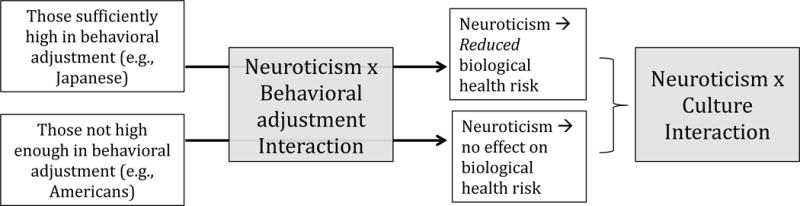
A theoretical scheme linking the Neuroticism × Behavioral adjustment interaction to the Neuroticism × Culture interaction. Japanese as a group showed a reliable association between high neuroticism and reduced biological health risk because they were sufficiently high in behavioral adjustment, whereas European Americans had no association between neuroticism and biological health risk because they were not high enough in behavioral adjustment.
Statistically, this model implies that the Neuroticism × Culture interaction on biological health risk should be mediated by the Neuroticism × Behavioral adjustment interaction. We formally tested this prediction by testing both interactions as simultaneous predictors of biological health risk. The Neuroticism × Culture interaction (the direct effect) was no longer significant, b = −.13, 95% CI [−.28, .02], t(1264) = −1.69, p = .091, while the Neuroticism × Behavioral adjustment interaction (the indirect effect) remained significant, b = −.10, 95% CI [−.18, −.02], t(1264) = −2.45, p = .014. A bootstrapping test with 2,000 replications indicated that the mediating path was statistically significant, 95% bias-corrected bootstrapping CI = [−.044, −.003] (see Fig. 1-C). We have therefore found support for the theoretical scheme illustrated in Fig. 2. Japanese as a group showed a reliable association between high neuroticism and reduced biological health risk because they were sufficiently high in behavioral adjustment, whereas European Americans had no association between neuroticism and biological health risk because they were not high enough in behavioral adjustment.
Analysis 3: Generalizability to Subjective Health
Next, we tested the two key interaction effects (Neuroticism × Behavioral adjustment, Neuroticism × Culture) on self-reported health measures to examine whether the moderation effects observed above using biomarkers would extend to subjective health. We expected that neuroticism should be associated with ill-health regardless of behavioral adjustment or culture.
We first examined whether neuroticism would interact with behavioral adjustment to predict the composite index of subjective health. The main effect of neuroticism was highly significant regardless of the covariates included (as in our Models 1–3), b = −.48, 95% CI [−.56, −.39], t(1268) = −11.01, p < .001 for Model 3. Unlike in our analyses on biomarkers, however, this effect was not significantly moderated by behavioral adjustment in any of the models, ts < 1.61, ps > .108. The consistent patterns of the results were shown when we examined each health measure separately. In all three models, neuroticism was linked to worse self-rated general health, b = −.65, 95% CI [−.80, −.50], t(1275) = −8.71, p < .001, a higher number of chronic conditions, b = .84, 95% CI [.64, 1.04], t(1273) = 8.33, p < .001, and greater functional limitations, b = .19, 95% CI [.13, .25], t(1272) = 6.47, p < .001 for Model 3. The Neuroticism × Behavioral adjustment interaction was statistically negligible on these variables, ts < −1.07, ps > .287.
Second, we tested the moderating effect of culture on the link between neuroticism and subjective health and similarly found that neuroticism was a significant predictor of ill-health in all three models, regardless of which marker of subjective health was tested, 4.19 < | ts | < 9.11, ps < .001. Although the relationship between neuroticism and ill-health was significant for both cultural groups, the magnitude of this effect was larger for Japanese than for European Americans when we examined self-rated general health, indicated by a significant Neuroticism × Culture interaction effect, b = −.45, 95% CI [−.76, −.15], t(1282) = −2.90, p = .004 for Model 3. The same interaction was not significant on chronic conditions and functional limitations, ts < 1.24, ps > .217.
Exploratory Analysis: Conscientiousness as a Moderator of the Link between Neuroticism and Biological Health Risk
As shown in Turiano et al. (2013), we duplicated the Neuroticism × Conscientiousness interaction on IL-6 among European Americans in all three models, b = −.07, 95% CI [−.13, −.00], t(946) = −2.02, p = .044 for Model 3. However, when our summary index of biological health risk was tested, the Neuroticism × Conscientious interaction became non-significant for European Americans in Model 3, b = −.10, 95% CI [−.26, .06], t(940) = −1.26, p = .209. Moreover, regardless of the index of biomarkers used (IL-6 or biological health risk), there was no Neuroticism × Conscientiousness interaction among Japanese, ts < 1.51, ps > .133. Hence, at this point, evidence is not strong for the proposition that neuroticism is “healthy” among those high in conscientiousness across cultures.
Discussion
Behavioral Adjustment, Culture, and Neuroticism
One key contribution of our work is to establish the crucial moderating role of behavioral adjustment for the effect of neuroticism on biological health risk. For people who were high in behavioral adjustment (1 SD higher than the mean), neuroticism was significantly linked to reduced biological health risk. In contrast, for those who were low in behavioral adjustment, this effect tended to be reversed. This latter effect was statistically significant for those who were at least 1.5 SD lower than the mean in behavioral adjustment. The Neuroticism × Behavioral adjustment interaction effect remained statistically significant even after controlling for all potentially relevant covariates (Model 3). Moreover, the pattern of the interaction was highly consistent across the four biomarkers and for both cultural groups. Taken together, these findings strongly suggest that a combination of high neuroticism and high behavioral adjustment is linked to low biological health risk.
Another key contribution of our work comes from an observation that behavioral adjustment is significantly higher among Japanese than among European Americans. This observation is consistent with prior cultural psychological work that demonstrates Japanese to be more interdependent and thus more prepared to fit in than Americans, who tend to be independent and thus less inclined to change the self to the demands of the situation (Morling et al., 2002; Tsai et al., 2007). Importantly, we showed that this cultural difference in the mean level of behavioral adjustment accounts for another cultural difference in the effect of neuroticism on biological health risk (i.e., Neuroticism × Culture interaction effect). As shown in Fig. 2, Japanese were relatively high in behavioral adjustment. Moreover, people high in behavioral adjustment showed salubrious health effects of neuroticism. As may be expected from these patterns, among Japanese, high neuroticism was linked to lowered biological health risk. In contrast, European Americans were relatively low in behavioral adjustment and, correspondingly, there was no significant relationship between neuroticism and biological health risk among them.
Subjective Health and Biological Health
Although the possibility that neuroticism could be healthy has previously been discussed (Friedman, 2011; Friedman & Kern, 2014; Turiano et al., 2013), our work is one of the first that provides strong evidence for the health-enhancing effect of neuroticism (see also Turiano et al., 2013). We suspect that previous work might have often failed to obtain evidence for it in part because it relied largely on Western participants, who are likely relatively low in behavioral adjustment and thus less likely to show an association between neuroticism and better health.
Another reason for the previous failure to show clear evidence for the salubrious effects of neuroticism may come from its nearly exclusive reliance on subjective measures of health such as perceived overall health, the number of reported symptoms and illness conditions, and pain. Although these measures are linked to biological, neuro-chemical, and molecular pathogenesis, the correspondence is not perfect. Thus, many illness cognitions only loosely capture biological conditions of the body. For example, there are no acute symptoms for many early-stage cancers. In these cases, biological pathogenesis has little or no subjective counterpart. Conversely, certain illness symptoms are no more than a “phantom” as in the phantom pain that is typical for those who have lost their limbs. Thus, subjective experience can occur in the absence of any biological entities to which the experience is attributed. To be sure, in many other cases, the link between subjective health and objective health does exist, but the correspondence may not always be strong.
Although subjective health is obviously important in many situations, for example, in cases involving conscious decisions and judgments (as in choosing or not choosing to seek treatment), it is potentially problematic in the context of neuroticism. Because it is defined primarily by negative emotions, neuroticism is inherently negative. Moreover, low subjective health is also inherently negative. The resulting semantic overlap alone could be sufficient to ensure that there is a strong link between neuroticism and lower subjective health. Thus, some of the link between neuroticism and lower subjective health could be due to this semantic artifact. In addition, high neuroticism is linked to attentional vigilance to threats including illness symptoms (Derryberry & Reed, 1994; Wilson et al., 2006). Because neuroticism may magnify the subjective appraisal of ill health, neuroticism may predict subjective ill health even after controlling for objective, biological pathogenic conditions. If the link between high neuroticism and low subjective health is mediated by the cognitive or attentional bias of neuroticism, there is no guarantee that the same relationship would exist at the level of objective health.
Indeed, previous evidence linking neuroticism to worse health and wellbeing is based mostly on self-report indicators of health such as self-rated health (Costa & McCrae, 1987; Okun & George, 1984) and chronic bodily conditions (Costa & McCrae, 1987). Similarly, when we tested three self-report indicators of health including self-rated general health, chronic conditions, and functional limitations, we consistently found negative health effects of neuroticism regardless of levels of behavioral adjustment or culture. Thus, the exclusive reliance on subjective markers of health could potentially obscure what might actually be happening at the biological level (Friedman, 2000; Watson & Pennebaker, 1989). Our work, then, underscores the urgent need to extend the current literature on neuroticism and health with objective measures of health such as markers of inflammation and cardiovascular malfunction.
Moderating Role of Conscientiousness
Turiano et al. (2013) found that neuroticism is related to reduced biological health risk for Americans who are high in conscientiousness. It is possible that high conscientiousness and associated orderliness and discipline may help individuals address threats resulting from problematic personal behaviors such as delay in loan payment, failure to keep appointed times, and the like. We duplicated the Neuroticism × Conscientiousness finding among European Americans although the interaction curiously disappeared with the current index of biological health risk. Moreover, regardless of the index of biomarker used, there was no Neuroticism × Conscientiousness interaction among Japanese. Altogether, the potential role of conscientiousness in moderating the link between neuroticism and biological health across cultures seems tenuous.
Limitations and Future Directions
Some limitations of the current work must be acknowledged. First, our work should be extended to other cultural and ethnic groups. Neuroticism may also be adaptive in certain subgroups of Americans who are behaviorally more adjusting than European Americans. Consistent with this view, a recent study (Campos et al., 2014) found that a maladaptive health effect of neuroticism is attenuated among Hispanic Americans—a group that is more interdependent than European Americans (Shkodriani & Gibbons, 1995; Triandis, 1983). Moreover, as people age, they may become increasingly more adjusting (Carstensen, 1992) and, if so, elderly may enjoy certain health benefits of neuroticism. Second, in the current work, neuroticism was assessed with an abbreviated 4-item measure. Future work must use a more extensive measurement instrument that enables one to assess separable facets of neuroticism and determine which facets might be most instrumental in forging health benefits to those who are high in the preparedness for behavioral adjustment. Third, we also tested behavioral adjustment with a brief, 5-item scale. It is well warranted to elaborate on the construct and develop a more extensive scale to measure it in future work. Fourth, the current work was cross-sectional, and a longitudinal extension of the current work (forthcoming in both MIDJA and MIDUS) will be informative.
Despite these limitations, our findings suggest that as long as biological health risks are concerned, high neuroticism may not be maladaptive; in fact, it may be adaptive in some contexts for some individuals. This offers some far-reaching implications. First, the current consensus that neuroticism is maladaptive must be revised. Second, it is the combination of high neuroticism and high behavioral adjustment that yields the reduction of biological health risk. Theoretically, this implies that to fully understand biological pathways of health, socio-cultural considerations are indispensable. Practically, our evidence suggests that certain therapeutic interventions designed to enhance one’s preparedness and skill set for behavioral adjustment could be effective in alleviating potentially negative effects of neuroticism.
Supplementary Material
Acknowledgments
This research was supported by a grant from the National Institute on Aging (5R37AG027343) to conduct a study of Midlife in Japan (MIDJA) for comparative analysis with MIDUS (Midlife in the U.S., P01-AG020166).
Appendix Behavioral Adjustment Scale
Instructions
The following questions are about how your views of yourself are linked to your relations with others. Please circle the number that corresponds to how much you agree or disagree with the following statements (where 1 = strongly disagree and 7 = strongly agree).
I usually follow the opinions of people I can respect.
When people have an opinion different from mine, I can adjust mine to theirs.
When values held by others sound more reasonable, I can adjust my values to theirs.
Once something has happened, I try to adjust myself to it because it is difficult to change it myself.
It is useless to try to change what is going to happen in life because it is impossible to predict it.
Footnotes
The choice of systolic blood pressure rather than diastolic blood pressure is arbitrary and non-consequential because these two assessments are typically highly correlated. Here we followed our earlier work to stay consistent (Kitayama et al., 2015).
Note that behavioral adjustment is likely to be related to, but distinct from interdependence. On the one hand, people may often be motivated to adjust their behaviors in order to maintain and enhance social harmony and, accordingly, we expect that behavioral adjustment would be positively associated with interdependence. On the other hand, behavioral adjustment may also be performed to meet other, non-social situational contingencies, and therefore, this construct is likely to be distinct from interdependence. In support of this proposition, we found that behavioral adjustment is positively associated with interdependent self-construal, assessed with the Singelis Self-Construal Scale (Singelis, 1994), both in Japan and the U.S., but the association is modest (rs = .341 and .256, ps < .001 in Japan and the U.S., respectively).
In an exploratory analysis, we tested our measures of health behaviors and health status as potential health risk factors that could be influenced by the interaction between neuroticism and behavioral adjustment or the interaction between neuroticism and culture. There emerged one significant interaction between neuroticism and culture on the use of antihypertensive medication, b = −.72, 95% CI [−1.35, −.09], Z = −2.25, p = .024. Neuroticism was linked to greater use of antihypertensive medication among European Americans, b = .49, 95% CI [.25, .73], Z = 3.99, p < .001, but not among Japanese, b = −.23, 95% CI [−.82, .35], Z = −.77, p = .440. This pattern was negligible on other variables, ts < −1.77, ps > .077. Note that in the analyses reported in the main text, these variables were controlled.
Results did not change when the Waist-to-Hip ratio was used in lieu of BMI.
In our previous studies, we tested two negative psychological states—i.e., negative affect (Curhan et al., 2014; Miyamoto et al., 2013) and anger expression (Kitayama et al., 2015)—and found that their effects on health are significantly moderated by culture. To sharpen the focus on neuroticism as potentially distinct from these constructs, we ran a set of supplementary analyses with these variables additionally controlled. The results from these analyses, displayed in Fig. S2, were no different from the results reported in the main text.
As noted earlier, two of our measures had low reliabilities; the reliability of the neuroticism scale was .56 for Japanese and the reliability of the behavioral adjustment scale was also below .6 for both cultural groups (αs = .54 and .59 for European Americans and Japanese, respectively). One way to address this issue is to use structural equation modeling (SEM), which allows us to explicitly model error variances associated with each of the scale items. Point estimates based on SEM are unbiased and tend to be associated with greater variance as the reliability of the scale decreases. This increase of the estimated variance of the point estimates does not occur in ordinary regression analysis. Hence, statistical tests based on SEM are suggested to be more conservative (Ledgerwood & Shrout, 2011). We thus used SEM to estimate the interaction effect between neuroticism and behavioral adjustment with the same set of the covariates included in Model 3 in our main analysis. We tested two models; a null model that does not estimate the interaction effect and an alternative model where the interaction effect is estimated. The log-likelihood ratio test comparing two models showed a significant result, D(1) = 5.41, p = .020, indicating that the alternative model provides a better fit to the data relative to the null model. Moreover, the interaction between neuroticism and behavioral adjustment proved statistically significant, b = −.21, SE = .10, p = .020, suggesting that the statistical conclusion remains unchanged when we use SEM. The results did not depend on the inclusion or exclusion of the control variables.
We also used SEM to estimate the interaction effect between neuroticism and culture with the same set of covariates included in Model 3. A comparison of the null model (without estimation of the interaction effect) with the alternative model (with estimation of the interaction effect) showed a significant result, D(1) = 4.87, p = .027, indicating that the alternative model provides a better fit to the data. Moreover, the Neuroticism × Culture interaction proved statistically significant, b = −.17, SE = .08, p = .029, suggesting that the key findings remain unchanged when the measurement errors of the neuroticism items were corrected using SEM.
References
- Armon G, Melamed S, Shirom A, Shapira I, Berliner S. Personality Traits and Body Weight Measures: Concurrent and Across-Time Associations. European Journal of Personality. 2013;27(4):398–408. http://doi.org/10.1002/per.1902. [Google Scholar]
- Bond R, Smith PB. Culture and Conformity: A Meta-Analysis of Studies Using Asch’s (1952b, 1956) Line Judgment Task. Psychological Bulletin. 1996;119(1):111–137. [Google Scholar]
- Brosschot JF, Godaert GL, Benschop RJ, Olff M, Ballieux RE, Heijnen CJ. Experimental stress and immunological reactivity: a closer look at perceived uncontrollability. Psychosomatic Medicine. 1998;60(3):359–361. doi: 10.1097/00006842-199805000-00024. [DOI] [PubMed] [Google Scholar]
- Campos B, Busse D, Yim IS, Dayan A, Chevez L, Schoebi D. Are the costs of neuroticism inevitable? Evidence of attenuated effects in U.S. Latinas. Cultural Diversity and Ethnic Minority Psychology. 2014;20(3):430–440. doi: 10.1037/a0035329. http://doi.org/10.1037/a0035329. [DOI] [PubMed] [Google Scholar]
- Carstensen LL. Social and emotional patterns in adulthood: Support for socioemotional selectivity theory. Psychology and Aging. 1992;7(3):331–338. doi: 10.1037//0882-7974.7.3.331. http://doi.org/10.1037/0882-7974.7.3.331. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. Dispositional optimism. Trends in Cognitive Sciences. 2014;18(6):293–299. doi: 10.1016/j.tics.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67(2):319–333. http://doi.org/10.1037/0022-3514.67.2.319. [Google Scholar]
- Cole SW, Levine ME, Arevalo JMG, Ma J, Weir DR, Crimmins EM. Loneliness, eudaimonia, and the human conserved transcriptional response to adversity. Psychoneuroendocrinology. 2015;62:11–17. doi: 10.1016/j.psyneuen.2015.07.001. http://doi.org/10.1016/j.psyneuen.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Neuroticism, Somatic Complaints, and Disease: Is the Bark Worse than the Bite? Journal of Personality. 1987;55(2):299–316. doi: 10.1111/j.1467-6494.1987.tb00438.x. http://doi.org/10.1111/j.1467-6494.1987.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Curhan KB, Sims T, Markus HR, Kitayama S, Karasawa M, Kawakami N, et al. Just How Bad Negative Affect Is for Your Health Depends on Culture. Psychological Science. 2014;25(12):2277–2280. doi: 10.1177/0956797614543802. http://doi.org/10.1177/0956797614543802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Temperament and attention: Orienting toward and away from positive and negative signals. Journal of Personality and Social Psychology. 1994;66(6):1128–1139. doi: 10.1037//0022-3514.66.6.1128. http://doi.org/10.1037/0022-3514.66.6.1128. [DOI] [PubMed] [Google Scholar]
- Epel ES, McEwen BS, Ickovics JR. Embodying psychological thriving: Physical thriving in response to stress. Journal of Social Issues. 1998;54(2):301–322. doi: 10.1111/j.1540-4560.1998.tb01220.x. [DOI] [Google Scholar]
- Friedman HS. Long-Term Relations of Personality and Health: Dynamisms, Mechanisms, Tropisms. Journal of Personality. 2000;68(6):1089–1107. doi: 10.1111/1467-6494.00127. [DOI] [PubMed] [Google Scholar]
- Friedman HS. Personality disease and self healing. The Oxford Handbook of Health Psychology. 2011 Retrieved from https://books.google.co.jp/books/about/The_Oxford_Handbook_of_Health_Psychology.html?id=J3-78PdF83kC.
- Friedman HS, Kern ML. Personality, Well-Being, and Health*. Annual Review of Psychology. 2014;65(1):719–742. doi: 10.1146/annurev-psych-010213-115123. http://doi.org/10.1146/annurev-psych-010213-115123. [DOI] [PubMed] [Google Scholar]
- Gale C, Cukic I, Batty GD, McIntosh AM, Weiss A, Deary IJ. When is higher Neuroticism protective against premature death? Findings from the UK Biobank. Psychological Science. 2017;28(9):1345–1357. doi: 10.1177/0956797617709813. https://doi.org/10.1177/0956797617709813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg LR. The development of markers for the Big-Five factor structure. Psychological Assessment. 1992;4(1):26–42. http://doi.org/10.1037/1040-3590.4.1.26. [Google Scholar]
- Gray JA. The neuropsychology of anxiety. British Journal of Psychology. 2011;69(4):417–434. doi: 10.1111/j.2044-8295.1978.tb02118.x. http://doi.org/10.1111/j.2044-8295.1978.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Heckhausen J, Schulz R. A life-span theory of control. Psychological Review. 1995;102(2):284–304. doi: 10.1037/0033-295x.102.2.284. http://dx.doi.org/10.1037/0033-295X.102.2.284. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Ruscio AM, Shear K, Wittchen HU. Epidemiology of Anxiety Disorders. Springer; Berlin Heidelberg: 2010. pp. 21–35. http://doi.org/10.1007/7854_2009_9. [PubMed] [Google Scholar]
- Kitayama S, Park J. Emotion and biological health: The socio-cultural moderation. Current Opinion in Psychology. 2017;17:99–105. doi: 10.1016/j.copsyc.2017.06.016. https://doi.org/10.1016/j.copsyc.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama S, Park H, Sevincer AT, Karasawa M, Uskul AK. A cultural task analysis of implicit independence: Comparing North America, Western Europe, and East Asia. Journal of Personality and Social Psychology. 2009;97(2):236–255. doi: 10.1037/a0015999. http://doi.org/10.1037/a0015999. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Park J, Boylan JM, Miyamoto Y, Levine CS, Markus HR, et al. Expression of Anger and Ill Health in Two Cultures: An Examination of Inflammation and Cardiovascular Risk. Psychological Science. 2015;26(2):211–220. doi: 10.1177/0956797614561268. http://doi.org/10.1177/0956797614561268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, Appraisal, and Coping. Springer; 1984. [Google Scholar]
- Ledgerwood A, Shrout PE. The trade-off between accuracy and precision in latent variable models of mediation processes. Journal of Personality and Social Psychology. 2011;101(6):1174–1188. doi: 10.1037/a0024776. http://doi.org/10.1037/a0024776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchetti M, Barkley JM, Stephan Y, Terracciano A, Sutin AR. Psychoneuroendocrinology. 2014:1–37. doi: 10.1016/j.psyneuen.2014.08.014. http://doi.org/10.1016/j.psyneuen.2014.08.014. [DOI] [PMC free article] [PubMed]
- Markus HR, Kitayama S. Culture and the self: Implications for cognition, emotion, and motivation. Psychological Review. 1991;98(2):224–253. [Google Scholar]
- Mendes WB, Blascovich J, Major B, Seery M. Challenge and threat responses during downward and upward social comparisons. European Journal of Social Psychology. 2001;31(5):477–497. http://doi.org/10.1002/ejsp.80. [Google Scholar]
- Mendes WB, Reis HT, Seery MD, Blascovich J. Cardiovascular correlates of emotional expression and suppression: Do content and gender context matter? Journal of Personality and Social Psychology. 2003;84(4):771–792. doi: 10.1037/0022-3514.84.4.771. http://doi.org/10.1037/0022-3514.84.4.771. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Boylan JM, Coe CL, Curhan KB, Levine CS, Markus HR, et al. Brain, Behavior, and Immunity. Brain Behavior and Immunity. 2013;34(C):79–85. doi: 10.1016/j.bbi.2013.07.173. http://doi.org/10.1016/j.bbi.2013.07.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morling B, Kitayama S, Miyamoto Y. Cultural Practices Emphasize Influence in the United States and Adjustment in Japan. Personality and Social Psychology Bulletin. 2002;28(3):311–323. http://doi.org/10.1177/0146167202286003. [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK. Heart disease and stroke statistics—2016 update: A report from the American Heart Association. Circulation. 2015;133(4):e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, Kemeny M, Epel ES. Stress appraisals and cellular aging: A key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain, Behavior, and Immunity. 2012;26(4):573–579. doi: 10.1016/j.bbi.2012.01.007. https://doi.org/10.1016/j.bbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun MA, George LK. Physician- and self-ratings of health, neuroticism and subjective well-being among men and women. Personality and Individual Differences. 1984;5(5):533–539. [Google Scholar]
- Oyserman D, Coon HM, Kemmelmeier M. Rethinking individualism and collectivism: Evaluation of theoretical assumptions and meta-analyses. Psychological Bulletin. 2002;128(1):3–72. http://doi.org/10.1037/0033-2909.128.1.3. [PubMed] [Google Scholar]
- Park J, Ayduk Ö, Kross E. Stepping back to move forward: Expressive writing promotes self-distancing. Emotion. 2016;16:349–364. doi: 10.1037/emo0000121. http://doi.org/10.1037/emo0000121. [DOI] [PubMed] [Google Scholar]
- Park J, Kitayama S, Karasawa M, Curhan K, Markus HR, Kawakami N, et al. Clarifying the links between social support and health: Culture, stress, and neuroticism matter. Journal of Health Psychology. 2013;18(2):226–235. doi: 10.1177/1359105312439731. http://doi.org/10.1177/1359105312439731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennebaker JW, Chung CK. Expressive writing, emotional upheavals, and health. In: Friedman HS, Silver RC, editors. Foundations of health psychology. Handbook of health psychology; 2007. pp. 263–284. [Google Scholar]
- Pennebaker JW, Kiecolt-Glaser JK, Glaser R. Disclosure of traumas and immune function: Health implications for psychotherapy. Journal of Consulting and Clinical Psychology. 1988;56(2):239–245. doi: 10.1037//0022-006x.56.2.239. http://doi.org/10.1037/0022-006X.56.2.239. [DOI] [PubMed] [Google Scholar]
- Rivkin ID, Taylor SE. The Effects of Mental Simulation on Coping with Controllable Stressful Events. Personality and Social Psychology Bulletin. 1999;25(12):1451–1462. http://doi.org/10.1177/01461672992510002. [Google Scholar]
- Rothbaum F, Weisz JR, Snyder SS. Changing the world and changing the self: A two-process model of perceived control. Journal of Personality and Social Psychology. 1982;42(1):5–37. http://dx.doi.org/10.1037/0022-3514.42.1.5. [Google Scholar]
- Shkodriani GM, Gibbons JL. Individualism and collectivism among university students in Mexico and the United States. The Journal of Social Psychology. 1995;135(6):765–772. [Google Scholar]
- Singelis TM. The measurement of independent and interdependent self-construals. Personality and Social Psychology Bulletin. 1994;20(5):580–591. [Google Scholar]
- Smith TW, MacKenzie J. Personality and Risk of Physical Illness. Annual Review of Clinical Psychology. 2006;2(1):435. doi: 10.1146/annurev.clinpsy.2.022305.095257. Annual Review of Clinical Psychology. [DOI] [PubMed] [Google Scholar]
- Smyth JM. Written emotional expression: Effect sizes, outcome types, and moderating variable. Journal of Consulting and Clinical Psychology. 2010;66(1):174–184. doi: 10.1037//0022-006x.66.1.174. http://doi.org/10.1080/026999396380079. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anger Expression Inventory. Hoboken, NJ, USA: John Wiley & Sons, Inc; 2010. http://doi.org/10.1002/9780470479216.corpsy0942. [Google Scholar]
- Suls J, Bunde J. Serials Solutions 360LINK. Psychological Bulletin 2005 [Google Scholar]
- Sutin AR, Terracciano A, Deiana B, Naitza S, Ferrucci L, Uda M, et al. High Neuroticism and low Conscientiousness are associated with interleukin-6. Psychological Medicine. 2009;40(09):1485–1493. doi: 10.1017/S0033291709992029. http://doi.org/10.1017/S0033291709992029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triandis HC. Allocentric vs idiocentric social behavior: A major cultural difference between Hispanics and the mainstream. (16) 1983;(16):1–81. Technical report. [Google Scholar]
- Tsai JL, Miao FF, Seppala E, Fung HH. Influence and adjustment goals: Souces of cultural differences in ideal affect. Journal of Personality and Social Psychology. 2007;92(6):1102–1117. doi: 10.1037/0022-3514.92.6.1102. [DOI] [PubMed] [Google Scholar]
- Turiano NA, Mroczek DK, Moynihan J, Chapman BP. Big 5 personality traits and interleukin-6: Evidence for “healthy Neuroticism” in a US population sample. Brain Behavior and Immunity. 2013;28:83–89. doi: 10.1016/j.bbi.2012.10.020. http://doi.org/10.1016/j.bbi.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Pennebaker JW. Health complaints, stress, and distress: exploring the central role of negative affectivity. Psychological Review. 1989;96(2):234–254. doi: 10.1037/0033-295x.96.2.234. [DOI] [PubMed] [Google Scholar]
- Wilson EJ, MacLeod C, Mathews A, Rutherford EM. The causal role of interpretive bias in anxiety reactivity. Journal of Abnormal Psychology. 2006;115(1):103–111. doi: 10.1037/0021-843X.115.1.103. http://doi.org/10.1037/0021-843X.115.1.103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


