Abstract
Background
In Colombia, taeniasis and cysticercosis have been significantly reduced over the past decades, however still reported with implications for public health and travel medicine.
Methods
An observational, retrospective study, in which the incidence of taeniasis and cysticercosis (ICD-10 codes B68s/B69s) in Colombia, 2009–2013, was estimated based on data extracted from the Individual Health Records System (Registro Individual de Prestación de Servicios, RIPS) was performed. The Geographic Information System (GIS) generated national maps showing the distribution of taeniasis and cysticercosis by department by year.
Results
During the period, 3626 cases were reported (median 796/year), for a cumulative crude national rate of 7.7 cases/100,000pop; 58.2% corresponded to male; 57% were < 40 year-old (10.2% < 9.9 year-old). Cases were 57.6% neurocysticercosis, the rest were taeniasis due to T. solium, T. saginata, ocular cysticercosis and cysticerci in other organs. Bolivar, a touristic department, had the highest cumulated incidence rate (16.17 cases/100,000pop), as also evident across the map series developed in this study.
Conclusion
Despite the limitations of this study, data presented provide recent estimates of national taeniasis and cysticercosis incidence in the country useful in public health and for travel medicine practitioners, as some highly touristic areas presented higher disease incidence. Improved control, particularly of taeniasis, should be an attainable goal, which among other strategies would require improved sanitation and health education to prevent transmission, but also enhanced surveillance.
Keywords: Taeniasis, Cysticercosis, Neurocysticercosis, Geographical information systems, Infectious diseases epidemiology, Colombia
1. Introduction
Parasitic zoonotic infections caused by Taenia saginata and Taenia solium are particular among helminths, because their life cycle involves humans as a definite host and depend on the interaction with cattle and pigs respectively [1–4]. Taeniasis is produced by both agents after accidental cysticerci ingestion in undercooked contaminated meat and minimal gastrointestinal clinical manifestations may occur. Moreover, when Taenia solium eggs are ingested, a systemic invasion occurs in several tissues called cysticercosis causing serious complications mostly in the central nervous system and invading also muscles, eyes, and subcutaneous tissue [5–7]. Exposure, given such conditions would be important in endemic areas, not just for people living there, but also for travelers and visitors, especially in touristic zones [3,6–10].
Taeniasis and cysticercosis are neglected tropical diseases according to the World Health Organization, because they persist as a public health problem in diverse regions in the world, including Latin America and the Caribbean. Neurocysticercosis (NCC) [9,10], is a preventable cause of epilepsy; 30% of cases live in many endemic areas where people and pigs live near. In addition, more than 80% of the world’s 50 million people who are affected by epilepsy live in low and lower-middle income countries [9,10]. Currently, high-income countries in North America and Europe have reported a rise in imported cases related to increased international migration. In 1993, The International Task Force for Disease Eradication identified T. solium as a “potentially eradicable parasite”, however, successful intervention programs have not yet been developed [8–11]. NCC in developed countries is considered to be an imported infectious disease brought by immigrants, refugees and by travelers who have visited endemic areas where pork, especially uncooked, is prepared in the backyard and served as a local traditional dish [12,13].
For control, prevention, treatment and monitoring of these diseases, interdisciplinary participation of institutions responsible for human health, food safety, animal care and growth and education are required [14–17]. Health education, adequate sanitary habits and wastewater disposal are critical strategies to mitigate the impact of the disease [5,6,18] and reduce economic burden related to losses in meat industry, medical costs and productivity of people affected, especially in countries with social and environmental risk factors such as extreme poverty [2,5,6,9,10,18,19].
In many countries reduction and eradication of both diseases have been significantly achieved [20], such as in Mexico [21] and Colombia [22–26]. Nonetheless transmission still occurs and cases are detected [22–26]. In other countries, such as is particularly the case of Venezuela, the rise in these and other parasitic and infectious diseases is clear and evident, reflecting the failure and negligence in the control of such diseases [15,27–29].
As part of an effort to enhance control and risk assessment for the residual number of cases of taeniasis and cysticercosis, the Regional Information System, the Universidad Tecnologica de Pereira (through the Research Group of Public Health and Infection) and the Ministry of Health, are working together on the academic analysis of epidemiological information of infectious diseases on a regional and national scale [30–34], including taeniasis and cysticercosis. In this setting, the present study aims to estimate incidence of taeniasis and cysticercosis between 2009 and 2013 for Colombia and to develop GIS-based epidemiological maps for this helminth disease in the country.
2. Methods
Colombia is a South American country constituted by 32 departments (main administrative level), and a total population of 48,747,632 for 2016. The Colombian territory has climatic, geographic, economic and epidemiological conditions adequate for the transmission of taeniasis, cysticercosis and other parasitic infections.
We performed an observational retrospective study. The epidemiological data were collected from Individual Health Records System (Registro Individual de Prestación de Servicios, RIPS). The International Classification of Diseases 10th revision (ICD-10) codes B680, B681, B689, B690, B691, B698, B699, were used to obtain the number of cases from each department of Colombia during 2009–2013, given the fact that taeniasis and cysticercosis are not under the surveillance system and this is a passive capture health information system. This information came from confirmed cases and the Colombian National Institute of Health reviewed data quality. Data were obtained with the agreement of the Ministry of Health and Social Protection through the Protection Information System (SISPRO) via a client access server: SISPRO RIPS. Data used for this study were obtained from confirmed cases that were reviewed in terms of data quality, initially from data from the National Institute of Health, Colombia and afterwards by SISPRO and its Data Cubes system. Data for this study came from 33 reference notification units, one per department, and were later consolidated and centralized in Bogotá onto the SISPRO system. Currently revised and consolidated data are available for the period 2009–2013. The quality of RIPS data in Colombia has been described elsewhere [32,35].
Official reference population data from National Administrative Department of Statistics (DANE) was used to estimate annual incidence rates for 32 Colombian departments and its capital district (per 100 000 population) by age and gender. In addition, we generated the spatial distribution for neurocysticercosis, non-organ specified cysticercosis and non-species specified taeniasis annual incidence rates per quartiles. The data were imported from Microsoft Excel 2013® to Geo Da 1.8® to perform the spatial analysis. We obtained the official shapefiles (.shp) for the country from the Instituto Geografico Agustin Codazzi (National Geographic Institute Agustin Codazzi, IGAC) website (http://www.igac.gov.co/wps/portal/igac/raiz/iniciohome/MapasdeColombia/Descargas).
3. Results
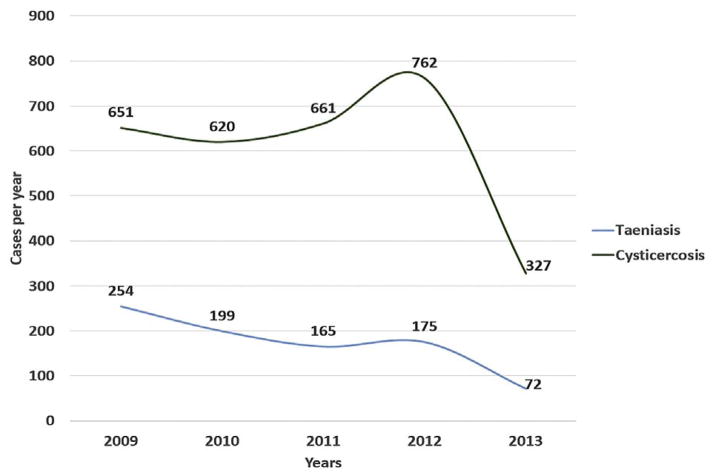
From 2009 to 2013, 3626 cases of taeniasis and cysticercosis were reported (Table 1), with an average of 725.2 cases per year (Fig. 1). National annual crude accumulated rate for taeniasis and cysticercosis combined was 7.7 cases/100,000 people; taeniasis due to T. solium (0.23 cases/100,000 pop), T. saginata (0.06 cases/100,000 pop) and taeniasis unspecified (1.55 cases/100,000 pop). Taeniasis and cysticercosis combined reduced from 1.9 cases/100,000 pop (in 2009) to 0.8 cases/100,000 pop (in 2013) (Fig. 1).
Table 1.
Number of cases and estimated incidence rate of taeniasis and cysticercosis (combined) in Colombia, 2009–2013, by territory.
| Department | 2009 | 2010 | 2011 | 2012 | 2013 | Total Period | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Cases | Rate | Casesa | Rateb | |
| Bolívar | 81 | 4.14 | 58 | 2.93 | 67 | 3.35 | 94 | 4.64 | 24 | 1.17 | 324 | 16.17 |
| Atlántico | 87 | 3.81 | 47 | 2.03 | 39 | 1.66 | 57 | 2.4 | 25 | 1.04 | 255 | 10.88 |
| Nariño | 29 | 1.79 | 35 | 2.13 | 40 | 2.41 | 47 | 2.8 | 20 | 1.18 | 171 | 10.30 |
| Sucre | 11 | 1.37 | 6 | 0.74 | 24 | 2.93 | 22 | 2.66 | 11 | 1.32 | 74 | 9.04 |
| Antioquia | 67 | 1.12 | 65 | 1.07 | 84 | 1.37 | 100 | 1.61 | 59 | 0.94 | 375 | 6.10 |
| Risaralda | 16 | 1.74 | 10 | 1.08 | 9 | 0.97 | 10 | 1.07 | 8 | 0.85 | 53 | 5.70 |
| Santander | 17 | 0.85 | 20 | 0.99 | 29 | 1.44 | 22 | 1.08 | 9 | 0.44 | 97 | 4.80 |
| Cesar | 9 | 0.94 | 8 | 0.83 | 5 | 0.51 | 17 | 1.71 | 2 | 0.2 | 41 | 4.19 |
| La Guajira | 4 | 0.51 | 5 | 0.61 | 5 | 0.59 | 16 | 1.83 | 2 | 0.22 | 32 | 3.78 |
| Caldas | 8 | 0.82 | 5 | 0.51 | 6 | 0.61 | 7 | 0.71 | 6 | 0.61 | 32 | 3.26 |
| Córdoba | 12 | 0.77 | 6 | 0.38 | 8 | 0.5 | 19 | 1.16 | 5 | 0.3 | 50 | 3.11 |
| Magdalena | 7 | 0.59 | 5 | 0.42 | 7 | 0.58 | 11 | 0.9 | 7 | 0.57 | 37 | 3.05 |
| Huila | 14 | 1.31 | 4 | 0.37 | 2 | 0.18 | 6 | 0.54 | 5 | 0.44 | 31 | 2.82 |
| Cauca | 5 | 0.38 | 9 | 0.68 | 8 | 0.6 | 13 | 0.97 | 2 | 0.15 | 37 | 2.78 |
| Bogotá, D.C. | 42 | 0.58 | 51 | 0.69 | 41 | 0.55 | 39 | 0.52 | 22 | 0.29 | 195 | 2.61 |
| Quindío | 5 | 0.91 | 1 | 0.18 | 2 | 0.36 | 5 | 0.9 | 1 | 0.18 | 14 | 2.53 |
| Valle del Cauca | 25 | 0.58 | 18 | 0.41 | 24 | 0.54 | 20 | 0.45 | 7 | 0.15 | 94 | 2.12 |
| Tolima | 4 | 0.29 | 8 | 0.58 | 3 | 0.22 | 9 | 0.64 | 4 | 0.29 | 28 | 2.01 |
| Norte de Santander | 8 | 0.62 | 3 | 0.23 | 8 | 0.61 | 4 | 0.3 | 3 | 0.23 | 26 | 1.99 |
| Caquetá | 0 | 0 | 1 | 0.22 | 1 | 0.22 | 5 | 1.09 | 2 | 0.43 | 9 | 1.98 |
| Boyacá | 3 | 0.24 | 4 | 0.32 | 4 | 0.32 | 8 | 0.63 | 3 | 0.24 | 22 | 1.73 |
| Cundinamarca | 5 | 0.21 | 8 | 0.32 | 6 | 0.24 | 6 | 0.23 | 3 | 0.12 | 28 | 1.11 |
| Guaviare | 0 | 0 | 0 | 0 | 1 | 0.95 | 0 | 0 | 0 | 0 | 1 | 0.95 |
| Meta | 2 | 0.23 | 3 | 0.34 | 0 | 0 | 3 | 0.33 | 0 | 0 | 8 | 0.9 |
| Putumayo | 1 | 0.31 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.3 | 2 | 0.61 |
| Casanare | 0 | 0 | 0 | 0 | 2 | 0.6 | 0 | 0 | 0 | 0 | 2 | 0.6 |
| Arauca | 0 | 0 | 0 | 0 | 1 | 0.4 | 0 | 0 | 0 | 0 | 1 | 0.4 |
Total number of cases during the period.
Total number of cases during the period divided the mean of the period population x 100,000 pop.
Fig. 1.
Number of taeniasis and cysticercosis cases in Colombia, 2009–2013.
Demographic features of taeniasis without species definition showed that 63% were female with an incidence rate of 1.15 cases/100,000 pop and the most affected age group was 0–10 years old (23%) (Tables 1 and 2).
Table 2.
Number of cases and estimated incidence rate of taeniasis and neurocysticercosis in Colombia, 2009–2013 by sex and age groups, cumulative during 5 years.
| Taeniasis | Neurocysticercosis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Cases | % | Rate (cases/100,000pop) | Cases | % | Rate (cases/100,000pop) | |
| Sex | ||||||
| Male | 544 | 62.96 | 1.15 | 1163 | 55.67 | 2.47 |
| Female | 320 | 37.04 | 0.68 | 926 | 44.33 | 1.97 |
| Age Group (years-old) | ||||||
| < 10 | 184 | 21.30 | 0.39 | 148 | 7.08 | 0.31 |
| 10–19 | 108 | 12.50 | 0.23 | 265 | 12.69 | 0.56 |
| 20–29 | 160 | 18.52 | 0.34 | 334 | 15.99 | 0.71 |
| 30–39 | 152 | 17.59 | 0.32 | 379 | 18.14 | 0.80 |
| 40–49 | 120 | 13.89 | 0.25 | 428 | 20.49 | 0.91 |
| 50–59 | 77 | 8.91 | 0.16 | 300 | 14.36 | 0.64 |
| 60–69 | 41 | 4.75 | 0.09 | 143 | 6.85 | 0.30 |
| 70–79 | 18 | 2.08 | 0.04 | 76 | 3.64 | 0.16 |
| ≥80 | 5 | 0.58 | 0.01 | 16 | 0.77 | 0.03 |
With regard to the systemic presence of infection due to T. solium cysticerci, neurocysticercosis (NCC), with a cumulative incidence of 4.43 cases/100,000 pop, was the most frequent; ocular cysticercosis showed a rate incidence of 0.19 cases/100,000 pop (Tables 1 and 2). NCC reduced from 1.04 cases/100,000 pop (in 2009) to 0.49 cases/100,000 pop (in 2013).
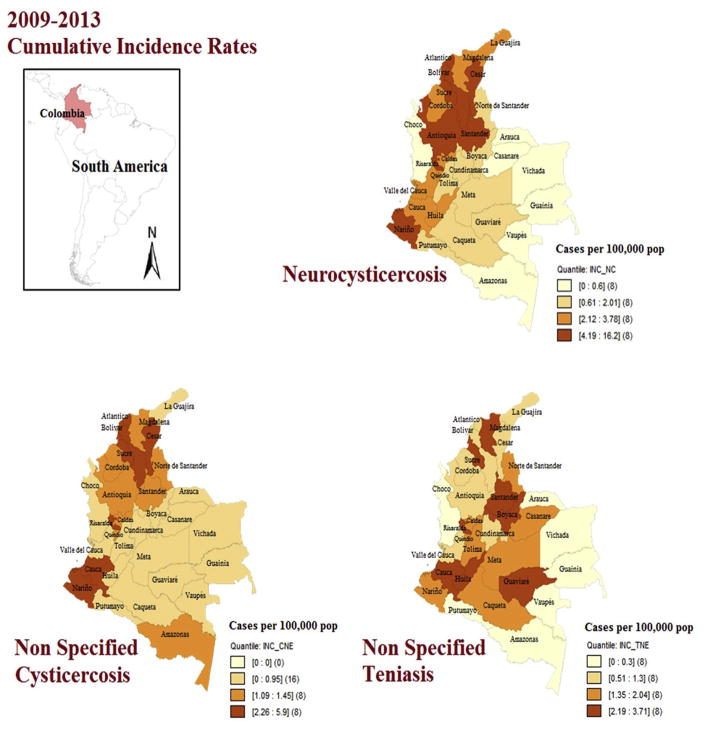
About NCC cases, 55.7% were female and the most affected age range was 40–49 years old in both sexes. Cases were reported in 28 departments. Antioquia reported most cases (375), however the departments with the highest accumulated incidence rates were Bolivar (16.2 cases/100,000 pop), Atlántico (10.9 cases/100,000 pop), Nariño (10.3 cases/100,000 pop), Sucre (9 cases/100,000 pop) and Antioquia (6 cases/100,000 pop) (Figs. 2 and 3).
Fig. 2.
Cumulative incidence rates for neurocysticercosis, non-specified cysticercosis and non-specified taeniasis in Colombia, 2009–2013 (by quartiles, Geo Da 1.8®).
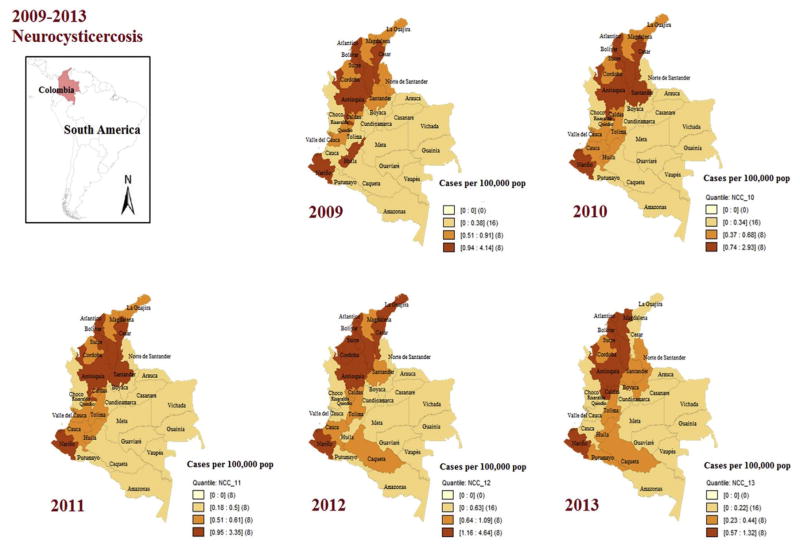
Fig. 3.
Annual incidence rates for neurocysticercosis in Colombia, 2009–2013 (by quartiles, Geo Da 1.8®).
At the Capital, Bogotá the incidence was 2.6 cases/100,000 pop. In the municipalities (third territory level) the highest incidence of NCC was at El Tablón de Gómez (60.3 cases/100,000 pop) in Nariño department; as well in San Francisco (52.3 cases/100,000 pop), Sabanalarga (48.8 cases/100,000 pop) and Montebello (44.6 cases/100,000 pop) in Antioquia department (Figs. 2 and 3).
GIS-based maps facilitated the observation that taeniasis is mainly distributed in the eastern lowland departments of Colombia and the southwestern area (Fig. 2), as well that NCC in northern and Caribbean coastal territories (Fig. 2), also that those departments, although still are reporting cases in 2013, were in significant reductions in the incidence rates since 2009 (Fig. 3).
4. Discussion
In Colombia, the incidence of taeniasis and cysticercosis indicates that these parasitic infections are still a health problem, although in reduction, from 1.9 cases/100,000pop in 2009 to 0.8 in 2013 (also in NCC from 1.04 to 0.49 in the same period). It is important to consider that during the study period (2009–2013), social conditions in the country evidently improved, moving from a Human Development Index of 0.695 in 2009 to 0.720 in 2013 (currently 0.727, 2017). Water supply and sanitation in the country also positively changed in many forms over the last years. Between 1990 and 2010, access to better sanitation increased from 67% to 82%, and access to clean water increased from 89% to 94%. These factors, associated to more surveillance and education regarding food safety would explain the reduction in the disease incidence. It is relevant to mention that data used in this study were not collected in an active survey, or active epidemiological surveillance, that would underestimate the real incidence of taeniasis and cysticercosis, the findings are consistent with previous small survey studies as well as with expected geographical distribution, e.g. in Bolivar, Antioquia, Boyacá, where previous reports identified these areas as endemic [22–26].
Official data and those [22–26] would suggest that in those areas, travel medicine practitioners and clinicians dealing with turists should be instructed regarding safe food consumption, hand washing and other sanitary measures in order to avoid taeniasis and cysticercosis. Medellin (Antioquia) and Villa de Leyva (Boyacá), a colonial city, have become among the most important touristic areas visited by international travelers with the consequent implications mentioned above. However, Bolivar, with its capital Cartagena de Indias, is recognized as the most relevant touristic area of the country, although it is the department with the highest cumulative incidence rate of taeniasis and cysticercosis (16.17 cases/100,000 pop), becoming a risk for travelers and travel medicine practitioners.
Furthermore, so far there are no recent publications regarding the nation wide epidemiology of these parasitic diseases [22–26]. These and other cestodes related diseases that can result in food-borne parasitic infections via the ingestion of infected fish and meats or contaminated vegetation are considered important not only for people living in endemic zones, but also for travelers [12,13]. Through globalization, travelers and immigrants are often at a greater risk for acquiring food-borne parasitic infections or bringing parasites to new locations [36]. In essence, parasites can be considered “travelers” as they are conveyed around in the world in people and animals as well as via meat, fish, and contaminated vegetation [12,13,36]. Appropriate meat inspection and food hygiene practices can go a long way to preventing many of these infections. However, no food can ever be guaranteed to be absolutely safe and travelers, as well as individuals living in countries that are endemic for these parasites, must remain ever vigilant [12,13,36].
Some studies made in Guatemala and Peru have suggested that tapeworm infections are more common among young people than older ones and especially among females [37–39]. Similar findings have been reported in Boyacá, Colombia, where older female are more likely to develop the infection [26]. These data are similar to our results.
Neurocysticercosis is the leading cause of acquired epilepsy in the world and it is considered still endemic in developing countries, in Latin America, Asia, Africa and Oceania [40,41]. Our study evidenced that half of the registered cases of cysticercosis in Colombia were related to NCC and is similar to the cases reported in Lima, Peru, where among 79% of seropositive patients, NCC was diagnosed by computed tomography [42]. Some studies in Colombia have reported positive results for taeniasis among epileptic and non-epileptic patients (12.69%), in Antioquia (capital Medellin), 23.3% of patients with convulsive syndrome in Nariño (capital Pasto) and 13% of patients with neurologic symptoms in Valle del Cauca (capital Cali) [20,23]. These data are concordant with findings reported by Flisser and Correa indicating that taeniasis is geographically associated to cysticercosis in humans and swine [21].
These findings show that complications due to cysticercosis are common and have an impact in public health in term of costs and estimates of disability adjusted life years (DALYs). Moreover the incidence is not yet determined in many regions and countries [43]. A study conducted at Mexico reported that the annual per patient direct cost charged to the social security administration was US$3,109 for patients with a history of hospitalization; also, it reported an annual indirect cost associated to productivity losses and transportation costs for the 224 patients of US$ 17,172 based on the opportunity cost method, US$ 41,841 based on the minimum wage approach, and US$ 44,406 based on the house cleaning approach [44]. In the same way, assessment of life quality in patients with NCC indicates negative changes in physical, mental, and social health [45].
Regarding mortality, a study undertaken in Brazil reported that cysticercosis was identified in 0.016% of all deaths during 2000–2011, and NCC was reported in 91.1% of those deaths due to cysticercosis [46]. The number of deaths due to NCC in our country is unknown, but it supports the idea that we are in risk of death, given the number of cases reported during the study period.
Nowadays, the prevalence of taeniasis and cysticercosis has risen in some developed countries because of the migration of infected persons from endemic areas, mainly those of low-income countries, as it happens in Europe where 74% of the imported cases of cysticercosis are from Latin America. According to a review published in Spain, the general incidence increased because of cases from Latin-America mostly from Ecuador, were almost 4% of the patients who were admitted in a hospital with neurologic symptoms and were diagnosed with NCC [47]. In Asia, there is a similar scenario to Latin America, where most of the cases of cysticercosis are original from the region, except in some countries like Japan and Oman where patients with cysticercosis, in contrast, have a history of traveling to endemic regions [48]. However, intensive work, research and improvement of socioeconomic conditions have demonstrated reduction of taeniasis and NCC, as has been reported in Mexico [5–7,21,41,43–45].
A systematic review recently published, shows that there were different risk factors for T. solium or T. saginata, being the only risk factor for T. saginata advanced age, while for T. solium risks were related to hygienic practices, sharing living space with infected pigs or owning pigs and some sociodemographic factors as being female, younger age and low education level [4,18,19,43]. Compared with our study we found that incidence rate for taeniasis and cysticercosis is higher among young people and men that may be more exposed to risk factors as being around infected pigs, eating outside, and low socio-economic conditions as it is previously mentioned [2,44].
It is important to point the relevance of regional costumes, hygienic and alimentary practices that are significant risk factors related to the persistence and transmission of the pathology [45–53]. Therefore veterinary controls and pig-meat inspection are interventions needed especially in places where meat is just for local consumption [18]. Also, international organizations and national ministries should be aware of prevention mechanisms by improving control measures to stop transmission among humans and pigs including the chance for swine vaccination programs among risk population that have shown to be very effective and provides significant levels of protection [41,49–51].
Finally, as has been shown in other epidemiological studies using GIS-based maps [32,33], in this case, these tools are useful in public health and parasitology for monitoring and observing the spatial distribution of disease and its forms, as well other parameters that can be used and linked in further studies (swine distribution, climate variables, sanitation indicators, socioeconomic variables, etc) [19,41,49]. These maps would be useful for travelers as well as for travel medicine practitioners regarding pre-travel advice and education in high risk areas, and on post-travel consultation when suspected cases of taeniasis and cysticercosis return from Colombia endemic areas. It is also important to consider eosinophilia as key element for the differential diagnosis of cysticercosis as was shown in Gnathostoma and other helminth infections, which have been reported in travelers returning from South America [54–56]. Eosinophilia has been associated to cysticercosis for a long time, and before imaging techniques, the presence of eosinophils was a presumptive diagnosis for cysticercois [56,57]. Maps provide relevant and detailed information to assess the risk as well as to integrate prevention and control strategies and proposals for public health policies, and for joint control of parasitic diseases in the country, such as taeniasis and cysticercosis and follow the example of Mexico in the control of this and other cestode diseases [52,53]. Interestingly the GIS-based map of cumulative incidence of cysticercosis and taeniasis in Colombia (Fig. 2) allowed differentiating the distributions of these diseases along the country and showed it is dissimilar, therefore distinctive control approaches should be targeted depending the location and the disease stage.
Acknowledgments
Funding
Training on GIS for Alfonso J. Rodriguez-Morales was funded by Universidad Tecnológica de Pereira, Pereira, Risaralda, Colombia. This study is part of the project “Desarrollo de Mapas Epidemiológicos a través de Sistemas de Información Geográfica para la Caracterización Geográfica de Enfermedades Infecciosas y Tropicales en el Eje Cafetero de Colombia” (Code 5-15-5 [2015–2018]), Vicerrectoría de Investigaciones, Innovación y Extensión, Universidad Tecnológica de Pereira, Pereira, Risaralda, Colombia.
Authors thank the support of the Dean of the Faculty of Health Sciences, of the Universidad Tecnológica de Pereira, Dr. Rodolfo Adrián Cabrales and the Council of the Faculty for partial travel support to the meetings where this study was presented (Santa Marta, Colombia and Salvador, Bahia, Brazil). Also to the Colombian Association of Infectious Diseases, Chapter Coffee-Triangle Region, for partial travel support.
Footnotes
This study was previously presented in part at the XVI Colombian Congress of Parasitology and Tropical Medicine, Santa Marta, Magdalena, Colombia, October 21–23, 2015, Poster Session; and in part at the XXIV Congress of the Brazilian Society of Parasitology (SBP) and XXIII Latin American Congress of Parasitology (FLAP), Salvador, Bahia, Brazil, October 27–31, 2015 (Poster P-675).
Conflicts of interest
The authors have no conflict of interests to declare.
References
- 1.Aiemjoy K, Gebresillasie S, Stoller NE, Shiferaw A, Tadesse Z, Chanyalew M, et al. Epidemiology of soil-transmitted helminth and intestinal Protozoan infections in preschool-aged children in the amhara region of Ethiopia. Am J Trop Med Hyg. 2017;96:866–72. doi: 10.4269/ajtmh.16-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gall S, Muller I, Walter C, Seelig H, Steenkamp L, Puhse U, et al. Associations between selective attention and soil-transmitted helminth infections, socioeconomic status, and physical fitness in disadvantaged children in Port Elizabeth, South Africa: an observational study. PLoS Neglected Trop Dis. 2017;11:e0005573. doi: 10.1371/journal.pntd.0005573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paige SB, Friant S, Clech L, Malave C, Kemigabo C, Obeti R, et al. Combining footwear with public health iconography to prevent soil-transmitted helminth infections. Am J Trop Med Hyg. 2017;96:205–13. doi: 10.4269/ajtmh.15-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salam N, Azam S. Prevalence and distribution of soil-transmitted helminth infections in India. BMC Publ Health. 2017;17:201. doi: 10.1186/s12889-017-4113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz-Rivera M, Vaughan G, Mendlovic F, Vergara-Castaneda A, Romero-Valdovinos M, Leon-Cabrera S, et al. Cytokine expression at the anchor site in experimental Taenia solium infection in hamsters. Vet Parasitol. 2014;200:299–302. doi: 10.1016/j.vetpar.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Flisser A. State of the art of Taenia solium as compared to Taenia asiatica. Kor J Parasitol. 2013;51:43–9. doi: 10.3347/kjp.2013.51.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salazar AM, Mendlovic F, Cruz-Rivera M, Chavez-Talavera O, Sordo M, Avila G, et al. Genotoxicity induced by Taenia solium and its reduction by immunization with calreticulin in a hamster model of taeniosis. Environ Mol Mutagen. 2013;54:347–53. doi: 10.1002/em.21782. [DOI] [PubMed] [Google Scholar]
- 8.Coyle CM, Mahanty S, Zunt JR, Wallin MT, Cantey PT, White AC, Jr, et al. Neurocysticercosis: neglected but not forgotten. PLoS Neglected Trop Dis. 2012;6:e1500. doi: 10.1371/journal.pntd.0001500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotez PJ. Neglected diseases and poverty in “The Other America”: the greatest health disparity in the United States? PLoS Neglected Trop Dis. 2007;1:e149. doi: 10.1371/journal.pntd.0000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotez PJ, Gurwith M. Europe’s neglected infections of poverty. Int J Infect Dis. 2011;15:e611–e619. doi: 10.1016/j.ijid.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Cantey PT, Coyle CM, Sorvillo FJ, Wilkins PP, Starr MC, Nash TE. Neglected parasitic infections in the United States: cysticercosis. Am J Trop Med Hyg. 2014;90:805–9. doi: 10.4269/ajtmh.13-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davaasuren A, Davaajav A, Ukhnaa B, Purvee A, Unurkhaan S, Luvsan A, et al. Neurocysticercosis: a case study of a Mongolian traveler who visited China and India with an updated review in Asia. Trav Med Infect Dis. 2017;20:31–6. doi: 10.1016/j.tmaid.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Ito A, Budke CM. Culinary delights and travel? A review of zoonotic cestodiases and metacestodiases. Trav Med Infect Dis. 2014;12:582–91. doi: 10.1016/j.tmaid.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Franco-Paredes C, Jones D, Rodriguez-Morales AJ, Santos-Preciado JI. Commentary: improving the health of neglected populations in Latin America. BMC Publ Health. 2007;7:11. doi: 10.1186/1471-2458-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benitez JA, Rodriguez-Morales AJ, Vivas P, Plaz J. Burden of zoonotic diseases in Venezuela during 2004 and 2005. Ann N Y Acad Sci. 2008;1149:315–7. doi: 10.1196/annals.1428.051. [DOI] [PubMed] [Google Scholar]
- 16.Toquero M, Morocoima A, Ferrer E. Seroprevalence and risk factors of cysticercosis in two rural communities in Anzoategui state, Venezuela. Biomedica. 2017;37:66–74. doi: 10.7705/biomedica.v37i2.2841. [DOI] [PubMed] [Google Scholar]
- 17.Noya O, Patarroyo ME, Guzman F, Alarcon de Noya B. Immunodiagnosis of parasitic diseases with synthetic peptides. Curr Protein Pept Sci. 2003;4:299–308. doi: 10.2174/1389203033487153. [DOI] [PubMed] [Google Scholar]
- 18.Zammarchi L, Strohmeyer M, Bartalesi F, Bruno E, Munoz J, Buonfrate D, et al. Epidemiology and management of cysticercosis and Taenia solium taeniasis in Europe, systematic review 1990–2011. PLoS One. 2013;8:e69537. doi: 10.1371/journal.pone.0069537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debacq G, Moyano LM, Garcia HH, Boumediene F, Marin B, Ngoungou EB, et al. Systematic review and meta-analysis estimating association of cysticercosis and neurocysticercosis with epilepsy. PLoS Neglected Trop Dis. 2017;11:e0005153. doi: 10.1371/journal.pntd.0005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serrano JA, Prada FH, Nlicholls RS, Duque S, Prada J, López MC. Determinación de la prevalencia de cisticercosis porcina en cuatro veredas del municipio de Coyaima. Biomedica. 1993;13:129–35. [Google Scholar]
- 21.Flisser A, Correa D. Neurocysticercosis may no longer be a public health problem in Mexico. PLoS Neglected Trop Dis. 2010;4:e831. doi: 10.1371/journal.pntd.0000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palacio LG, Jimenez I, Garcia HH, Jimenez ME, Sanchez JL, Noh J, et al. Neurocysticercosis in persons with epilepsy in Medellin, Colombia. The neuroepidemiological research group of Antioquia. Epilepsia. 1998;39:1334–9. doi: 10.1111/j.1528-1157.1998.tb01333.x. [DOI] [PubMed] [Google Scholar]
- 23.Agudelo Florez P, Palacio LG. Prevalence of Taenia solium antibodies in humans and in pigs in an endemic area of Colombia. Rev Neurol. 2003;36:706–9. [PubMed] [Google Scholar]
- 24.Botero D, Castano S. Treatment of cysticercosis with praziquantel in Colombia. Am J Trop Med Hyg. 1982;31:811–21. doi: 10.4269/ajtmh.1982.31.811. [DOI] [PubMed] [Google Scholar]
- 25.Vaughn JB, Newell KW, Brayton JB, Barth RA, Gracian M. A zoonotic survey in abattoirs in Colombia. Bol Officina Sanit Panam. 1967;63:17–30. [PubMed] [Google Scholar]
- 26.Florez AC, Pastrán SM, Vargas NS, Peña AP, Benavides A, Villarreal A, et al. Cisticercosis en Boyacá, Colombia: estudio de seroprevalencia. Acta Neurol Colomb. 2011;27:9–18. [Google Scholar]
- 27.Strauss RA, Castro JS, Reintjes R, Torres JR. Google dengue trends: an indicator of epidemic behavior. The Venezuelan Case. Int J Med Inf. 2017;104:26–30. doi: 10.1016/j.ijmedinf.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Morales AJ, Paniz-Mondolfi AE. Venezuela’s failure in malaria control. Lancet. 2014;384:663–4. doi: 10.1016/S0140-6736(14)61389-1. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Morales AJ, Paniz-Mondolfi AE. Venezuela: far from the path to dengue and chikungunya control. J Clin Virol. 2015;66:60–1. doi: 10.1016/j.jcv.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Ramirez LM, Giraldo-Pulgarin JY, Agudelo-Marin N, Holguin-Rivera YA, Gomez-Sierra S, Ortiz-Revelo PV, et al. Geographical and occupational aspects of leptospirosis in the coffee-triangle region of Colombia, 2007–2011. Recent Pat Anti-Infect Drug Discov. 2015;10:42–50. doi: 10.2174/1574891x10666150410130425. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Morales AJ, Galindo-Marquez ML, Garcia-Loaiza CJ, Sabogal-Roman JA, Marin-Loaiza S, Ayala AF, et al. Mapping Zika virus disease incidence in Valle del Cauca. Infection. 2017;45:93–102. doi: 10.1007/s15010-016-0948-1. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Morales AJ, Granados-Alvarez S, Escudero-Quintero H, Vera-Polania F, Mondragon-Cardona A, Diaz-Quijano FA, et al. Estimating and mapping the incidence of giardiasis in Colombia, 2009–2013. Int J Infect Dis. 2016;49:204–9. doi: 10.1016/j.ijid.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Morales AJ, Orrego-Acevedo CA, Zambrano-Munoz Y, Garcia-Folleco FJ, Herrera-Giraldo AC, Lozada-Riascos CO. Mapping malaria in municipalities of the coffee triangle region of Colombia using geographic information systems (GIS) J Infect Public Health. 2015;8:603–11. doi: 10.1016/j.jiph.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Morales AJ, Cardenas-Giraldo EV, Montoya-Arias CP, Guerrero-Matituy EA, Bedoya-Arias JE, Ramirez-Jaramillo V, et al. Mapping chikungunya fever in municipalities of one coastal department of Colombia (Sucre) using geographic information system (GIS) during 2014 outbreak: implications for travel advice. Trav Med Infect Dis. 2015;13:256–8. doi: 10.1016/j.tmaid.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Bernal O, Forero JC, Villamil Mdel P, Pino R. Data availability and morbidity profile in Colombia. Rev Panam Salud Públic. 2012;31:181–7. doi: 10.1590/s1020-49892012000300001. [DOI] [PubMed] [Google Scholar]
- 36.Ito A, Wandra T, Li T, Dekumyoy P, Nkouawa A, Okamoto M, et al. The present situation of human taeniases and cysticercosis in Asia. Recent Pat Anti-Infect Drug Discov. 2014;9:173–85. doi: 10.2174/1574891x10666150410125711. [DOI] [PubMed] [Google Scholar]
- 37.Allan JC, Velasquez-Tohom M, Garcia-Noval J, Torres-Alvarez R, Yurrita P, Fletes C, et al. Epidemiology of intestinal taeniasis in four, rural, Guatemalan communities. Ann Trop Med Parasitol. 1996;90:157–65. doi: 10.1080/00034983.1996.11813039. [DOI] [PubMed] [Google Scholar]
- 38.Allan JC, Velasquez-Tohom M, Torres-Alvarez R, Yurrita P, Garcia-Noval J. Field trial of the coproantigen-based diagnosis of Taenia solium taeniasis by enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1996;54:352–6. doi: 10.4269/ajtmh.1996.54.352. [DOI] [PubMed] [Google Scholar]
- 39.Garcia HH, Gilman RH, Gonzalez AE, Verastegui M, Rodriguez S, Gavidia C, et al. Hyperendemic human and porcine Taenia solium infection in Peru. Am J Trop Med Hyg. 2003;68:268–75. [PubMed] [Google Scholar]
- 40.Garcia HH, Gonzalez AE, Evans CA, Gilman RH. Cysticercosis working group in P. Taenia solium cysticercosis. Lancet. 2003;362:547–56. doi: 10.1016/S0140-6736(03)14117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flisser A, Rodriguez-Canul R, Willingham AL., 3rd Control of the taeniosis/cysticercosis complex: future developments. Vet Parasitol. 2006;139:283–92. doi: 10.1016/j.vetpar.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 42.Bern C, Garcia HH, Evans C, Gonzalez AE, Verastegui M, Tsang VC, et al. Magnitude of the disease burden from neurocysticercosis in a developing country. Clin Infect Dis. 1999;29:1203–9. doi: 10.1086/313470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhattarai R, Budke CM, Carabin H, Proano JV, Flores-Rivera J, Corona T, et al. Estimating the non-monetary burden of neurocysticercosis in Mexico. PLoS Neglected Trop Dis. 2012;6:e1521. doi: 10.1371/journal.pntd.0001521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhattarai R, Carabin H, Proano JV, Flores-Rivera J, Corona T, Flisser A, et al. Cost of neurocysticercosis patients treated in two referral hospitals in Mexico City, Mexico. Trop Med Int Health. 2015;20:1108–19. doi: 10.1111/tmi.12497. [DOI] [PubMed] [Google Scholar]
- 45.Bhattarai R, Budke CM, Carabin H, Proano JV, Flores-Rivera J, Corona T, et al. Quality of life in patients with neurocysticercosis in Mexico. Am J Trop Med Hyg. 2011;84:782–6. doi: 10.4269/ajtmh.2011.10-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martins-Melo FR, Ramos AN, Jr, Cavalcanti MG, Alencar CH, Heukelbach J. Neurocysticercosis-related mortality in Brazil, 2000–2011: epidemiology of a neglected neurologic cause of death. Acta Trop. 2016;153:128–36. doi: 10.1016/j.actatropica.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Rojo Marcos G, Cuadros González J, Arranz Caso A. Enfermedades infecciosas importadas en España. Med Clínica. 2008;131:540–50. doi: 10.1157/13127586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajshekhar V, Joshi DD, Doanh NQ, van De N, Xiaonong Z. Taenia solium taeniosis/cysticercosis in Asia: epidemiology, impact and issues. Acta Trop. 2003;87:53–60. doi: 10.1016/s0001-706x(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 49.Braae UC, Devleesschauwer B, Gabriel S, Dorny P, Speybroeck N, Magnussen P, et al. CystiSim - an agent-based model for Taenia solium transmission and control. PLoS Neglected Trop Dis. 2016;10:e0005184. doi: 10.1371/journal.pntd.0005184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flisser A, Gauci CG, Zoli A, Martinez-Ocana J, Garza-Rodriguez A, Dominguez-Alpizar JL, et al. Induction of protection against porcine cysticercosis by vaccination with recombinant oncosphere antigens. Infect Immun. 2004;72:5292–7. doi: 10.1128/IAI.72.9.5292-5297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lightowlers MW, Donadeu M. Designing a minimal intervention strategy to control Taenia solium. Trends Parasitol. 2017;33:426–34. doi: 10.1016/j.pt.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Flisser Maravilla P, Mata-Miranda P, Martinez-Hernandez F, Rodriguez-Morales AJ, editors. Current topics in echinococcosis. Croatia: InTech; Sep, 2015. http://dx.doi.org/10.5772/60868 Available at: https://www.intechopen.com/books/current-topics-in-echinococcosis/echinococcosis-in-mexico-a-story-worth-sharing. [Google Scholar]
- 53.Rodriguez-Morales AJ, Calvo-Betancourt LS, Alarcón-Olave C, Bolívar-Mejía A. Echinococcosis in Colombia — a neglected zoonosis? In: Rodriguez-Morales AJ, editor. Current topics in echinococcosis. Croatia: InTech; Sep, 2015. http://dx.doi.org/10.5772/60731 Available at: http://www.intechopen.com/books/current-topics-in-echinococcosis/echinococcosis-in-colombia-a-neglected-zoonosis. [Google Scholar]
- 54.Theunissen C, Bottieau E, Van Gompel A, Siozopoulou V, Bradbury RS. Presumptive gnathostoma binucleatum-infection in a Belgian traveler returning from South America. Trav Med Infect Dis. 2016;14(2):170–1. doi: 10.1016/j.tmaid.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Norman FF, López-Vélez R. Immigration, helminths and eosinophilia: a complex triad. Trav Med Infect Dis. 2015;13(4):283–4. doi: 10.1016/j.tmaid.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Salas-Coronas J, Cabezas-Fernández MT, Vázquez-Villegas J, Soriano-Pérez MJ, Lozano-Serrano AB, Pérez-Camacho I, et al. Evaluation of eosinophilia in immigrants in Southern Spain using tailored screening and treatment protocols: a prospective study. Trav Med Infect Dis. 2015;13(4):315–21. doi: 10.1016/j.tmaid.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Ali-Khan Z, Siboo R, Meerovitch E, Faubert G, Faucher MG. Cysticercus racemosus in an eosinophilic phlegmon in the brain. Trans Roy Soc Trop Med Hyg. 1981;75(6):774–9. doi: 10.1016/0035-9203(81)90408-9. [DOI] [PubMed] [Google Scholar]